
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 10 October 2022
Sec. Environmental Health and Exposome
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.978338
 Yang Chen1,2,3†
Yang Chen1,2,3† Xudong Shen1,2,3†
Xudong Shen1,2,3† Guoxiang Li1,2,3†
Guoxiang Li1,2,3† Shaoyu Yue1,2,3
Shaoyu Yue1,2,3 Chaozhao Liang1,2,3*
Chaozhao Liang1,2,3* Zongyao Hao1,2,3*
Zongyao Hao1,2,3*Environmental pollution sources may play a key role in the pathogenesis of nephrolithiasis, although the link between environmental aldehyde exposure and the incidence of nephrolithiasis is unclear. The researchers in this study set out to see whether adult kidney stone formation was linked to environmental aldehydes. We examined data from 10,175 adult participants over the age of 20 who took part in the 2013–2014 National Health and Nutrition Examination Survey (NHANES), which was a cross-sectional research. A logistic regression model was employed in this work to examine the relationship between aldehyde exposure and kidney stones, machine learning was utilized to predict the connection of different parameters with the development of kidney stones, and a subgroup analysis was performed to identify sensitive groups. After controlling for all confounding variables, the results revealed that isopentanaldehyde, benzaldehyde, and hexanaldehyde were risk factors for kidney stone formation, with odds ratio (OR) of 2.47, 1.12, and 1.17, respectively, and 95 percent confidence intervals (95% CI) of 1.15–5.34, 1.02–1.22, and 1.00–1.36. Kidney stones may be a result of long-term exposure to aldehydes, which may cause them to form. Environmental pollution-related aldehyde exposure might give a novel notion and direction for future study into the process of kidney stone production, even if the cause is yet unknown.
Kidney stones are one of the most common diseases of the genitourinary system, endangering human health, seriously interfering with people's daily work and life, and imposing a significant economic burden on the health-care system, particularly in industrialized countries, as a result of lifestyle and diet. Because of a shift in lifestyle, the prevalence of urolithiasis has progressively grown over the last several decades (1). It's worth mentioning that kidney stone production rates vary greatly across nations and areas. According to current epidemiological research, the prevalence of kidney stones in the United States is more than 10% (2), whereas it is 9 percent in Europe (3) and 5.8 percent in China (4). Unfortunately, its prevalence is anticipated to rise further due to a variety of variables, including global warming, since stone disease is more likely to develop in warmer places (5, 6), and other environmental pollution-related factors also lead to kidney stones. Rising incidence, such as increased cadmium exposure (7), increased lead, mercury, and arsenic exposure (8), as well as ethnic origin, age, and gender, all impact the incidence of kidney stones (9). It is true that urinary calculi are benign lesions in the short term, but they may cause serious consequences, such as blockage of the urinary system and infection, which can lead to death. As a result, figuring out the causes of nephrolithiasis and how to prevent it is a top priority for public health issue.
Few studies have examined the role of external environmental elements in kidney stone development in recent years, despite there have been an increasing number of research on the mechanism of kidney stone development in recent years. As previously said, exposure to certain harmful compounds in the environment influences the occurrence of kidney stones, and these toxic substances present in every aspect of our everyday lives, making us impossible to avoid them. As a result, environmental contamination was proven to cause kidney stones in the everyday lives of individuals. The search for a probable explanation for the rise in cases is essential.
Aldehydes are common organic molecules with a CHO group to which organisms are regularly exposed, both exogenously and endogenously formed. Exogenous aldehydes may be obtained from a variety of sources, including the combustion of organic compounds such as gasoline, nicotine, food additives (10), and so on. Paints, radiators, synthetic carpets, and other sources of indoor aldehydes are the most common (11). Fire and agricultural combustion (12), as well as odor exposure at trash transfer facilities (13), are the primary producers of aldehydes in the air. In metropolitan areas, automobile exhaust gas directly emits aldehydes and hydrocarbons into the air, resulting in aldehydes in the air. Hydrocarbons, an important source of chemicals, are transformed into aldehydes by photochemical oxidation processes (14, 15). Furthermore, research have demonstrated that aldehyde exposure is widespread during laboratory dissection experiments (16). Endogenous aldehyde sources are generated by normal cellular metabolic processes such as lipid oxidation, glucose metabolism, histone demethylation, and so on. Endogenous aldehyde compounds such as 4-hydroxynonenal and malondialdehyde may be formed by lipid peroxidation of mitochondria and plasma membranes under oxidative stress conditions (17, 18). Aldehydes are highly reactive electrophilic compounds that are potentially carcinogenic and mutagenic (19–21), and aldehyde exposure can harm human health by causing allergic hypersensitivity diseases, liver disease, neurodegenerative disease (22), cardiovascular disease (23), and diabetes (24), but few studies have linked aldehyde exposure to kidney stones. As a consequence, this research investigates the possible link between aldehydes and kidney stone production based on the NHANES database in order to uncover preventative and therapy targets for kidney stone epidemic illness.
Data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional survey of the general population conducted at non–profit organizations in order to collect nationally representative data on population health and nutritional status. Data from a survey other data included demographics, body measures, blood pressure, creatinine-urine, standard biochemistry profiles, and urological data. Data on aldehydes were only available for the 2013-2014 cycle. The NHANES website (www.cdc.gov/nchs/nhanes/) has further information on this data. The Institutional Review Board of the Ethics Review Board of the National Center for Health Statistics provided ethical permission for this research, which utilized previously gathered public data.
In 2013-2014, a total of 10,175 participants took part in the NHANES survey. The following were among the exclusion criteria: 1. No one knew about kidney stones (n = 4,417); 2. At least one aldehyde or more was present (n = 4,983); a total of 775 individuals were included in the final research, including 68 with kidney stones.
Automated analytical methods combining solid-phase microextraction (SPME), gas chromatography (GC), and high-resolution mass spectrometry (HRMS) with selective ion mass detection and isotope dilution techniques were used to assess aldehyde levels in blood. Only six aldehydes were found in more than 75 percent of the subjects (23, 25). Therefore, this investigation included isopentanaldehyde, benzaldehyde, butyraldehyde, heptanaldehyde, hexanaldehyde, and propanaldehyde.
Direct interviews and medical center examinations were used to obtain covariates that may influence the association between aldehyde concentrations and the risk of kidney stones, such as age, gender, race, education level, marital status, physical activity, water intake, household poverty-to-income ratio (PIR), body mass index (BMI), drinking and smoking status, history of diabetes, history of hypertension, and laboratory test results. Age and PIR in the household were handled as continuous factors. The following categorical variables were used: Gender (Female, Male), Race (Mexican American, White, Black, Other), Educational Level (High school or equivalent, College or above), Physical Activity (None, Moderate, Vigorous), Alcohol Consumption (No, Yes), Diabetes History (No, Yes, Cutoff), Hypertension History (No, Yes), and Marriage (no, yes). Body mass index (kg/m2) is computed by dividing one's body weight (kg) by one's height (m) squared.
Categorical, dichotomous, or continuous variables are used to record data. The standard deviation of the mean is used to represent distributed continuous data; count proportions are used for dichotomous and categorical variables. The chi-square test (categorical variables), one-way ANOVA (normally distributed continuous variables), or Kruskal-Wallis' H test were used to identify differences in clinical features across groups (skewed distribution continuous variables).
To examine the risk of variables related with kidney stone development, we applied machine learning to predict the influence of each research variable on kidney stone formation. In this investigation, we developed three logistic regression models to evaluate independent associations: (1) unadjusted, (2) slightly adjusted, and (3) adjusted for all covariates. All the covariates were selected on the basis of their clinical importance, the estimated variables change of at least 10% of potential confounding effects. The probable relationship of each aldehyde and kidney stones was then shown using a smooth curve fit. We used hierarchical multiple logistic regression to uncover sensitive groups using subgroup analysis. In addition, during the model development phase, we constructed an XGBoost algorithm model to predict the relative importance of the selected variables. We implemented the XGBoost model to analyze the contribution (gain) of each variable to the prevalence of kidney stones (26, 27). R 3.5.3 (http://www.r-project.org/) and Empower Stats software (http://www.empowerstats.com) were used for statistical analysis, and a p value of 0.05 was deemed statistically significant.
The main features of baseline data between study arms are shown in Table 1. A total of 775 people took part in the study, including 68 kidney stone sufferers. The non-nephrolithic and nephrolithic populations had mean ages of 48.37 17.03 and 54.75 15.65 years, respectively. In terms of racial distribution, white participants made up the lion's share of the NHANES population. Non-nephrolithic and nephrolithic subjects differed considerably in terms of age, gender, blood pressure, and serum creatinine levels. Three aldehydes, Benzaldehyde (ng/mL), Hexanaldehyde (ng/mL), and Isopentanaldehyde (ng/mL), were found to be considerably higher in stone sufferers.
All variables were included in the machine learning model (28), and sorted according to the impact results, the results show that the top 10 factors influencing the formation of kidney stones include (Figure 1): urinary creatinine, water intake, Hexanaldehyde (ng/ml), Benzaldehyde (ng/ml) ml), Serum Creatinine (mg/dl), Age, Butyraldehyde (ng/ml), Isopentanaldehyde (ng/ml), Propanaldehyde (ng/ml), Heptanaldehyde (ng/ml).
The major goal of this research was to determine the link between aldehyde exposure and the prevalence of kidney stone formation. A multiple logistic regression analysis was carried out. Three models were created using the Reporting of Strengthening Epidemiological Observational Studies (STROBE) standards (29): 1. Unadjusted model; 2. Model with minor adjustments; 3. Model with all variables adjusted. Table 2 shows that isopentanaldehyde/benzaldehyde and hexanaldehyde were strongly linked with the incidence of kidney stones, with OR, 95 percent CIs of 2.47 (1.15, 5.34) /1.12 (1.02, 1.22) /1.17 (1.00, 1.36), respectively. We also flattened the curve fitting in order to examine the association between the three aldehydes and kidney stones. The findings revealed that isopentanaldehyde and benzaldehyde had a linear connection (Figure 2), but hexanaldehyde had a non–linear relationship, with no evident threshold impact.
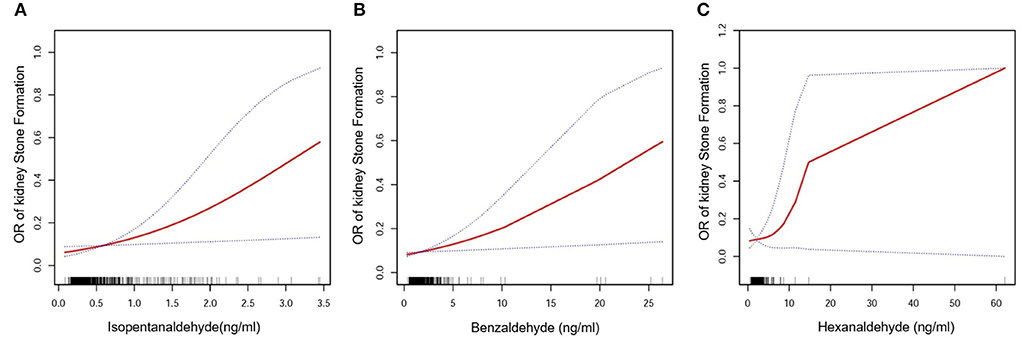
Figure 2. Isopentanaldehyde and total kidney stone formation dose-response relationship (A). Benzaldehyde and total kidney stone formation dose-response relationship (B). Hexanaldehyde and total kidney stone formation dose-response relationship (C). The area between the upper and lower dashed lines is indicated as the 95% CI. The red line is connected by the magnitude of the aldehyde concentration into a continuous line. Adjustments were made for all covariates except for effect modifiers.
We did a subgroup analysis of the three aldehydes based on the aforesaid findings (Table 3). The findings revealed that white people (OR = 5.08, 95% CI: 1.83–14.09) and those over the age of 50 (OR = 5.38, 95% CI: 1.39–20.78) were more likely to be white. The level of Isopentanaldehyde in the population was connected with the risk of kidney stones; female (OR = 1.15, 95% CI: 1.04–1.28), white (OR = 1.13, 95% CI: 1.04–1.28), and the level of Benzaldehyde in the population was associated with the risk of kidney stones. Only the female population in the Hexanaldehyde group was favorably linked with the incidence of nephrolithiasis (OR = 1.16, 95% CI: 1.03–1.56).
Kidney stones, as we are all aware, are a common medical condition. People's eating habits and way of life are continually evolving in today's quickly emerging industrialized nations as a result of economic growth and rising living standards. Kidney stones are becoming more and more common. The prevalence of kidney stones is constantly rising. This not only causes a great deal of problems and annoyance in people's job and lives, but it also causes pain and devastation to health, as well as a significant strain on the social economy and the medical and health system. Although medical technology is advancing by leaps and bounds with the advancement of life science, clinical diagnosis and treatment methods for diseases are also changing with each passing day, and the pain and trauma suffered by clinical patients in the treatment of diseases is becoming smaller and smaller. However, medical science Progress has not slowed the rising occurrence of kidney stones from year to year. Many investigations on the mechanism of kidney stone formation have also been conducted, including the theory of renal calcium plaques, the theory of supersaturated crystallization, the theory of matrix, the theory of inhibitor shortage, and the theory of immunological damage (30). The hypothesis of supersaturation crystallization is the most researched mechanism, and its particular processes include supersaturation, crystal nucleation, aggregation, crystal development, deposition, and stone production (31). However, the specific mechanism of formation is still unknown, and these studies on the mechanism of formation of kidney stones are all at the molecular biology level, whereas there are few reports on the impact of the external environment that is closely related to human life on the formation of kidney stones, and it has not piqued people's interest.
Aldehydes are organic chemicals found in abundance in nature, and their origins are classified as exogenous and endogenous. Exogenous aldehydes enter the human body mostly via the respiratory system and the digestive tract (32), and are prevalent in people's everyday lives and workplaces. Endogenous aldehydes may be produced by lipid peroxidation, carbohydrate metabolism or ascorbic acid autoxidation, cytochrome P-450S, or metabolic activation mediated by myeloperoxidase (17, 18). Previous research has discovered that aldehyde exposure has negative effects on human health, including carcinogenicity, mutagenicity, cardiovascular disease, liver disease, embryotoxicity/teratogenicity, diabetes/hypertension, cerebral ischemia/toxicity in neurodegenerative diseases, and other aging-related diseases (19, 20). However, there have been no studies linking aldehyde exposure to kidney stones.
Serum aldehydes and kidney stones have never before been studied in a representative sample of the American population, according to our literature analysis. After controlling for all possible confounders, we discovered that Isopentanaldehyde/Benzaldehyde and Hexanaldehyde were positively linked with the incidence of kidney stones. Isopentanaldehyde/Benzaldehyde and Hexanaldehyde were shown to be linearly and non-linearly linked with the incidence of nephrolithiasis, respectively, in the findings.
Isopentanaldehyde has been linked to the onset of a variety of disorders in recent years, including obesity (25), cardiovascular disease (23), diabetes (24), and others. There is evidence that high-fat diets cause kidney injury in animals. Urinary oxalate and calcium levels were elevated as a result of crystal retention in the urothelium (33), and male Otsuka Long-Evans Tokushima fat (OLETF) rats were used to create an animal model of metabolic syndrome after drinking 1.0 percent ethylene glycol. The rats in the model group developed higher calcium oxalate crystal deposits after being exposed to water (34). Interestingly, it has been shown that the generation of glyoxal, a substrate for the synthesis of oxalic acid molecules, is increased during diabetic atherosclerosis. Furthermore, lipids include unsaturated fatty acids, and lipids are oxidized to create hydroperoxides, which are then converted to glyoxal (35). Oxalic acid levels that are too high may induce kidney injury and inflammation, both of which are required for the production of kidney stones. Furthermore, triglyceride production in the liver is linked to the de novo synthesis of purines, which are catabolized to generate uric acid, and metabolic syndrome is related with higher plasma uric acid levels (36). The elevated uric acid level promotes the production of uric acid crystals and the creation of uric acid stones. Although uric acid is often utilized as an antioxidant, it should be highlighted that xanthine oxidase (XO) produces reactive oxygen species during the synthesis of uric acid, which leads to the development of kidney stones (37). Furthermore, in patients with cardiovascular disease, there is a systemic imbalance between the essential fatty acid-3 and−6 pathways, which is thought to lead to increased levels of arachidonic acid phospholipids as well as hypercalciuria and hyperoxaluria, both of which are prerequisites for kidney stone formation. Obesity was shown to be positively linked with kidney stone development in the Taylor trial, with men with a BMI of 30 or higher having a 1.33 risk factor for kidney stones compared to men with a BMI of 21 to 22.9, while the risk factor for the same category of BMI in elderly and young women was 1.90 (38). And other studies have shown that a greater BMI has been linked to an increased risk of kidney stones (39), as have studies showing that those with diabetes are more likely to develop kidney stones (40). Finally, the data suggests that obesity, cardiovascular disease, and diabetes are risk factors for the development of kidney stones, and that isopentanaldehyde is linked to the development of these disorders. According to our findings, isopentanaldehyde is also a risk factor for the development of kidney stones. We suspect that it is the explanations might be multi-system and multi-faceted, and the precise process must be validated via additional in vitro and in vivo investigations.
Weng and colleagues used data from 1,795 participants in the National Health and Nutrition Examination Survey (NHANES) from 2013 to 2014 to conduct a multivariate logistic regression analysis of the relationship between aldehydes and diabetes. They discovered that benzaldehyde and hexanaldehyde may increase the risk of diabetes. Women's diabetes development is accelerated by their diabetes risk (24). Our findings reveal that benzaldehyde and hexanaldehyde are risk factors for kidney stone development, and that diabetes is also a risk factor. The underlying process of stone formation might be linked to the development of diabetes, and the particular molecular mechanism has to be confirmed.
To find out whether particular characteristics affect the likelihood of kidney stones, this research applied machine learning. Machine learning is widely used because of its advantages such as easy identification of trends and patterns, no human intervention, and continuous improvement. In this study we used XGboost machine learning to predict the relative importance of the effect of incorporated variables on the prevalence of kidney stones. Data may be ranked using machine learning depending on the significance of the information. For example, urine creatinine, daily water consumption, benzaldehyde and butyraldehyde concentrations as well as serum creatinine and age all play an important role in the kidney stone formation model. According to machine learning findings, the link between Butyraldehyde and the incidence of kidney stones is stronger than the correlation between Isopentanaldehyde and the occurrence of kidney stones. However, the results of our traditional logistic regression analysis indicate that butyraldehyde has no correlation with the occurrence of kidney stones. We hypothesize that this result is due to the small sample size used in the statistics, and to compare the performance of machine learning methods and traditional regression techniques. There have been several studies conducted on the advantages and disadvantages. However, the findings achieved so far have been rather disparate. Some studies have demonstrated that logistic regression may be as accurate or even more accurate than other machine learning techniques (28). Some research has indicated that machine learning approaches are more reliable than classical regression analysis, however. A distinct set of conclusions may be drawn when using the same strategy to other study subjects and datasets. A larger sample size might provide more accurate results, but this study's findings are also reassuring.
As a worldwide epidemic illness with a high prevalence, kidney stones have a significant influence on human health, but aldehyde exposure in environmental pollution and the formation of kidney stones have been overlooked. This research conclusion provides a new way of thinking and direction for the majority of researchers. An inadequate etiology for nephrolithiasis has been shown in this research, due to its cross-sectional design and the inability to establish a causal association between serum aldehydes and kidney stones. We must also take into account that our statistical findings can be influenced by the size of the sample and the sources of serum aldehydes. A rise in serum aldehydes can't be traced back to a specific source despite exposure to both internal and external sources of aldehydes, as shown by NHANES data.
It's possible that aldehyde exposure is a contributing factor in kidney stones. Research on kidney stone formation's process may provide a fresh concept and direction if it focuses on aldehyde exposure caused by environmental pollutant pollution.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/.
YC: conceptualization, methodology, and software. XS: data curation and writing original draft. GL: visualization and investigation. SY: supervision and software. CL and ZH: writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (82070724).
The authors are grateful for the invaluable support and useful discussions with other members of the urological department.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Scales CD, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
2. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D'andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007–2018 data. Eur Urol Focus. (2020) 7:1468–75. doi: 10.1016/j.euf.2020.08.01
3. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan J. Epidemiology of stone disease across the world. World J Urol. (2017) 35:1301–20. doi: 10.1007/s00345-017-2008-6
4. Zeng G, Mai Z, Xia S, Wang Z, Zhang K, Wang L, et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. (2017) 120:109–16. doi: 10.1111/bju.13828
5. Hesse A, Brändle E, Wilbert D, Köhrmann K, Alken K. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. (2003) 44:709–13. doi: 10.1016/S0302-2838(03)00415-9
6. Strohmaier WL. Economics of stone disease/treatment. Arab J Urol. (2012) 10:273–8. doi: 10.1016/j.aju.2012.02.002
7. Guo Z, Wang J, Gong L, Gan C, Gu S, Wang S. Association between cadmium exposure and urolithiasis risk: a systematic review and meta-analysis. Medicine(Baltimore). (2018) 97:e9460. doi: 10.1097/MD.0000000000009460
8. Sun Y, Zhou Q, Zheng J. Nephrotoxic metals of cadmium, lead, mercury and arsenic and the odds of kidney stones in adults: an exposure-response analysis of NHANES 2007-2016. Environ Int. (2019) 132:105115. doi: 10.1016/j.envint.2019.105115
9. Wróbel G, Kuder T. The role of selected environmental factors and the type of work performed on the development of urolithiasis - a review paper. Int J Occup Med Environ Health. (2019) 32:761–75. doi: 10.13075/ijomeh.1896.01491
10. Silva LK, Hile GA, Capella KM, Espenship MF, Smith MM, Jesús VR, et al. Quantification of 19 aldehydes in human serum by headspace SPME/GC/High-resolution mass spectrometry. Environ Sci Technol. (2018) 52:10571–9. doi: 10.1021/acs.est.8b02745
11. Hang SS, Simon HS, Fai K, Ting LP, Chan CS, Dai W, et al. Hazardous airborne carbonyls emissions in industrial workplaces in China. J Air Waste Manag Assoc. (2013) 63:864–77. doi: 10.1080/10962247.2013.797519
12. Gavin P, Daniel M, Danielle L, Alexander C, Kenneth W. Airborne contamination during post-fire investigations: hot, warm and cold scenes. J Occup Environ Hyg. (2022) 19:35–49. doi: 10.1080/15459624.2021.2002343
13. Chai F, Li P, Li L, Han Y, Yang K. Dispersion, olfactory effect, and health risks of VOCs and odors in a rural domestic waste transfer station. Environ Res. (2022) 209:112879. doi: 10.1016/j.envres.2022.112879
14. Cecinato A, Yassaa N, Palo V, Possanzin M. Observation of volatile and semi-volatile carbonyls in an Algerian urban environment using dinitrophenylhydrazine/silica-HPLC and pentafluorophenylhydrazine/silica-GC-MS. J Environ Monit. (2002) 4:223–8. doi: 10.1039/b110616n
15. Destaillats H, Spaulding R, Charles M. Ambient air measurement of acrolein and other carbonyls at the Oakland-San Francisco Bay Bridge toll plaza. Environ Sci Technol. (2002) 36:2227–35. doi: 10.1021/es011394c
16. Izabela G, Camila R, Rafael O. Formaldehyde exposure and atmospheric biomonitoring with lichen Cladonia verticillaris in an anatomy laboratory. Environ Sci Pollut Res Int. (2021) 28:48569–80. doi: 10.1007/s11356-021-14036-9
17. Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. (2008) 283:15539–43. doi: 10.1074/jbc.R800001200
18. Mali VR, Palaniyandi SS. Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radical Res. (2014) 48:251–63. doi: 10.3109/10715762.2013.864761
19. Singh S, Brocker C, Koppaka V, Chen Y, Jackson BC, Matsumoto A, et al. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic Biol Med. (2013) 56:89–101. doi: 10.1016/j.freeradbiomed.2012.11.010
20. Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, et al. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol Cell. (2015) 60:177–88. doi: 10.1016/j.molcel.2015.08.020
21. Costa S, Costa C, Madureira J, Valdiglesias V, Teixeira-Gomes A, Pinho PG, et al. Occupational exposure to formaldehyde and early biomarkers of cancer risk, immunotoxicity and susceptibility. Environ Res. (2019) 179:108740. doi: 10.1016/j.envres.2019.108740
22. Peter J, Arno G, Nandita S. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. (2005) 35:609–62. doi: 10.1080/10408440591002183
23. Xu C, Liang J, Xu S, Liu Q, Xu J, Gu A. Increased serum levels of aldehydes are associated with cardiovascular disease and cardiovascular risk factors in adults. J Hazard Mater. (2020) 400:123134. doi: 10.1016/j.jhazmat.2020.123134
24. Weng X, Chen J, Fei Q, Guo X, Liu S, Wen L, et al. The association of aldehydes exposure with diabetes mellitus in US population: NHANES 2013-2014. Chemosphere. (2022) 291:133019. doi: 10.1016/j.chemosphere.2021.133019
25. Liao S, Wu N, Gong D, Tang X, Yin T, Zhang H, et al. Association of aldehydes exposure with obesity in adults. Ecotoxicol Environ Saf. (2020) 201:110785. doi: 10.1016/j.ecoenv.2020.110785
26. Zhang ZH, Ho KM, Hong YC. Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit Care. (2019) 23:112. doi: 10.1186/s13054-019-2411-z
27. Livne M, Boldsen JK, Mikkelsen IK, Fiebach JB, Sobesky J, Mouridsen K. Boosted tree model reforms multimodal magnetic resonance imaging infarct prediction in acute stroke. Stroke. (2018) 49:912–8. doi: 10.1161/STROKEAHA.117.019440
28. Matthew MC, Trevor CY, Christopher W, David O, Michael WK. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. (2016) 44:368–74. doi: 10.1097/CCM.0000000000001571
29. Von EE, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. (2007) 85:867–72. doi: 10.2471/BLT.07.045120
30. Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation. Int J Mol Med. (2021) 48:149. doi: 10.3892/ijmm.2021.4982
31. Chen Y, Wei P, Meng J, Zhang L, Liang C, Hao Z. Harnessing calcium-oxalate- (CaOx-) nanocrystal-induced prodeath autophagy for attenuating human renal proximal tubular epithelial cell injury. Part Part Syst Charact. (2019) 36:1900083. doi: 10.1002/ppsc.201900083
32. Moeller BC, Kun Lu, Doyle-Eisele M. Determination of N2-hydroxymethyl-dG adducts in the nasal epithelium and bone marrow of nonhuman primates following 13CD2-formaldehyde inhalation exposure. Chem Res Toxicol. (2011) 24:162–4. doi: 10.1021/tx1004166
33. Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. (2002) 30:35–47. doi: 10.1007/s00240-001-0228-z
34. Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T. Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol. (2010) 17:996–1003. doi: 10.1111/j.1442-2042.2010.02661.x
35. Kumskova EM, Antonova OA, Balashov SA, Tikhaze AK, Melkumyants AM, Lankin VZ. Malonyldialdehyde and glyoxal act differently on low-density lipoproteins and endotheliocytes. Mol Cell Biochem. (2014) 396:79–85. doi: 10.1007/s11010-014-2144-x
36. Matsubara K, Matsuzawa Y, Jiao S, Takama T, Kubo M, Tarui S. Relationship between hypertriglyceridemia and uric acid production in primary gout. Metabolism. (1989) 38:698–701. doi: 10.1016/0026-0495(89)90110-8
37. Ogura J, Kuwayama K, Sasaki S, Kaneko K, Koizumi T, Yabe K, et al. Reactive oxygen species derived from xanthine oxidase interrupt dimerization of breast cancer resistance protein, resulting in suppression of uric acid excretion to the intestinal lumen. Biochem Pharmacol. (2015) 97:89–98. doi: 10.1016/j.bcp.2015.06.021
38. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. (2005) 293:455–62. doi: 10.1001/jama.293.4.455
39. Yoshimura E, Sawada SS, Lee IM, Gando Y, Kamada M, Matsushita M, et al. Body mass index and kidney stones: a cohort study of Japanese men. J Epidemiol. (2016) 26:131–6. doi: 10.2188/jea.JE20150049
Keywords: environmental pollution, aldehydes, kidney stones, isopentanaldehyde, benzaldehyde
Citation: Chen Y, Shen X, Li G, Yue S, Liang C and Hao Z (2022) Association between aldehyde exposure and kidney stones in adults. Front. Public Health 10:978338. doi: 10.3389/fpubh.2022.978338
Received: 25 June 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Ciro Fernando Bustillo-Lecompte, Ryerson University, CanadaReviewed by:
Zeinab A. Kasemy, University of Menoufia, EgyptCopyright © 2022 Chen, Shen, Li, Yue, Liang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongyao Hao, aGFvem9uZ3lhb0AxNjMuY29t; Chaozhao Liang, bGlhbmdfY2hhb3poYW9AYWhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.