- Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, Translation Medicine Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Considering that the previously developed mortality prediction models have limited applications to the Chinese population, a questionnaire-based prediction model is of great importance for its accuracy and convenience in clinical practice.
Methods: Two national cohort, namely, the China Health and Nutrition Survey (8,355 individual older than 18) and the China Health and Retirement Longitudinal Study (12,711 individuals older than 45) were used for model development and validation. One hundred and fifty-nine variables were compiled to generate predictions. The Cox regression model and six machine learning (ML) models were used to predict all-cause mortality. Finally, a simple questionnaire-based ML prediction model was developed using the best algorithm and validated.
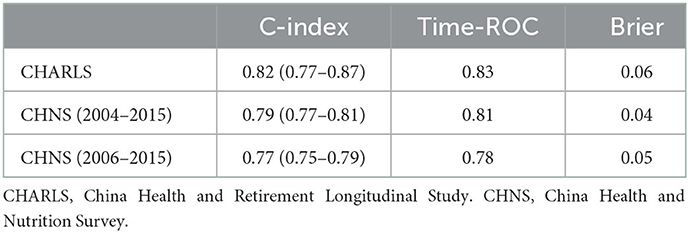
Results: In the internal validation set, all the ML models performed better than the traditional Cox model in predicting 6-year mortality and the random survival forest (RSF) model performed best. The questionnaire-based ML model, which only included 20 variables, achieved a C-index of 0.86 (95%CI: 0.80–0.92). On external validation, the simple questionnaire-based model achieved a C-index of 0.82 (95%CI: 0.77–0.87), 0.77 (95%CI: 0.75–0.79), and 0.79 (95%CI: 0.77–0.81), respectively, in predicting 2-, 9-, and 11-year mortality.
Conclusions: In this prospective population-based study, a model based on the RSF analysis performed best among all models. Furthermore, there was no significant difference between the prediction performance of the questionnaire-based ML model, which only included 20 variables, and that of the model with all variables (including laboratory variables). The simple questionnaire-based ML prediction model, which needs to be further explored, is of great importance for its accuracy and suitability to the Chinese general population.
Introduction
Accurate identification of individuals at high risk of mortality is valuable for both medical care and public health policymaking (1). Recently, a few prognostic indices and prediction models with variable predictors have been developed to predict an individual's probability of death (2–6). However, most of the current mortality prediction models have been developed using data from the United States and Western Europe (5–7), which may greatly limit their validity for Chinese. With the change of lifestyle in recent years, the number of premature deaths caused by non-communicable diseases has increased. However, the limited mortality prediction research on Chinese mostly focuses on elderly or those suffering from specific diseases, which may not be suitable for the general population (8–10). Prediction of young people's death is also noteworthy for early death of youngers brings more losses to individuals, families, and society. The need for laboratory test data is another reason that limits the application of the current mortality prediction models (2). As a country with a large population and lack of medical resources, the death prediction model based on questionnaire may be more valuable. Thus, developing a questionnaire-based all-cause mortality prediction model for the general population of China is of great significance.
Traditionally, mortality prediction models have only applied logistic or Cox regression models on limited variables, resulting in low predictive performance (6, 11). Over the past decades, many advanced machine learning (ML) techniques have further expanded the traditional medical prediction owing to their ability in processing large-scale data and identifying hidden risk factors of diseases (12). A large number of variables can be simultaneously included in prediction models using ML algorithms. The ability to automatically model non-linear correlations and interactions between different risk factors may allow ML models to outperform statistical models in terms of calibration and discrimination (13, 14). Despite claims that the ML algorithm can revolutionize risk prediction and replace traditional statistical models, the performances of ML algorithms may differ in different areas (15, 16). At present, all-cause mortality prediction models developed using the ML algorithm are still rare. Moreover, studies comparing the performances of different ML algorithms with those of traditional Cox regression models in predicting all-cause mortality are still lacking.
To address these important knowledge gaps, the present study aimed to develop a questionnaire-based mortality prediction model for Chinese by comparing the performances of six ML approaches with that of the commonly used Cox regression model using data from the China Health and Nutrition Survey (CHNS).
Materials and methods
Study design and participants
Two national surveys, namely, the CHNS and the China Health and Retirement Longitudinal Study (CHARLS) were used for model development and validation. The details of the CHNS and CHARLS design have been described previously and are discussed in the Supplementary Data (17, 18). Briefly, the CHNS is a national, longitudinal, open cohort study that started in 1989 and has been followed up every 2–4 years. In the 2009 record of the CHNS, the data of subjects included both questionnaire and laboratory variables. The CHARLS is a nationally representative longitudinal survey of the social, economic, and health circumstances of individuals older than 45 in China.
To compare the predictive effect of the laboratory and questionnaire variables on mortality, the model development data of this study were extracted from the 2009 to 2015 survey of the CHNS, which included 12,178 participants. The exclusion criteria were as follows: age < 18 years; no follow-up data; pregnant or breastfeeding; with a history of myocardial infarction, stroke, or any type of tumor; and with more than 30% missing variables. The CHNS cohorts were randomly split into training (80%) and internal validation (20%) datasets to develop the 6-year mortality prediction models. The 2004–2015 CHNS dataset, 2006–2015 CHNS dataset, and the dataset derived from CHARLS were used to externally validate the performance of the model in predicting 11-, 9- and 2-year mortality, separately.
Mortality ascertainment
The mortality status and date of mortality were confirmed according to the information reported by household members. Years of follow-up were calculated from the time of the baseline to death or censoring in the end survey wave, whichever came first.
Predictors
All candidate predictors were extracted from the CHNS data. Trained staff administered a standardized questionnaire to collect information on individuals, households, and communities. Trained clinicians performed physical examinations, including measurements of height, weight, and blood pressure. Blood samples were collected from an empty stomach after the participants maintained a regular pattern of life for at least 3 days. The details of all candidate predictors can be found on the following website: http://www.cpc.unc.edu/projects/china/data/datasets. Variables that were missing in more than 30% of the participants were excluded. Ultimately, 159 independent predictors, selected from 11 commonly investigated domains, were measured in the present study. The 11 domains were as follows: (i) demographics, (ii) family relations, (iii) community score, (iv) activity and time spent, (v) socioeconomic status, (vi) macronutrient intakes and dietary behaviors, (vii) lifestyle, (viii) diet and exercise knowledge, (ix) health condition, (x) physical examination, and (xi) laboratory examination. Complete details are provided in the Appendix.
Development and comparison of models
The Cox regression model and six ML approaches, namely, the least absolute shrinkage and selection operator (LASSO) regression (17), survival tree (19), random survival forest (RSF) (20), conditional inference forest (CIF) (21), boosted generalized linear model (glmBoost) (22), and gradient boosting (GBM) (23, 24), were developed separately to predict the risk of 6-year all-cause mortality. The description of ML algorithms has been described previously and are provided in the Supplementary Data (25, 26). Cross-validation was used to limit mean cross-validation error. To evaluate the performance of the model with different variables, three different sets of variables were separately compiled to generate predictions. The three sets of variables included the 159 variables, all laboratory variables, and all variables except laboratory variables. To develop the questionnaire-based prediction model, the performance of the best ML algorithm was further compared with those of models derived using a similar approach: (1) with the top 20 questionnaire variables, (2) with the top 10 questionnaire variables, and (3) with all variables. Each variable was ranked using the variable importance (VIMP) metric in the RSF algorithm (20). To achieve a simple model, the variables that could be directly obtained through questionnaires without calculation were compiled to generate predictions.
Sensitivity analysis
To assess the generalizability of the ML-based algorithms in predicting long-term mortality, sensitivity analyses were performed on data that were followed up for 9 and 11 years. Two cohorts were recruited separately from the 2004 and 2006 surveys of the CHNS, followed to the 2015 survey. The inclusion and exclusion criteria used in the sensitivity analysis cohorts were the same as those used in the model development cohort. The variables in the two cohorts were all the questionnaire variables, which were the same as those in the model development cohort. All variables were used to develop the Cox regression model and six ML models to separately predict 9- or 11-year all-cause mortality. The performances of all models were compared.
Model validation
All models were validated in the 2009 CHNS internal validation cohort to predict the risk of 6-year all-cause mortality. The simple questionnaire-based model was further externally validated in three independent cohorts, namely, the 2004–2015 CHNS dataset, 2006–2015 CHNS dataset, and the dataset derived from the CHARLS.
Statistical analysis
The missing values for each predictor were imputed using an iterative imputation method based on a random forest algorithm (23). The mean ± standard deviation or median with interquartile range was calculated for continuous variables. Totals and percentages were calculated for categorical variables.
The model discriminatory performance was measured using the time-dependent receiver operating characteristic (ROC) curve and Harrell concordance index (C-index) (27, 28). The ROC curve is a statistical tool used to evaluate the discriminative capacity of a diagnostic test. C statistics, ranging from 0.5 to 1, measure the ability of a model to rank patients from high to low risk. The differences between the C-indices of the different models were determined using the DeLong test (29). Calibration of the mortality prediction model was assessed with the Brier score, with a value of Brier score < 0.25 indicating adequate calibration (30, 31).
All analyses were conducted using the R software (version 4.1). The R packages used included “survival,” “gbm,” “grid,” “party,” “pec,” “mboost,” “glmnet,” and “randomForestSRC.” The statistical significance was set at P < 0.05.
Results
Participants and predictors
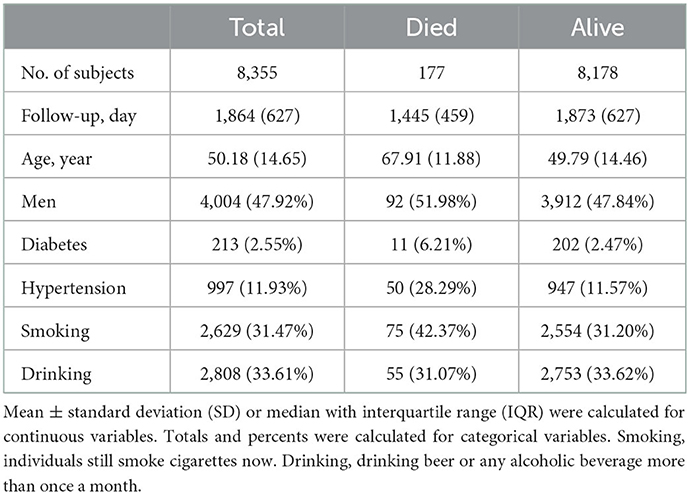
A total of 8,355 individuals recruited in the development cohort of 2009–2015 CHNS and 177 participants died during the 6-year follow-up (Supplementary Figure 1, Table 1). The validation cohort of 2006–2015 CHNS, consisted of 8,126 individuals, and 495 died after 9 years of follow-up. The validation cohort of 2004–2015 CHNS, consisted of 8,827 individuals, and 665 died after 11 years of follow-up. The validation cohort of CHARLS consisted of 12,711 individuals, and 262 died during the 2-year follow-up.
In total, 159 variables collected at the baseline were considered candidates for developing the models. The descriptive statistics for variables are presented in Supplementary Tables 1, 2.
Model performance
Supplementary Table 3 displays the discrimination and calibration of models in the training cohort. The performance metrics in the internal validation cohort of all models are listed in Table 2. All ML models performed better than the traditional Cox model. The RSF model had the highest C-index [0.86 (95%CI: 0.80–0.92)] and area under the ROC curve (0.85) (Figure 1). And there were significant differences between the C-index of RSF and that of other models which indicating that the RSF model has the best discrimination ability in the present study. The C-indices of models without laboratory variables did not significantly decrease compared with the models with all variables (p > 0.05). Compared with the performance of the models that included all variables, those of the models that only included laboratory variables decreased significantly. The ML models demonstrated improved calibration compared with the Cox model (Table 2 and Supplementary Figures 2–4).
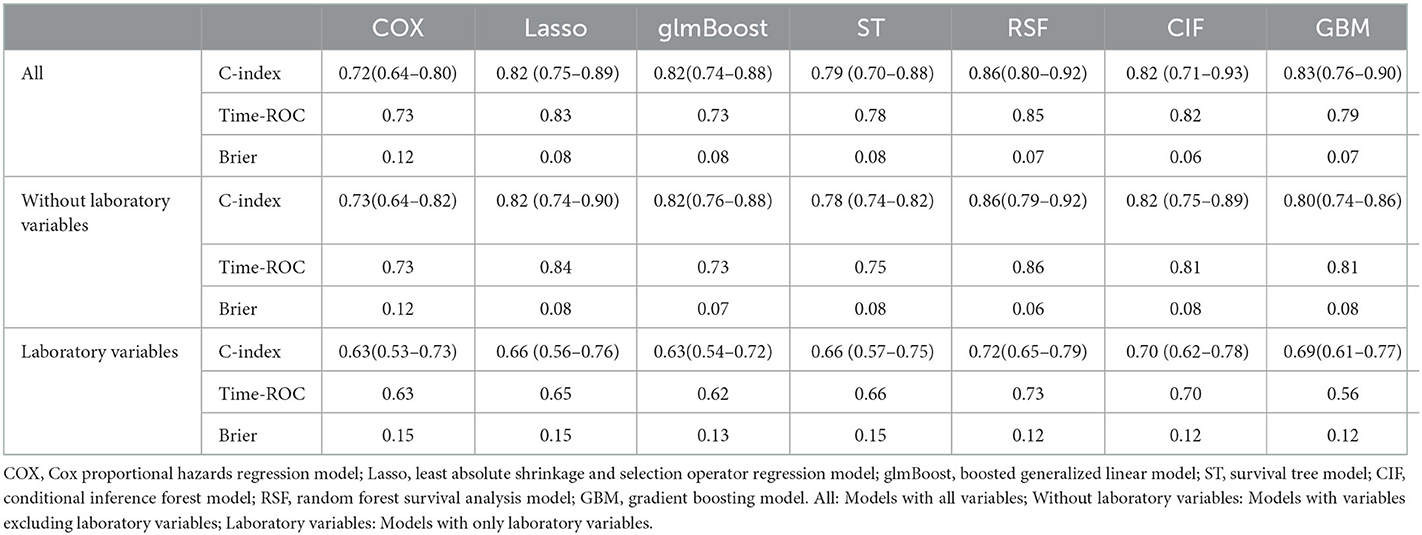
Table 2. Performance of the models for predicting all-cause mortality in internal validation cohort.
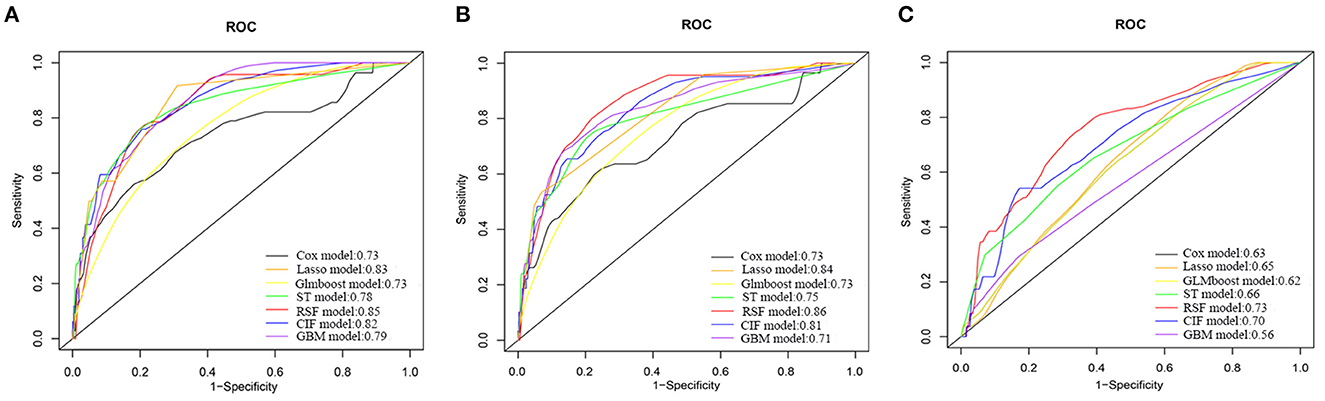
Figure 1. Receiver operating characteristic curves of different models for predicting 6-year all-cause mortality in Internal Validation Cohort. (A) Models with all variables. (B) Models with variables excluding laboratory variables. (C) Models with only laboratory variables. COX, Cox proportional hazards regression model; Lasso, least absolute shrinkage and selection operator regression model; GLMBoost, boosted generalized linear model; ST, survival tree model; CIF, conditional inference forest model; RSF, random forest survival analysis model; GBM, gradient boosting model.
Sensitivity analyses
In the dataset of the 2006–2015 CHNS, the best performer was the RSF model, which had the highest C-index value 0.85 (95%CI: 0.82–0.88), and the C-index for the Cox regression model was only 0.79 for estimation of the risk of 9-year all-cause mortality. Similarly, the RSF algorithm had the highest C-index value, and the Cox model had the lowest C-index when predicting the 11-year all-cause mortality in the dataset of the 2004–2015 CHNS (Supplementary Tables 4, 5).
Derivation of questionnaire-based ML models
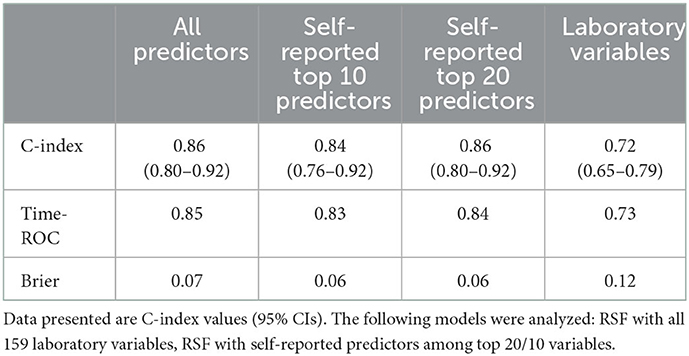
Considering its best performance among all the algorithms, the RSF algorithm was used to develop questionnaire-based models. Supplementary Figure 5 and Supplementary Table 6 show the relative importance of the top 20 variables in the RSF model (without laboratory variables). The C-index for the RSF with the top 20 self-reported predictor model was 0.86 (95%CI: 0.80–0.92), which was higher than the model with 10 variables. Meanwhile, the discrimination and calibration of the RSF with the top 20 self-reported predictor model was similar to that of the RSF model with all predictors (Table 3 and Supplementary Figure 5). To improve the clinical utility of the prediction model, we implemented the simple ML model which included 20 variables on a publicly available website (https://chnsmortalityprediction.shinyapps.io/mortality-20/) (Supplementary Figure 7). Furthermore, simple ML model which included 10 variables was also implemented on a publicly available website (https://chnsmortalityprediction.shinyapps.io/mortality-10/) (Supplementary Figure 8).
External validation of the questionnaire-based ML prediction model
In the present study, three external cohorts were used to validate the questionnaire-based model. In the CHNS 2004–2015 cohort, the overall C-index for 11-year mortality was 0.79 (95%CI: 0.77–0.81) and the time-dependent ROC value was 0.81. The overall C-index for 9-year mortality in the CHNS 2006–2015 cohort was 0.77 (95%CI: 0.75–0.79) and the time-dependent ROC value was 0.78. The overall C-index for 2-year mortality in the CHARLS was 0.82 (95%CI: 0.77–0.87). The calibration of all three external validations was acceptable, with a Brier score of < 0.6 (Table 4 and Supplementary Figures 9–11).
Discussion
In the present study, we compared the performances of six ML approaches with that of the commonly used Cox proportional hazards regression model for predicting all-cause mortality in Chinese and developed a questionnaire-based ML prediction model based on the best-performing approach. Several key inferences can be drawn from the results. First, ML algorithms can improve traditional approaches used for creating all-cause mortality prediction tools, and the RSF model has the best performance among all models in the present study. Furthermore, the performance of the model based on only 20 questionnaire variables is similar to that of the model with all variables which included laboratory variables. Finally, the questionnaire-based ML model developed from the CHNS data can estimate all-cause mortality with good discrimination for Chinese.
To limit the burden on already strained health systems and improve efficiency, identifying individuals with a high risk of death to provide more targeted prevention and treatment is of great significance. Most of the available mortality prediction models are constructed using traditional statistical techniques (2, 3, 5, 7, 10, 32). Because of the limited variables included and the simple model fitting method, the performances of the prediction models constructed using traditional methods may be greatly limited. Recently, ML has gradually attracted interest because of its powerful ability to identify potential variables and fit models (33–36). Many studies have found that prediction models constructed using ML algorithms have higher performances than traditional statistical methods in predicting the occurrence of clinical events, such as hypoglycemia and cardiovascular events, and prognosis of tumors (20, 37–39). However, it is worth noting that ML techniques are not always better than “classical” statistical methods (40). The effectiveness of ML algorithms on specific data and problems must be evaluated separately. Recently, some studies on the application of ML in determining all-cause mortality have been conducted (41–45). However, most of these studies regarded death as a binary event rather than a time-to-event survival outcome. Directly applying popular ML models to data without censoring substantially biases risk predictions (16). To the best of our knowledge, there is no comprehensive comparative study between ML and traditional methods on the premise of taking death as a time-to-event outcome. In the present study, six ML algorithms derived from three major approaches to predict survival events were compared with the traditional Cox model in the prediction of all-cause mortality. The results indicated that all ML methods performed better than the traditional Cox model. This finding suggests that ML algorithms can improve traditional approaches if the same data are used for mortality prediction. Meanwhile, the RSF model has the highest C-index, which is higher than those of the CIF and GBD models, indicating that a complex model does not necessarily result in a higher predictive power.
Another important advantage of ML algorithms is that they rely on machine-guided data-driven methods instead of experience-guided data analysis to identify risk factors and generate the best fit for the data. Traditional mortality prediction models only empirically incorporate a limited number of variables. Even if more factors are included, the non-linear relationship cannot be identified because of the characteristics of the algorithm. Therefore, it is possible to ignore factors that have important impacts on death. ML algorithms have great advantages in dealing with a wide range of complex datasets with multifactor causality and potentially non-intuitive interactions (46). In the present study, we used an ML algorithm to incorporate 159 factors and explored the potential factors that might affect death. The results lay a foundation for further exploration of the risk factors of all-cause mortality.
Recently, many clinical prediction models have been developed. However, the applications of these tools are limited. One major reason is the inclusion of laboratory variables in the current prediction models (4, 5). Simple questionnaire questions may be closely related to all-cause mortality. Recently, there have been a few questionnaire-based all-cause mortality prediction model which the C-indexes of model is higher than 0.7 (4, 5). However, these prediction models are established by traditional COX model. At present, there is no questionnaire-based all-cause mortality prediction model based on machine learning algorithm for the general population in China. In the present study, there is no significant difference between ML-based models with and without laboratory variables. There may be several reasons for this. First, although laboratory variables, such as glucose, are closely related with disease which are associated with mortality, the level of laboratory variable included in the study cannot fully reflect the situation of diseases for that patients might have normal level of laboratory variable during treatment. Second, the laboratory examination indicators involved in our study only include basic blood indexes (such as glucose, red blood cell count, hemoglobin, index for lipid metabolism). The prediction efficiency of a single variable may be limited in the general population when predicting all-cause mortality. Therefore, the prediction effect of basic blood indicators involved in our study on all-cause mortality may be limited. Previous studies indicated that the c-index for predicting all-cause mortality using basic blood indicators only reached 0.7 (2, 47). Third, in terms of predicting all-cause death, the prediction ability of socioeconomic indicators, lifestyle and disease history is not weaker than that of basic blood indicators (7). Consistent with previous reports, the present results in our study suggest that the prediction performance of a model may not be significantly improved by further adding laboratory variables when sufficient variables are included in the process of fitting the model with an ML algorithm. To reduce the complexity of the model and increase its clinical practicability, we further developed a simple prediction model that only included 20 variables. The performance of the simple questionnaire-based ML model was not significantly different from those of models with all variables. To further improve the application of the model, a web page for our simple questionnaire-based ML model was developed. Therefore, anyone can use this ML-based model to improve their self-awareness of health status.
Another important factor that affects model application is the development data of the model. No predictive model can be used in clinical practice if its effectiveness is not yet tested in other populations (48). The prediction model developed for a certain population may not achieve a good performance when applied to other populations. For example, the performance of a model developed using data from the United States may deteriorate when it is applied to Britons (49). Therefore, most of the available mortality prediction models developed using data from European and American countries may not be suitable for China because of the large differences in lifestyle, culture, and genes (4, 25, 32). In this mortality prediction model study, to develop a prediction model for Chinese, data from the CHNS, which comprehensively contain information on diet, exercise, and lifestyle of Chinese, were used. To the best of our knowledge, previous all-cause mortality prediction models for Chinese have mostly focused on specific groups, such as the elderly and those with diabetes (8–10). There is no all-cause mortality prediction model for the general population in China. In this study, we used data from all adults to predict all-cause mortality for the general population. More importantly, our prediction model was further validated using external datasets. Owing to the limited follow-up time and size of the cohort, the number of deaths in the development cohort of the questionnaire-based model was small, which might have influenced the accuracy of the model. To further evaluate the effectiveness of the questionnaire-based model, cohorts with longer follow-up times and larger number of deaths were used as external validation datasets. The questionnaire-based model performed well-during the external validation. Moreover, the model satisfactorily performed in predicting short-term mortality in the external validation. These results indicate that the questionnaire-based ML model can estimate all-cause mortality with good discrimination for Chinese. Compared with previous death prediction models, our prediction model comes from the general Chinese population and improves the prediction ability of questionnaire variables through machine learning algorithm, which may be more suitable for application in primary health care of China.
Strengths and limitations
Our study has several strengths. First, although a few ML prediction studies have been conducted on all-cause mortality, most of them considered death as a binary outcome rather than a time-to-event outcome. Our study considered the censoring of death, which reduced the miscalibration of the models. Second, all three approaches mainly used for predicting survival events, namely, penalized regression, boosting, and tree or forest, were included in this study. Furthermore, we comprehensively compared ML methods with traditional ones. Third, owing to the characteristics of the CHNS data, many variables for Chinese, such as lifestyle, activities, and health status, are considered in the process of developing the model. Therefore, the prediction model may be more suitable for Chinese. Fourth, to be more convenient to use, we developed a simple prediction model with only 20 questionnaire variables, and the performance of this model was not significantly different from those of the models with all variables. Fifth, although the development of our model was based on 6-year mortality data, three external validation cohorts were used to assess the predictive effectiveness of the model on long- and short-term mortalities, which showed that the model has good application potential for Chinese. Finally, we created a webpage for the ML-based model to enhance its application.
The present study has some shortcomings. First, although we included as many candidate factors as possible in the CHNS, several factors that cause important death burdens, such as environmental factors, were not included because of the research design of the CHNS. Second, there were no causes of death in this study due to data limitations. The issue on whether there are differences in the prediction of different types of death by the prediction model still needs to be further explored. Third, because we excluded pregnant and nursing women and individuals with myocardial infarction, stroke, or tumor during the model development, our model is only applicable to the general population of China. Although our model has been validated in the CHARLS cohort of people over 45 years of age, further study is still necessary to determine whether the model is applicable to other special populations. Fourth, this model has not been verified based on data in other countries; therefore, it is not certain whether it can be applied to other populations. Fifth, the cause of death in our study including accidents or unnatural death which may add a degree of randomness.
Conclusions
In this prospective population-based study, ML algorithms were shown to improve traditional approaches used for creating all-cause mortality prediction tools, and the model based on RSF analysis performed best among all models. Furthermore, no significant difference between the prediction performance of the questionnaire-based ML model, which only included 20 variables, and that of the model with all variables that included laboratory variables was found. The simple questionnaire-based ML prediction model, which needs to be further explored, is of great importance for its accuracy and suitability to the Chinese general population.
Data availability statement
The datasets presented in this study can be found in online repositories. The data and research materials supporting the findings of this study can be found on the CHNS official website (http://www.cpc.unc.edu/projects/china) and CHARLS official website (http://charls.pku.edu.cn/).
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill, National Institute of Nutrition and Food Safety, and Chinese Center for Disease Control and Prevention. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HZ and YL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. HZ, ZL, and YL planned the concept of this study. HZ, ZL, NY, and YL conducted data extraction and analysis. LH, JW, FP, and WL carried out data cleaning and material support. All authors critically reviewed, revised, and contributed to the final manuscript.
Funding
This work was supported by grants from the Beijing Municipal Natural Science Foundation (No. M22014), the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-015), the CAMS Innovation Fund for Medical Sciences (No. 2021-1-I2M-002), the National Natural Science Foundation of China (No. 91846106), and the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019XK320029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1033070/full#supplementary-material
References
1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
2. Fischer K, Kettunen J, Würtz P, Haller T, Havulinna AS, Kangas AJ, et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. (2014) 11:e1001606. doi: 10.1371/journal.pmed.1001606
3. Liao J, Muniz-Terrera G, Scholes S, Hao Y, Chen YM. Lifestyle index for mortality prediction using multiple ageing cohorts in the USA, UK and Europe. Sci Rep. (2018) 8:6644. doi: 10.1038/s41598-018-24778-1
4. Suemoto CK, Ueda P, Beltrán-Sánchez H, Lebrão ML, Duarte YA, Wong R, et al. Development and validation of a 10-year mortality prediction model: meta-analysis of individual participant data from five cohorts of older adults in developed and developing countries. J Gerontol A Biol Sci Med Sci. (2017) 72:410–6. doi: 10.1093/gerona/glw166
5. Ganna A, Ingelsson E. 5-year mortality predictors in 498 103 UK Biobank participants: a prospective population-based study. Lancet. (2015) 386:533–40. doi: 10.1016/S0140-6736(15)60175-1
6. Häppölä P, Havulinna AS, Tasa T. A data-driven medication score predicts 10-year mortality among aging adults. Sci Rep. (2020) 10:15760. doi: 10.1038/s41598-020-72045-z
7. Walter S, Mackenbach J, Vokó Z. Genetic, physiological, and lifestyle predictors of mortality in the general population. Am J Public Health. (2012) 102:e3–10. doi: 10.2105/AJPH.2011.300596
8. Li TC, Li CI, Liu CS. Derivation and validation of 10-year all-cause and cardiovascular disease mortality prediction model for middle-aged and elderly community-dwelling adults in Taiwan. PLoS ONE. (2020) 15:e0239063. doi: 10.1371/journal.pone.0239063
9. Lee WJ, Peng LN, Chiou ST, Chen LK. Physical health indicators improve prediction of cardiovascular and all-cause mortality among middle-aged and older people: a national population-based study. Sci Rep. (2017) 7:40427. doi: 10.1038/srep40427
10. Chiu SY, Chen YI, Lu JR. Developing a prediction model for 7-year and 10-year all-cause mortality risk in type 2 diabetes using a hospital-based prospective cohort study. J Clin Med. (2021) 10:4779. doi: 10.3390/jcm10204779
11. Kotwal AA, Lee SJ, Dale W. Integration of an objective cognitive assessment into a prognostic index for 5-year mortality prediction. J Am Geriatr Soc. (2020) 68:1796–802. doi: 10.1111/jgs.16451
12. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. (2020) 395:1579–86. doi: 10.1016/S0140-6736(20)30226-9
13. Fan K, Cheng L, Li L. Artificial intelligence and machine learning methods in predicting anti-cancer drug combination effects. Brief Bioinform. (2021) 22:bbab271. doi: 10.1093/bib/bbab271
14. Slomka PJ, Kolli KK, Kumar A, Slomka PJ, Kolli KK, Kumar A, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. (2019) 40:1975–86. doi: 10.1093/eurheartj/ehy404
15. Hinton G. Deep learning-a technology with the potential to transform health care. JAMA. (2018) 320:1101–2. doi: 10.1001/jama.2018.11100
16. Li Y, Sperrin M, Ashcroft DM, van Staa TP. Consistency of variety of machine learning and statistical models in predicting clinical risks of individual patients: longitudinal cohort study using cardiovascular disease as exemplar. BMJ. (2020) 371:m3919. doi: 10.1136/bmj.m3919
17. Wang W, Liu W. Integration of gene interaction information into a reweighted Lasso-Cox model for accurate survival prediction. Bioinformatics. (2020) btaa1046. doi: 10.1093/bioinformatics/btaa1046
18. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. (2014)43:61–8. doi: 10.1093/ije/dys203
19. Linden A, Yarnold PR. Modeling time-to-event (survival) data using classification tree analysis. J Eval Clin Pract. (2017) 23:1299–308. doi: 10.1111/jep.12779
20. Segar MW, Jaeger BC, Patel KV, Nambi V, Ndumele CE, Correa A, et al. Development and validation of machine learning-based race-specific models to predict 10-year risk of heart failure: a multicohort analysis. Circulation. (2021) 143:2370–83. doi: 10.1161/CIRCULATIONAHA.120.053134
21. Nasejje JB, Mwambi H, Dheda K, Lesosky M. A comparison of the conditional inference survival forest model to random survival forests based on a simulation study as well as on two applications with time-to-event data. BMC Med Res Methodol. (2017) 17:115. doi: 10.1186/s12874-017-0383-8
22. Zhao L, Dong Q, Luo C. DeepOmix: a scalable and interpretable multi-omics deep learning framework and application in cancer survival analysis. Comput Struct Biotechnol J. (2021) 19:2719–25. doi: 10.1016/j.csbj.2021.04.067
23. Karami G, Giuseppe Orlando M, Delli Pizzi A. Predicting overall survival time in glioblastoma patients using gradient boosting machines algorithm and recursive feature elimination technique. Cancers. (2021)13:4976. doi: 10.3390/cancers13194976
24. Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med. (2018) 44:884–92. doi: 10.1007/s00134-018-5208-7
25. Puterman E, Weiss J, Hives BA, Gemmill A, Karasek D, Mendes WB, et al. Predicting mortality from 57 economic, behavioral, social, and psychological factors. Proc Natl Acad Sci U S A. (2020) 117:16273–82. doi: 10.1073/pnas.1918455117
26. Ma B, Meng F, Yan G, Yan H, Chai B, Song F. Diagnostic classification of cancers using extreme gradient boosting algorithm and multi-omics data. Comput Biol Med. (2020) 121:103761. doi: 10.1016/j.compbiomed.2020.103761
27. Beyene KM, El Ghouch A. Time-dependent ROC curve estimation for interval-censored data. Biom J. (2022) 64:1056–74. doi: 10.1002/bimj.202000382
28. Pencina MJ, D'Agostino RB. Evaluating discrimination of risk prediction models: the C statistic. JAMA. (2015) 314:1063–4. doi: 10.1001/jama.2015.11082
29. Samuel LT, Sultan AA, Kheir M, Villa J, Patel P, Parvizi J, Higuera CA. Positive alpha-defensin at reimplantation of a two-stage revision arthroplasty is not associated with infection at 1 year. Clin Orthop Relat Res. (2019) 477:1615–21. doi: 10.1097/CORR.0000000000000620
30. Rahman MS, Ambler G, Choodari-Oskooei B, Omar RZ. Review and evaluation of performance measures for survival prediction models in external validation settings. BMC Med Res Methodol. (2017) 17:60. doi: 10.1186/s12874-017-0336-2
31. Amini B, Bassett RL, Haygood TM, McEnery KW, Richardson ML. Confidence calibration: an introduction with application to quality improvement. J Am Coll Radiol. (2020) 17:620–8. doi: 10.1016/j.jacr.2019.12.009
32. Bérard E, Bongard V, Arveiler D, Amouyel P, Wagner A, Dallongeville J, et al. Ten-year risk of all-cause mortality: assessment of a risk prediction algorithm in a French general population. Eur J Epidemiol. (2011) 26:359–68. doi: 10.1007/s10654-010-9541-6
33. Greener JG, Kandathil SM, Moffat L, Jones DT. A guide to machine learning for biologists. Nat Rev Mol Cell Biol. (2022) 23:40–55. doi: 10.1038/s41580-021-00407-0
34. Pencina M, Goldstein BA, D'Agostino RB. Prediction models - development, evaluation, and clinical application. N Engl J Med. (2020) 382:1583–6. doi: 10.1056/NEJMp2000589
35. D'Ascenzo F, De Filippo O, Gallone G, Mittone G, Deriu MA, Iannaccone M, et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet. (2021) 397:199–07. doi: 10.1016/S0140-6736(20)32519-8
36. Chen JH, Asch SM. Machine learning and prediction in medicine - beyond the peak of inflated expectations. N Engl J Med. (2017) 376:2507–9. doi: 10.1056/NEJMp1702071
37. Ruan Y, Bellot A, Moysova Z, Tan GD, Lumb A, Davies J, et al. Predicting the risk of inpatient hypoglycemia with machine learning using electronic health records. Diabetes Care. (2020) 43:1504–11. doi: 10.2337/dc19-1743
38. Adeoye J, Hui L, Koohi-Moghadam M, Tan JY, Choi S-W, Thomson P. Comparison of time-to-event machine learning models in predicting oral cavity cancer prognosis. Int J Med Inform. (2022) 157:104635. doi: 10.1016/j.ijmedinf.2021.104635
39. Li R, Zhu J, Zhong WD, Jia Z. Comprehensive evaluation of machine learning models and gene expression signatures for prostate cancer prognosis using large population cohorts. Cancer Res. (2022) 82:1832–43. doi: 10.1158/0008-5472.CAN-21-3074
40. Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. (2019) 110:12–22. doi: 10.1016/j.jclinepi.2019.02.004
41. Clift AK, Lannou EL, Tighe CP, Shah SS, Beatty M, Hyvärinen A, et al. Development and validation of risk scores for all-cause mortality for a smartphone-based “general health score” app: prospective cohort study using the UK biobank. JMIR Mhealth Uhealth. (2021) 9:e25655. doi: 10.2196/25655
42. Tedesco S, Andrulli M, Larsson MÅ, Kelly D, Alamäki A, Timmons S, et al. Comparison of machine learning techniques for mortality prediction in a prospective cohort of older adults. Int J Environ Res Public Health. (2021) 18:12806. doi: 10.3390/ijerph182312806
43. Sakr S, Elshawi R, Ahmed AM, Qureshi WT, Brawner CA, Keteyian SJ, et al. Comparison of machine learning techniques to predict all-cause mortality using fitness data: the henry ford exercIse testing (FIT) project. BMC Med Inform Decis Mak. (2017) 17:174. doi: 10.1186/s12911-017-0566-6
44. Ajnakina O, Agbedjro D, McCammon R, Faul J, Murray RM, Stahl D, et al. Development and validation of prediction model to estimate 10-year risk of all-cause mortality using modern statistical learning methods: a large population-based cohort study and external validation. BMC Med Res Methodol. (2021) 21:8. doi: 10.1186/s12874-020-01204-7
45. Weng SF, Vaz L, Qureshi N, Kai J. Prediction of premature all-cause mortality: a prospective general population cohort study comparing machine-learning and standard epidemiological approaches. PLoS ONE. (2019) 14:e0214365. doi: 10.1371/journal.pone.0214365
46. Bello GA, Dumancas GG, Gennings C. Development and validation of a clinical risk-assessment tool predictive of all-cause mortality. Bioinform Biol Insights. (2015) 9:1–10. doi: 10.4137/BBI.S30172
47. Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. (2019) 20:e262–73. doi: 10.1016/S1470-2045(19)30149-4
48. Yourman LC, Lee SJ. Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. (2012) 307:182–92. doi: 10.1001/jama.2011.1966
Keywords: mortality, machine learning, prediction model, personalized prediction, questionnaire-based
Citation: Li Z, Yang N, He L, Wang J, Ping F, Li W, Xu L, Zhang H and Li Y (2023) Development and validation of questionnaire-based machine learning models for predicting all-cause mortality in a representative population of China. Front. Public Health 11:1033070. doi: 10.3389/fpubh.2023.1033070
Received: 31 August 2022; Accepted: 11 January 2023;
Published: 27 January 2023.
Edited by:
Hongyu Miao, Florida State University, United StatesReviewed by:
Ruonan Duan, Sichuan University, ChinaHuijuan Ma, Hebei General Hospital, China
Mingxia Yuan, Affiliated Beijing Friendship Hospital, Capital Medical University, China
Copyright © 2023 Li, Yang, He, Wang, Ping, Li, Xu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huabing Zhang,  aHVhYmluZ3poYW5nY2huQDE2My5jb20=; Yuxiu Li,
aHVhYmluZ3poYW5nY2huQDE2My5jb20=; Yuxiu Li,  bGl5dXhpdUBtZWRtYWlsLmNvbS5jbg==
bGl5dXhpdUBtZWRtYWlsLmNvbS5jbg==
 Ziyi Li
Ziyi Li Na Yang
Na Yang Liyun He
Liyun He Jialu Wang
Jialu Wang Fan Ping
Fan Ping Wei Li
Wei Li Lingling Xu
Lingling Xu Huabing Zhang
Huabing Zhang Yuxiu Li
Yuxiu Li