
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 23 January 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1022735
Introduction: Frailty is a complex condition that is highly associated with health decline and the loss of independence. Home-delivered meal programs are designed to provide older adults with health and nutritional support that can attenuate the risk of frailty. However, home-delivered meal agencies do not routinely assess frailty using standardized instruments, leading to uncertainty over the longitudinal impact of home-delivered meals on frailty levels. Considering this knowledge gap, this study aimed to facilitate home-delivered meal staff's implementation of a standardized frailty instrument with meal clients as part of routine programming. This article (a) describes the use of Implementation Mapping principles to develop strategies supporting frailty instrument implementation in one home-delivered meal agency and (b) examines the degree to which a combination of strategies influenced the feasibility of frailty instrument use by home-delivered meal staff at multiple time points.
Methods and materials: This retrospective observational study evaluated staff's implementation of the interRAI Home Care Frailty Scale (HCFS) with newly enrolled home-delivered meal clients at baseline-, 3-months, and 6-months. The process of implementing the HCFS was supported by five implementation strategies that were developed based on tenets of Implementation Mapping. Rates of implementation and reasons clients were lost to 3- and 6-month follow-up were evaluated using univariate analyses. Client-level data were also examined to identify demographic factors associated with attrition at both follow-up time points.
Results: Staff implemented the HCFS with 94.8% (n = 561) of eligible home-delivered meal clients at baseline. Of those clients with baseline HCFS data, staff implemented the follow-up HCFS with 43% of clients (n = 241) at 3-months and 18.0% of clients (n = 101) at 6-months. Insufficient client tracking and documentation procedures complicated staff's ability to complete the HCFS at follow-up time points.
Discussion: While the HCFS assesses important frailty domains that are relevant to home-delivered meal clients, its longitudinal implementation was complicated by several agency- and client-level factors that limited the extent to which the HCFS could be feasibly implemented over multiple time points. Future empirical studies are needed to design and test theoretically derived implementation strategies to support frailty instrument use in the home- and community-based service setting.
Home-delivered meal programs provide community-dwelling older adults with health and nutritional support to optimize wellness and reduce the need for more advanced healthcare services (1, 2). Programming typically targets older adults who are unable to safely and independently perform routine mealtime activities (e.g., shopping, meal preparation), who live alone and below the poverty line, and experience fair-to-poor health (3, 4). In a recent nationwide sample from the United States, 76% of home-delivered meal clients had at least one activity of daily living (ADL) impairment, 74% had five or more reported health conditions, and 33% experienced difficulty affording food items on a routine basis (5).
The aforementioned characteristics of home-delivered meal clients also place them at elevated risk for frailty (6–8). Operationally defined, frailty is a recognizable state of marked vulnerability resulting from age-related declines in physiological health (9). While the term “frailty” used to be synonymous with “disability,” more refined definitions of frailty have recently emerged with greater consideration for the accumulation of physical, social, and cognitive factors that contribute to physiological decline (10, 11). As defined by Fried at al., presence of three of the following five criteria are indicative of frailty: low grip strength, low energy levels, slowed walking speed, low physical activity, and unintentional weight loss (9, 12). Frailty-related health declines drastically minimize older adults' ability to tolerate health stressors (e.g., acute illness), leading to poorer health outcomes and individual healthcare costs that can total over $30,000 annually (13, 14). Globally, up to 27% of community-dwelling older adults experience frailty (10)–15% in the United States—with frailty being more prevalent among women, racial and ethnic minorities, and people of lower income (15).
Although trained health professionals (e.g., physicians, nurse practitioners) can address frailty and its associated risk factors (16), the assessment of frailty can be time- and resource-intensive (17, 18), particularly with older adults, such as home-delivered meal clients, who present with complex needs and chronic comorbidities (3, 19). Given that over 70% of home-delivered meal clients experience frailty (20), innovative approaches are needed to regularly assess and monitor the frailty levels of older adults enrolled in home-delivered meal programs. Routine evaluation of frailty can alert home-delivered meal staff of changes in frailty levels, which may warrant additional services or interventions. Timely intervention can potentially reverse the severity of frailty, thereby reducing clients' risk of further decline and institutionalization (21).
Frailty has previously been assessed in the home-delivered meal setting by means of secondary data analyses (e.g., chart review) (15) but has yet to be examined longitudinally through the use of standardized frailty instruments administered directly to clients. The implementation, also referred to as “uptake” or “use,” of such instruments by home-delivered meal staff has the potential to provide home-delivered meal agencies with metrics representing clients' improvement or maintenance of frailty levels—metrics that are necessary for demonstrating the valuable impact of these meal programs overtime (22, 23).
Despite the high prevalence of frailty and the importance of monitoring frailty levels, there is little guidance for how home-delivered meal staff members can effectively implement frailty instruments, particularly when those instruments are implemented at multiple time points (e.g., baseline, 3-month, and/or 6-month follow-up). Accordingly, the purpose of this paper is to (a) describe the use of Implementation Mapping (24) principles to develop strategies supporting frailty instrument implementation in one home-delivered meal agency and (b) examine the degree to which a combination of implementation strategies influenced the feasibility of frailty instrument use by home-delivered meal staff at multiple time points. Insights from agency staff and leadership also illuminate challenges and opportunities for implementing frailty instruments within the home-delivered meal context. This work underscores practical considerations for how home-delivered meal providers may assess frailty and continuously monitor health status changes among a highly vulnerable group of community-dwelling older adults.
To evaluate our implementation strategies, we used a retrospective observational design and examined home-delivered meal staff's implementation of the interRAI Home Care Frailty Scale (HCFS) (25) at baseline (meal program enrollment), 3-months, and 6-months.
The agency partner for this study was a not-for-profit organization that provided home-delivered meals and nutritional support services to older adults, age 60 and over, in the five surrounding counties of Columbus, Ohio. With a staff of over 200 full- and part-time individuals, our partner agency employed a diverse group of staff members representing the fields of social work, nursing, community health, and dietetics, as examples.
The HCFS is a 30-point scale developed from a secondary analysis of client-level interRAI Home Care data (25). HCFS items cover the following five domains: function, movement, cognition and communication, social interaction, and nutrition, and it also assesses the presence of common clinical conditions (e.g., renal failure, pneumonia), with higher total HCFS scores indicating greater levels of frailty. Agency staff members and leaders, in collaboration with our research team, selected to implement the HCFS given its perceived ease of use by clinical and non-clinical staff, implementability via telephone, and evidence of acceptable criterion-related validity (average Kappa values for each domain: function = 0.59; movement = 0.32; cognition and communication = 0.51; social interaction = 0.28; nutrition = 0.20) (25). Unlike the agency's standard in-take assessment that was implemented with clients upon enrollment and every 12-months thereafter, staff implemented the HCFS at 3-month and 6-month follow-up points for the purposes of the present study. At all time points, the HCFS was administered via telephone as agency staff were not permitted to complete in-home assessments with clients given COVID-19 restrictions.
Implementation Mapping is a systematic, theory- and evidence-informed process designed to guide the development of implementation strategies—or the approaches used to support the uptake of high-quality interventions, assessments, programs, or practices (24, 26). It consists of a series of tasks that culminate in implementation strategy deployment and the evaluation of implementation of outcomes (e.g., feasibility, adoption, fidelity) (27). The manner in which these tasks were applied to HCFS implementation is described below and expands upon prior methods used to develop implementation strategies in the community-based setting (28). All Implementation Mapping tasks (Figure 1) were co-led by agency partners (assistant director of nutrition programs, a case manager, and three administrators) in collaboration with our research team.
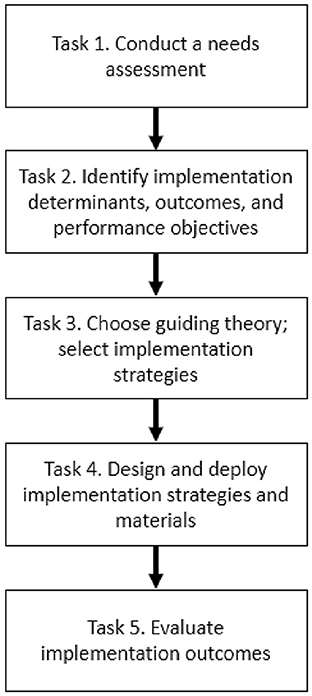
Figure 1. Implementation mapping steps informed by Fernandez et al. (24).
Our needs assessment was conducted in two phases. Phase 1 involved 1-on-1 interviews and focus groups with home-delivered meal staff as well as personal care assistants, homemakers, nurses, and dietitians employed by our partner agency. Interview and focus group guides were structured to evaluate the factors (i.e., determinants or barriers and facilitators) influencing evidence-based practice implementation in the context of home- and community-based services more broadly. Qualitative data underwent directed content analysis to identify key determinants of evidence implementation, and complete methodological details are reported elsewhere (29). In Phase 2, we held 3, 1-h meetings with agency leadership and staff to understand current workflow procedures and how those procedures may be altered as a result of implementing the HCFS with home-delivered meal clients.
Through our needs assessment, we identified that perceived determinants at the agency-level—rather than policy-level, staff-level, or client-level—served as major determinants of HCFS implementation. In recognition of these determinants, home-delivered meal program directors, assessment staff, and the research team established the target outcomes (27) and performance objectives that needed to be achieved in order for HCFS implementation to be successful. Establishing target outcomes and performance objectives also informed the research team's selection of data sources available within the agency that were needed for our outcome evaluation.
The identification of perceived determinants (Task 2) was informed by the Consolidated Framework for Implementation Research (CFIR)—a meta-theoretical framework of constructs representing the dynamic context within which organizations may implement new practices (30). Thus, the CFIR also guided the research team's selection of HCFS implementation strategies that were vetted and confirmed by agency partners. Strategies were drawn from the Expert Recommendations for Implementing Change (ERIC) taxonomy (31) using the CFIR-ERIC matching tool (32). Whereas, the CFIR provides uniform nomenclature to define implementation barriers and facilitators, the ERIC taxonomy is a compilation of over 70 implementation strategies hypothesized to promote the uptake of evidence-based practices into routine care. The CFIR-to-ERIC matching tool uses expert opinion data to generate a rank-ordered list of specific strategies to support evidence-based practice implementation.
Our team began designing our implementation strategies and materials over the course of 5-months prior to HCFS implementation. Strategy development was led primarily by the agency's assistant director of nutrition programs as well as our research team. All strategies were designed and operationalized according to recommendations by Proctor et al. (26). These recommendations include: clearly identifying the individuals involved in providing (actors) and receiving each strategy (action targets), describing how the strategy is delivered (action), and establishing the strategy's main goal (outcome), justification (rationale), and frequency (temporality, dosage).
To evaluate HFCS implementation outcomes, data were collected retrospectively from our agency's custom HCFS documentation website from the 12-month time period of June 1, 2020–May 31, 2021, as per chart audit recommendations for implementation studies (33). HCFS data were examined monthly by the research team to determine rates of HCFS implementation for individual clients at baseline, 3-months, and 6-months. Rates were established by calculating the proportion of clients who had documentation of the HCFS being completed compared to the total number of clients eligible for the HCFS. Documented reasons why staff were unable to complete the HCFS with clients were analyzed descriptively. We also conducted univariate analyses to assess staff member's rates of implementation at all three time points, and used bivariate analyses to identify demographic characteristics associated with client attrition at 3-months and 6-months. All associated research activities were approved by the Institutional Review Board at The Ohio State University (#2020E1238).
Our needs assessment found three key, agency-level determinants influencing the implementation of evidence-based practices in the home-delivered meal setting. These determinants, as defined by the CFIR (30), were: (1) networks and communications, (2) available resources, and (3) compatibility (29). Networks and communications referred to the nature and quality of how HCFS data were documented and shared within the agency; available resources included the time, staff, and equipment needed to implement the HCFS; and compatibility referred to the perceived “fit” of the HCFS with the agency's existing workflow, standard practices, and values. Our meetings with agency leadership and staff also allowed our team to gather robust understanding of standard in-take assessment procedures and the extent to which these procedures would be altered by implementing the HCFS at multiple time points. Figure 2 compares staff's processes of implementing both the standard in-take assessment and the HCFS.
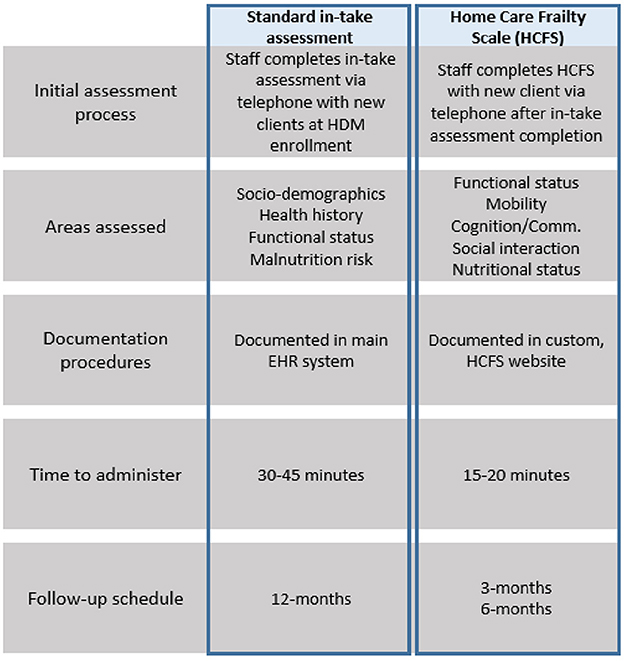
Figure 2. Comparison of standard in-take assessment with HCFS at baseline, 3-month, and 6-months; HDM, home-delivered meals; EHR, electronic health record.
Determinants of implementation were identified through the completed needs assessment (see above). Consensus from agency leadership and staff indicated their primary outcome of interest was staff's feasibility of implementing the HCFS longitudinally. For the present study, feasibility was defined as the utility or suitability of an evidence-based innovation for everyday use, which can be measured through the collection and analysis of administrative or health record data (27, 34). Lastly, agency partners identified the following, single performance objective for staff: To implement the HCFS with 100% of home-delivered meal clients—funded through Title-IIIC—at baseline as well as 3-months and 6-months after program enrollment, for all clients still enrolled in a meal plan.
Identified determinants from the CFIR, recommendations from the CFIR-ERIC matching tool, and input from agency leadership and staff informed our selection of five implementation strategies to address the determinants of networks and communications, available resources, and compatibility. These included (a) conduct ongoing training, (b) identify and prepare a HCFS champion, (c) complete pilot testing, (d) change record systems, and (e) perform chart audits and provide feedback.
The five implementation strategies designed and deployed by our team are described below and specified in (Table 1).
When developing the structure for HCFS staff training, our team purchased the HCFS training manual ($65) (35), which contained instructions for how to administer and interpret each of the 29 HCFS items. We then converted the training manual into a presentation format that was delivered to home-delivered meal staff members during an initial training session. Initial training consisted of an in-depth review of all HCFS items, examples of how to score the HCFS, demonstration of how to document the HCFS (see “Change record systems” description below), and a question-and-answer session. Five months after initial training, a 1-h follow-up “booster” training session was held. Training materials were updated with additional examples of how to administer and interpret client responses to individual HCFS items. Staff were also provided a “cheat sheet” document for interpretation and scoring of item responses.
The agency's assistant director of nutrition programs held the role of HCFS champion. In addition to their leadership within the agency, the champion had extensive knowledge of agency workflow and oversaw assessment procedures completed by staff. In this role, the HCFS champion received advanced training in administering and interpreting the HCFS, facilitated our research team's receipt of monthly HCFS data files for auditing and analysis, and maintained a tracking log of clients to indicate when each baseline HCFS was completed as well as anticipated dates for 3-month and 6-month HCFS collection. Each month, the HCFS champion emailed staff the updated tracking log and also sent weekly emails containing a list of clients due to have their HCFS completed. Staff were responsible for administering the HCFS within a 14-day window of clients' estimated 3-or 6-month HCFS follow-up date. Moreover, the champion held biweekly phone calls with our research team to discuss concerns with HCFS implementation and to clarify discrepancies in staff's interpretation of individual HCFS items.
After initial HCFS training, home-delivered meal staff (n = 7) pilot tested the HCFS with a minimum of 10 clients over a 30-day period. Piloting the HCFS allowed for home-delivered meal staff and leadership to gain comfort with its administration and allowed our research team to clarify any challenges with HCFS interpretation prior to formally rolling out the HCFS with all home-delivered meal staff and clients. Results from pilot testing also informed how our research team structured “booster” training sessions with staff, such as by including specific examples of how to interpret/score responses to each HCFS item.
As part of our agency's routine operating procedures, all standard in-take assessments were completed by staff and entered into the agency's main EHR system. Building HCFS items into the main EHR required involvement from programmers external to the agency, thus complicating the extent to which staff could feasibly document the HCFS electronically. As a solution to this documentation issue, the agency's information technology (IT) department developed their own HCFS website that allowed staff to document HCFS data electronically but separately from the agency's EHR system. Staff accessed the HCFS website to enter the following data: (a) client ID, (b) date HCFS was attempted, (c) HCFS completion [yes/no], (d) reason if HCFS was not completed [unable to reach client after three attempts, client unenrolled from services, client deceased, client on hold, etc.], (e) responses to all 29 HCFS items, (f) date follow-up HCFS was expected, and (g) name of staff member completing the HCFS.
The HCFS champion shared monthly data sets with our research team who monitored the extent to which staff completed the HCFS at baseline, 3-month, and 6-month time periods. Implementation rates were reported to agency leadership during each monthly team call. When rates of implementation fell below 60–70%, the HCFS champion provided additional reminders to staff via email and encouraged staff to share any challenges they experienced relative to HCFS use or scoring. Further details on this audit-and-feedback approach and our four additional implementation strategies are described in Table 1.
A total of 13 staff members implemented the HCFS with home-delivered meal clients between June 2020–May 2021 with higher rates of implementation noted among staff members with the most years of service to our partner agency (Table 2). Analyses from our retrospective chart review indicated that rates of implementation were highest in June 2020 (94.6%) and lowest in May 2021 (57.1%) (Figure 3). Staff completed the HCFS with 94.8% of eligible clients at the baseline timepoint. Of those who completed the HCFS at baseline, however, staff were only able to obtain HCFS data from 43% of clients at 3-months and 18% of clients at 6-months.
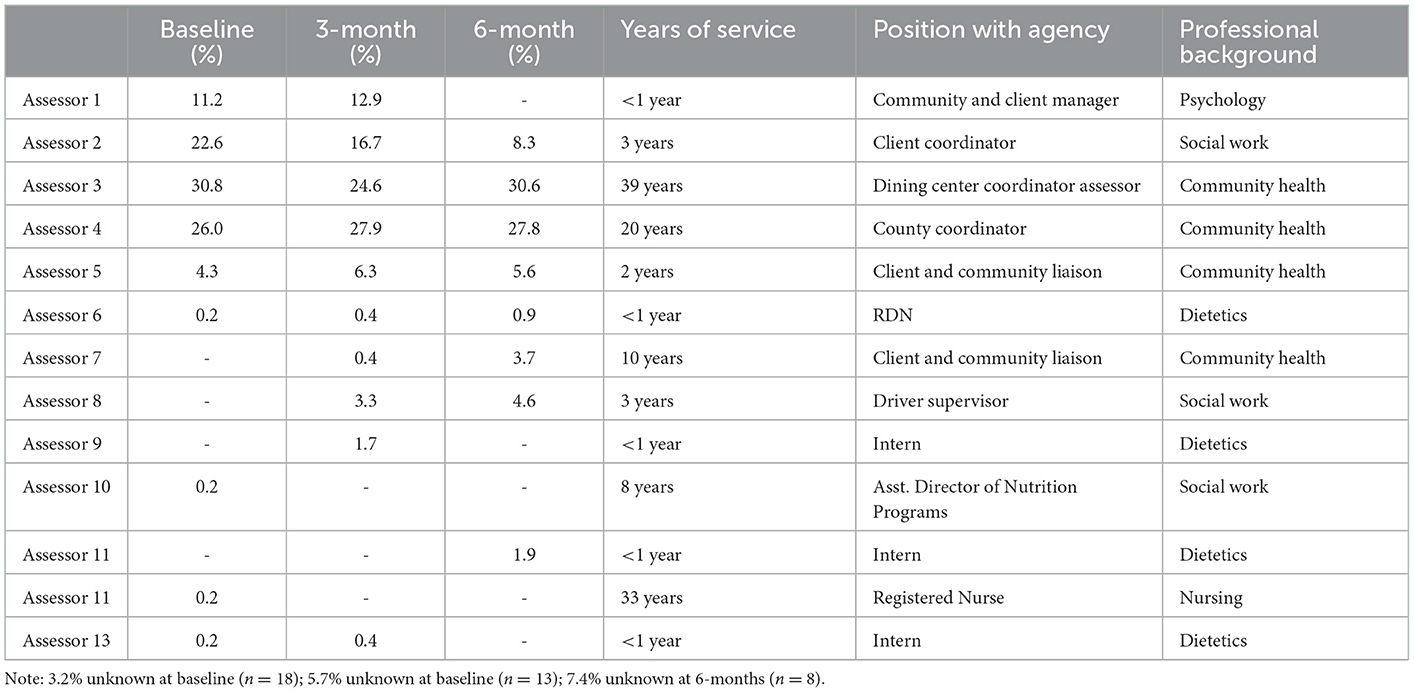
Table 2. Agency assessment staff and rates of Home Care Frailty Scale implementation at baseline, 3-months, and 6-months.
At baseline, the most common reason staff were unable to complete the HCFS was attributed to clients (n = 13) being “on hold” as they were recently hospitalized or admitted to a care facility (e.g., rehabilitation facility), had a family member who could temporarily provide nutritional support, or were in the process of relocating. At 3-month and 6-month follow-up, clients having “unenrolled” from their meal plan was the most frequently documented reason staff could not complete the HCFS (n = 77 at 3-months; n = 24 at 6-months). Overwhelmingly, however, staff did not or were not able to document the reasons clients were lost to follow-up (Figure 4).
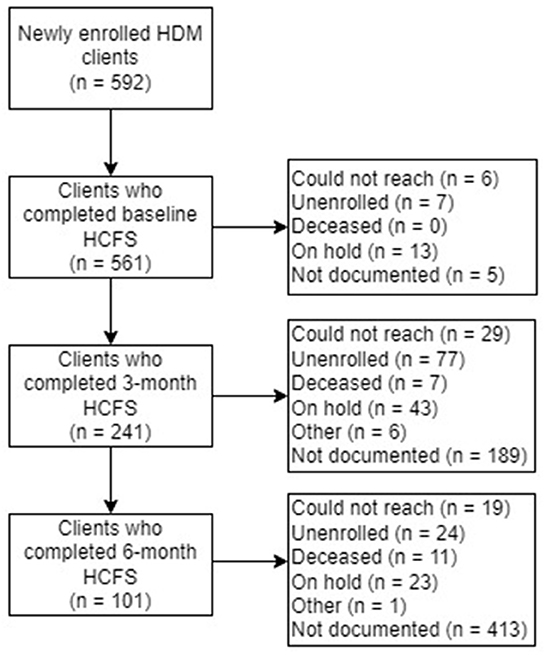
Figure 4. Home-delivered meals (HDM) clients who completed the Home Care Frailty Scale (HCFS) at baseline, 3-months, and 6-months.
At the client-level, while age was not associated with baseline or 3-month HCFS completion, 6-month attrition was significantly more likely among older clients (p < 0.01). Relatedly, there was no significant difference by race between those who completed and those who did not complete the baseline or 3-month HCFS; however, there was a significant difference at the 6-month time point in that clients who identified as African-American or black were more likely to complete the 6-month HCFS compared to those who identified as white (p = 0.04). Lastly, clients living with relatives were significantly more likely to complete the baseline HCFS (p = 0.03) as compared to clients who indicated an “other” household composition. There were no significant differences in HCFS follow-up based on gender, marital status, or county of residence (i.e., rural-urban residence).
Clients who completed the HCFS at 3-months but not 6-months had significantly higher 3-month total HCFS scores (p = 0.02). In other words, those with greater frailty at the 3-month time point were significantly less likely to complete the 6-month HCFS (Table 3). Table 4 presents the average baseline, 3-month, and 6-month HCFS scores by domain, including total HCFS scores. With the exception of the social interaction domain score at the 6-month time point, all domain scores and total HCFS scores indicated a downward trend—or improvement in frailty—based on available data.
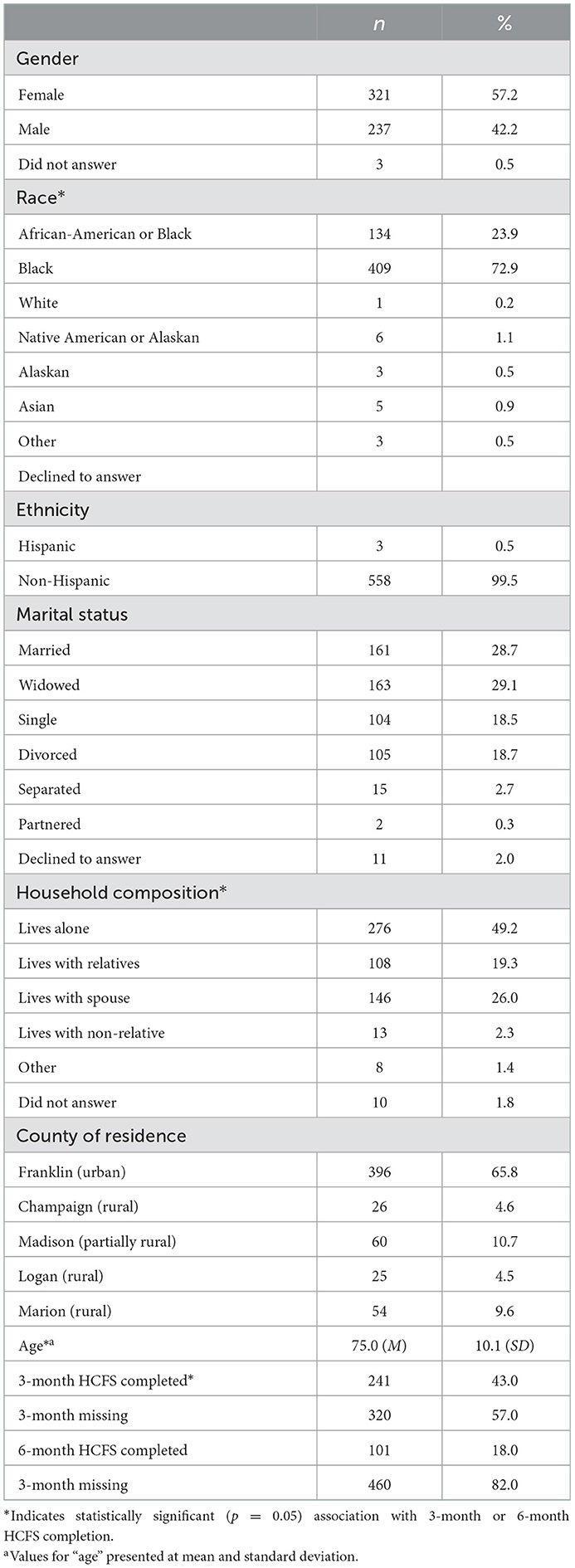
Table 3. Demographic characteristics of clients who completed the baseline home care frailty scale (HCFS) (n = 561).
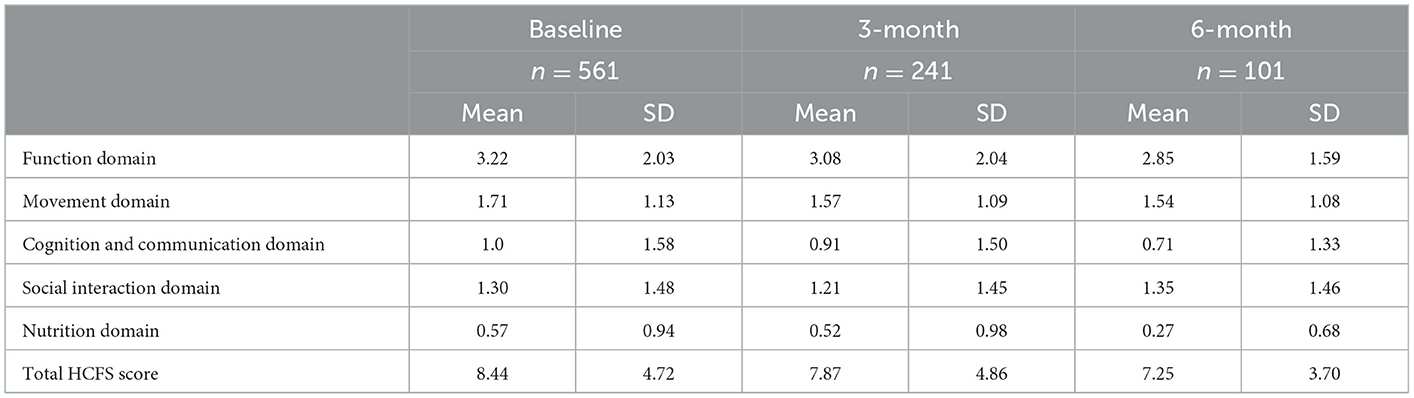
Table 4. Home care frailty scale (HCFS) scores at baseline, 3-month, and 6-month time points by domain.
This study examined one home-delivered meal agency's process of implementing the HCFS—an instrument for measuring the frailty levels of home-delivered meal clients at multiple time points. Although our strategies to support HCFS use by home-delivered meal staff were systematically developed using principles from Implementation Mapping (24, 28), our strategies did not lead to the feasible collection of HCFS data from clients overtime. Though staff demonstrated a high rate of HCFS implementation at baseline (94.8%), our findings also indicated major challenges to collecting follow-up HCFS data at 3-month and 6-month time points for reasons such as staff being unable to contact clients, clients having meal deliveries placed on temporary “holds,” or clients becoming unenrolled from services. Our analyses also indicated that certain demographic factors, particularly older age, race, and household composition, were associated with attrition at either the 3-month or 6-month time point. Importantly, clients with greater frailty scores (or worse frailty) at 3-months were significantly less likely to complete the 6-month HCFS, potentially indicating that clients with greater 3-month HCFS scores may be more vulnerable to health decline and in need of additional services or interventions, though these findings require further investigation to understand reasons for attrition.
Beyond factors at the client-level, there were certainly agency-level obstacles that influenced the feasibility of HCFS implementation. Insights provided by our agency partners, described below, shed light on these complex challenges and opportunities for improvement given the importance of monitoring the frailty levels of home-delivered meal clients over time. Specifically, these insights draw attention to the intricate details that may have been overlooked when our agency and research team members were designing strategies to support HCFS implementation. Consistent with our guiding framework, insights are organized by constructs from the CFIR (30).
Perhaps the most significant agency-level factor influencing HCFS implementation was the manner in which it was documented and how HCFS data were communicated across the agency. Given that modifications within the main EHR system could not be made directly by the agency, the agency's IT department built a custom website—accessible only to agency staff—for HCFS documentation. Though this solution was initially a viable option to support HCFS use, it ultimately posed challenges for our team over the course of our study period. For instance, as indicated in Figure 2, both the in-take assessment and HCFS evaluated areas related to functional status and nutrition. These areas of overlap were duplicative and interrupted the flow of staff's assessment procedures, especially as staff were required to access two different documentation systems to complete all in-take and baseline HCFS items. This interruption also hindered staff's natural course of conversation in the first encounter during which staff could build rapport with clients. Further, staff found substantial difficulty in tracking which clients needed to be contacted for their 3-month and 6-month HCFS. Although the HCFS champion's tracking log and reminder emails helped alert staff when they needed to contact clients for follow-up, these reminders did not indicate if clients were still actively receiving meals, nor did they list client phone numbers. Accordingly, staff were expected to log into their EHR system account, verify that the client had an active meal plan, and obtain the client's phone number, increasing the total amount of time staff were expected to dedicate to HCFS follow-up activities.
Our agency's lack of integrated documentation systems also limited the extent to which HCFS data could be entered longitudinally for individual clients. To accommodate for this barrier, the research team had one dedicated member who was responsible for merging baseline, 3-month, and 6-month HCFS data together for individual clients, but this was not a sustainable solution for tracking client frailty levels. An additional, though minor, barrier to HCFS implementation was staff's lack of access to dual-monitor computers during standard in-take assessments. After completing in-take assessments in the EHR system, staff immediately transitioned to implementing the baseline HCFS with clients. However, given the challenge of logging out of the EHR system, opening a web browser, and accessing the custom HCFS website on the same computer, staff often completed the HCFS on a paper form and entered client responses—at a later time—through the HCFS website. The timing of data entry, though, was occasionally delayed given staff's other demands and work responsibilities.
The concept and format of the HCFS were initially perceived to be compatible by our agency's administrative leaders. Despite these perceptions, leaders later expressed their concern that the information collected via the HCFS was being underutilized internally. Once HCFS implementation began, agency staff and leaders quickly learned of the additional needs of clients (e.g., mobility needs, social interaction needs) that were not necessarily being met by meal delivery alone. Our agency partners expressed their discomfort with assessing but not addressing these needs that were revealed as a result of implementing the HCFS longitudinally.
Given that documentation was arguably the primary barrier to HCFS implementation, integrating the 29 HCFS items directly into the agency's main EHR system could have likely streamlined documentation, particularly during the baseline period where staff completed both the in-take assessment and HCFS. Going forward, centralizing this information in one location has the opportunity to decrease staff burden, improve assessment workflow, and enhance staff's interaction with clients (36). The return to in-person baseline assessments, pending statewide adjustments to COVID-19 restrictions, may also facilitate more streamlined assessments of frailty as staff can leverage their professional judgment and observational skills to determine the extent to which frailty domains (e.g., mobility, ADLs) are impaired (37, 38).
Notably, staff who implemented the HCFS were partially compensated through a demonstration project grant which reduced the agency's expenditures toward implementation activities. As these funds were temporary, alternative strategies are needed to support staff's future ability to implement the HCFS feasibly and more consistently. Integrating HCFS items into the agency's main EHR system is a first step toward minimizing assessment and documentation burden on staff. Though, while customized changes to EHR systems have shown promise for improving the quality of staff documentation behaviors (39), these system-level changes may need to be augmented by additional sources of support to promote assessment implementation (40). One additional option for this support is through clinical alerts which have served as effective reminders for staff who are involved in client documentation activities (41, 42). These alerts may take the forms of e-mails, electronic “flags” directly within client charts, and/or pop-up notices within the EHR. Such alert systems can also be configured to deliver text message reminders to staff if documentation is not completed for clients on a specified date (43). While these alert systems have led to improvements in documentation, there is also the threat of “alert fatigue” which may negatively impact staff job performance and satisfaction (44). Thus, use of these alerts, how often they are triggered and under what circumstances should be thoughtfully considered in collaboration with staff and leaders involved in documentation procedures (45).
Implementation of the HCFS revealed frailty-related needs (e.g., fall risk factors, mental health concerns) that staff did not feel fully equipped to address. Although home-delivered meal providers can serve as “gatekeepers” to other community-based services and supports for older adults (46), our agency's staff were not sufficiently aware of recommendations and local resources that could be shared with clients who indicated specific frailty needs. Cataloging these resources for staff, prior to the study period, may have facilitated their ability to make recommendations or referrals to other health and nutrition services, thereby improving the “fit” of the HCFS with the mission of our partner agency to maximize older adult health and wellbeing (19, 47).
Drawing from our experiences and from the existing literature, we have identified several actionable steps to improve HCFS implementation over time. Our first step is to re-evaluate the “fit” of the HCFS within the workflow and available staff of our partner agency. Given that the time required for staff to complete the HCFS with clients ranged from 15 to 20 min, a shorter scale with similar psychometric strengths may be more compatible with our agency's resources. As one example, the FRAIL scale is a validated tool that can predict functional decline in older adults and can be administered over the phone in < 5 min (48, 49), though it has not yet been implemented specifically in the home-delivered meal context. Implementing a shorter, simpler frailty instrument, such as the FRAIL scale, may also enable staff to monitor frailty more frequently, optimizing the completeness of data collection. With our high rate of attrition based on a variety of reasons (e.g., unable to reach clients; clients unenrolling from meal services), an increase in the frequency of frailty instrument administration may provide our partner agency, and other peer agencies, with more robust data that could be used to examine changes in frailty more consistently and identify clients in need of more comprehensive care. Operationally, the implementation of a simpler frailty tool could serve as a supplement to the monthly socialization “check-in” phone calls that our partner agency has previously completed with clients. Frequent monitoring of frailty may be particularly beneficial with clients who live in the more rural counties of our partner agency's service area. For instance, several rural clients receive frozen meals delivered once every 1–2 weeks as compared to urban-dwelling clients who often receive daily-delivered meals. As previously studied, clients who received daily-delivered meal services—and had more regular contact with drivers compared to frozen meal clients–experienced improved health outcomes (e.g., reduced falls; reduced feelings of loneliness) (50, 51), which has implications for rural-dwelling older adults who experience frailty at a higher rate than national estimates (52). Though daily-delivered meals may not be a feasible option for our partner agency to provide to all their rural clients, more frequent check-ins, such as through phone-based screenings of frailty, may help capture the frailty-related needs of these high-risk clients and inform care planning and service delivery. Notably, our analysis did not yield associations between rurality and HCFS attrition, but this may be attributed to clients' closer proximity to the greater metropolitan area of Columbus, Ohio.
To administer a simpler frailty tool, however, we must ensure that our partner agency has the sufficient capacity to do so. In addition to appointing specific staff members to be responsible for tool administration and adjusting electronic documentation systems, staff should have access to initial training as well as ongoing consultations to confirm procedures for administration, documentation, and interpretation of results. The combination of training and follow-up consultations has been previously found effective for supporting the uptake of evidence-based interventions and assessments (53, 54), particularly in the community setting. Lastly, of the available data our partner agency's staff collected from clients beyond May 2021, we identified that clients had unmet needs, especially in the domains of function and mobility, that could not be addressed through home-delivered meal services alone. Thus, our partner agency and research team are currently in the process of developing a data-driven care model that will specifically address the functional- and mobility-related needs of clients at greatest risk for malnutrition.
Although this study makes several, unique contributions to the understudied home-delivered meal context, it is not without limitations. First, our application of Implementation Mapping principles could have been strengthened by selecting a behavioral change theory or adult learning theory to guide predictions about staff's HCFS use. While our implementation strategy selection was informed by the CFIR (30)—a comprehensive implementation framework—frameworks are not explanatory in nature and can rarely help predict relationships among theoretical constructs (55, 56). Second, we also recognize that our strategies only targeted agency-level implementation determinants whereas policy-level and staff-level determinants may have also played an influential role in our implementation efforts. Third, given that this was a retrospective, observational study, we did not conduct an a priori power analysis but rather assessed the rate at which the HCFS was implemented with clients who were served over a 12-month time frame, as recommended for studies of implementation that include chart review methodology (33). Fourth, though the HCFS has evidence of acceptable criterion-related validity, additional psychometric properties (e.g., predictive validity, interrater reliability) are unknown (25). Lastly, we did specify our five implementation strategies (Table 1) per expert recommendations, yet more robust details are needed to understand the mechanisms that promoted—or hindered—HCFS implementation (56, 57). In addition to our own future work, we encourage other teams to routinely track their implementation activities to obtain thorough information on the types of implementation activities completed, their purpose, their duration, and the individuals involved (58, 59).
Home-delivered meal agencies are essential for providing health and nutrition services to a population of older adults at great risk for frailty-related health decline. Frailty instruments, such as the Home Care Frailty Scale (25), can serve as tools to help home-delivered meal staff assess and monitor the frailty levels of their clients. However, prior to adopting such instruments, home-delivered meal providers are strongly encouraged to comprehensively evaluate and address barriers that pertain to the longitudinal electronic documentation of frailty data, the staff and resources needed to implement frailty instruments consistently, and the extent to which instruments “fit” within agency workflow, standard practices, and values.
The datasets presented in this article are not readily available because data collected and analyzed for this manuscript are not publicly available as to comply with our partner agency's data sharing policies; however, materials used to deploy our implementation strategies with staff are available upon request made to the corresponding author. Requests to access the datasets should be directed to bGlzYS5qdWNrZXR0QG9zdW1jLmVkdQ==.
The studies involving human participants were reviewed and approved by the Institutional Review Board at The Ohio State University. The Ethics Committee waived the requirement of written informed consent for participation.
LJ conceptualized this study, led study activities, led manuscript development, and performed statistical analyses. HO completed data management and preliminary data analysis activities. GH assisted with developing the study design and developed the approach to data analysis. LB and AD completed project management activities and provided agency insights, as described in the Discussion Section. All authors contributed to manuscript development and approved the final submitted version.
This project was supported, in part by grant number 90INNU0016, from the Administration for Community Living, U.S. Department of Health and Human Services, Washington, D.C. 20201. Grantees undertaking projects with government sponsorship are encouraged to express freely their findings and conclusions. Points of view or opinions do not, therefore, necessarily represent official ACL policy.
The authors extend their sincere gratitude to Charles W. Gehring and John R. Gregory for their support throughout this project and their dedication to improving the health and nutrition of older Central Ohioans. We also thank Dr. Heather Hutchins-Wiese for her recommendation to consider monitoring frailty among home-delivered meal clients.
LB and AD were employed by the company Lifecare Alliance.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Berkowitz SA, Terranova J, Hill C, Ajayi T, Linsky T, Tishler LW, et al. Meal delivery programs reduce the use of costly health care in dually eligible medicare and medicaid beneficiaries. Health Aff. (2018) 37:535–42. doi: 10.1377/hlthaff.2017.0999
2. Lloyd JL, Wellman NS. Older Americans act nutrition programs: a community-based nutrition program helping older adults remain at home. JNutrGerontolGeriatr. (2015) 34:90–109. doi: 10.1080/21551197.2015.1031592
3. Choi NG, Sullivan JE, Marti CN. Low-income homebound older adults receiving home-delivered meals: physical and mental health conditions, incidence of falls and hospitalizations. Health Soc Care Commun. (2019) 27:e406–16. doi: 10.1111/hsc.12741
4. Sonnega A, Robinson K, Levy H. Home and community-based service and other senior service use: prevalence and characteristics in a national sample. Home Health Care Serv Q. (2017) 36:16–28. doi: 10.1080/01621424.2016.1268552
5. Codebook_Home_Meals_2018.pdf. Available online at: https://agid.acl.gov/ (accessed January 6, 2021).
6. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
7. Costenoble A, Knoop V, Vermeiren S, Vella RA, Debain A, Rossi G, et al. Comprehensive overview of activities of daily living in existing frailty instruments: a systematic literature search. Gerontologist. (2021) 61:e12–22. doi: 10.1093/geront/gnz147
8. Welstead M, Jenkins ND, Russ TC, Luciano M, Muniz-Terrera G, A. Systematic review of frailty trajectories: their shape and influencing factors. Gerontologist. (2021) 61:e463–75. doi: 10.1093/geront/gnaa061
9. Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. (2011) 27:1–15. doi: 10.1016/j.cger.2010.08.009
10. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. (2018) 34:25–38. doi: 10.1016/j.cger.2017.09.004
11. Mendiratta P, Schoo C, Latif R. Clinical Frailty Scale. StatPearls Publishing. (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK559009/ (accessed December 29, 2022).
12. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–156. doi: 10.1093/gerona/56.3.m146
13. Mondor L, Maxwell CJ, Hogan DB, Bronskill SE, Campitelli MA, Seitz DP, et al. The incremental health care costs of frailty among home care recipients with and without dementia in Ontario, Canada: a cohort study. Med Care. (2019) 57:512–20. doi: 10.1097/MLR.0000000000001139
14. Evenhuis HM, Hermans H, Hilgenkamp TIM, Bastiaanse LP, Echteld MA. Frailty and disability in older adults with intellectual disabilities: results from the healthy ageing and intellectual disability study. J Am Geriatr Soc. (2012) 60:934–8. doi: 10.1111/j.1532-5415.2012.03925.x
15. Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. (2015) 70:1427–34. doi: 10.1093/gerona/glv133
16. Macdonald SH-F, Travers J, Shé ÉN, Bailey J, Romero-Ortuno R, Keyes M, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS ONE. (2020) 15:e0228821. doi: 10.1371/journal.pone.0228821
17. Abbasi M, Khera S, Dabravolskaj J, Garrison M, King S. Identification of frailty in primary care: feasibility and acceptability of recommended case finding tools within a primary care integrated seniors' program. Gerontol Geriatr Med. (2019) 5:2333721419848153. doi: 10.1177/2333721419848153
18. Karunananthan S, Bergman H. Managing frailty in primary care: evidence gaps cannot be ignored. CMAJ. (2018) 190:E1122–3. doi: 10.1503/cmaj.181151
19. Morris AM, Engelberg JK, Schmitthenner B, Dosa D, Gadbois E, Shield RR, et al. Leveraging home-delivered meal programs to address unmet needs for at-risk older adults: preliminary data. J Am Geriatr Soc. (2019) 67:1946–52. doi: 10.1111/jgs.16013
20. Hutchins-Wiese HL, Walsh SE. Frailty and nutrition risk screening in home-delivered meal clients. J Nutr Gerontol Geriatr. (2020) 39:114–30. doi: 10.1080/21551197.2020.1719258
21. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. (2019) 12:23–30. doi: 10.2147/RMHP.S168750
22. Campbell A, Godfryd A, Buys D. Does participation in home-delivered meals programs improve outcomes for older adults? Results of a systematic review. J Nutri. (2015) doi: 10.1080/21551197.2015.1038463
23. Thomas KS. Outcomes matter: the need for improved data collection and measurement in our nation's home-delivered meals programs. J Nutr Gerontol Geriatr. (2015) 34:85–9. doi: 10.1080/21551197.2015.1031591
24. Fernandez ME, ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. (2019) 7:158. doi: 10.3389/fpubh.2019.00158
25. Morris JN, Howard EP, Steel KR. Development of the interRAI home care frailty scale. BMC Geriatr. (2016) 16:188. doi: 10.1186/s12877-016-0364-5
26. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation Science. (2013) 8:139. doi: 10.1186/1748-5908-8-139
27. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
28. Kang E, Foster ER. Use of implementation mapping with community-based participatory research: development of implementation strategies of a new goal setting and goal management intervention system. Front Public Health. (2022) 10:4473. doi: 10.3389/fpubh.2022.834473
29. Juckett LA, Bunger AC, Jarrott SE, Dabelko-Schoeny HI, Krok-Schoen J, Poling RM, et al. Determinants of fall prevention guideline implementation in the home-and community-based service setting. Gerontologist. (2021) 61:942–53. doi: 10.1093/geront/gnaa133
30. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:50. doi: 10.1186/1748-5908-4-50
31. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. refined compilation of implementation strategies: results from the expert recommendations for implementing change. (ERIC) Project Implement Sci. (2015) 10:21. doi: 10.1186/s13012-015-0209-1
32. Waltz TJ, Powell BJ, Fernández ME, Abadie B, Damschroder LJ. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci. (2019) 14:42. doi: 10.1186/s13012-019-0892-4
33. Prusaczyk B, Fabbre V, Carpenter CR, Proctor E. Measuring the delivery of complex interventions through electronic medical records: challenges and lessons learned. EGEMS. (2022) 6:230. doi: 10.5334/egems.230
34. Lengnick-Hall R, Gerke DR, Proctor EK, Bunger AC, Phillips RJ, Martin JK, et al. Six practical recommendations for improved implementation outcomes reporting. Implement Sci. (2022) 17:16. doi: 10.1186/s13012-021-01183-3
35. interRAI, Home Care. (HC) Assessment Form and User's Manual,. (Standard English Edition), 9.1.2 | interRAI Catalog. Available online at: https://catalog.interrai.org/content/interrai-home-care-hc-assessment-form-and-users-manual-standard-english-edition-912 (accessed June 29, 2022).
36. Guo U, Lu Chen, Mehta PH. Electronic health record innovations: helping physicians—One less click at a time. Health Inform Manag J. (2017) 46:140–4. doi: 10.1177/1833358316689481
37. Feuering R, Vered E, Kushnir T, Jette AM, Melzer I. Differences between self-reported and observed physical functioning in independent older adults. Disabil Rehabil. (2014) 36:1395–401. doi: 10.3109/09638288.2013.828786
38. Nielsen LM, Kirkegaard H, Østergaard LG, Bovbjerg K, Breinholt K, Maribo T. Comparison of self-reported and performance-based measures of functional ability in elderly patients in an emergency department: implications for selection of clinical outcome measures. BMC Geriatr. (2016) 16:199. doi: 10.1186/s12877-016-0376-1
39. Richardson KJ, Sengstack P, Doucette JN, Hammond WE, Schertz M, Thompson J, et al. Evaluation of nursing documentation completion of stroke patients in the emergency department: a pre-post analysis using flowsheet templates and clinical decision support. Comput Inform Nurs. (2016) 34:62–70. doi: 10.1097/CIN.0000000000000206
40. Carpenter JG, Scott WJ, Kononowech J, Foglia MB, Haverhals LM, Hogikyan R, et al. Evaluating implementation strategies to support documentation of veterans' care preferences. Health Serv Res. (2022) 1:3958. doi: 10.1111/1475-6773.13958
41. Giovannelli J, Coevoet V, Vasseur C, Gheysens A, Basse B, Houyengah F. How can screening for malnutrition among hospitalized patients be improved? An automatic e-mail alert system when admitting previously malnourished patients. Clin Nutr. (2015) 34:868–73. doi: 10.1016/j.clnu.2014.09.008
42. Hamade N, Terry A, Malvankar-Mehta M. Interventions to improve the use of EMRs in primary health care: a systematic review and meta-analysis. BMJ Health Care Inform. (2019) 26:0. doi: 10.1136/bmjhci-2019-000023
43. Tollinche LE, Shi R, Hannum M, McCormick P, Thorne A, Tan KS, et al. The impact of real-time clinical alerts on the compliance of anesthesia documentation: a retrospective observational study. Comput Methods Programs Biomed. (2020) 191:105399. doi: 10.1016/j.cmpb.2020.105399
44. Kizzier-Carnahan V, Artis KA, Mohan V, Gold JA. Frequency of passive EHR alerts in the ICU: another form of alert fatigue? J Patient Saf. (2019) 15:246–50. doi: 10.1097/PTS.0000000000000270
45. Shultz CG, Malouin JM, Green LA, Plegue M, Greenberg GM, A. Systems approach to improving tdap immunization within 5 community-based family practice settings: working differently. (and Better) by transforming the structure and process of care. Am J Public Health. (2015) 105:1990–7. doi: 10.2105/AJPH.2015.302739
46. Thomas KS, Smego R, Akobundu U, Dosa D. Characteristics of older adults on waiting lists for meals on wheels: identifying areas for intervention. J Appl Gerontol. (2017) 36:1228–42. doi: 10.1177/0733464815614918
47. Juckett LA, Bunger AC, Bunck L, Balog EJ. Evaluating the implementation of fall risk management practices within home-delivered meal organizations. J Gerontol Soc Work. (2021) 64:372–87. doi: 10.1080/01634372.2021.1894521
48. Morley Je M, Miller Dk. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. Af Am J Nutr Health Aging. (2012) 16:601–8. doi: 10.1007/s12603-012-0084-2
49. Kan GA, van, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Direct Assoc. (2008) 9:71–2. doi: 10.1016/j.jamda.2007.11.005
50. Thomas KS, Parikh RB, Zullo AR, Dosa D. Home-delivered meals and risk of self-reported falls: results from a randomized trial. J Appl Gerontol. (2018) 37:41–57. doi: 10.1177/0733464816675421
51. Thomas KS, Akobundu U, Dosa D. More than a meal? A randomized control tria l comparing the effects of home-delivered meals programs on participants' feelings of loneliness. J Gerontol Series B: Psychol Sci Soc Sci. (2016) 71:1049–58. doi: 10.1093/geronb/gbv111
52. Xu R, Li Q, Guo F, Zhao M, Zhang L. Prevalence and risk factors of frailty among people in rural areas: a systematic review and meta-analysis. BMJ Open. (2021) 11:e043494. doi: 10.1136/bmjopen-2020-043494
53. Lyon AR, Liu FF, Connors EH, King KM, Coifman JI, Cook H, et al. How low can you go? Examining the effects of brief online training and post-training consultation dose on implementation mechanisms and outcomes for measurement-based care. Implement Sci Commun. (2022) 3:79. doi: 10.1186/s43058-022-00325-y
54. Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. (2012) 63:660–5. doi: 10.1176/appi.ps.201100401
55. Birken SA, Rohweder CL, Powell BJ, Shea CM, Scott J, Leeman J, et al. T-CaST: an implementation theory comparison and selection tool. Implement Sci. (2018) 13:143. doi: 10.1186/s13012-018-0836-4
56. Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, Walsh-Bailey C, Weiner B. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health. (2018) 6:136. doi: 10.3389/fpubh.2018.00136
57. Lewis CC, Boyd MR, Walsh-Bailey C, Lyon AR, Beidas R, Mittman B, et al. A systematic review of empirical studies examining mechanisms of implementation in health. Implement Sci. (2020) 15:21. doi: 10.1186/s13012-020-00983-3
58. Bunger AC, Powell BJ, Robertson HA, MacDowell H, Birken SA, Shea C. Tracking implementation strategies: a description of a practical approach and early findings. Health Res Policy Sys. (2017) 15:15. doi: 10.1186/s12961-017-0175-y
Keywords: evidence-based practice, home-and community-based care and services, evaluation, nutrition, implementation science, knowledge translation, pragmatic trials
Citation: Juckett LA, Oliver HV, Hariharan G, Bunck LE and Devier AL (2023) Strategies for implementing the interRAI home care frailty scale with home-delivered meal clients. Front. Public Health 11:1022735. doi: 10.3389/fpubh.2023.1022735
Received: 18 August 2022; Accepted: 05 January 2023;
Published: 23 January 2023.
Edited by:
Danielle Hitch, Deakin University, AustraliaReviewed by:
David Hollar, Mercer University School of Medicine, United StatesCopyright © 2023 Juckett, Oliver, Hariharan, Bunck and Devier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa A. Juckett,  bGlzYS5qdWNrZXR0QG9zdW1jLmVkdQ==
bGlzYS5qdWNrZXR0QG9zdW1jLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.