- National Clinical Research Center for Metabolic Diseases, Key Laboratory of Diabetes Immunology, Ministry of Education, and Department of Metabolism and Endocrinology, The Second Xiangya Hospital of Central South University, Changsha, China
Objective: Regarding the effects and practical application of insulin pumps on patients with type 1 diabetes mellitus (T1DM), the real-world evidence is limited especially concerning the incidence of hypoglycemia. This study aimed to compare the efficacy of continuous subcutaneous insulin infusion (CSII) therapy with multiple daily injection (MDI) therapy on glycemic metrics evaluated by retrospective continuous glucose monitoring (CGM) in Chinese patients with T1DM.
Methods: In total, 362 T1DM Chinese patients from the outpatient department of the Second Xiangya Hospital, Central South University, who underwent intensive insulin therapy and used a retrospective CGM system were included in this retrospective cross-sectional study. Comprehensive analysis of clinical and biological features and retrospective CGM derived-metrics was performed on the 362 enrolled T1DM patients who underwent CSII (n = 61) or MDI (n = 301) therapy (defined as 4 or more insulin injections per day).
Results: Our findings demonstrated that patients who underwent CSII therapy, compared with those who received MDI therapy, had lower levels of hemoglobin A1c (HbA1c) and fasting blood glucose; moreover, CSII therapy was associated with better glycemic outcomes in terms of increasing time in range (TIR), decreasing time above range (TAR), and achieving CGM-associated targets of TIR ≥70% and TAR <25%. However, patients who underwent CSII therapy did not experience decreasing time below range (TBR), achieving CGM-associated targets of TBR <4%, and reduction of the risk of hypoglycemia as evidenced by comparing TBR and low blood glucose index (LBGI) between the two treatment regimens. The parameters of glycemic variability, such as standard deviation of glucose (SD), mean amplitude glycemic excursion (MAGE), and large amplitude glycemic excursion (LAGE) in T1DM patients who underwent CSII therapy outperformed.
Conclusion: Our results provided further evidence that CSII therapy is safe and effective for management of Chinese T1DM patients, which was confirmed by a lower HbA1c level and better CGM-derived metrics but no demonstration of improvment in the risk of hypoglycemia. To achieve more satisfactory glycemic outcomes through the utilization of CSII therapy for Chinese T1DM patients, a strong physician-patient relationship is essential.
Introduction
Type 1 diabetes mellitus (T1DM) is a progressive disease as a result of the severe destruction of islet β-cell function, and exogenous insulin is not only essential for more efficiently control of blood glucose levels, but also for T1DM patients to stay in life. (1). In the 100 years since the discovery of insulin, a great progress has been made in our ability to manage T1DM effectively, largely because of the improvements in insulin formulation and delivery (2). Daily multiple insulin injections (MDI) therapy is a well-established intensive therapy, and this basal-bolus therapy is the best therapeutic option for patients with T1DM for a long time, until emergence and development of continuous subcutaneous insulin infusion (CSII) using insulin pumps. An insulin pump mimics the physiological situation by combining a continuous insulin infusion rate to cover the basal insulin requirements with additional bolus deliveries to cover prandial insulin requirements. To date, a number of randomized clinical trials (RCTs) suggested lower levels of hemoglobin A1c (HbA1c) with CSII than with MDI (3). However, whether CSII increase the risk of hypoglycemic events are controversial. With the increasing use of insulin pumps in real-world, the frequency of hypoglycemic events in patients with T1DM has noticeably increased, and the safety of insulin pump therapy has also been concerned (4, 5). Several meta-analyses showing that CSII does not increase the risk of adverse events (maybe with the exception of diabetic ketoacidosis), additionally, and some researches also suggested that a reduction in the incidence of hypoglycemia with pump use compared to MDI (6–10).Although the use of insulin pumps for intensive insulin therapy among patients with T1DM has substantially increased in several developed countries (11), CSII has not been widely used in China (12). A recent multicenter survey of T1DM in Chinese children indicated that only 15.21% of participants received insulin pump therapy. The application of insulin pump therapy varies widely that could be related to the following factors: local economic level, social health insurance, and concerns associating with therapeutic efficacy and hypoglycemic risk in clinical practice.
Regarding the applicability of insulin pump therapy for Chinese patients with T1DM, the real-world evidence is limited, especially concerning hypoglycemia. A recent study examined the effects of CSII therapy on HbA1c level of Chinese patients with T1DM, and found that CSII therapy was associated with a better blood glucose control characterized by a lower HbA1c level. HbA1c level has long been regarded as the gold standard for long-term glycemic control. However, there are several HbA1c-associated limitations, particularly because HbA1c does not always provide an accurate measure of average glucose level and HbA1c does not reflect glycemic excursions (13). Compared with HbA1c, continuous glucose monitoring (CGM) can provide a better and more complete assessment of the glycemic outcomes. In this scenario, HbA1c and CGM-derived metrics are both equally used in T1DM management. CGM has been demonstrated to be clinically valuable, reducing risks of hypoglycemia and hyperglycemia, glycemic variability, and improving patient quality of life for a wide range of patient populations and clinical indications (14–16). Retrospective CGM is a masked device without visual indication of immediate feedback on blood glucose level for patients when they wear CGM, thus, the actual glucose outcomes of interventional measures can be obtained. This feature enables patients less likely to have some unknown behaviors according to the real-time glucose level that may affect the actual glycemic outcome. The present study aimed to compare the efficacy of CSII therapy with MDI therapy on glycemic metrics evaluated by the retrospective CGM in Chinese patients with T1DM.
Subjects and methods
Study population
In the present retrospective cross-sectional study, a total of 362 T1DM patients (61 were treated with CSII therapy and 301 with MDI therapy) were admitted to the Second Xiangya Hospital of Central South University (Changsha, Hunan, China) from October 2019 to December 2021 and used a retrospective CGM system (Medtronic plc, Northridge, CA, USA) were enrolled. The study protocol was approved by the Ethics Committees of the Second Xiangya Hospital of Central South University, and it was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment. The inclusion criteria were as follows: (1) patients who met the 1999 World Health Organization (WHO) diagnostic criteria for diabetes; (2) insulin-dependent diagnosis for T1DM; and (3) patients who were treated with intensive insulin therapy administered by either CSII or MDI, in form of 4 or more insulin injections per day. The exclusion criteria were as follows: (1) utilization of other types of CGM systems during the study period; (2) recently occurrence of complications, including diabetic ketoacidosis, acute infection, chronic infection, surgical complication, trauma, etc.; (3) long-term use of glucocorticoids or immunomodulators; (4) unwilling to wear a CGM device or being allergic to the device; (5) acute and chronic hepatic and renal insufficiency; and (6) presence of autoimmune diseases, such as abnormal thyroid function.
Data collection
Continuous glucose monitoring was performed using iPro2® as the recorder and an En-lite® glucose sensor (Medtronic plc). The CGM system was placed in the abdominal area and the lateral upper arms according to the manufacturer's instructions. Standard POC capillary blood glucose measurements were carried out by Gold AQ glucometers (Sinocare Co., Ltd., Shanghai, China) for three times/day before breakfast, lunch and dinner to calibrate the CGM system. CGM data were collected from study enrollment until discharge at a week-long study session, and we analyzed average glucose level, estimated HbA1c (eHAb1c) level, glucose variability (calculated as the coefficient of variation, CV; MAGE, mean amplitude glycemic excursion; LAGE, large amplitude glycemic excursion; LBGI, low blood glucose index; HBGI, high blood glucose index), time in range (TIR, 3.9-10.0 mmol/l), time above range (TAR, >10.0 mmol/l), and time below range (TBR, <3.9 mmol/l). The targets were set according to the international consensus guidelines on CGM as follows: TIR ≥ 70%, TAR <25%, TBR <4% and CV <36%, and target HbA1c level of <7.5% was recommended (17).
Statistical analysis
Normally distributed measurement data were presented as the mean ± standard deviation (SD), and skewed data after normality testing (Shapiro-Wilk test) were expressed as the median and interquartile range (IQR). The independent-sample t-test or the Mann–Whitney U test was used to compare differences between groups. Assessment of differences in proportions between two groups was performed by the Chi-square test. A two-tailed P < 0.05 was considered statistically significant. SPSS 26.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis.
Results
Patients' characteristics
In total, 362 patients with T1DM who were admitted to the outpatient department, Second Xiangya Hospital of Central South University were included in this study, of whom 61 patients received CSII (CSII group) and 301 patients underwent MDI therapy (MDI group). The median duration of time (before enrollment) that the CSII was used by the participants included in the study was 11.5 months. There was a roughly equal proportion of female and male patients (51.9 vs. 48.1%). The mean age was 26 (15, 39) years old. The average duration of T1DM was 2.7 (0.8, 7.0) years. The mean body mass index (BMI) was 20.4 ± 3.2 kg/m2. Patients had a mean HbA1c value of 8.0% (7.0%, 9.9%). Patients' demographic and clinical data are presented in Table 1.
As shown in Table 1, there was no significant difference in age, gender, duration of disease, and daily insulin dosage between the CSII and MDI groups (All P > 0.05). However, fasting C-peptide (FCP) level in the CSII group was significantly lower than that in the MDI group (43.0 vs. 68.3 pmol/L, P < 0.001). As for clinical indicators, it was revealed that although the FCP level was lower in the CSII group than that in the MDI group, the HbA1c rate (8.1 vs. 8.7%, P = 0.004) and fasting blood glucose (FBG) level (7.5 vs. 9.6 mmol/L, P = 0.004) were significantly lower, and the HbA1c rate <7.5% (52.5 vs. 32.9%, P <0.001) was significantly higher in the CSII group. Besides, there were no significant difference in BMI, waist-to-hip ratio, and lipid metabolic parameters between the two groups (All P > 0.05).
CGM-derived metrics
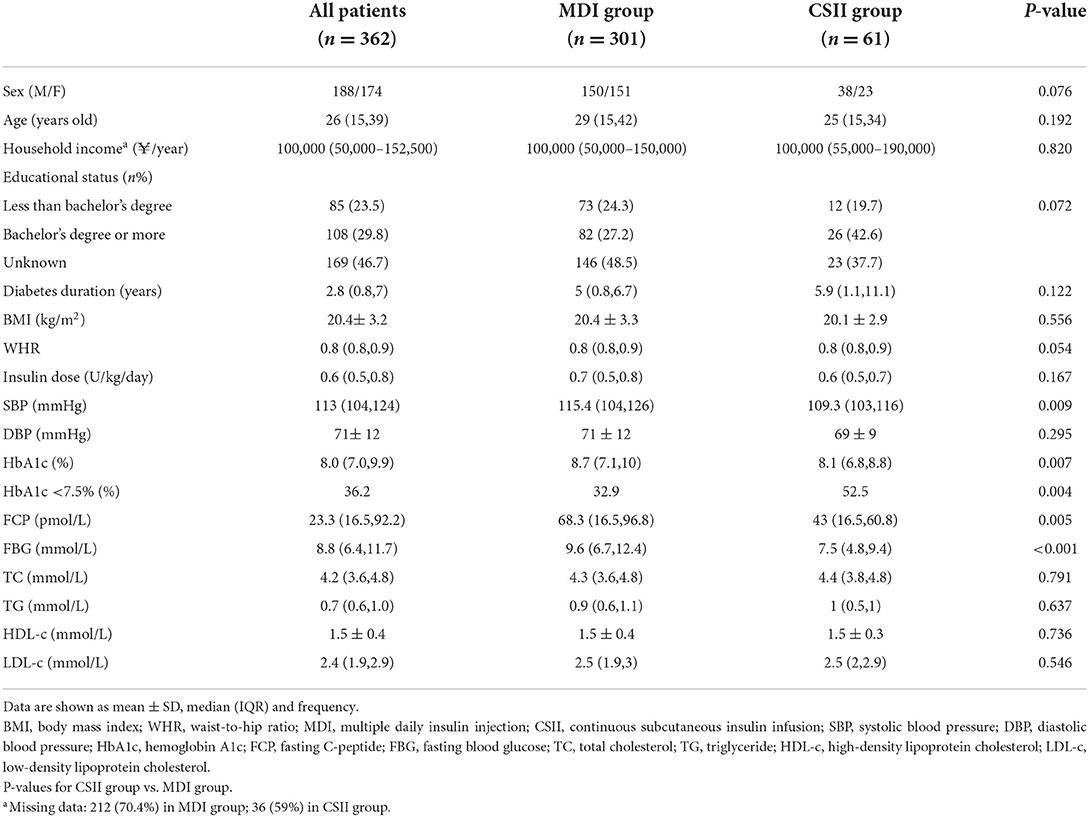
The CGM-derived metrics between the two groups was shown in Table 2. The estimated HbA1c (6.9 vs. 7.5%, P < 0.001) reflected the mean blood glucose level (8.3 vs. 9.4 mmol/l, P < 0.001), also known as glucose management index, which was significantly lower in the CSII group, and this trend is in line with that of the HbA1c rate. Although no significant difference was found in the CV of glucose between the two groups, the other glycemic variability-related parameters, such as SD (2.9 vs. 3.5 mmol/L, P = 0.019), MAGE (6.5 vs. 7.3 mmol/L, P = 0.019), and LAGE (15.6 vs. 16.4 mmol/L, P = 0.038) of glucose were significantly lower in the CSII group. The TIR was significantly higher in the CSII group than that in the MDI group (44.3 vs. 18.3%, P < 0.001). Compared with patients in the MDI group, those patients in the CSII group had significantly lower TAR (24.8 vs. 39.0%, P < 0.001) and HBGI (7.5 vs. 11.3, P < 0.001). Further investigation indicated that the proportions of time spent within the glucose range of 10.0–13.9 mmol/L (20.0 vs. 24.9%, P = 0.019) and >13.9 mmol/L (4.8 vs. 11.1%, P < 0.001) were lower in the CSII group. However, no significant difference was found in the TBR and LBGI between the two groups. The results showed that there was no significant difference in the proportions of time spent within the glucose range of 3.0–3.9 mmol/L or < 3.0 mmol/L between the two groups (Figure 1).
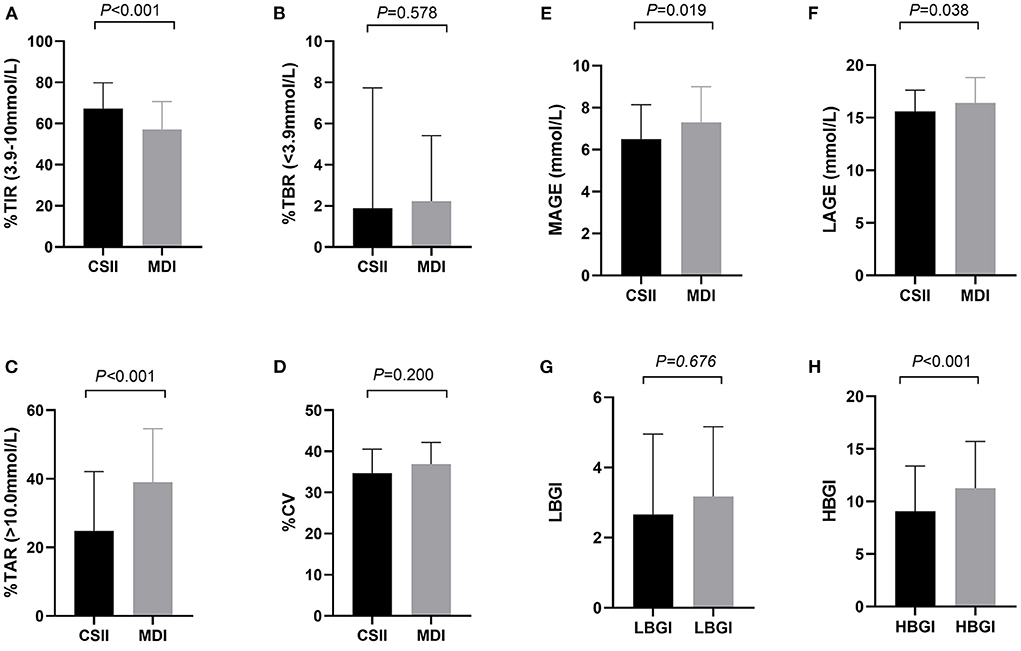
Figure 1. CGM-derived metrics of patients in CSII group and MDI group (n = 362). (A) TIR, (B) TAR, (C) TBR, (D) CV, (E) MAGE, (F) LAGE, (G) LBGI, (H) HBGI. Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily insulin injection; CSII, continuous subcutaneous insulin infusion; TIR, time in range; TAR, time above range; TBR, time below range; CV, coefficient of variation; MAGE, mean amplitude of glucose excursions; LAGE, mean amplitude of glucose excursions; LBGI, low blood glucose index; HBGI, high blood glucose index. Data are shown as median (IQR). P values for CSII group vs. MDI group.
CGM-associated target achievement
The results indicated differences in rates of achieving the CGM targets according to the treatment regimens (Figure 2). Compared with T1DM patients in the MDI group, those patients in the CSII group had a remarkably higher proportion of TAR <25% (50.8 vs. 27.9%, P < 0.001), and higher rates of achieving the targets of TIR ≥ 70 (44.3 vs. 18.3%, P < 0.001). In contrast, proportion of TBR >4% was comparable between the two groups. The proportion of CV <36% was higher in the CSII group than that in the MDI group, while the difference was not statistically significant (57.4 vs. 44.2 %, P = 0.060).
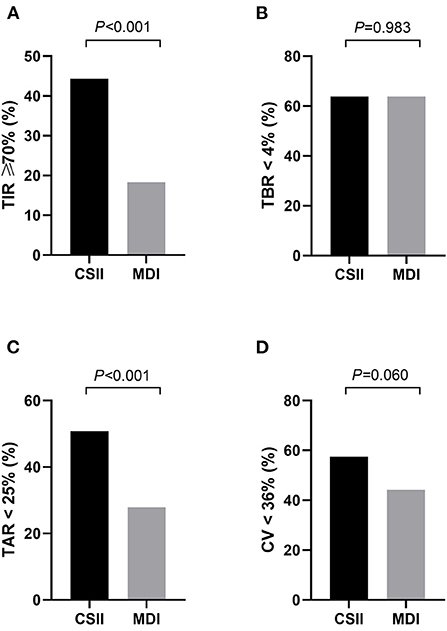
Figure 2. The proportion of patients who achieved the CGM-associated targets in CSII group and MDI group (n = 362). (A) TIR ≥ 70%, (B) TAR < 25%, (C) TBR < 4%, (D) CV < 36%. MDI, multiple daily insulin injection; CSII, continuous subcutaneous insulin infusion; TIR, time in range; TAR, time above range; TBR, time below range; CV, coefficient of variation. Data are shown as frequency. P-values for CSII group vs. MDI group.
Discussion
To our knowledge, this is the first study that compared the effectiveness of CSII therapy with that of the MDI therapy on glycemic outcomes evaluated using the retrospective CGM in Chinese patients with T1DM. The main finding of our study is that prescribed CSII therapy was significantly associated with better glycemic outcomes in Chinese patients with T1DM, rather than being correlated with the lower HbA1c, and the better CGM-derived metrics are noteworthy. Among patients with T1DM enrolled in our study, the use of CSII, compared with MDI, resulted in lower levels of HbA1c and FBG, while insulin dose reduction was not significant; moreover, CSII outperformed in increasing TIR, decreasing TAR, and achieving CGM-associated targets of TIR ≥ 70% and TAR < 25%. However, patients who received CSII therapy have not shown decreasing TBR, increasing achieving CGM-associated targets of TBR <4%, and improving the risk of hypoglycemia indicted by comparable LBGI between the two treatment regimens. CGM has traditionally been regarded as the best tool to evaluate short-term glycemic variability in T1DM management, in which the parameters, such as SD, MAGE, and LAGE in patients who received CSII therapy showed better outcomes. Although previous studies have indicated benefits of CSII therapy on glucose variability in patients with T1DM, the real-world evidence is limited and no study evaluated T1DM patients in China using the retrospective CGM to determine glycemic outcomes.
In our study, T1DM patients who received CSII therapy had a significantly better overall glycemic control with the exception of hypoglycemia evaluated by the retrospective CGM. Meta-analyses of the benefits of RCTs demonstrated that CSII therapy compared with MDI therapy mainly exhibited a better HbA1c and a lower risk of severe hypoglycemia (9, 10). In the recent decades, numerous researches have concentrated on the practical application of insulin pumps, and different and even contradictory conclusions in different countries have been reached in a real-world setting (18–21). In the present study, the utilization rate of insulin pumps by T1DM patients was 16.9%, which was relatively consistent with the recently reported findings in China (22, 23). A study that compared CSII and MDI in terms of benefits, safety, and cost-effectiveness showed no significant difference in the level of HbA1c and the incidence of severe hypoglycemia between the two groups, and the cost of CSII treatment was higher (3). The benefits of CSII for long-term glycemic management need to be validated by additional studies in clinical practice. A number of Chinese scholars who concentrated on T1DM patients demonstrated that utilization of insulin pumps is associated with a lower level of HbA1c (12, 24–26). Further cost-effectiveness analysis of CSII therapy vs. MDI therapy also suggested that CSII therapy should be considered as a preferred alternative to MDI therapy for Chinese T1DM patients (12, 26). Our results provided further evidence that CSII therapy is effective for management of Chinese T1DM patients, which was confirmed by a lower HbA1c level and better CGM-derived metrics.
However, there is no evidence indicating the correlation of the utilization of insulin pumps with a lower or a higher hypoglycemia risk based on retrospective CGM-associated metrics in the present study. Correlation of insulin pumps with hypoglycemia has been reported in several RCTs, real-world studies, and meta-analyses, while the results were inconsistent (4, 5, 27–29). Based on the hypoglycemia-related metrics evaluated by the retrospective CGM, the use of insulin pumps is safe as they may not increase or decrease risk of hypoglycemia in our real-world research. Previous real-world studies demonstrated the association of the utilization of insulin pumps with a reduction of adverse hypoglycemic events in patients undergoing CSII therapy, as well as a lower total daily insulin dose compared with patients receiving MDI therapy (11, 30). The daily insulin may have a slightly protective effect on the development of hypoglycemia, which was comparable between the two treatment regimens in our study. Accordingly, it was speculated that daily insulin dose, prandial to total insulin ratio and utilization of rapid-acting insulin analogs can influence hypoglycemia. Therefore, for Chinese T1DM patients, in order to assess the potential role of insulin pumps in reducing the risk of hypoglycemia, the insulin delivery and insulin regimen are equally important.
Chinese T1DM patients undergoing CSII therapy benefited from a better glycemic control compared with those receiving MDI therapy in the present study. In our study, we found an unsatisfactory general rates of target HbA1c level <7.5% and CGM-associated target in Chinese T1DM patients treated with CSII therapy. Therefore, the safety of insulin pumps has been paid intensive attention in China. To achieve desired glycemic targets in T1DM patients undergoing CSII therapy, only dependency on technology is not recommended; the accurate management and utilization of insulin pumps would be the most important steps toward controlling the glycemic outcomes in T1DM patients (31). In addition, an intensive contact between physicians and T1DM patients undergoing CSII therapy is essential to achieve a greater level of knowledge to appropriately carry out insulin dose adjustment for dietary intake, specifically carbohydrate counting, lifestyle factors, and in particular, disease management. An intensive educational program has been developed by our research team via a structured T1DM self-management educational system, namely “Type 1 Diabetes Education in Lifestyle and Self Adjustment' (TELSA)”, which could be adapted to medical, social, and cultural practices in China (32). The TELSA program and other intensive educational programs may improve outcomes in Chinese T1DM patients undergoing CSII therapy (33). Moreover, recent studies have demonstrated that intensive insulin therapy of T1DM patients using real-time CGM or flash glucose monitoring is associated with better glycemic outcomes (34–39). In China, the individual version of flash glucose monitoring (Free-Style Libre; Abbott Diabetes Care, Witney, UK) is the most conventional type of CGMs for patients with T1DM caring out of hospitals. A recent research indicated that flash glucose monitoring could be considered as a cost-effective strategy compared with self-monitoring of blood glucose for Chinese T1DM patients receiving insulin therapy (40). Therefore, flash glucose monitoring can be a promising option to improve glycemic control in Chinese T1DM patients undergoing CSII therapy. Further research is required to identify barriers to the effectiveness of insulin pumps for Chinese T1DM patients, in order to provide more targeted measures.
There are some limitations in the present study. The limitations mainly include the lack of the analysis of physical activity, sleep quality, dietary habits, diabetic education, motivation, family support, and mental health factors, influencing the glycemic control of T1DM patients undergoing intensive insulin therapy. Importantly, the observational nature of the study might cause selection bias. Besides, the use of CSII or CGM was highly dependent on patients' and their family member's preferences, economic considerations, or treatment expectancy. In addition, the lack of a long-term study comparing the effects of CSII or MDI therapy on glycemic outcomes in T1DM patients is noteworthy. Therefore, further research should be conducted to eliminate the above-mentioned shortcomings and to confirm our findings.
Conclusions
In summary, using CSII therapy was significantly associated with better glycemic outcomes compared with MDI therapy in Chinese T1DM patients, which not only would be reflected by the lower HbA1c level, but also by the better outcomes of the most of CGM-derived metrics with comparable hypoglycemia-related parameters. However, the general rates of target HbA1c level <7.5% and CGM-associated target were unsatisfactory in Chinese T1DM patients who underwent both treatment regimens, indicating the necessity of strengthening publicity and educational programs to improve the management of T1DM patients undergoing intensive insulin therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YL and ZZ designed the study. GK and LJ conducted the data analysis and drafted the manuscript. GK, ZL, YJ, FL, DZ, ZQ, and LX collected data. All the authors read and approved the submitted version of the manuscript.
Funding
This study was financially supported by the National Key R&D Program of China (Grant No. 2018YFC2001005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo K, Tian Q, Yang L, Zhou Z. The role of glucagon in glycemic variability in type 1 diabetes: a narrative review. Diabetes Metab Syndr Obes. (2021) 14:4865–73. doi: 10.2147/DMSO.S343514
2. Sims EK, Carr ALJ, Oram RA, DiMeglio LA, Evans-Molina C. 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat Med. (2021) 27:1154–64. doi: 10.1038/s41591-021-01418-2
3. Blair JC, McKay A, Ridyard C, Thornborough K, Bedson E, Peak M, et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomised controlled trial and economic evaluation. BMJ. (2019) 365:l1226. doi: 10.1136/bmj.l1226
4. Karges B, Rosenbauer J, Kapellen T, Wagner VM, Schober E, Karges W, et al. Hemoglobin A1c Levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. (2014) 11:e1001742. doi: 10.1371/journal.pmed.1001742
5. Fredheim S, Johansen A, Thorsen SU, Kremke B, Nielsen LB, Olsen BS, et al. Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol. (2015) 52:591–9. doi: 10.1007/s00592-014-0697-5
6. Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. (2008) 51:941–51. doi: 10.1007/s00125-008-0974-3
7. Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine. (2017) 55:77–84. doi: 10.1007/s12020-016-1039-x
8. Dos Santos TJ, Donado Campos JM, Argente J, Rodríguez-Artalejo F. Effectiveness and equity of continuous subcutaneous insulin infusions in pediatric type 1 diabetes: a systematic review and meta-analysis of the literature. Diabetes Res Clin Pract. (2021) 172:108643. doi: 10.1016/j.diabres.2020.108643
9. Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol. (2019) 56:973–80. doi: 10.1007/s00592-019-01326-5
10. Pease A, Lo C, Earnest A, Kiriakova V, Liew D, Zoungas S. The efficacy of technology in type 1 diabetes: a systematic review, network meta-analysis, and narrative synthesis. Diabetes Technol Ther. (2020) 22:411–21. doi: 10.1089/dia.2019.0417
11. Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. (2017) 318:1358–66. doi: 10.1001/jama.2017.13994
12. Hu S, Yang H, Chen Z, Leng X, Li C, Qiao L, et al. Clinical outcome and cost-effectiveness analysis of CSII versus MDI in children and adolescent with type 1 diabetes mellitus in a public health care system of China. Front Endocrinol (Lausanne). (2021) 12:604028. doi: 10.3389/fendo.2021.604028
13. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA(1c) alone to assess glycemic control can be misleading. Diabetes Care. (2017) 40:994–9. doi: 10.2337/dc17-0636
14. Weinstein JM, Kahkoska AR. Association of continuous glucose monitoring use and hemoglobin A1c levels across the lifespan among individuals with type 1 diabetes in the US. JAMA Netw Open. (2022) 5:e2223942. doi: 10.1001/jamanetworkopen.2022.23942
15. Yoshii H, Mita T, Katakami N, Okada Y, Osonoi T, Aso K, et al. The importance of continuous glucose monitoring-derived metrics beyond HbA1c for optimal individualized glycemic control. J Clin Endocrinol Metab. (2022) 31:dgac459. doi: 10.1210/clinem/dgac459
16. Teo E, Hassan N, Tam W, Koh S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia. (2022) 65:604–19. doi: 10.1007/s00125-021-05648-4
17. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. (2017) 40:1631–40. doi: 10.2337/dc17-1600
18. Kramer G, Kloos C, Müller UA, Wolf G, Kuniss N. Metabolic control and hypoglycaemia in people with type 1 diabetes: insulin pump therapy vs. intensified insulin therapy in an unselected cohort in routine care. Diabetol Metab Syndr. (2021) 13:80. doi: 10.1186/s13098-021-00700-0
19. Karagianni P, Sampanis C, Katsoulis C, Miserlis G, Polyzos S, Zografou I, et al. Continuous subcutaneous insulin infusion versus multiple daily injections. Hippokratia. (2009) 13:93–6.
20. Quirós C, Giménez M, Ríos P, Careaga M, Roca D, Vidal M, et al. Long-term outcome of insulin pump therapy: reduction of hypoglycaemia and impact on glycaemic control. Diabet Med. (2016) 33:1422–6. doi: 10.1111/dme.13094
21. Korkmaz Ö, Demir G, Çetin H, Mecidov I, Atik Altinok Y, Özen S, et al. Effectiveness of continuous subcutaneous insulin infusion pump therapy during five years of treatment on metabolic control in children and adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. (2018) 10:147–52. doi: 10.4274/jcrpe.5117
22. Hou L, Li X, Liu L, Wei H, Xiong F, Du H, et al. A multicenter survey of type I diabetes mellitus in chinese children. Front Endocrinol (Lausanne). (2021) 12:583114. doi: 10.3389/fendo.2021.583114
23. Huo L, Deng W, Shaw JE, Magliano DJ, Zhang P, McGuire HC, et al. Factors associated with glycemic control in type 1 diabetes patients in China: a cross-sectional study. J Diabetes Investig. (2020) 11:1575–82. doi: 10.1111/jdi.13282
24. Blair J, McKay A, Ridyard C, Thornborough K, Bedson E, Peak M, et al. Continuous subcutaneous insulin infusion versus multiple daily injections in children and young people at diagnosis of type 1 diabetes: the SCIPI RCT. Health Technol Assess. (2018) 22:1–112. doi: 10.3310/hta22420
25. Zhang Y, Xu W, Ai H, Lü J, Zhu Y, Yang D, et al. [Factors associated with glycemic control in adults with type 1 diabetes mellitus on an insulin pump]. Zhonghua Yi Xue Za Zhi. (2014) 94:3488–91.
26. Zhang L, Leng X, Tian F, Xiao D, Xuan J, Yang H, et al. Cost-effectiveness analysis of continuous subcutaneous insulin infusion versus multiple daily insulin for treatment of children with Type 1 diabetes. Postgrad Med. (2022). doi: 10.1080/00325481.2022.2088938
27. Misso ML, Egberts KJ., Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. (2010) (1):Cd005103. doi: 10.1002/14651858.CD005103.pub2
28. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. (2010) 363:311–20. doi: 10.1056/NEJMoa1002853
29. Rosenlund S, Hansen TW, Rossing P, Andersen S. Effect of sensor-augmented pump treatment versus multiple daily injections on albuminuria: a 1-year randomized study. J Clin Endocrinol Metab. (2015) 100:4181–8. doi: 10.1210/jc.2015-2839
30. Guzmán G, Martínez V, Yara JD, Mina MA, Solarte JS, Victoria AM, et al. Glycemic control and hypoglycemia in patients treated with insulin pump therapy: an observational study. J Diabetes Res. (2020) 2020:1581726. doi: 10.1155/2020/1581726
31. REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. (2017) 356:j1285. doi: 10.1136/bmj.j1285
32. Xie Y, Liu F, Huang F, Lan C, Guo J, He J, et al. Establishment of a type 1 diabetes structured education programme suitable for Chinese patients: type 1 diabetes education in lifestyle and self adjustment (TELSA). BMC Endocr Disord. (2020) 20:37. doi: 10.1186/s12902-020-0514-9
33. Liu F, Xie YT, Xu R, Fan L, Li J, Li X, et al. [Application of TELSA structured education program in adults with type 1 diabetes mellitus]. Zhonghua Yi Xue Za Zhi. (2022) 102:1202–8.
34. Matsuda E, Brennan P. The effectiveness of continuous subcutaneous insulin pumps with continuous glucose monitoring in outpatient adolescents with type 1 diabetes: a systematic review. JBI Libr Syst Rev. (2012) 10:1–10. doi: 10.11124/jbisrir-2012-170
35. Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess. (2016) 20:v–xxxi, 1–251. doi: 10.3310/hta20170
36. Viñals C, Quirós C, Giménez M, Conget I. Real-life management and effectiveness of insulin pump with or without continuous glucose monitoring in adults with type 1 diabetes. Diabetes Ther. (2019) 10:929–36. doi: 10.1007/s13300-019-0599-1
37. Wan W, Skandari MR, Minc A, Nathan AG, Winn A, Zarei P, et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. (2018) 41:1227–34. doi: 10.2337/dc17-1821
38. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. (2018) 137:37–46. doi: 10.1016/j.diabres.2017.12.015
39. Šoupal J, PetruŽelková L, Flekač M, Pelcl T, Matoulek M, Danková M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. (2016) 18:532–8. doi: 10.1089/dia.2016.0171
Keywords: type 1 diabetes mellitus, continuous subcutaneous insulin infusion, multiple daily insulin injection, continuous glucose monitoring, glycemic control
Citation: Keyu G, Jiaqi L, Liyin Z, Jianan Y, Li F, Zhiyi D, Qin Z, Xia L, Lin Y and Zhiguang Z (2022) Comparing the effectiveness of continuous subcutaneous insulin infusion with multiple daily insulin injection for patients with type 1 diabetes mellitus evaluated by retrospective continuous glucose monitoring: A real-world data analysis. Front. Public Health 10:990281. doi: 10.3389/fpubh.2022.990281
Received: 09 July 2022; Accepted: 11 August 2022;
Published: 25 August 2022.
Edited by:
Ping Wang, Michigan State University, United StatesReviewed by:
Theocharis Koufakis, Aristotle University of Thessaloniki, GreecePranjali Sharma, Parkview Health System, United States
Copyright © 2022 Keyu, Jiaqi, Liyin, Jianan, Li, Zhiyi, Qin, Xia, Lin and Zhiguang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Lin, eWFuZ2xpbl9uZm1AY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Guo Keyu
Guo Keyu Li Jiaqi†
Li Jiaqi† Ye Jianan
Ye Jianan Fan Li
Fan Li Li Xia
Li Xia Yang Lin
Yang Lin Zhou Zhiguang
Zhou Zhiguang