
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 11 October 2022
Sec. Occupational Health and Safety
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.978590
 Ambadasu Bharatha1†
Ambadasu Bharatha1† Kandamaran Krishnamurthy2*†
Kandamaran Krishnamurthy2*† Damian Cohall1†
Damian Cohall1† Sayeeda Rahman3†
Sayeeda Rahman3† Corey A. Forde4
Corey A. Forde4 Rhea Corbin-Harte5
Rhea Corbin-Harte5 Nkemcho Ojeh1†
Nkemcho Ojeh1† Russell Kabir6†
Russell Kabir6† Ali Davod Parsa7†
Ali Davod Parsa7† Ahbab Mohammad Fazle Rabbi8
Ahbab Mohammad Fazle Rabbi8 Md Anwarul Azim Majumder1†
Md Anwarul Azim Majumder1†Background: The use of personal protective equipment (PPE) reduces the risk of transmission of infectious agents significantly among healthcare workers (HCWs). The study aimed to investigate the prevalence and characteristics of PPE-related adverse skin reactions among HCWs working at the main COVID-19 isolation center in Barbados.
Methods: A cross-sectional web-based online survey was conducted during April to June 2021 which recorded demographic information, details of PPE use and adverse skin reactions including severity and duration of onset of symptoms.
Results: Most of the respondents used PPE for consecutive days (77.9%), 1–6 h/day (59.2%), and more than a year (62.5%). Fewer than half of the participants (45.6%) experienced adverse skin reactions from the use of PPE. The reactions were mostly observed in the cheeks (40.4%) and nose bridges (35.6%). Females had more reactions than their male counterparts (p = 0.003). The use of N95 masks and a combination of surgical and N95 masks produced adverse effects predominantly in the ears (60%) and cheeks (56.4%). Binary logistic regression showed that female HCWs (OR = 5.720 95% CI: 1.631, 20.063), doctors (OR = 5.215 95% CI: 0.877, 31.002), and longer duration of PPE use (>1 year) (OR = 2.902 95% CI: 0.958, 8.787) caused a significantly higher prevalence of adverse skin reactions.
Conclusion: The PPE-related skin reactions were common among HCWs which mainly occurred due to prolonged use. Preventive measures inclusive of appropriate training of HCWs on the use of PPE are recommended to minimize these adverse events.
Frontline healthcare workers (HCWs) have faced numerous challenges while treating and managing COVID-19 patients during the pandemic (1–5). These frontline HCWs are susceptible to infection, and they account for 10% of the COVID-19 cases (1). During the Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) outbreaks, a quarter of infected cases were among HCWs (6, 7). Studies showed that the use of personal protective equipment (PPE) could reduce the risk of infection significantly among HCWs (8, 9). Standard measures such as the use of gloves, gowns, and eye protection are recommended by the Centers for Disease Control and Protection (CDC) (10) and the World Health Organization (WHO) (11). Furthermore, the CDC recommends the use of N95 filtering facepiece respirators by all COVID-19 patients (both suspected and confirmed). Also, the WHO recommends the use of surgical masks by HCWs providing care to COVID-19 patients along with the exclusive use of respirators by HCWs for aerosol-generating procedures (12).
The mode of transmission of COVID-19 has been shown to be respiratory droplets and contact with fomites. The use of PPEs is critical to reducing cross-transmission of the infection (6–8). The PPE provides a layer of safety for HCWs by limiting contact between clinical staff and patients (13). HCWs require appropriate PPE training and consistent guidance to protect their health and well-being (14). Several studies have already highlighted the high frequency of physical health issues, including skin abnormalities due to PPE use among HCWs (15–17). These are mainly due to the long-term wearing, inappropriate re-use, ill-fitting PPE and PPE shortages during the COVID-19 pandemic (16–19). Moreover, PPE may generate a series of skin lesions due to (i) long-term sealing caused by poor air permeability, (ii) friction-induced skin conditions such as erythema, blisters or ulcers, associated with pain and even secondary infection, and (iii) pressure on the skin (20, 21). There is an increasing incidence of occupational dermatoses due to facial PPE, including adverse cutaneous reactions, irritant contact dermatitis, allergic contact dermatitis, acneiform eruptions, and contact urticaria (22). Appropriate strategies need to be taken to prevent PPE-related adverse events by supplying an adequate number of PPEs and organizing training on the proper use of PPE by HCWs (16, 19).
The first COVID-19 case was identified in Barbados on 17 March 2020 (2). As of Jan 09, 2022, 11% of the Barbadian population tested positive for COVID-19, and the death rate was reported at 0.93 per 1,000 people (23). The aim of this study is to investigate the prevalence and characteristics of adverse facial skin reactions due to the use of PPE among HCWs working in a COVID-19 isolation center in Barbados and suggest potential risk factors and management strategies for these reactions.
Barbados is the most southeastern island in the Caribbean and spans an area of 432 km2 (166 sq mi) with a population of 287,000. The Queen Elizabeth Hospital (QEH) is the only tertiary care hospital on the island with a bed capacity of 519. Four isolation centers were established to combat the COVID-19 pandemic as the caseload on the island increased: Harrison Point, Enmore Center, Psychiatric Hospital and the Sunbay Hotel. The largest isolation facility is the Harrison Point, which was established in a refurbished military base with a capacity of 200 beds. The facility has been staffed with 30 nurses, 18 physicians, 45 housekeeping and 18 orderlies and subdivided into primary, secondary and tertiary care units. The tertiary care unit accommodates asymptomatic but confirmed COVID positive cases, the secondary unit admits mild cases where patients require oxygen for management, and moderate to severe cases are managed in the primary care unit. The Enmore Center is a 3-bedded intensive care unit located just opposite the QEH. Patients at this site are transferred to the Harrison Point isolation center once they are stabilized. The Enmore Center is staffed with 10 nurses, 2 physicians and 4 housekeeping staff to cover the 24 h schedule at the facility. The other two isolation centers are the Psychiatric Hospital and the Sunbay Hotel where mild cases are managed. Each center is staffed with ~15 nurses, 4 physicians, 4 orderlies, and 8 housekeeping staff.
The study used purposive sampling of all HCWs working at the COVID-19 main isolation center in Barbados. HCWs were invited to complete a cross-sectional online survey assessing adverse skin reactions using PPE. Inclusion criteria for the study are staff working in areas deemed necessary for PPE usage, namely staff caring for suspected and infected COVID-19 patients or staff at the frontline hospitality services, receptionists and ushers. Email invitations were sent with a Google Forms survey link from 1 April 2021 to 21 June 2021.
The study used a validated questionnaire developed by Abiakam et al. (17). The modified questionnaire was pretested, and the final version was approved for use to conduct the survey. Items were grouped in the following sections: (i) demographic information, (ii) occupational related information, and (iii) adverse skin reactions (Supplementary material). To assess the effect of PPE on the skin, two different measures were recorded for each participant. The respondents' perception of their skin health was assessed prior to and after the use of PPE by self-reports. The measurement of pain due to the use of PPE was recorded on a scale of 0 (no pain) to 10 (highest pain). The perceived skin health before and after PPE use was assessed by a Likert scale of 1 (The worst skin health you can imagine) to 10 (The best skin health you can imagine).
Participation was purely voluntary and informed consent was implied by completing the questionnaire.
The study was approved by the University of the West Indies, Cave Hill Campus/Barbados Ministry of Health and Wellness Research Ethics Committee/Institutional Review Board (IRB No. 210322-B).
Demographic data and some data on adverse effects due to the use of PPE were analyzed using descriptive statistics mainly. Frequency distributions were obtained to analyze the incidence and prevalence of skin reactions that occurred due to PPE use during the period of observation. Bivariate correlations were examined between the average duration of PPE use (also with the history of using PPE), demographic characteristics, and different skin effects of PPE. To assess the significant association between two variables, chi-square statistics with p-values were calculated. All statistical analysis was performed using IBM SPSS 20.0.
Out of 215 HCWs, 104 completed the survey and the response rate was 48.4%. The majority of the respondents were females (71.2%) and the nurses represented the largest group (45.2%) of HCWs. The majority of the study respondents were aged between 25 and 34 years (41.3%) (Table 1). In addition, more than half of the respondents (52.4%) were employed in public /government institutes. Eighty percent of the respondents reported that they had no chronic diseases. Only 40.4% of respondents attended a PPE fit testing appointment. Most of the respondents felt comfortable (86.5%), safe (99%), and able to breathe easily (82.5%) while using PPE (Table 2). Gender specific influence on the use of protective equipments is shown in Table 3.
More than three-quarters of the respondents (77.9%) used PPE for consecutive days, more than half (59.2%) used it for 1–6 h/day and approximately two-thirds used it for more than a year (62.5%) (Table 1). The average number of working days per week reported by the respondents was 4. In relation to protective equipment, 98% used a face shield as an eye protection instrument. Majority of the respondents (41.3%) reported that they used the surgical mask, followed by HCWs using N95 masks (37.5%) and those using both surgical and N95 masks (19.2%) (Table 2).
The use of PPE caused red lesions (35.9%), indentation lesions (36.5%), and broken skin (2.9%) in facial areas. More than 35% of respondents stated that they had the best skin health prior to using PPE, while 28.1% had the same skin health after PPE use. On a scale of 1 (worst) to 10 (best), ~24 and 32% scored between 1 and 5 to indicate their skin health before and after using the PPE, respectively.
More than 45% (n = 47) of participants experienced adverse skin reactions from the use of PPE. Redness, itchiness, rash, pressure damage and dry skin were the different reactions reported by the respondents. These occurred specifically at five face locations, namely the forehead, the nose, cheeks, ears and under lips as summarized in Table 4. The use of face masks caused skin reactions mainly at the cheeks (40.4%), nose (35.6%), ears (34.6%), and under lips (19.2%). The respondents who were using a face shield as eye protection suffered skin reactions on the forehead (34.6%).
Female professionals had more adverse outcomes for using PPE than their male counterparts with a significant statistical association (p = 0.003). More than half of the respondents reported that their cheeks (56.4%), nose bridge (51.3%) and ear (51.3%) were affected by using N95 masks (Figure 1). The use of both surgical masks and N95 masks produced adverse effects predominantly at the ears (60%) and cheeks (50%). When asked about the overall pain due to the use of PPE in a scale of 0 (no pain) to 10 (highest pain), more than half of respondents (54.4%) reported no pain (Figure 2).
To check whether various demographic variables and use pattern of PPE have any effect on the skin, we used binary logistic regression. The findings are summarized in Table 5. Here the dependent variable is whether the HCWs have any adverse effect on the skin due to using PPE. Gender, profession, and duration of PPE use were found to have a significant adverse skin reaction. Female HCWs were 5.7 times more likely to have adverse skin reactions than their male counterpart. Doctors are 5.2 times more risk than the nurses to develop adverse reactions. The use of PPE for longer was also found to be significant in this context, those who are using PPE for more than 1 year were 2.9 times more likely to have adverse skin reactions than those who were using less than a year.
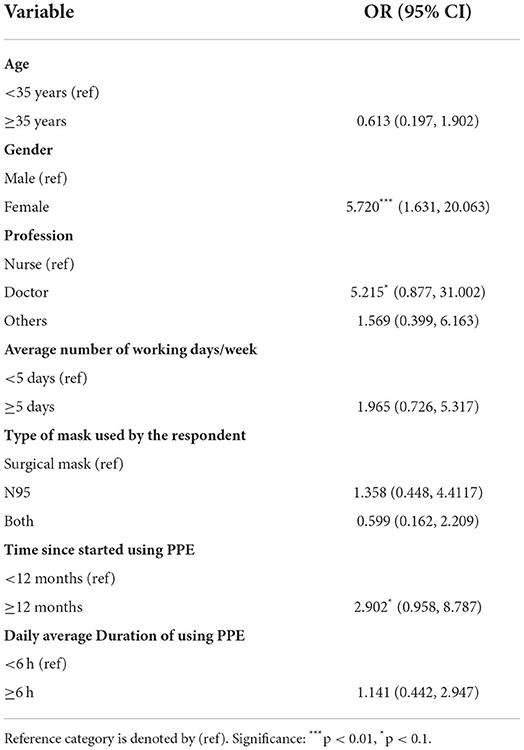
Table 5. Logistic regression coefficients and odds ratios (95% CI) for determinants adverse reactions.
This study was conducted in Harrison's Point, the main COVID-19 isolation center of Barbados, to assess facial skin reactions due to the use of PPE by HCWs. Less than half of the participants (45.6%) reported changes in skin health as a direct result of PPE usage. A systematic review analyzed 14 studies (n = 11,746 HCWs) from 16 countries and reported that the prevalence of skin changes due to the use of PPE among HCWs was 78% with a range from 42.8 to 95.1% reported among the studies (15). The causes of a wide range of prevalence include the use of PPE for varied periods due to the PPE demand and supply challenges, the increased workload of HCWs, lack of training and awareness regarding PPE usage, and not wearing (donning/doffing) PPE in the appropriate way (24–26). Our study found that doctors had a higher risk of developing adverse skin reactions compared to nurses. This may be attributable to prolonged wear of PPE by doctors due to longer clinical shift work and on-call hours. We also found that female professionals suffered more skin reactions when using PPE than their male counterparts (p = 0.003) and they were 5.7 times more likely to have adverse skin reactions than their male counterparts (OR = 5.720 95% CI: 1.631, 20.063). Our finding is consistent with the findings of some recent studies conducted during the COVID-19 pandemic (21, 27–29). More skin reactions among females may be explained by genetic factors, hormonal differences, physical activity, hygiene practices and the use of skin care products (15). Moreover, PPE is typically designed for white male size and shape and incorrect fitting for the anthropometric facial features of Black, Asian, and Minority (BAME) ethnicities has been reported elsewhere (17, 30). Hence, in this study, ill-fitted PPE, due to gender differences in facial features, may account for the increased skin reactions seen among females when worn for extended periods.
The current study found compelling evidence that the use of PPE for prolonged periods without skin relief is linked to the occurrence of adverse skin reactions. Our findings also demonstrated that those HCWs using PPE for a longer duration (>1 year) may be more likely to suffer adverse skin reactions, than those who were using less than a year. The majority of the respondents in our study who used PPE for more than a year, consecutive days or an average of 4 days per week and 1–6 h per day suffered skin reactions. The most commonly affected areas were the cheeks and the nose (Table 3), which is consistent with previous findings (29, 31–33). The use of PPE for longer periods causes compression injuries and excessive sweating; both stimulating the skin reactions in the above-mentioned body areas (31, 34–36). The use of PPE also causes skin barrier dysfunction due to prolonged contact time or increased frequency of use (37, 38). Specifically, prolonged exposure of PPE to the skin surface can cause pressure, friction and shear forces which can inflict skin and underlying tissue damage. Moreover, the combination of excessive sweating and moisture compromises the epidermal stratum corneum and reduces the mechanical load tolerance of the skin thus compromising the barrier function of the skin and providing grounds for injury (17) and secondary infection (39). Profuse sweating can also induce skin itching, pain and redness (15). Pressure damage to the skin behind the ears can be the result of repetitive friction attributable to the face masks string around the ears (16). The itching experienced may be due to the humid microenvironment that is generated under the PPE along with discomfort after extended PPE usage. The mask material may also trigger an allergic reaction which can give rise to contact dermatitis (21). A systematic review conducted by Keng et al. (39) using sixteen studies (n = 3,958) found that the most affected sites were nose or nose bridge (24.7%) and cheeks (21.3%). Another systematic review (16 studies) identified irritant contact dermatitis due to pressure and friction which were common on the cheeks and nasal bridge (40). Several studies showed that HCWs who wore PPE more than 6 h daily were at increased risk of adverse skin reactions (31, 37, 41).
It was found that wearing an N95 mask and a combination of both surgical and N95 masks were associated with a higher incidence of adverse skin reactions than wearing a surgical mask (Figure 1). Battista et al. noted that the incidence of adverse skin reactions was higher in the healthcare staff wearing N95 masks (41). Another study by Hu et al. (21) also reported a higher incidence (95.1%) of the skin reactions provoked by using an N95 mask. An Indian study recorded adverse skin reactions in the nasal bridge (76.64%) and ears (66.42%) after wearing N95 masks (16). Similar findings were echoed in China (21) and Singapore (42) among nurses and other HCWs, respectively. The skin reactions of N95 masks occur as HCWs have to tie the mask tightly using the metal clip to protect themselves from the COVID-19 infection (16). This may cause physical problems (e.g., headache, nausea, vomiting, etc.) due to hypoxemia and hypercapnia (43) especially in the warm and humid climate prevailing in the Caribbean countries (44). In addition, the combination of heat, humidity and moisture generated under N95 masks along with prolonged pressure and friction on the skin surface creates a microenvironment that raises the risks of skin reactions (45).
Only 40.4% of study respondents reported that they attended the PPE fit testing. Although most respondents reported being comfortable, safe and able to breathe easily while wearing PPE, a narrative review (46) states that fit testing is recommended by various international and national bodies to ensure respirators fit appropriately for the individual HCW and also to avoid transmission rates. Fit testing is also important as a training measure and can improve the mental wellness of HCWs. One study demonstrated a correlation between mental health and the number of skin reactions caused by the use of PPE (47). Furthermore, training also reduces the occurrence of adverse skin reactions and improves mental/physical health and boosts the morale and quality of life of HCWs (48, 49). Long-term studies should be conducted to evaluate the burnout, anxiety, depression, and emotional effects due to the use of the PPE and problems experienced by the HCWs providing patient care. We also found that there was a 20% reduction in the HCWs' perception of their facial skin health after their use of PPE. Adequate skin care before and after using PPE and applying barrier protectors and moisturizers on a regular basis is recommended (50).
Various studies suggest that HCWs should perform regular skin checks during and between periods of wearing PPE (17). HCWs wearing PPE should maintain hygiene and apply skin protectors or moisturizers to the contact areas with the PPE (50). After applying PPE, check for a “good” fit and ensure there are no areas of extra pressure. To relieve pressure, persons should provide frequent relief from PPE when safe, especially during long clinical shifts (51). As soon as adverse reactions are observed, switching the PPE device is recommended to avoid exposing vulnerable skin sites (17). It is reassuring that approximately three-quarters of the respondents in our study had consented to monitor their skin health on a weekly basis.
One of the important limitations of the present study was the small sample size and the use of HCWs in a single isolation center which may impact generalizability, as the study did not include other isolation or healthcare centers (selection bias). In addition, “recall” and “answer” biases cannot be ruled out as HCWs responded to a self-administered questionnaire and provided self-reported assessments of adverse reactions. Previous skin problems were not explored prior to the study nor were formal verification or diagnoses of the skin reactions ascertained in this study. Therefore, the factors associated with the causes and severity of these adverse effects were not assessed independently. Additionally, the present study failed to appraise the HCWs' psychological effects, quality of life and morale due to PPE induced skin problems. As this is the first study of its kind in the Caribbean region, it sets the scene to investigate the incidence of adverse skin reactions which can be caused by the long-term and inappropriate use of PPE by the HCWs.
The study identified several PPE-related skin reactions which were common among HCWs working in a COVID isolation center in Barbados. These were induced due to prolonged and inappropriate use of PPE. The cheeks and nasal bridge were the most affected areas and female HCWs were more susceptible to adverse effects than males. HCWs who wore an N95 mask or a combination of surgical and N95 masks had a higher rate of adverse skin reactions than those who wore a surgical mask alone. To reduce the risk of skin reactions of frontline HCWs, there is an urgent need to improve PPE guidelines and the design/materials used to manufacture protective equipment. Moreover, preventive measures and appropriate training to counter these adverse events are recommended.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the University of the West Indies, Cave Hill Campus/Barbados Ministry of Health, and Wellness Research Ethics Committee/Institutional Review Board (IRB No. 210322-B). The patients/participants provided their written informed consent to participate in this study.
AB, KK, DC, and MM contributed to the conceptualization of the project. KK, CF, AB, MM, and RC-H contributed to the methodology and data collection. AB, KK, DC, SR, and MM wrote original draft. AR, AB, and MM analyzed the data. AB, KK, DC, NO, and MM contributed to the project administration. RK, AP, NO, and AR critically read, edited the manuscript, and provided useful suggestions on data analysis. AB, KK, and MM have full access to all the data and take responsibility for the integrity of the data. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.978590/full#supplementary-material
1. Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers - a systematic review and meta-analysis. Am J Emerg Med. (2020) 38:1727–31. doi: 10.1016/j.ajem.2020.05.113
2. Krishnamurthy K, Sobers N, Kumar A, Ojeh N, Scott A, Cave C, et al. COVID-19 vaccine intent among health care professionals of Queen Elizabeth Hospital, Barbados. J Multidiscip Healthc. (2021) 14:3309–19. doi: 10.2147/JMDH.S336952
3. Alam A, Azim Majumder MA, Haque M, Ashraf F, Khondoker MU, Mashreky SR, et al. Disproportionate COVID-19 vaccine acceptance rate among healthcare professionals on the eve of nationwide vaccine distribution in Bangladesh. Expert Rev Vaccines. (2021) 20:1167–75. doi: 10.1080/14760584.2021.1951248
4. Ashok N, Krishnamurthy K, Singh K, Rahman S, Majumder MAA. High COVID-19 vaccine hesitancy among healthcare workers: should such a trend require closer attention by policymakers? Cureus. (2021) 13:e17990. doi: 10.7759/cureus.17990
5. Majumder MAA, Lutfor AB, Rabbi AMF, Alam A, Rahman M, Saha N, et al. Prevalence of COVID-19 vaccine reactogenicity among Bangladeshi physicians. FASEB Bioadv. (2022) 4:379–90. doi: 10.1096/fba.2021-00158
6. Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. (2020) 49:717–26. doi: 10.1093/ije/dyaa033
7. Hsin DH, Macer DR. Heroes of SARS: professional roles and ethics of health care workers. J Infect. (2004) 49:210–5. doi: 10.1016/j.jinf.2004.06.005
8. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intens Care Med. (2020) 46:854–87. doi: 10.1007/s00134-020-06022-5
9. Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic - a narrative review. Anaesthesia. (2020) 75:920–7. doi: 10.1111/anae.15071
10. Guidance for the Selection and Use of Personal Protective Equipment (PPE) in Healthcare Settings. Department of Health and Human Services Centers for Disease Control and Prevention (2021). Available online at: https://www.cdc.gov/hai/pdfs/ppe/ppeslides6-29-04.pdf.
11. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations During Severe Shortages. World Health Organisation (2020).
12. Cheng VC, Wong SC, Chuang VW, So SY, Chen JH, Sridhar S, et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. (2020) 81:107–14. doi: 10.1016/j.jinf.2020.04.024
13. Honda H, Iwata K. Personal protective equipment and improving compliance among healthcare workers in high-risk settings. Curr Opin Infect Dis. (2016) 29:400–6. doi: 10.1097/QCO.0000000000000280
14. Hoernke K, Djellouli N, Andrews L, Lewis-Jackson S, Manby L, Martin S, et al. Frontline healthcare workers' experiences with personal protective equipment during the COVID-19 pandemic in the UK: a rapid qualitative appraisal. BMJ Open. (2021) 11:e046199. doi: 10.1136/bmjopen-2020-046199
15. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Impact of personal protective equipment use on health care workers' physical health during the COVID-19 pandemic: a systematic review and meta-analysis. Am J Infect Control. (2021) 49:1305–15. doi: 10.1016/j.ajic.2021.04.084
16. Jose S, Cyriac MC, Dhandapani M. Health problems and skin damages caused by personal protective equipment: experience of frontline nurses caring for critical COVID-19 patients in intensive care units. Indian J Crit Care Med. (2021) 25:134–9. doi: 10.5005/jp-journals-10071-23713
17. Abiakam N, Worsley P, Jayabal H, Mitchell K, Jones M, Fletcher J, et al. Personal protective equipment related skin reactions in healthcare professionals during COVID-19. Int Wound J. (2021) 18:312–22. doi: 10.1111/iwj.13534
18. Park C-Y KK, Roth S, Beck S, Kang JW, Tayag MC. Global shortage of personal protective equipment amid COVID-19: supply chains, bottlenecks, and policy implications. Asian Dev Bank. (2020) 130:1–10. doi: 10.22617/BRF200128-2
19. Cohen J, Rodgers YVM. Contributing factors to personal protective equipment shortages during the COVID-19 pandemic. Prev Med. (2020) 141:106263. doi: 10.1016/j.ypmed.2020.106263
20. Zhou NY, Yang L, Dong LY, Li Y, An XJ, Yang J, et al. Prevention and treatment of skin damage caused by personal protective equipment: experience of the first-line clinicians treating 2019-nCoV infection. Int J Dermatol Venereol. (2020) 3:70–75. doi: 10.1097/JD9.0000000000000085
21. Hu K, Fan J, Li X, Gou X, Li X, Zhou X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine. (2020) 99:e20603. doi: 10.1097/MD.0000000000020603
22. Yu J, Goldminz A, Chisolm S, Jacob SE, Zippin JH, Wu PA, et al. Facial personal protective equipment: materials, resterilization methods, and management of occupation-related dermatoses. Dermatitis. (2021) 32:78–85. doi: 10.1097/DER.0000000000000699
23. Coronavirus in Barbados. Covid Observer. (2021). Available online at: https://covid.observer/bb/ (accessed November 25, 2021).
24. Hakim M, Khattak FA, Muhammad S, Ismail M, Ullah N, Atiq Orakzai M, et al. Access and use experience of personal protective equipment among frontline healthcare workers in Pakistan during the COVID-19 emergency: a cross-sectional study. Health Secur. (2021) 19:140–9. doi: 10.1089/hs.2020.0142
25. Liu Q, Luo D, Haase JE, Guo Q, Wang XQ, Liu S, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. (2020) 8:e790–8. doi: 10.1016/S2214-109X(20)30204-7
26. Iwu CJ, Jordan P, Jaca A, Iwu CD, Schutte L, Wiysonge CS. Cochrane corner: personal protective equipment for preventing highly infectious diseases such as COVID-19 in healthcare staff. Pan Afr Med J. (2020) 37:148. doi: 10.11604/pamj.2020.37.148.24934
27. Metin N, Turan Ç, Utlu Z. Changes in dermatological complaints among healthcare professionals during the COVID-19 outbreak in Turkey. Acta Dermatovenerol Alp Pannonica Adriat. (2020) 29:115–22. doi: 10.15570/actaapa.2020.25
28. Çiriş Yildiz C, Ulaşli Kaban H, Tanriverdi F. COVID-19 pandemic and personal protective equipment: evaluation of equipment comfort and user attitude. Arch Environ Occup Health. (2020) 77:1–8. doi: 10.1080/19338244.2020.1828247
29. Lin P, Zhu S, Huang Y, Li L, Tao J, Lei T, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. (2020) 183:190–2. doi: 10.1111/bjd.19089
30. Green S, Gani A, Bailey M, Brown O, Hing CB. Fit-testing of respiratory protective equipment in the UK during the initial response to the COVID-19 pandemic. J Hosp Infect. (2021) 113:180–6. doi: 10.1016/j.jhin.2021.04.024
31. Lan J, Song Z, Miao X, Li H, Li Y, Dong L, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol. (2020) 82:1215–6. doi: 10.1016/j.jaad.2020.03.014
32. Daye M, Cihan FG, Durduran Y. Evaluation of skin problems and dermatology life quality index in health care workers who use personal protection measures during COVID-19 pandemic. Dermatol Ther. (2020) 33:e14346. doi: 10.1111/dth.14346
33. Kiely LF, Moloney E, O'Sullivan G, Eustace JA, Gallagher J, Bourke JF. Irritant contact dermatitis in healthcare workers as a result of the COVID-19 pandemic: a cross-sectional study. Clin Exp Dermatol. (2021) 46:142–4. doi: 10.1111/ced.14397
34. Gefen A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 1. Nurs Stand. (2009) 23:64:6, 8 passim. doi: 10.7748/ns2009.07.23.45.64.c7115
35. Grap MJ, Munro CL, Wetzel PA, Schubert CM, Pepperl A, Burk RS, et al. Tissue interface pressure and skin integrity in critically ill, mechanically ventilated patients. Intensive Crit Care Nurs. (2017) 38:1–9. doi: 10.1016/j.iccn.2016.07.004
36. Jiang Q, Song S, Zhou J, Liu Y, Chen A, Bai Y, et al. The prevalence, characteristics, and prevention status of skin injury caused by personal protective equipment among medical staff in fighting COVID-19: a multicenter, cross-sectional study. Adv Wound Care. (2020) 9:357–64. doi: 10.1089/wound.2020.1212
37. Yan Y, Chen H, Chen L, Cheng B, Diao P, Dong L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease (2019). Dermatol Ther. (2020) 33:e13310. doi: 10.1111/dth.13310
38. Darlenski R, Tsankov N. COVID-19 pandemic and the skin: what should dermatologists know? Clin Dermatol. (2020) 38:785–7. doi: 10.1016/j.clindermatol.2020.03.012
39. Keng BMH, Gan WH, Tam YC, Oh CC. Personal protective equipment-related occupational dermatoses during COVID-19 among health care workers: a worldwide systematic review. JAAD Int. (2021) 5:85–95. doi: 10.1016/j.jdin.2021.08.004
40. Yu J, Chen JK, Mowad CM, Reeder M, Hylwa S, Chisolm S, et al. Occupational dermatitis to facial personal protective equipment in health care workers: a systematic review. J Am Acad Dermatol. (2021) 84:486–94. doi: 10.1016/j.jaad.2020.09.074
41. Battista RA, Ferraro M, Piccioni LO, Malzanni GE, Bussi M. Personal Protective Equipment (PPE) in COVID 19 pandemic: related symptoms and adverse reactions in healthcare workers and general population. J Occup Environ Med. (2021) 63:e80–5. doi: 10.1097/JOM.0000000000002100
42. Foo CC, Goon AT, Leow YH, Goh CL. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome–a descriptive study in Singapore. Contact Dermatitis. (2006) 55:291–4. doi: 10.1111/j.1600-0536.2006.00953.x
43. Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand. (2006) 113:199–202. doi: 10.1111/j.1600-0404.2005.00560.x
44. Williams WJ, Cichowicz JK. Heat Stress Imposed by PPE Worn in Hot and Humid Environments. CDC (2020). Available online at: https://blogs.cdc.gov/niosh-science-blog/2020/08/06/ppe-heat-stress/ (accessed January 8, 2022).
45. Yuan X, Xi H, Le Y, Xu H, Wang J, Meng X, et al. Online survey on healthcare skin reactions for wearing medical-grade protective equipment against COVID-19 in Hubei Province, China. PLoS ONE. (2021) 16:e0250869. doi: 10.1371/journal.pone.0250869
46. Regli A, Sommerfield A, von Ungern-Sternberg BS. The role of fit testing N95/FFP2/FFP3 masks: a narrative review. Anaesthesia. (2021) 76:91–100. doi: 10.1111/anae.15261
47. Hu D, Kong Y, Li W, Han Q, Zhang X, Zhu LX, et al. Frontline nurses' burnout, anxiety, depression, and fear statuses and their associated factors during the COVID-19 outbreak in Wuhan, China: a large-scale cross-sectional study. EClinicalMedicine. (2020) 24:100424. doi: 10.1016/j.eclinm.2020.100424
48. Dhandapani M, Dhandapani S. Challenges posed by COVID-19 and neurosurgical nursing strategies in developing countries. Surg Neurol Int. (2020) 11:441. doi: 10.25259/SNI_677_2020
49. Dubaniewicz MT, Rottach DR, Yorio PL. Quality assurance sampling plans in US stockpiles for personal protective equipment: a computer simulation to examine degradation rates. Health Secur. (2019) 17:324–33. doi: 10.1089/hs.2019.0042
50. LeBlanc KCH, Butt B, Bresnai-Harris J, Wiesenfeld L. Prevention and Management of Skin Damage Related to Personal Protective Equipment (PPE): Hospital News, Canada's Health Care News and Best Practices, Vertical Media. Available online at: https://hospitalnews.com/prevention-and-management-of-skin-damage-related-to-personal-protective-equipment-ppe/ (accessed January 8, 2022).
Keywords: COVID-19, PPE, adverse skin reactions, healthcare workers, Barbados
Citation: Bharatha A, Krishnamurthy K, Cohall D, Rahman S, Forde CA, Corbin-Harte R, Ojeh N, Kabir R, Parsa AD, Rabbi AMF and Majumder MAA (2022) Personal protective equipment (PPE) related adverse skin reactions among healthcare workers at the main COVID-19 isolation center in Barbados. Front. Public Health 10:978590. doi: 10.3389/fpubh.2022.978590
Received: 28 June 2022; Accepted: 12 September 2022;
Published: 11 October 2022.
Edited by:
Fabriziomaria Gobba, University of Modena and Reggio Emilia, ItalyReviewed by:
Venerando Rapisarda, University of Catania, ItalyCopyright © 2022 Bharatha, Krishnamurthy, Cohall, Rahman, Forde, Corbin-Harte, Ojeh, Kabir, Parsa, Rabbi and Majumder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kandamaran Krishnamurthy, a2FuZGFtYXJhbi5rcmlzaG5hbXVydGh5QGNhdmVoaWxsLnV3aS5lZHU=
†ORCID: Ambadasu Bharatha orcid.org/0000-0003-0287-3959
Kandamaran Krishnamurthy orcid.org/0000-0001-5592-7020
Damian Cohall orcid.org/0000-0002-2173-7322
Sayeeda Rahman orcid.org/0000-0002-7005-8801
Nkemcho Ojeh orcid.org/0000-0002-2507-2209
Russell Kabir orcid.org/0000-0001-9257-2775
Ali Davod Parsa orcid.org/0000-0001-7390-4308
Md Anwarul Azim Majumder orcid.org/0000-0003-3398-8695
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.