
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 23 February 2023
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1043696
 Joud Mohammed Alkhalifah1*†
Joud Mohammed Alkhalifah1*† Ahad Al Seraihi2
Ahad Al Seraihi2 Jaffar A. Al-Tawfiq3,4,5†
Jaffar A. Al-Tawfiq3,4,5† Badr Fadhel Alshehri1
Badr Fadhel Alshehri1 Alhanouf Hani Alhaluli1
Alhanouf Hani Alhaluli1 Naif Mansour Alsulais1
Naif Mansour Alsulais1 Mohammed Mesfer Alessa1
Mohammed Mesfer Alessa1 Waleed Seddiq6,7
Waleed Seddiq6,7 Thamer Aljeri2
Thamer Aljeri2 Mohammad Hassan Qahtani2
Mohammad Hassan Qahtani2 Mazin Barry8,9†
Mazin Barry8,9† Maram Al-Otaiby2,10*
Maram Al-Otaiby2,10*Background: Vaccination against coronavirus disease 2019 (COVID-19) is the most effective way to end the pandemic. Any development of adverse events (AEs) from various vaccines should be reported. We therefore aimed to explore major and minor AEs among vaccinated individuals in Saudi Arabia.
Methods: This is a nationwide report based on the Saudi Arabian Ministry of Health (MOH) registry. It included those who received COVID-19 vaccines from 17th December 2020 to 31st December 2021. The study included spontaneous self-reported adverse effects to COVID-19 vaccines where the study participants used a governmental mobile app (Sehhaty) to report their AEs following vaccination using a checklist option that included a selection of side-effects. The primary outcome was to determine AEs reported within 14 days of vaccination which included injection site itching, pain, reaction, redness, swelling, anxiety, dizziness, fever, headache, hoarseness, itchiness, loss of consciousness, nausea, heartburn, sleep disruption, fatigue, seizures, anaphylaxis, shortness of breath, wheezing, swelling of lips, face, and throat, loss of consciousness, and admissions into the intensive care unit (ICU).
Results: The study included a total number of 28,031 individuals who reported 71,480 adverse events (AEs); which were further classified into minor and major adverse events including ICU admissions post vaccination. Of the reported AEs, 38,309 (53. 6%) side-effects were reported following Pfizer-BioNTech, 32,223 (45%) following Oxford-AstraZeneca, and 948 (1.3%) following Moderna. The following reported AEs were statistically significant between the different vaccine types: shortness of breath\difficulty of breathing, dizziness, fever above 39°C, headache, hoarseness, injection site reactions, itchiness, nausea, sleep disruption, fatigue, wheezing, swelling of lips/face and\or throat, and loss of consciousness (p-value < 0.05). Fever and seizure were the only statistically significant AEs amongst the number of vaccine doses received (p-value < 0.05). Ten ICU admissions were reported in the 14 days observation period post-COVID-19 vaccination with the following diagnoses: acute myocardial infarction, pneumonia, atherosclerosis, acute respiratory failure, intracranial hemorrhage, grand mal seizure, Guillain-Barré syndrome, abnormal blood gas levels, and septic shock.
Conclusion: This study demonstrated that the most prevalent SARS-CoV-2 vaccine side-effects among adults in Saudi Arabia were mild in nature. This information will help reduce vaccine hesitancy and encourage further mass vaccination to combat the COVID-19 pandemic, especially as booster doses are now available. Further studies are warranted to obtain a better understanding of the association between risk factors and the experiencing of side-effects post vaccination.
The coronavirus disease (COVID-19) pandemic has caused a serious threat and catastrophes worldwide. As of May 20, 2022, the virus had caused more than 521 million confirmed cases, with 6 million deaths worldwide as reported by the World Health Organization (WHO) (1). In Saudi Arabia, however, COVID-19 has infected 762,575 people and resulted in 9,128 deaths, according to the Saudi Ministry of Health (MOH) COVID-19 dashboards.
To combat the pandemic, multiple vaccines have been developed. The most used vaccines include Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), and Oxford–AstraZeneca (ChAdOx1). As of May 20, 2022, more than 11 billion vaccine doses have been administered globally (1). Post-vaccination adverse events (AEs) reporting and safety monitoring are recommended by multiple institutions worldwide, especially during a pandemic (2).
The Kingdom of Saudi Arabia (KSA) has implemented multiple strategies to mitigate the impact of COVID-19 pandemic and was indeed one of the first countries to launch a nationwide mass vaccination campaign against COVID-19 (3–5). Indeed, The Saudi Food and Drug Authority (SFDA) granted emergency use authorization for the use of Pfizer-BioNTech COVID-19 vaccine on the same day the Phase III trial results were published (6, 7). Six days later, on December 17, 2020, Saudi Arabia received its first shipments of BNT162b2 and immediately started the mass vaccination campaign based on a phased approach to vaccinate the adult citizens and residents free of charge (3). Subsequently on February 19, 2021, Saudi Arabia authorized the use of AstraZeneca-ChAdOx1 vaccine for emergency use, which was then followed by the approval of Moderna-mRNA-1273 vaccine on July 09, 2021. The vaccine distribution was based on high-risk categories identified by Saudi MOH, as follows; the first phase targeted high risk groups which included citizens over 65 years old, professionals who are most vulnerable to infection, obese people with a BMI >40 kg/m2, immunocompromised citizens and citizens with two or more of the following chronic conditions: asthma, diabetes, chronic kidney disease, chronic heart disease, including coronary artery disease, and chronic obstructive pulmonary disease, and those with a previous stroke. The second phase targeted citizens over 50 years old, and those with one of the chronic illnesses mentioned above. The third phase included all citizens and residents 16 years or older that wish to be vaccinated (8). The estimated period for vaccination completion was July 2021 for phase one and two, and September 2021 for phase three (5). On June 27, 2021, the SFDA approved Pfizer-BioNTech COVID-19 vaccine for Saudi citizens and residents between 12 and 18 years old, and the Saudi MOH started campaigns to encourage adolescents to register for vaccination (9).
In order to combat the SARS-CoV-2 pandemic, the Kingdom has developed their digital health system through multiple applications that target different facets of managing this ongoing pandemic, one of these applications named “Sehhaty” was created to manage to aid users in managing their COVID-19 related services, including booking vaccination appointments, COVID-19 testing appointments, viewing testing results, reporting any side-effects and many other services (10). The WHO reported that as of May 14, 2022, more than 64 million vaccine doses were administered in KSA (1).
Vaccine hesitancy is one of the main barriers of vaccine uptake. One study from KSA showed that one-third of healthcare workers (HCWs) received the COVID-19 vaccine during the first month of launching the vaccine campaign (11). Another study of 23,000 surveyed HCWs in KSA showed that the most common reason for vaccine hesitancy was fear of potential AE as reported by 60% of respondents (12). Few studies reported such occurrences of AEs following COVID-19 in KSA and these studies were limited to single centers and to specific types of vaccines (13–15). However, this study represented the first national report of vaccine related AEs across the Kingdom using a national database in relation to the different vaccine types and different number of vaccine doses.
This is a retrospective, population-based, observational study examining the patterns of AEs secondary to COVID-19 vaccines as reported by vaccine recipients over the period from 17th December 2020 to 31st December 2021 to the Saudi MOH. The self-reported data of adverse vaccine side-effects were obtained from the Sehhaty mobile application and integrated with the national vaccination registry (NVR), and the health electronic surveillance network (HESN) databases. Furthermore, the ICU patient data were obtained from a national database called Ehalat and were further integrated with the aforementioned databases. Data privacy and confidentiality were maintained throughout the study. Informed consent was waived due to the retrospective and observational nature of the study.
The Sehhaty application was launched under the umbrella of digital transformation initiatives by the Saudi MOH aiming to provide access to several healthcare services for individuals in the Kingdom. These services included consultations, PCR testing for SARS-CoV-2, booking COVID-19 vaccine appointments, and reporting COVID-19 vaccines side-effects through the option of manual entering and/or drop box option selection (16). Indeed, the Sehhaty application offers a checkbox option to report symptoms for the received vaccine. The following symptoms were available for users to select from in the following order: injection site itching, wheezing, fatigue, hoarseness, low fever, anxiety, fever above or below 39°C, heartburn, injection site swelling, headache, loss of consciousness, nausea, injection site redness, injection site pain, itchiness other than injection site, seizure, dizziness, difficulty or shortness of breath, sleep disruption, swelling of lips, face, and throat, and intensive care unit (ICU) admission. The study participants were provided with a checkbox list that included all of the aforementioned side-effects and were given the option to select from the list if they experienced any of them. Another helpful feature was the registration of the date and the start of symptoms, which allowed for filtering and limiting the studied side-effects to be within a window of 14 days post-vaccination (7). Participants' demographics data were collected and included: age, gender, nationality, region, COVID-19 vaccine type, the number of vaccine doses, date of vaccination, and date of ICU admission.
The study included all vaccinated female and male individuals in Saudi Arabia of any ethnicity, who were 12–96 years of age and spontaneously self-reported their side-effects through the Sehhaty app after receiving at least one dose of the three available COVID-19 vaccines: Pfizer–BioNTech (BNT162b2), Oxford–AstraZeneca (ChAdOx1-S), and/or Moderna (mRNA-1273) within 14 days of receiving COVID-19 vaccination over the period from 17th December 2020 to 31st December 2021 (7). The ICU records were considered post vaccine events if they happened within 14 days post COVID-19 vaccination. The exclusion criteria entailed individuals with wrong or missing data entry and/or misidentified unique IDs, individuals who are under 12 years or above 96 years old, those who did not receive COVID-19 vaccination, or who received COVID-19 vaccines other than Pfizer–BioNTech, Oxford–AstraZeneca or Moderna vaccines, and those who reported their side-effects outside of the 14-day window. We excluded those with pre-existing comorbidities and/or autoimmune diseases, as they could confound the results of the self-reported vaccines adverse events. The primary objective was to determine the patterns of minor side-effects: injection site (itchiness, pain, reaction, redness, swelling, and other), anxiety, dizziness, fever above or below 39°C, headache, hoarseness, itchiness other than injection site, loss of consciousness, nausea, heartburn, sleep disruption, and fatigue. In addition, we examined the pattern of reported major COVID-19 vaccine side-effects: seizures, shortness of breath, wheezing, swelling of lips, face, and throat, ICU admission, and loss of consciousness. The AEs were classified as local, systemic, or allergic, or as mild or moderate.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 26.0 statistical software tool (IBM Statistics, Armnok, NJ, USA). Data were represented using descriptive statistics including (frequency, percentage tables, means, and standard deviations (SD), accordingly. The Chi-square test was used to determine statistically significant differences between vaccine types and number of doses, and the major and minor vaccine side-effects. Logistic regression analyses with the determinants of p-value and 95% confidence intervals (CIs) were then performed to compare demographic variables with the pattern of vaccine minor and/or major adverse events. A p-value of < 0.05 was used to report the statistical significance.
This study was ethically approved by the Institutional Review Board (IRB) committee from the General Directorate for Research and Studies (GDRS) (IRB No. 21-50M) at the Saudi MOH, KSA, with a waiver of the need for informed consent.
The total number of COVID-19 vaccine recipients who spontaneously reported any AE was 28,031 between 17 December 2020 and 31 December 2021 with an estimated reported incidence of 4.2 AEs per 10,000 doses. Table 1 represents the baseline demographic characteristics of the study participants in which the majority (n = 24,683; 88.1%) were Saudi nationals. A slightly higher proportion of the participants were females (54.3%; n = 15,231) compared with (45.7%; n = 12,800) males. Of the participants, the highest percentage of respondents was 8,791 (31.4%) from 30-39 years of age, whilst participants between 90 and 96 years and between 12 and 19 years were 20 (0.07%), and 1,043 (3.7%), respectively. The geographic distribution of the study participants varied between the 13 Saudi regions; 38.6% were from Riyadh followed by Makkah Al-Mukarramah (27.9%), and the Eastern Region (15.5%). Of the different COVID-19 vaccines, 15,111 (53.9%) received the Pfizer-BioNTech vaccine, and 12,601 (45%) received the Oxford–AstraZeneca vaccine, whilst only 319 (1.1%) received the Moderna vaccine. Of the included respondents, 21,126 (75.4%) received the first vaccine dose, 6,822 (24.3%) received the second dose, and only 83 (0.3%) reported receiving the third vaccine dose during the study period (Table 1).
The study included a total number of 28,031 individuals who reported 71,480 AEs. Of the reported AEs, 38,309 (53.6%), 32,223 (45%), and 948 (1.3%) were after receiving the Pfizer-BioNTech, Oxford-AstraZeneca, and Moderna vaccines, respectively (Table 2). The most common reported AEs after Pfizer-BioNTech vaccine were fatigue 7,028 (18.35%), others 5,838 (15.24%), fever < 39°C 5,151 (13.45%), and headache 5,081 (13.26%). For Astra-Zeneca vaccine, the following were the most commonly reported AEs: fatigue 6,256 (19.41%), others 5,186 (16.09%), fever < 39°C 4,431 (13.75%), and injection site reactions 4,063 (12.61%). For the Moderna vaccine, however, the following were the most commonly reported AEs: others 200 (21.1%), fatigue 164 (17.3%), injection site reactions 119 (12.55%), and fever < 39°C 113 (11.92%). The following reported AEs were statistically significant between the different vaccine types received: shortness of breath\difficulty of breathing, dizziness, fever above 39°C headache, hoarseness, injection site reactions, itchiness other than injection site, nausea, sleep disruption, fatigue, wheezing, swelling of lips/face and\or throat, and loss of consciousness (p-value < 0.05). The rate of reported major AEs were as follows: 300 (0.42%), 270 (0.83%), and 13 (1.37%) after receiving the Pfizer-BioNTech, Oxford-AstraZeneca, and Moderna vaccines, respectively (Table 2). The rate of intensive care admission after vaccination was only 10 (0.03%) of all cases.
The reported AEs in relation to the doses were as follows: 53,910 (75.42%), 17,369 (24.30%) and 201 (0.28%) after receiving the first, second, and third vaccine doses, respectively (Table 3). The most common AEs reported after receiving the first dose were: fatigue 10,174 (18.87%), others 8,451(15.68%), fever < 39°C 7,395 (13.72%), headache 6,965 (12.92%), and injection site reactions 6,735 (12.49%). For the second dose, the most common AEs were fatigue 3,233(18.61%), others 2,745 (15.8%), fever < 39°C 2,271 (13.08%), headache 2,245(12.93%), and injection site reactions 2,143 (12.34%). For the third dose, the most common AEs were: fatigue 41 (20.4%), fever < 39°C 29 (14.43%), other AEs 28 (13.93%) and headache 25 (12.44%). The occurrences of severe AEs were reported as follows: 444 (0.82%), 138 (0.79%), and 1 (0.50%), after receiving the first, second, and third vaccine doses, respectively. Fever and epileptic seizure were the only statistically significant AEs amongst the number of vaccine doses received (p-value < 0.05), whereby the occurrence of these vaccine side-effects was higher in the first dose recipients, followed by the second and then third doses. However, seizure was a very rare occurrence and constituted only 0.08% of all reported AEs (Table 3).
A logistic regression analysis was performed to explore the associations between AEs and the following variables: age, gender, vaccine dosage, and vaccine type. Interestingly, the majority of these significantly associated vaccine side-effects were minor. The following AEs were significantly associated with age: injection site pain (P < 0.001), injection site redness (P = 0.008), headache (P < 0.001), fatigue (P = 0.001), and sleep disturbances (P = 0.029). Whilst itching was significantly associated with age (P = 0.034), Pfizer-BioNTech (P < 0.001), and Oxford-AstraZeneca (P = 0.009), low fever was significantly associated with age (P < 0.001), gender (P = 0.019), and second vaccine dose (P = 0.039) (Table 4).
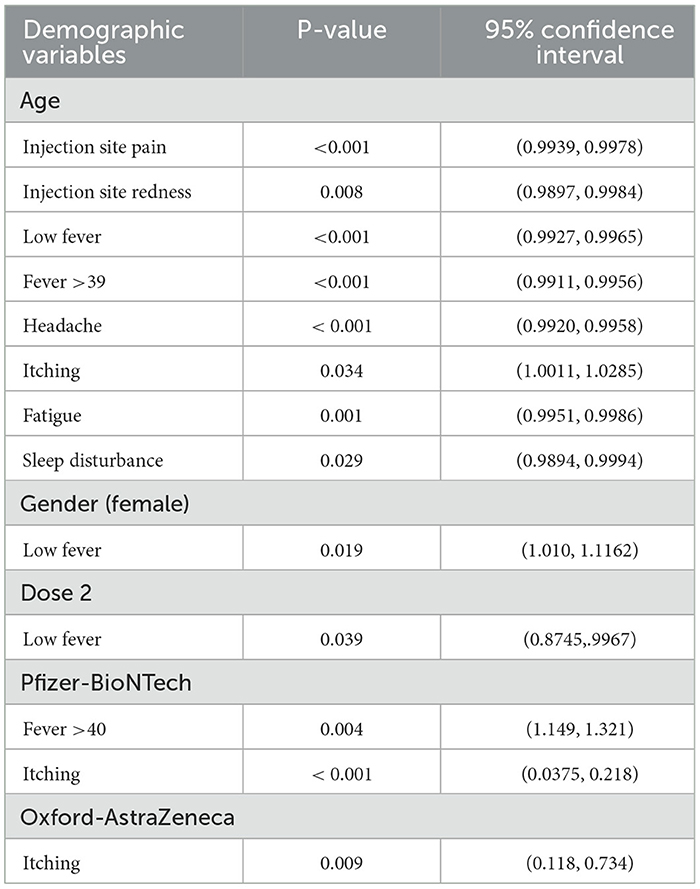
Table 4. Logistic regression analysis for associations between vaccine minor and/or major adverse events and demographic variables.
There were 10 ICU admissions post COVID-19 vaccination (five males and five females) with a mean age of 38.4 ± 13.8 years of age. All cases required mechanical ventilation. Three patients had comorbidities. The mean of days between vaccination and ICU admission was 8.2 ±3.8 days.
As shown in Table 5, five ICU cases were reported after receiving the first dose of Oxford-AstraZeneca. Two cases had COVID-19 pneumonia 8–14 days after vaccination. One case of acute myocardial infarction in a previously healthy 32 years old male 4 days following the first dose of Oxford-AstraZeneca. One case experienced acute respiratory failure 11 days after vaccine administration. One case of Guillain-Barré syndrome was reported in a previously healthy 39 years old male 10 days following his first dose of Pfizer-BioNTech vaccine. A case of septic shock was reported in a 60-year male with a coronary arterial disease, 5 days following the first dose of Pfizer-BioNTech. A case of grand mal seizure was reported 7 days following the second dose of Pfizer-BioNTech. One patient aged 28 years suffered from intracranial hemorrhage 12 days following her second dose of Pfizer-BioNTech (Table 5).
In this study, we examined the spectrum and occurrence of AEs in relation to COVID-19 vaccine types and number of doses. The majority of reported AEs were mild and were not associated with hospitalization. The COVID-19 pandemic has caused a global crisis on all fronts including health, society, and economy. To combat the pandemic that has impacted the daily lives of many people, the FDA has granted emergency use authorization for Pfizer-BioNTech, Oxford-AstraZeneca, and Moderna vaccines. Indeed, with a record that beats the fastest vaccine that had previously been developed and approved for mumps virus in the 1960's which took four years, this in turn has raised some concerns regarding the safety and efficacy of these COVID-19 vaccines and the potential adverse events (12, 15, 17). According to the Saudi MOH databases, up to 23.18 million individuals completed the initial COVID-19 vaccination protocol (two vaccine doses) and 2.7 million individuals completed the third vaccine “booster” doses during the study period. The findings from this retrospective nationwide study, with a sample size of 28,031 participants who self-reported their side-effects through digital governmental platforms, represent important insights at the times of modern medicine. Indeed, this study emphasizes the importance of patient-centered care and the employment of technology in telemedicine which can facilitate health-care providers and patients alike.
Notably, the results of this study align with the low occurrence of major AEs after COVID-19 vaccination (18–23). The reported AEs in relation to vaccine doses were 53,910 (75.42%), 17,369 (24.30%), and 201 (0.28%) after the first, second, and third vaccine dose, respectively. The low number of AEs reported following the third dose might be associated with a lower uptake for the third dose. However, the higher rate of reporting of AEs after the first dose may reflect the type of the vaccine administered or could be related to reporting bias as people may tend to be more motivated to report AEs initially with the increase in vaccine uptake and safety. In one study, the rate of AEs was lower with heterologous vaccination than homologous vaccination (24).
There had been no reported anaphylaxis after COVID-19 vaccines in this study. Shimabukuro et al. delineated that 21 cases of anaphylaxis were reported in the United States following the first dose of Pfizer-BioNTech vaccine in December 2020 with a rate of 11.1 cases per million vaccine doses administered (25). In a systematic review, the occurrence of anaphylactic reaction after Pfizer-BioNTech and Moderna vaccines was associated with an overall pooled prevalence estimate of 5.0 (95% CI 2.9 to 7.2, I2 = 81%, p = < 0.0001) (26). Furthermore, Cirillo et al. stated that various vaccine side-effects affecting the orofacial (mouth and face area) were reported after receiving both Pfizer-BioNTech and Oxford-AstraZeneca vaccines. The authors stated that these events were rare, which included acute peripheral facial paralysis (Bell's palsy), facial swelling, and swelling of the lips, face or tongue associated with anaphylaxis (27).
In our study, two of the major reasons for ICU admissions following COVID-19 vaccination were the development of acute myocardial infarction in one patient and Guillain-Barré in another patient who received AstraZeneca and Pfizer-BioNTech vaccines respectively. Indeed, the occurrence of myocardial infarction and acute myocarditis following COVID-19 vaccination had been reported in several case reports and case series following different types of COVID-19 vaccines (28–32). In a case-control study, the incidence of myocardial infarction and stroke was 6.18 vs. 5.49 per 1,000,000 person-days in those who were never vaccinated vs. fully vaccinated individuals (33). Another study showed no significant increased risk of myocardial infarction in the first 14 days following COVID-19 vaccine (34). The occurrence of Guillain-Barré syndrome after COVID-19 vaccination had been reported in case series and reports (35, 36). In a head-to-head comparison, the adjusted relative risk was 20.56 (95% CI, 6.94–64.66) in Ad.26.COV2.S vs. mRNA vaccines (36). One study showed that the occurrence of Guillain-Barré syndrome 1–21 days after COVID-19 vaccination with mRNA vaccines was 1.3 per 100,000 person-years. This rate was not different from the usual rate in the community (37). Finally, two ICU cases in our study developed COVID-19 pneumonia 8–14 days after vaccination. It is worth noting here that none of the vaccines administered were live-attenuated, and therefore, it would be medically implausible to assume that the vaccines induced COVID-19.
To the best of our knowledge, this is the first nationwide registry-based study in KSA that provided insights into the patterns and spectrum of COVID-19 vaccine side-effects derived from self-reported data. Indeed, the present study has constituted a relatively large sample size (>28,000 study participants) who reported vaccine side-effects through the Sehhaty mobile application. However, the bias of self-reporting could not be ruled out. In addition, the majority of the participants were apparently healthy as only a small number has reported comorbidities. This may bias the spectrum of reported AEs. Moreover, the data was based on the use of an online mobile application, and so the collected data may not be representative of the whole population in KSA, especially in regions with limited internet connection. Also, the reported vaccine side-effects were based on a limited checkbox selection questionnaire which was not verified by a healthcare professional.
In conclusion, our findings revealed that the most prevalent COVID-19 vaccine side-effects in Saudi Arabia were mild in their severity; adverse events to COVID-19 vaccines at any dose were extremely rare and only 0.03% required intensive care admission. Indeed, this information will help reduce vaccine hesitancy amongst the wider community and encourage further mass vaccination and continued advocacy for vaccination to combat and end the COVID-19 pandemic, especially as second booster doses are now available. Further studies are warranted to obtain a better understanding of the association between risk factors and the experiencing of side-effects post-vaccination.
The datasets presented in this article are not readily available because restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from the Saudi MOH. Requests to access the datasets should be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) committee from the General Directorate for Research and Studies (GDRS) (IRB No. 21-50M) at the Saudi Ministry of Health (MOH), Riyadh, Saudi Arabia. The Ethics Committee waived the requirement of written informed consent for participation.
JMA and AA: conceptualization, data collection, design methodology and analysis, manuscript writing, and editing. JAA: conceptualization, manuscript writing, data analysis, and interpretation. BFA: data collection, analysis, methodology, and manuscript writing. AHA, NMA, MMA, and WS: data collection, analysis, and manuscript editing. TA and MHQ: data collection and interpretation. MB and MA: conceptualization, manuscript editing, methodology, and supervision. All authors reviewed and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. WHO Coronavirus Disease Dashboard. WhoInt. (2022). Available online at: https://covid19.who.int/ (accessed February 2, 2022).
2. Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. (2021) 384:643–9. doi: 10.1056/nejmra2035343
3. Barry M, Bahammam AS. COVID-19 vaccine in the Kingdom of Saudi Arabia: a true operation warp speed. J Nat Sci Med. (2021) 4:92–8. doi: 10.4103/jnsm.jnsm_8_21
4. Barry M, Ghonem L, Alsharidi A, Alanazi A, Alotaibi NH, Al-shahrani FS, et al. Coronavirus disease-2019 pandemic in the kingdom of Saudi Arabia: mitigation measures and hospital preparedness. J Nat Sci Med. (2020) 3:155–8. doi: 10.4103/JNSM.JNSM_29_20
5. Assiri A, Al-Tawfiq JA, Alkhalifa M, Al Duhailan H, Al Qahtani S, Dawas RA, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Travel Med Infect Dis. (2021) 43:102119. doi: 10.1016/j.tmaid.2021.102119
6. Interim guidelines for the use of SARS-CoV-2 vaccine. Saudi Public Health Authority. (2021). Available online at: https://covid19.cdc.gov.sa/professionals-health-workers/interim-guidelines-for-the-use-of-SARS-CoV-2-vaccine/ (accessed November 27, 2022).
7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/nejmoa2034577
8. MOH. MOH: COVID-19 vaccine to be received in phases. Ministry Of Health Saudi Arabia. Ministry of Health. (2020). Available online at: https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2020-12-13-007.aspx (accessed October 9, 2022).
9. MOH. MOH begins vaccinating 12–18 age group with Pfizer vaccine. Ministry Of Health Saudi Arabia. Ministry of Health. (2021). Available online at: https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2021-06-27-008.aspx (accessed October 9, 2022).
10. Alkhalifah JM, Seddiq W, Alshehri BF, Alhaluli AH, Alessa MM, Alsulais NM. The role of the COVID-19 pandemic in expediting digital health-care transformation: Saudi Arabia's experience. Inform Med Unlock. (2022) 3:101097.
11. Barry M, Temsah MH, Aljamaan F, Saddik B, Al-Eyadhy A, Alenezi S, et al. COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout. Vaccine. (2021) 39:5762–8. doi: 10.1016/J.VACCINE.2021.08.083
12. Elharake JA, Galal B, Alqahtani SA, Kattan RF, Barry MA, Temsah MH, et al. COVID-19 vaccine acceptance among health care workers in the Kingdom of Saudi Arabia. Int J Infect Dis. (2021) 109:286–93. doi: 10.1016/j.ijid.2021.07.004
13. Al Bahrani S, Albarrak A, Alghamdi OA, Alghamdi MA, Hakami FH, Al Abaadi AK, et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int J Infect Dis. (2021) 110:359–62. doi: 10.1016/j.ijid.2021.07.052
14. Almohaya AM, Qari F, Zubaidi GA, Alnajim N, Moustafa K, Alshabi MM, et al. Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev Med Reports. (2021) 24:101595. doi: 10.1016/j.pmedr.2021.101595
15. Alharbi NK, Al-Tawfiq JA, Alghnam S, Alwehaibe A, Alasmari A, Alsagaby SA, et al. Outcomes of single dose COVID-19 vaccines: 8-month follow-up of a large cohort in Saudi Arabia. J Infect Public Health. (2022) 15:573–7. doi: 10.1016/j.jiph.2022.04.001
16. Saudi Ministry of Health. Help & Support - MOH Apps for Smartphones. (n.d.). Available online at: https://www.moh.gov.sa/en/Support/Pages/MobileApp.aspx (accessed August 5, 2022).
17. Ball P. The lightning-fast quest for COVID vaccines-and what it means for other diseases. Nature. (2021) 589:16–8. doi: 10.1038/d41586-020-03626-1
18. Medeiros KS, Costa APF, Sarmento ACA, Freitas CL, Gonçalves AK. Side effects of COVID-19 vaccines: a systematic review and meta-analysis protocol of randomized trials. BMJ Open. (2022) 12:278. doi: 10.1136/bmjopen-2021-050278
19. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. (2021) 4:e2140364–e2140364. doi: 10.1001/jamanetworkopen.2021.40364
20. Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. (2021) 106:376–81. doi: 10.1016/j.ijid.2021.04.047
21. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. (2021) 36:1–9. doi: 10.3346/jkms.2021.36.e115
22. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. (2021) 25:1663–79. doi: 10.26355/eurrev_202102_24877
23. Kim SH Wi YM, Yun SY Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of chadox1 Ncov-19 Or Bnt162b2 Mrna Covid-19 vaccination: a single center experience. J Korean Med Sci. (2021) 36:1–8. doi: 10.3346/jkms.2021.36.e107
24. Shaw RH, Stuart A, Greenland M, Liu X, Van-Tam JSN, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. (2021) 397:2043–6. doi: 10.1016/S0140-6736(21)01115-6
25. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. (2021) 21:939–49. doi: 10.1016/S1473-3099(21)00224-3
26. Alhumaid S, Al Mutair A, Al Alawi Z, Rabaan AA, Tirupathi R, Alomari MA, et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis. Allergy, Asthma Clin Immunol. (2021) 17:28. doi: 10.1186/s13223-021-00613-7
27. CDC COVID-19 Response Team. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:46–51. doi: 10.15585/mmwr.mm7002e1
28. Fazlollahi A, Zahmatyar M, Noori M, Nejadghaderi SA, Sullman MJM, Shekarriz-Foumani R, et al. Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev Med Virol. (2021) 32:2318. doi: 10.1002/rmv.2318
29. Iqbal S, Adnan G, Farhad A, Ahmed I, Rahman MN. Acute myocardial infarction after coronavirus vaccine: a rare adverse effect. Cureus. (2022) 14:21544. doi: 10.7759/cureus.21544
30. Boivin Z, Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. (2021) 13:13651. doi: 10.7759/cureus.13651
31. Sung JG, Sobieszczyk PS, Bhatt DL. Acute myocardial infarction within 24 h after COVID-19 vaccination. Am J Cardiol. (2021) 156:129–31. doi: 10.1016/j.amjcard.2021.06.047
32. Kumar B, Sabbarwal V, Nigam A, Gore P, Chauhan G, Darbari A. Two case reports of acute ST-elevation myocardial infarction after COVID-19 vaccination: co-incidence or causal-association? J Heal Soc Sci. (2021) 6:293–8. doi: 10.19204/2021/twcs14
33. Kim YE, Huh K, Park YJ, Peck KR, Jung J. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. (2022) 3:12992. doi: 10.1001/JAMA.2022.12992
34. Jabagi MJ, Botton J, Bertrand M, Weill A, Farrington P, Zureik M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA - J Am Med Assoc. (2022) 327:80–2. doi: 10.1001/jama.2021.21699
35. Chun JY, Park S, Jung J, Kim SH, Kim TS, Choi YJ, et al. Guillain-Barré syndrome after vaccination against COVID-19. Lancet Neurol. (2022) 21:117–9. doi: 10.1016/S1474-4422(21)00416-6
36. Shao SC, Wang CH, Chang KC, Hung MJ, Chen HY, Liao SC. Guillain-Barré syndrome associated with COVID-19 vaccination. Emerg Infect Dis. (2021) 27:3175–8. doi: 10.3201/eid2712.211634
Keywords: COVID-19, vaccines, side-effects, adverse events, self-reported, cohort, registry, Saudi Arabia
Citation: Alkhalifah JM, Al Seraihi A, Al-Tawfiq JA, Alshehri BF, Alhaluli AH, Alsulais NM, Alessa MM, Seddiq W, Aljeri T, Qahtani MH, Barry M and Al-Otaiby M (2023) Pattern of self-reported adverse events related to COVID-19 vaccines in Saudi Arabia: A nationwide study. Front. Public Health 11:1043696. doi: 10.3389/fpubh.2023.1043696
Received: 13 September 2022; Accepted: 07 February 2023;
Published: 23 February 2023.
Edited by:
Fuqiang Cui, Peking University, ChinaReviewed by:
Farman Ullah Khan, Xi'an Jiaotong University, ChinaCopyright © 2023 Alkhalifah, Al Seraihi, Al-Tawfiq, Alshehri, Alhaluli, Alsulais, Alessa, Seddiq, Aljeri, Qahtani, Barry and Al-Otaiby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maram Al-Otaiby,  YWxvdGFpYnltYUBtb2guZ292LnNh;
YWxvdGFpYnltYUBtb2guZ292LnNh;  bWFvdGFpYnlAa3N1LmVkdS5zYQ==; Joud Mohammed Alkhalifah,
bWFvdGFpYnlAa3N1LmVkdS5zYQ==; Joud Mohammed Alkhalifah,  SnVkZS5BbGtoYWxpZmFoQGdtYWlsLmNvbQ==
SnVkZS5BbGtoYWxpZmFoQGdtYWlsLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.