- 1Department of Maternal, Child & Adolescent Health, School of Public Health, Anhui Medical University, Key Laboratory of Population Health Across Life Cycle (Anhui Medical University), Ministry of Education of the People's Republic of China, NHC Key Laboratory of Study on Abnormal gametes and Reproductive Tract, Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, China
- 2Scientific Research Center in Preventive Medicine, School of Public Health, Anhui Medical University, Hefei, China
Background: Elective cesarean section (ECS) primarily contributes to the rising cesarean section (CS) rate, and much attention has been attracted to its health consequences. The association between ECS and overweight and obesity in children has been controversial, and few studies distinguished ECS with medical indications from those without indications. Based on a large sample birth cohort, we aim to examine the association of ECS with or without medical indications on children's physical development by using repeated anthropometric data from birth to 6 years of age.
Methods: A total of 2304 mother-child pairs with complete data on delivery mode and children's anthropometric measurements were recruited from the Ma'anshan-Anhui Birth Cohort (MABC) in China. ECS was the main exposure in this study, and the primary outcomes were children's growth trajectories and early adiposity rebound (AR). Children's BMI trajectories were fitted by using group-based trajectory models and fractional polynomial mixed-effects models. The association between ECS and children's growth trajectories and early AR was performed using multiple logistic regression models.
Results: Among 2,304 mother-child pairs (1199 boys and 1105 girls), 1088 (47.2%) children were born by CS, including 61 (5.6%) emergency CS, 441 (40.5%) ECS with medical indications, and 586 (53.9%) ECS without medical indications. After adjusting for potential confounders, it was found that ECS with medical indications was associated with a “high level” of BMI trajectory (OR = 1.776; 95% CI: 1.010–3.123), and ECS without medical indications was associated with early AR (OR = 1.517; 95% CI: 1.123–2.050) in girls. In boys, we found that ECS without medical indications was unlikely to experience an accelerated growth trajectory (OR = 0.484; 95%CI: 0.244–0.959).
Conclusions: ECS may be related to girls' “high level” BMI trajectories and early AR. If causal, the findings will provide an evidence-based reference for early life interventions for childhood obesity.
Introduction
With the development of perinatal medicine, cesarean section (CS) has become an effective means of saving the lives of women and babies in the event of serious obstetric complications. Recent data (2010–2018) based on 154 countries covering 94.5% of live births worldwide shows that, on average, 21.1% of women have childbirth by CS. It is estimated that 28.5% of women worldwide will deliver by CS by 2030 (1). The domestic cesarean rate increased from 28.8% in 2008 to 36.7% in 2018 (2).
CS is divided into emergency CS and elective CS (ECS) according to the presence or absence of labor, and ECS is subdivided into ECS with medical indications and ECS without medical indications. Studies have shown that ECS without medical indication is the main contributor to the increasing CS rate (3).
At present, there is no evidence that CS without medical indications benefits the mother and the baby. Also, WHO states that CS rates above 10% are no longer associated with a reduction in maternal and infant mortality. Actually, CS carries risks and may have health consequences for women and children (4). Infants born via CS have different hormonal and microbial exposures from vaginal delivery, and that can subtly alter the physiology of the newborn (5). The health effects of CS on children have been shown to include altered immune development, increased risk of allergy and asthma, and reduced gut microbial diversity (5).
The increasing prevalence of childhood obesity has become a worldwide public health problem, with 39 million children under 5 years of age being overweight or obesity worldwide in 2020 (6). Obesity children would experience respiratory distress, increased risk of fracture, hypertension, early signs of cardiovascular disease, insulin tolerance and psychological problems. They are also at great risk of obesity, premature death and disability in adulthood (6).
The high rate of CS and the simultaneous high prevalence of obesity have led scholars to become interested in the effect of CS on offspring's overweight and obesity. Previous studies have looked closely at the association between CS and offspring's overweight and obesity, but the findings are controversial. Mulleer NT et al (7) found that CS was associated with higher rates of weight gain in the first year of life in offspring compared to vaginal delivery. Evidence from American GUTS cohort showed that cesarean delivery was associated with 15% increased risk of offspring's obesity (8). Chinese studies similarly revealed that CS was correlated with overweight and obesity in offspring (9, 10). However, a cohort study in Ireland showed no association between mode of delivery and obesity in children aged 2 and 5 years (11). However, when examining the association between CS and offspring's overweight/obesity, few studies have distinguished emergency CS from ECS, and the findings are conflicting. A Singaporean study (12) found that ECS was significantly associated with the risk of overweight in infants, whereas Masukume G et al indicated a statistically significant association between ECS and obesity in children at 24 months of age, but the association was not observed at 54 months of age (13). More importantly, there were no studies that intensively looked into the respective effect of ECS with or without medical indications on children's overweight/obesity. ECS without medical indications, without a doubt, obtains the great significance of preventability and intervention.
It has been well known that body mass index (BMI) fluctuates early in life (14). Tracking trajectory patterns over time during child development no longer focuses only on BMI at one point in time and can account for dynamic changes in size (15, 16). Compared to qualitative definition on overweight/obesity, BMI trajectories may better reflect the underlying dynamic patterns of individual's physical development over the life course. Adiposity rebound (AR) is defined as a second rise in BMI, usually occurring in early childhood. Studies have shown that early adiposity rebound (EAR) increased the risk of childhood obesity and is strongly associated with metabolic syndrome (17, 18) and cardiovascular diseases (19, 20). A recent meta-analysis found an increasing trend in the prevalence of EAR, suggesting that the age of AR in humans may be advancing compared to the past (21). AR, which relies on BMI trajectories, may be an early sensitive indicator of child physical growth and development.
The association between ECS and child growth trajectory, particularly with AR, has not been reported previously. Based on a large-sample birth cohort, this study aims to investigate the association between ECS and childhood BMI trajectories and early AR.
Methods
Study population
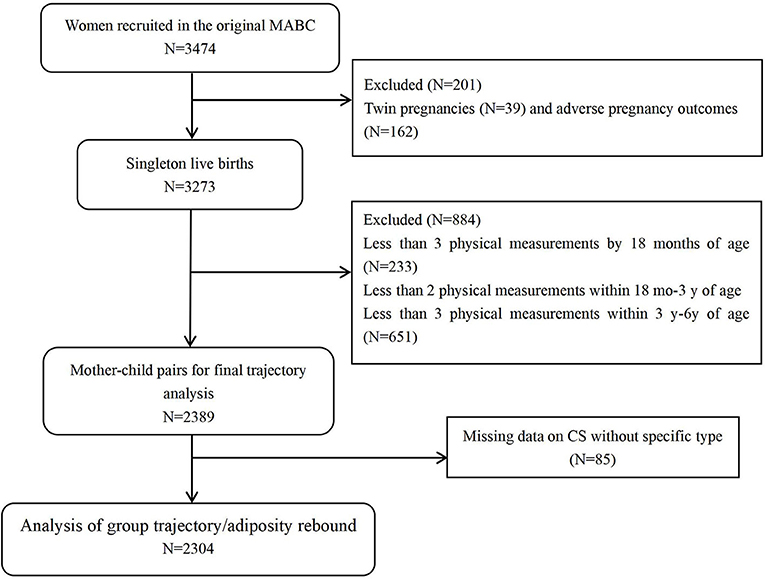
The participants were from the Ma'anshan-Anhui Birth Cohort (MABC) in China. Pregnant women were consecutively recruited from antenatal clinics of the Maternal and Child Health Care Center in Ma'anshan, Anhui Province, from May 2013 to September 2014 by trained investigators. The center contains about eighty percent of all pregnant women in Ma'anshan city every year. The inclusion criteria for women were (1) within 14 weeks of pregnancy; (2) planned to have antenatal checkups and childbirth in the hospital; (3) being able to understand and complete the questionnaires; and (4) willing to participate in follow-ups during childhood. A total of 3474 eligible women were recruited. After excluding 39 twins and 162 adverse pregnancy outcomes, 3273 women with live singleton births were obtained.
The study was approved by the ethical committee of Anhui Medical University (20131401). Written informed consents were obtained from all participants.
Delivery mode
Data on delivery mode was extracted from the obstetrical records. Delivery mode was classified as CS and vaginal delivery (VD), thereafter CS was classified as ECS when it was performed before labor and emergency CS when it was performed after an attempt of vaginal delivery. Then ECS was further categorized as ECS with medical indicators and ECS without indicators.
Assessment of children's BMI
Children's BMI was calculated as body weight (in kilograms) divided by body length or body height (in meters squared). Birth weight and birth length were extracted from the obstetrical records during childbirth. Children's body weight and length/height were measured by trained child healthcare providers. The infant's weight was measured using a pan-type lever scale, accurate to 0.01 kg. The toddler's weight was measured using a seated lever scale, accurate to 0.05 kg. The infant's length is measured by using a standard measuring bed, accurate to 0.1 cm. And the child's height and weight were measured using a mechanical child height and weight scale (Model: RRZ-50-RP) with an accurate reading of 0.1 kg for weight and 0.1 cm for height.
Children's body length/height and weight were repeatedly measured at 42 postnatal days, and 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, 72 months after birth. The raw BMI values in each age group were used for trajectory modeling.
Covariates
Confounding variables were identified by directed acyclic graph (DAG) (22) and included maternal age, education level, place of residence, monthly household income per capita, smoking status, parity, pre-pregnancy BMI, weight gain during pregnancy, gestational diabetes mellitus (GDM) and hypertensive disorders in pregnancy (HDP).
Data on maternal age, education level, place of residence, family income, parity and smoking status were collected during the women's first antenatal visit by questionnaire survey. In this visit, women's body weight and height were measured, and the weight was regarded as pre-pregnancy body weight. Thus pre-pregnancy BMI was calculated from weight (kg) divided by the square of height (m2). Based on the guideline of Cooperative Meta-analysis Group of Working Group on Obesity in China (WGOC), BMI < 18.5 kg/m2 was defined as underweight, 18.5–23.9 kg/m2 as normal weight,24-27.9 kg/m2 as overweight and ≥28 kg/m2 for obesity for Chinese adults (23). Gestational weight gain was calculated by weight recorded shortly before childbirth subtracting the weight before pregnancy. GDM and HDP were abstracted from obstetrical notes.
Children's birth weight, gestational age, and exclusive breastfeeding were used in sensitivity analyses. Data on gestational age and birth weight were abstracted from obstetrical notes. A newborn with a birth weight below the 10th percentile for gestational age was defined as small for gestational age (SGA), between the 10th and 90th percentile for gestational age was defined as appropriate for gestational age (AGA) and above the 90th percentile for gestational age was defined as large for gestational age (LGA).
Exclusive breastfeeding was extracted from children's follow-up questionnaire survey. In this study, taking into account the actual situation of the Chinese population, exclusive breastfeeding was defined as infants who were not given any liquid or solid food, including water juice or vitamins other than breast milk.
Statistical analysis
Trajectory modeling
Sample selection
Typically, trajectory fitting requires a minimum of 3 BMI measurements. This is because fewer measurements can limit the modeling capabilities and, therefore, the type and number of trajectories that can be generated (16, 24, 25). To ensure the quality of the trajectory fitting, among the 3273 newborns included in the MABC, 233 children were excluded because BMI data was < 3 times before 18 months. As data for AR was requested more stringently after 18 months, 651 children were excluded because of < 2 BMI measurements between 18 months and 3 years and < 3 measurements between 3 and 6 years, leading to a sample of 2389 children.
Furthermore, infants with missing data on CS cause (n = 85) were further excluded, resulting in a complete sample of 2304 mother-infant pairs (Figure 1). Trajectories fitting was performed by using Stata 15.1 software.
Group-based trajectory
A group-based trajectory model (GBTM) was used to identify distinctive patterns of children's growth observed from birth to 6 years. GBTM is a latent class growth model designed to identify groups of trajectories, i.e., groups of individuals that follow similar trajectories over time. In GBTM, the optimal number of trajectory groups with different trajectory patterns was determined by the Bayesian information criterion (BIC). The group with the highest BIC value and polynomial are basically chosen in order. Usually, objective criteria are used for modeling, but subjective judgment is also essential (26). The number of groups or the polynomial order can be specified with professional knowledge. The final model should be simple while adequately presenting the data's characteristics (26) Therefore, the GBTM was used to fit the trajectories of children aged 0–6 years. Due to the large number of age groups involved and the large temporal variability of BMI in the early stages of child development, high-order terms (4 order terms) were used to fit the trajectories. The optimal model was selected based on the model parameters. For the category fitting, the number of categories increased from 2, based on the BIC of each fitted model, until a suitable trajectory category and a suitable combination of higher terms were selected.
Individual-specific trajectories
Individual-specific BMI trajectories were fitted from month 1 to month 72 of age by using a fractional polynomial mixed-effects model. Specific steps have been described in detail in wen's study (27). Briefly, children's BMI trajectory was firstly modeled as a function of age using a fractional polynomial approach. The expected value of BMI was modeled as , where m was the degree of the model, and powers Pj were selected from a fixed set of 8 candidate values, including −2, −1, −0.5, 0 or log, 0.5, 1, 2, and 3. Since the trajectory of children's BMI firstly rises, and falls after 1 year of age and then rises again after 4–5years of age, we set the minimum model degree m = 3. There are 219 candidate models, including 56 models of 3rd-degree, 70 models of 4th-degree, 56 models of 5th-degree, 28 models of 6th-degree, 8 models of 7th-degree, and 1 model of 8th-degree. Afterward, BMI trajectories were fitted using mixed-effects models. The models specified fixed effects for each fractional polynomial term, which reflected the overall average trend, and random effects for each term for each child as well, which had modeled individual differences. Then the trajectory characteristics of the candidate models were combined with the BIC value to select the best model. The smaller the BIC value was, the better the model fit would be. Estimates for AR were obtained from the BMI trajectories for each child. In the present study, age at AR before 54 months was defined as early AR. According to the estimated AR for each child, therefore, children were divided into early AR and non-early AR.
Association between ECS and children's growth trajectories and AR
IBM SPSS Statistics 23.0 was used to analyze the association between ECS and children's growth trajectories and AR. Multiple logistic regression models were adopted and stratifying by children's sex. For the association between ECS and children's growth trajectories, trajectory 2 was regarded as the reference. The independent variables were divided into ECS with medical indicators, ECS without indicators, emergency CS and VD, with vaginal delivery being as reference.
For the association between ECS and AR, non-early AR was regarded as the reference in the dependent variable. P-value of < 0.05 was considered significant.
We had performed two sensitivity analyses: First, there is an interaction between gestational week-birth weight and mode of delivery, and SGA is associated with catch-up growth leading to overweight/obesity (28). Thus, gestational week-birth weight may be a confounder and mediator in the association between cesarean delivery and child physical development. In a second sensitivity analysis, breastfed children exhibit lower trajectories of BMI-Z scores (29), and early access to complementary foods is associated with obesity in later childhood (30). Thus, exclusive breastfeeding is not a strict confounder but a precision variable.
Results
Basic characteristics of participants
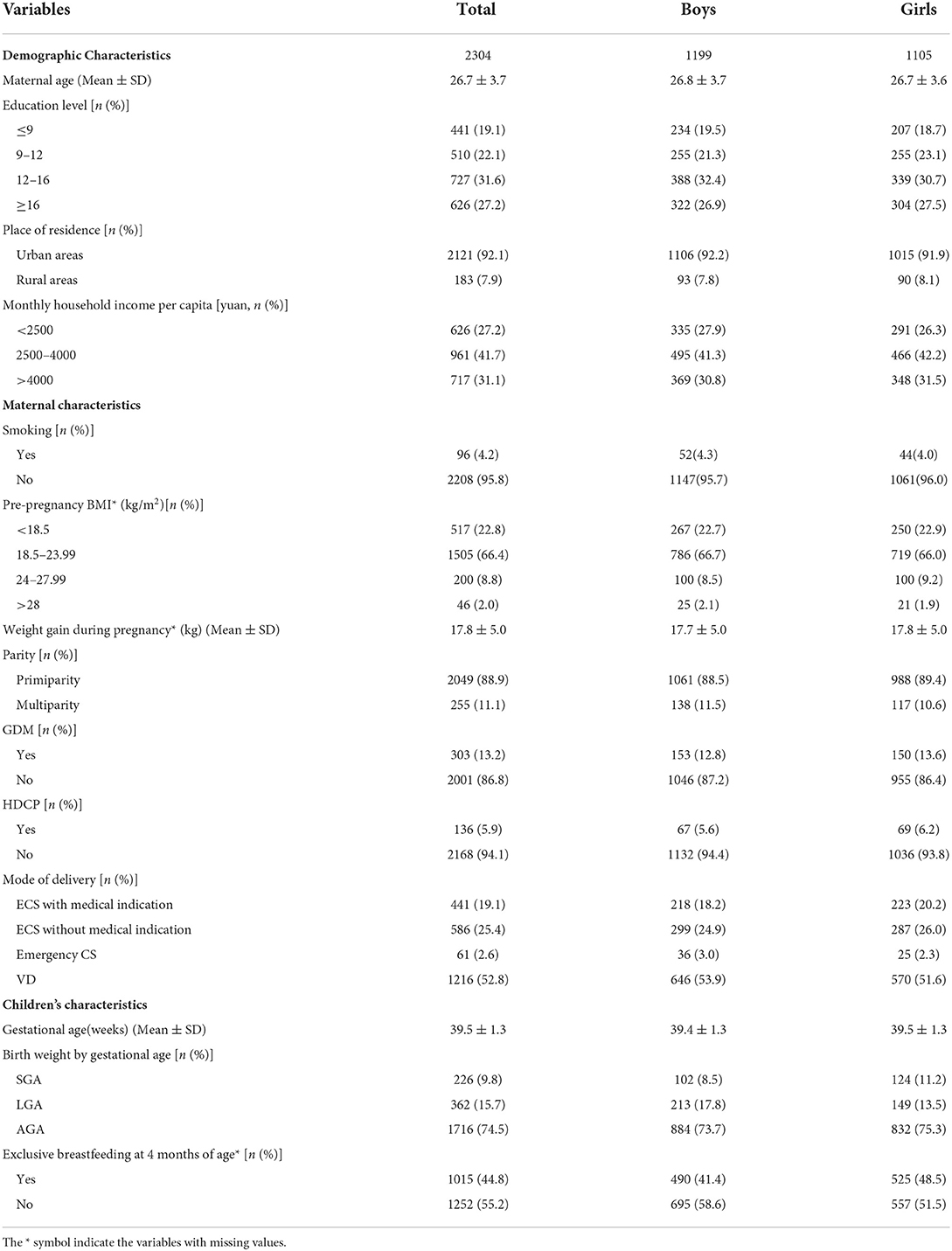
Among 2304 children, 1216 (52.8%) were born vaginally and 1088 (47.2%) were born by CS, including 61 (5.6%) emergency CS, 441 (40.5%) ECS with medical indications, and 586 (53.9%) ECS without medical indications. A higher rate of exclusive breastfeeding in the first 4 months was found in girls than in boys (48.5% vs. 41.4%), and a higher proportion of girls were SGA (11.2% v.8.5%) and a higher proportion of boys were LGA (17.8% vs. 13.5%) (Table 1)
Grouping of BMI trajectories
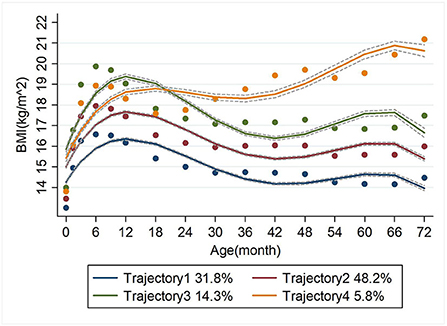
Four BMI trajectory groups were identified. Trajectory 1 was the 'low level' group, representing a consistently low level of BMI with increasing age, accounting for 31.8%. Trajectory 2 was the 'middle level' group, representing a stable medium BMI with age, accounting for 48.2%. Trajectory 3 was the “high level” group, representing a consistently high level of BMI with age and accounted for 14.3%. And trajectory 4 was the 'accelerated growth' group accounting for 5.8% (Figure 2).
Distribution of children's BMI trajectories and EAR under different delivery modes
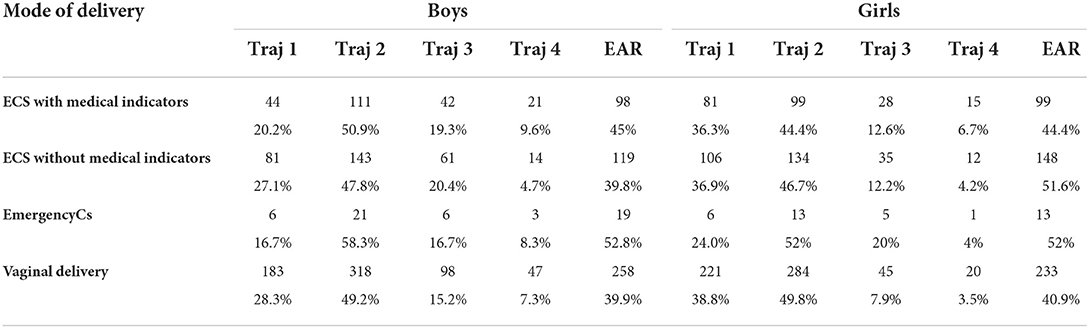
As shown in Table 2, in boys, boys born via ECS without medical indicators and girls born by emergency CS had the most proportion of the “high level” BMI trajectory. In both boys and girls, those born via ECS with medical indications were more likely to have the 'accelerated growth' trajectory.
Basically, children born by CS had higher rate of EAR than those born by VD. The prevalence of EAR was higher in children born by emergency CS in both boys and girls.
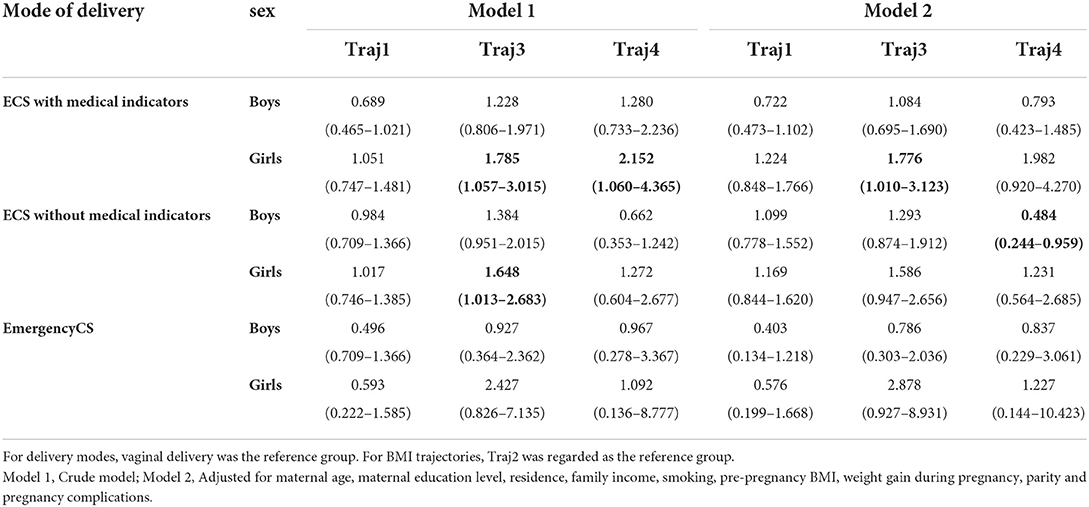
Association between ECS and children's BMI trajectories in boys and girls
As shown in Table 3, after adjusting for potential confounders, it was found that, among girls, ECS with medical indications births significantly associated with the “high level” trajectory (trajectory 3) (OR = 1.776, 95% CI: 1.010–3.123). Boys born by ECS without medical indication were less likely to have an “accelerated growth” trajectory (trajectory 4) (OR = 0.484, 95%CI: 0.244–0959).
Sensitivity analysis did not change the main findings (Supplementary Tables S1–S2)
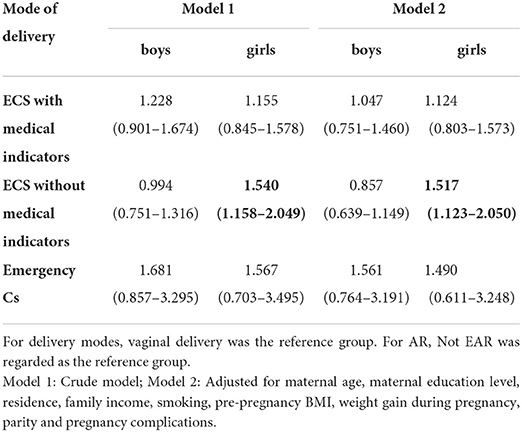
Association between ECS and children's EAR in boys and girls
After adjusting for potential confounders, in girls, ECS without medical indicators was associated with the occurrence of the EAR (OR = 1.517 95%CI = 1.123-2.050). No significant association was observed between ECS and boys' prevalence of EAR (OR=.0994 95%CI=0.751-1.316 for ECS with medical indicators; OR = 0.857 95%CI=0.639-1.149 for ECS without medical indicators) (Table 4). Sensitivity analysis did not change the main findings fundamentally (Supplementary Tables S3–S4).
Discussion
In the current study, ECS was found to be associated with girl's BMI trajectory and timing of AR. In detail, ECS with medical indications was associated with “high level” BMI trajectory, and ECS without medical indication was related with girls' early AR.
Previous studies have reported the association between CS and children's BMI trajectories. Begum et al. (31)showed that the pooled OR of “high level” trajectory in offspring delivered by CS was 1.72 (95% CI: 1.28–2.31) compared with those born via VD. Zhang et al. (32) found that children delivered by CS (without distinguishing ECS and non-ECS) more possibly had a “medium to high increase” trajectory from 3 to 60 months of age and a “low to high increase” trajectory from 3 to 60 months of age. Similar to previous studies, we found an increased possibility of “high level” BMI trajectory in children born via ECS with medical indicators. “High level” trajectories are reported to have predictive values for obesity in later life (15). We did not find any association of emergency CS on children's BMI trajectories. Emergency CS is a procedure preceded by labor, where the membrane usually ruptures, resulting in exposure of the infant to vaginal microbiota. ECS, however, is performed before the onset of labor. A lack of infants' exposure to these microbes at birth may increase the risk of obesity later in life. Studies have found that CS lead to lower gut microbial diversity in children (33), with few species of the Bacteroides (34), a kind of beneficial bacteria. Decrease in the Bacteroides may increase the risk of obesity (35). Stress hormones triggered by different modes of delivery may also play a role. A normal increase in the stress hormone, cortisol, during childbirth facilitates the adaptation of the newborn to the extra-uterine environment (36). Studies have shown that infants born through ECS have the lowest stress response, lower response in emergency CS, and higher response in VD (37, 38). Reduced fetal cortisol levels in the ECS may lead to metabolic disorders at birth and future obesity (39).
In the current study, particularly, we found that girls born via ECS without medical indications were more possibly to have early AR. It is worth noting that ECS with medical indications is an effective means of saving women and children's life. It is necessary, and must be performed even if it would have certain impact on children's later health. ECS without medical indications is a non-essential procedure and is modifiable through informed choice by the health providers and health users. This is why we need to examine the respective effect of ECS with and without medical indications on maternal and children's health. Studies have proven that childhood early AR serves as an early predictor of obesity in later life (14, 17). Therefore, understanding the determinants of early AR, especially the modifiable factors, may improve the overall effectiveness of childhood obesity prevention. Due to ethical considerations, it is unfeasible to conduct randomized controlled trials to identify the impact of CS on maternal and infant health. In this study, findings of the association between ECS without medical indications and the risk of early AR and possible later onset of obesity will provide additional evidence-based advice to clinicians and women in their informed choice of delivery mode. It will also help to decrease the waste of medical resources for unnecessary CS and achieve much earlier prevention and control of childhood obesity.
The findings that ECS related with high level BMI trajectory and early AR were observed only in girls. This may be related to boys' and girls' physiology. Girls are genetically determined to be more prone to fat accumulation and obesity than boys (40), and have higher body fat than boys beginning in the second trimester of gestation and extending throughout life (41). The animal model also found that mice with two X chromosomes gained almost twice as much fat as mice with X and Y chromosomes (42).
Our study has some strengths. Firstly, to our knowledge, this is the first time that ECS has been subdivided into ECS with medical indicators and ECS without medical indicators in examining the association of ECS on children's physical development. As has not been previously reported, we found ECS without medical indication related with girls' early AR. ECS without medical indicators may violate eugenic principles and adversely affect offspring's physical development, and this type of delivery can exactly be avoided by rational informed consent toward women. In the second, there were exceedingly frequent follow-ups with children. Follow-up visits were conducted every 3 months until 1 year of age and every 6 months after 1 year of age. To date, we have collected children's complete data from 15 follow-up visits. This would permit a more accurate fitting for children's growth trajectory (15). In the third, based on a prospective birth cohort, information on exposure, outcome and confounders could be accurately collected, which decreased the recall bias. Meanwhile, precision variables were considered to effectively enhance the precision of the findings.
Some limitations must be acknowledged. Other postnatal factors, such as children's activities, sleep, diet also affect their physical development. Low activity levels and short sleep duration are reported to be associated with high BMI in pre-adolescent children (43, 44). These factors, however, had not been addressed in our study. Furthermore, paternal genetic factors may play vital roles in influencing children's physical development. For instance, paternal overweight and obesity and their potential inheritance have been shown to be a risk factor for children's high BMI (45). There was still a lack of father's anthropometric indicators in the current study.
In conclusion, in this population-based longitudinal study, ECS with medical indication was found to be associated with “high level” BMI trajectory, and ECS without medical indication was found to be associated with early AR in girls.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee of Anhui Medical University (20131401). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
SZ collated, analyzed the data, and was the lead author of the first draft. JZ and XT were involved in the data analysis. MY was involved in the cohort data collection. FZ was involved in the literature collection. FT was the cohort leader. KH was the cohort site leader, reviewed, revised the first draft, supervised, and led the study. All authors contributed to the article and approved the submitted version.
Funding
National Natural Science Foundation of China (81872630), The University Synergy Innovation Program of Anhui Province (GXXT-2020-067), Sci-tech Basic Resources Research Program of China (2017FY101107), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT310002) and Research Fund of Anhui Institute of translational medicine (ZHYX2020A001).
Acknowledgments
The authors thank all the women and children participated in the birth cohort. We are also grateful for all researchers and medical workers involved in the study at the Ma'anshan Maternal and Child Health Care Center for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.985851/full#supplementary-material
Abbreviations
CS, Cesarean Section; ECS, Elective Cesarean Section; VD, Vaginal Delivery; AR, Adiposity Rebound; EAR, Early adiposity rebound; BMI, Body Mass Index; MABC, Ma'anshan-Anhui Birth Cohort; GDM, G estational Diabetes Mellitus; HDP, Hypertensive Disorders in Pregnancy; SGA, Small for Gestational Age; AGA, Appropriate for Gestational Age; LGA, Large for Gestational Age; GBTM, Group-Based Trajectory Model; BIC, Bayesian information criterion.
References
1. Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. (2021) 6:e005671. doi: 10.1136/bmjgh-2021-005671
2. Li HT, Hellerstein S, Zhou YB, Liu JM, Blustein J. Trends in Cesarean delivery rates in China, 2008-2018. JAMA. (2020) 323:89–91. doi: 10.1001/jama.2019.17595
3. Deng R, Tang X, Liu J, Gao Y, Zhong X. Cesarean delivery on maternal request and its influencing factors in Chongqing, China. BMC Pregnancy Childbirth. (2021) 21:384. doi: 10.1186/s12884-021-03866-7
5. Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. (2018) 392:1349–57. doi: 10.1016/S0140-6736(18)31930-5
6. WHO. Obesity and Overweight. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 9 June 2021).
7. Mueller NT, Zhang M, Hoyo C, Østbye T, Benjamin-Neelon SE. Does cesarean delivery impact infant weight gain and adiposity over the first year of life? Int J Obes (Lond). (2019) 43:1549–55. doi: 10.1038/s41366-018-0239-2
8. Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. (2016) 170:e162385. doi: 10.1001/jamapediatrics.2016.2385
9. Chu S, Zhang Y, Jiang Y, Sun W, Zhu Q, Liu S, et al. Cesarean section and risks of overweight and obesity in school-aged children: a population-based study. QJM. (2018) 111:859–65. doi: 10.1093/qjmed/hcy195
10. Rutayisire E, Wu X, Huang K, Tao S, Chen Y, Tao F. Cesarean section may increase the risk of both overweight and obesity in preschool children. BMC Pregnancy Childbirth. (2016) 16:338. doi: 10.1186/s12884-016-1131-5
11. Masukume G, McCarthy FP, Baker PN, Kenny LC, Morton SM, Murray DM, et al. Association between caesarean section delivery and obesity in childhood: a longitudinal cohort study in Ireland. BMJ Open. (2019) 9:e025051. doi: 10.1136/bmjopen-2018-025051
12. Cai M, Loy SL, Tan KH, Godfrey KM, Gluckman PD, Chong YS, et al. Association of Elective and Emergency Cesarean Delivery With Early Childhood Overweight at 12 Months of Age. JAMA Netw Open. (2018) 1:e185025. doi: 10.1001/jamanetworkopen.2018.5025
13. Masukume G, McCarthy FP, Russell J, Baker PN, Kenny LC, Morton SM, et al. Caesarean section delivery and childhood obesity: evidence from the growing up in New Zealand cohort. J Epidemiol Community Health. (2019) 73:1063–70. doi: 10.1136/jech-2019-212591
14. Rolland-Cachera MF, Deheeger M, Bellisle F, Sempé M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. (1984) 39:129–35. doi: 10.1093/ajcn/39.1.129
15. Mattsson M, Maher GM, Boland F, Fitzgerald AP, Murray DM, Biesma R. Group-based trajectory modelling for BMI trajectories in childhood: a systematic review. Obes Rev. (2019) 20:998–1015. doi: 10.1111/obr.12842
16. Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. (2015) 19:1–336. doi: 10.3310/hta19430
17. Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Pediatrics. (2014) 133:e114–9. doi: 10.1542/peds.2013-0966
18. González L, Corvalán C, Pereira A, Kain J, Garmendia ML, Uauy R. Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int J Obes (Lond). (2014) 38:1299–304. doi: 10.1038/ijo.2014.97
19. Aris IM, Rifas-Shiman SL Li LJ, Kleinman KP, Coull BA, Gold DR, et al. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int J Epidemiol. (2019) 48:157–67. doi: 10.1093/ije/dyy286
20. Sovio U, Kaakinen M, Tzoulaki I, Das S, Ruokonen A, Pouta A, et al. How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? Findings from the Northern Finland Birth Cohort 1966 Study. Int J Obes (Lond). (2014) 38:53–9. doi: 10.1038/ijo.2013.165
21. Zhou J, Zhang F, Qin X, Li P, Teng Y, Zhang S, et al. Age at adiposity rebound and the relevance for obesity: a systematic review and meta-analysis. Int J Obes (Lond). (2022)46:1413–1424. doi: 10.1038/s41366-022-01120-4
22. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. (1999) 10:37–48. doi: 10.1097/00001648-199901000-00008
23. Cooperative Meta-analysis Group of Working Group on Obesity in China. Prospective study for cut-off points of body mass index in Chinese adults. Chin J Epidemiol. (2002) 6:431–4.
24. Aris IM, Rifas-Shiman SL, Li LJ, Kleinman K, Coull BA, Gold DR, et al. Pre-, perinatal, and parental predictors of body mass index trajectory milestones. J Pediatr. (2018) 201:69–77.e8. doi: 10.1016/j.jpeds.2018.05.041
25. Cissé AH, Lioret S, de Lauzon-Guillain B, Forhan A, Ong KK, Charles MA, et al. Association between perinatal factors, genetic susceptibility to obesity and age at adiposity rebound in children of the EDEN mother-child cohort. Int J Obes (Lond). (2021) 45:1802–10. doi: 10.1038/s41366-021-00847-w
27. Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol. (2012) 12:38. doi: 10.1186/1471-2288-12-38
28. Wu D, Zhu J, Wang X, Shi H, Huo Y, Liu M, et al. Chen J. Rapid BMI increases and persistent obesity in small-for-gestational-age infants. Front Pediatr. (2021) 4:9:625853. doi: 10.3389/fped.2021.625853
29. Zheng M, Campbell KJ, Baur L, Rissel C, Wen LM. Infant feeding and growth trajectories in early childhood: the application and comparison of two longitudinal modelling approaches. Int J Obes (Lond). (2021) 45:2230–7. doi: 10.1038/s41366-021-00892-5
30. Morgen CS, Ängquist L, Baker JL, Andersen AN, Sørensen TIA, Michaelsen KF. Breastfeeding and complementary feeding in relation to body mass index and overweight at ages 7 and 11 y: a path analysis within the Danish National Birth Cohort. Am J Clin Nutr. (2018) 107:313–22. doi: 10.1093/ajcn/nqx058
31. Begum T, Fatima Y, Perales F, Anuradha S, Mamun A. Associations of caesarean section with body mass and waist circumference trajectories from age 2 to 13 years: a nationally representative birth cohort study in Australia. Pediatr Obes. (2021) 16:e12769. doi: 10.1111/ijpo.12769
32. Zhang L, Huang L, Zhao Z, Ding R, Liu H, Qu W, et al. Associations between delivery mode and early childhood body mass index Z-score trajectories: a retrospective analysis of 2,685 children from mothers aged 18 to 35 years at delivery. Front Pediatr. (2020) 8:598016. doi: 10.3389/fped.2020.598016
33. Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. (2014) 63:559–66. doi: 10.1136/gutjnl-2012-303249
34. Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574:117–21. doi: 10.1038/s41586-019-1560-1
35. Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. (2018) 14:501–9. doi: 10.1089/chi.2018.0040
36. Tribe RM, Taylor PD, Kelly NM, Rees D, Sandall J, Kennedy HP. Parturition and the perinatal period: can mode of delivery impact on the future health of the neonate? J Physiol. (2018) 596:5709–22. doi: 10.1113/JP275429
37. Miller NM, Fisk NM, Modi N. Stress responses at birth: determinants of cord arterial cortisol and links with cortisol response in infancy. BJOG. (2005) 112:921–6. doi: 10.1111/j.1471-0528.2005.00620.x
38. Schuller C, Känel N, Müller O, Kind AB, Tinner EM, Hösli I, et al. Stress and pain response of neonates after spontaneous birth and vacuum-assisted and cesarean delivery. Am J Obstet Gynecol. (2012) 207:416.e1–6 doi: 10.1016/j.ajog.2012.08.024
39. Shokry E, Marchioro L, Uhl O, Bermúdez MG, García-Santos JA, Segura MT, et al. Investigation of the impact of birth by cesarean section on fetal and maternal metabolism. Arch Gynecol Obstet. (2019) 300:589–600. doi: 10.1007/s00404-019-05213-w
40. Link JC, Reue K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu Rev Nutr. (2017) 37:225–45. doi: 10.1146/annurev-nutr-071816-064827
41. Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients. (2020) 12:2735. doi: 10.3390/nu12092735
42. Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. (2012) 8:e1002709. doi: 10.1371/journal.pgen.1002709
43. Koning M, Hoekstra T, de Jong E, Visscher TL, Seidell JC, Renders CM. Identifying developmental trajectories of body mass index in childhood using latent class growth (mixture) modelling: associations with dietary, sedentary and physical activity behaviors: a longitudinal study. BMC Public Health. (2016) 16:1128. doi: 10.1186/s12889-016-3757-7
44. Seegers V, Petit D, Falissard B, Vitaro F, Tremblay RE, Montplaisir J, et al. Short sleep duration and body mass index: a prospective longitudinal study in preadolescence. Am J Epidemiol. (2011) 173:621–9. doi: 10.1093/aje/kwq389
Keywords: cesarean section, BMI trajectory, adiposity rebound, birth cohort, repeated anthropometric data
Citation: Zhang S, Zhou J, Yang M, Zhang F, Tao X, Tao F and Huang K (2022) Sex-specific association between elective cesarean section and growth trajectories in preschool children: A prospective birth cohort study. Front. Public Health 10:985851. doi: 10.3389/fpubh.2022.985851
Received: 04 July 2022; Accepted: 25 August 2022;
Published: 20 September 2022.
Edited by:
Hong Jiang, Fudan University, ChinaReviewed by:
Xu Xiong, Tulane University School of Public Health and Tropical Medicine, United StatesYongfu Yu, Fudan University, China
Copyright © 2022 Zhang, Zhou, Yang, Zhang, Tao, Tao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Huang, YWhtdWh1YW5na0AxNjMuY29t
 Shanshan Zhang1
Shanshan Zhang1 Kun Huang
Kun Huang