
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 11 August 2022
Sec. Public Health Education and Promotion
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.974923
This article is part of the Research TopicPublic Perspectives on Health and WellbeingView all 24 articles
Background: As the incidence of gastric cancer (GC) increases sharply in adults aged over 40 years, screening of this high-risk population is important. This study aimed to explore knowledge level of GC related risk factors and symptoms, and to identify influencing factors associated with intention toward GC screening among people aged 40 years old and above in China.
Methods: A cross-sectional, web-based survey was conducted among people aged 40 years old and above between October 2021 and March 2022 in Southeastern China. The participants' knowledge was assessed by a series of questions about risk factors (24-item scale) and warning symptoms (14-item scale).
Results: A total of 2547 complete responses were received. The mean age was 47.72 (±7.20) years and near 60% were male. Respondents had a moderate level of knowledge about risk factors and warning symptoms of GC. The total mean knowledge score was 23.9 (±9.8) out of a possible score of 38. Majority (80%) of respondents reported intention to be screened for GC in the next 5 years. The most influential predictors of screening intention were income level (OR = 2.13, 95% CI: 1.36–3.32), perceived benefits (OR = 1.99, 95% CI: 1.33–2.73), perceived severity (OR = 1.68, 95% CI: 1.20–2.34), ever took GC screening (OR = 1.63, 95% CI: 1.28–2.08), perceived poor overall health (OR = 1.59, 95% CI: 1.19–2.11), and perceived barriers (OR = 1.56, 95% CI: 1.17–2.09). Other significant factors were ever diagnosed with chronic gastric diseases, total knowledge score, and cues-to-action. The major reasons for not willing to take screening were “endoscopy is uncomfortable” (29.6%), “worry about screening results” (23.6%), and “have no symptoms” (21.3%).
Conclusion: High-risk population aged 40 years and above expressed high intention to receive GC screening. Intervention to improve health promotion and reduce the barriers to uptake of GC screening among high-risk populations in China is warranted.
Gastric cancer (GC) remains an important cancer worldwide and is responsible for over one million new cases in 2020 and an estimated 769,000 deaths (1). Eastern Asia and Central and Eastern Europe are regions with the highest incidence rate of GC in the world (2). In China, although the incidence and mortality have slightly decreased in the past two decades, high burden of GC still persists (3). The incidence and mortality rates of GC in China account for a staggering near 50% of the global burden (3, 4). GC is often asymptomatic in early stage, and the majority of patients were diagnosed with advanced stage, usually after they seek medical advice due to symptoms present (5). Likewise in China, more than 90% (6) of GC patients in clinics were presented at an advanced stage, in which the 5-year survival rate was only 35.1% (7). In contrast, the 5-year survival rate of patients with early GC after treatment exceeds 90% and can even be cured (8, 9).
Early detection of GC has great potential to improve survival and reduce disease mortality. Endoscopic screening for GC in moderate to high risk populations was found to be cost-effective (10), and it had been implemented in many countries with high incidence of GC (11, 12). Because the incidence of GC sharply increases after 40 years of age, regular screening is recommended for this target population in countries with high incidence of GC, such as Korea, Japan and China. The 5-year survival rate of GC is significantly lower in China than that of Japan and Korea (13), suggesting diagnosis delays among Chinese patients (14). Differences in screening rate coverage might partly explain the intercountry discrepancies of diagnosis delays. In Korea, National Cancer Screening Program (NCSP) has been initiated since 1999 to provide GC screening for patients 40 years or older every 2 years at no charge or 10% co-payment, depending on their insurance or income stratum (15). Upon implementation of GC screening program, screening rate in Korean has increased from 7.5% in 2002 to 47.3% in 2012 (16). As a result, more than 50% of GC in Korea were diagnosed at an early stage, compared to fewer than 10% in Western countries and China (17). Despite the serious burden of GC, there are no nationwide screening programs in China (14). Opportunistic screening with endoscopy in asymptomatic people is the primary practice in China (18). Compared with organized screening, opportunistic screening involves fewer formal decisions about whether to screen, whom to screen and at what intervals screening should be done (19). In 2005, China launched National Key Public Health Projects, and provided free endoscopic screenings for upper gastrointestinal cancer in more than 110 high-risk areas throughout the country. However, the estimated compliance rate (33.5%) was low (20). The national GC screening rate is still unknown in China. According to a recent cross-sectional study, the ever-screening rate of GC among adult Chinese was only 15.2% (21).
Similar to many countries worldwide, China has faced many obstacles in the introduction of GC screening, such as lack of knowledge related to GC and screening, high cost of screening, and negative attitude toward screening (21). GC is a multifactorial, multistep process (22). Host factors include blood group A, pernicious anemia, prior gastric surgery, family history, hereditary diffuse GC, and genetic syndromes. Smoking, salt, salty and smoked food, red meat, obesity, and low socioeconomic status are environmental factors. Moreover, infection with Helicobacter pylori and Epstein–Barr virus also play a role in gastric carcinogenesis (22, 23). Information on these risk factors helps characterize individuals at risk of GC during their lifetime and promote health-related behavior change. A recent survey from Korea (24) demonstrated that people with lower perceived risk of GC are less likely to take screening. This may primarily due to the fact that knowledge of the risk factors is a vital aspect in developing cancer risk perceptions and further influencing the participation in cancer screening (25, 26). In addition, knowledge about warning symptoms is critical for patients' timely medical care-seeking behavior. A recent study showed that knowledge about warning symptoms can lead to earlier presentation to medical care, which could result in earlier diagnosis and better outcomes (27). The presence of an abdominal lump, abdominal fullness and pain are typical warning symptoms of GC (28), which are easily mistaken as mild gastrointestinal disease. Economic problem was also suggested as a significant barrier. People in the lowest income level were less likely to undergo GC screening (21). Furthermore, negative attitudes toward GC screening, such as fear of screening procedure, fear of finding tumor, may also cause ignorance about screening (21, 29).
Fujian province, located in Southeastern part of China, is a well-known high-risk region of GC in China with higher incidence rate than the average national level (33.1/100,000 vs. 30.0/100,000) (30). Several cities in Fujian province have reported a 2-fold higher mortality rate than the national average level (49.47/100,000 vs. 21.9/100,000) (31). According to expert consensus in China, individuals aged at least 40 years from high-risk regions can be grouped as high-risk population of GC and regular screenings are recommended (6). To the best of our knowledge, no study on GC screening intention was carried out in high-risk population of China. Thus, the current study mainly aimed to investigate knowledge level of GC risk factors and symptoms as well as intention toward screening in Fujian province of China. Accurate information on factors associated with screening behaviors has important implications for health-related behavior change and may strengthen GC prevention and control.
We commenced a cross-sectional, web-based anonymous survey using an online questionnaire during October 2021 and March 2022. Convenience sampling was conducted to recruit subjects for this study. The research team used WeChat (the most popular social media platform in China) to advertise and circulate the survey link to their network members. Network members were requested to distribute the survey invitation to all their contacts that satisfy the inclusion criteria. The inclusion criteria were that (1) aged 40 years and above; (2) living in Fuzhou, Putian, Quanzhou, Xiamen, and Zhangzhou city of Fujian province; (3) having no history of cancer. Upon completing the survey, each respondent providing a valid questionnaire was awarded an incentive of 5 Chinese Yuan (equivalent to 0.75 USD). In an attempt to reach a more comprehensive recipient coverage, we also encouraged participants to disseminate the survey link to all their contacts with a thank you note at the end. The participants were informed that their participation was voluntary, and consent was implied through their completion of the questionnaire. The reason for selecting these five cities was due to they are the major cities with the highest incidence of GC in Fujian Province. In total, the accumulated population of these five cities accounts for 73.43% of the total population in Fujian province (32).
The questionnaire was self-developed and pilot tested. Local experts of both epidemiologists and clinicians validated the content of the questionnaire. The survey consisted of four sections, which mainly assessed (1) demographic and general health; (2) knowledge about GC-related risk factors and warning symptoms; (3) history of treatment-seeking, and (4) attitudes and intention toward GC screening.
The first section of the questionnaire assessed participants' demographic characteristics such as age, gender, height, weight, highest education level, marital status, current residing location (urban/rural), current residing city, occupational types, and monthly average income. Participants were also asked if they ever knew any first-degree relatives, or any friends, neighbors, or colleagues who have been diagnosed with GC. For general health status, participants were asked if they “Ever diagnosed with chronic gastric diseases (e.g., chronic gastritis, gastric ulcer, etc.)”, perceived overall health, smoking, alcohol drinking, health insurance, and if they ever took GC screening.
The participants' knowledge was assessed by a series of questions about risk factors (24-item scale) and warning symptoms (14-item scale). The response options were “true,” “false,” or “don't know.” A correct response was given a score of one, and an incorrect or “don't know” response was scored zero. The total possible knowledge scores ranged from 0 to 38, with higher scores representing higher levels of knowledge. The median score was used to divide participants into high or low knowledge groups.
Health beliefs about GC screening was measured using the constructs from the Health Belief Model (HBM) (33). The questions probed perceived susceptibility to GC (three items), perceived severity of GC (three items), perceived benefits of GC screening (two items), perceived barriers to conduct GC screening (five items), and cues-to-action (three items). Perceived susceptibility queried participants about (1) general risk of a person having GC in their lifetime; (2) general risk of a person contracting Helicobacter Pylori in their lifetime, and (3) their own perceived risk of having GC. Perceived severity assessed participants' perception of harm of GC. Questions evaluating perceived benefits queried participants their views about the benefit of GC screening in early diagnosis and treatment of GC, and prognosis. Perceived barriers to conduct GC screening explored participants' concerns/hesitations when thinking of having screening. Cues-to-action questioned participants about motivation to conduct screening. The response options were “strongly agree,” “agree,” “disagree,” and “strongly disagree.”
A four-point scale was also used for questions about participants' intention to take GC screening in the next 5 years, namely “certainly yes,” “probably yes,” “probably no,” and “certainly no.” The domain reason for not being willing to take screening was also queried. Respondents were also requested to report their preferences of screening method by selecting one of the following options: “endoscopy,” “blood test,” “fecal examination,” and “none of them.”
The minimal sample size was calculated based on the formula N = [ × π × (1–π)]/δ2. The prevalence rate was 15% (π) based on the GC screening rate reported in the previous study (21), with a significant level set to be 0.05 (α), and allowable error as 0.03 (δ). The estimated minimal sample size was 544. In consideration of non-response rate, invalid questionnaire of 40%, a final target sample of 800 was determined.
The reliability of the knowledge score was evaluated by assessing the internal consistency of the items representing the knowledge scores. Multivariable logistic regression was used to determine the factors influencing screening intention. All factors found to be statistically significant (p-value < 0.05) in the univariate regression analysis were entered into multivariable logistic regression analyses using a simultaneous forced-entry method. Odds ratio (OR), 95% confidence interval (95% CI) and p-values were calculated for each independent variable. The model fit of multivariable logistic regression analysis was assessed using the Hosmer-Lemeshow goodness-of-fit test (34). All p-values are based on a two-sided test with a statistical test level of α set at 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 26.0.
Following the standards of Helsinki Declaration and its corresponding modifications or similar ethical principles, this study was carried out. The data was collected through an online survey where written informed consent was taken from each participant. Respondents who expressed their consent, after reading the aforementioned, to take part in the study by clicking either “Yes” or “No” were included in the study. Those who did not consent by clicking “No” were not included in the study. Ethics approval and permission for data collection were granted by the Medical Ethics Committee at the Fujian Medical University (FJMU No. 2020 [53]).
Between October 2021 and March 2022, a total of 2,547 completed responses were received. Supplementary Table 1 shows the demographics of our study participants compared with the adults aged 40 years and older population in Fujian. A summary of the characteristics of the participants is provided in the Table 1 and second column of Table 3. The mean age of study participants was 47.72 years (±7.20). A large proportion of participants were aged 40–50 years (78.2%). Near half of the participants lived in urban (56.9%) and had monthly average income > 5,000 RMB (750 USD) (48.0%). The highest education level is distributed nearly even in secondary school and below (33.3%), high school/technical school (35.1%), and university and above (31.6%). Only 18.8% of participants reported first-degree relatives had GC, while 40.6% were aware of their friends, neighbor, or colleagues had ever been diagnosed with GC. A total of 40.0% of participants reported a history of chronic gastric disease and 42.6% ever took GC screening.
Figure 1 and Table 2 show the proportion of correct responses to all 38 knowledge items (24 items of risk factors and 14 items of warning symptoms). The 38 items for knowledge scores had a reliability (Cronbach's α) of 0.954. The mean and standard deviation (SD) for the total knowledge score was 23.9 (SD ± 9.8) out of a possible score of 38. The median was 25 (interquartile range, IQR, 17–33). Knowledge scores were categorized high or low based on median split; as such, a total of 1,209 (47.5%) were categorized as having a high score (25 to 38) and 1,338 (52.5%) had a low score (0–24).
The most highly recognized risk factors were “irregular diet habit” (75.0%), and “alcohol drinking” (74.9%), followed by “history of digestive disease” (72.6%), “consumption of pickled food” (71.6%), “consumption of smoked food” (71.2%), “aged 40 years and above” (70.9%), and “stomach ulcer” (70.9%). The least recognized risk factor was “male” (46.3%). In particular, only 56.7% of male respondents (data not shown) were aware of this inherent risk. Meanwhile, majority of participants wrongly regarded “too short/long sleeping time” (55.0%) as a risk factor of GC. The most highly recognized warning symptoms were “gastrointestinal bleeding” (77.1%), followed by “chronic gastritis can't be cured for a long time” (72.4%), “upper abdominal pain” (72.0%) and “recurrent nausea and vomiting” (71.5%). The least recognized warning symptoms were “early satiety” (57.4%) and “hypoferric anemia” (49.5%), while 70.2% of respondents wrongly considered “gastric perforation” (70.2%) as a warning symptom.
Figure 2 shows the proportions of intention to take screening in the next 5 years. In total, 80.0% (n = 2,038) of participants reported “certainly yes/probably yes” and 20.0% (n = 509) reported “certainly no/probably no” regarding their intention to screen in the next 5 years (Figure 2).
Results of univariate and multivariable logistic regression were presented in Table 3. Multivariable logistic regression showed that monthly income > 5,000 RMB (OR = 2.13, 95% CI: 1.36–3.32) was the most robust factor associated with screening intention. Respondents that perceived their own overall health as “fair/poor/very poor” (OR = 1.59, 95% CI: 1.19–2.11), ever took GC screening (OR = 1.63, 95% CI: 1.28–2.08) had more than 50% higher odds of intention to conduct screening. The odds of intention to conduct screening were also higher among respondents who were ever diagnosed with chronic gastric diseases (OR = 1.30, 95% CI: 1.01–1.68), and those had high score of total knowledge (OR = 1.46, 95% CI: 1.16–1.84). Results of HBM indicate that the following five components were significantly associated with screening intention, including perceived susceptibility (risk of getting GC is high, OR = 1.44, 95% CI: 1.11–1.85), perceived severity (afraid of getting GC, OR = 1.68, 95% CI: 1.20–2.34), perceived benefit (GC screening is effective in saving life, OR = 1.99, 95% CI: 1.33–2.73), perceived barriers (endoscopy is uncomfortable, OR = 1.56, 95% CI: 1.17–2.09), and cues-to-action (only take screening when it is free of charge, OR = 1.47; 95% CI: 1.08–2.00).
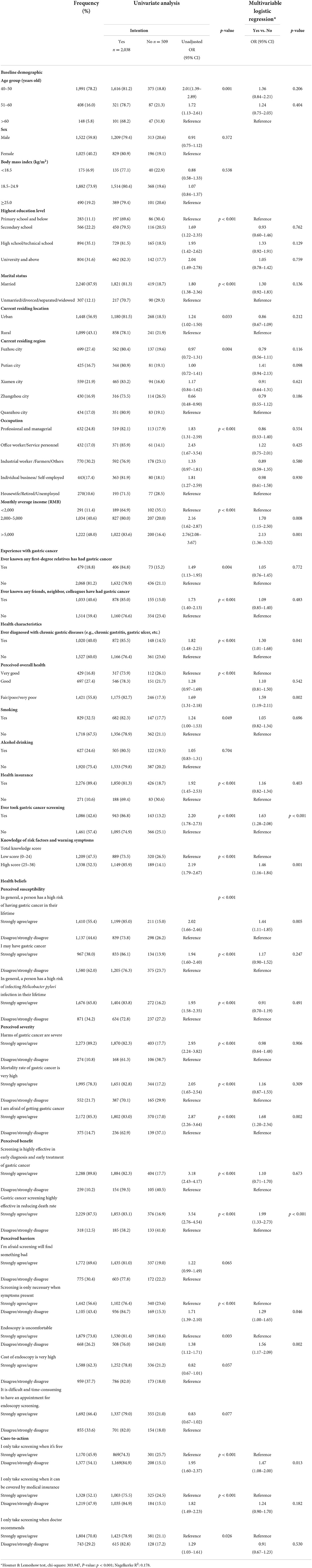
Table 3. Factors associated with intention to take gastric cancer screening in the next 5 years (N = 2,547).
The domain reasons for not willing to take screening in the next 5 years are shown in Figure 3. Among respondents who reported probably yes/certainly no/probably no (n = 2,058), the three most common reasons, in descending order, were “endoscopy is uncomfortable” (29.6%), “worried about screening results” (23.6%), and “no symptoms” (21.3%). Other reasons included “no time” (8.3%), “don't know the benefits of screening” (6.9%), “screening cost is too high” (5.5%), and “believe that gastric cancer cannot be cured even detected by screening” (3.4%).
Figure 4 presents respondents' preferences of screening method, grouping by if they ever took GC screening. For those who had ever taken GC screening, the most preferred screening method is endoscopy (52.3%), followed by blood test (35.9%), and fecal examination (10.5%). In contrast, among respondents who never took GC screening, the most favorite screening method was blood test (50.8%), followed by endoscopy (21.5%), and fecal examination (21.3%).
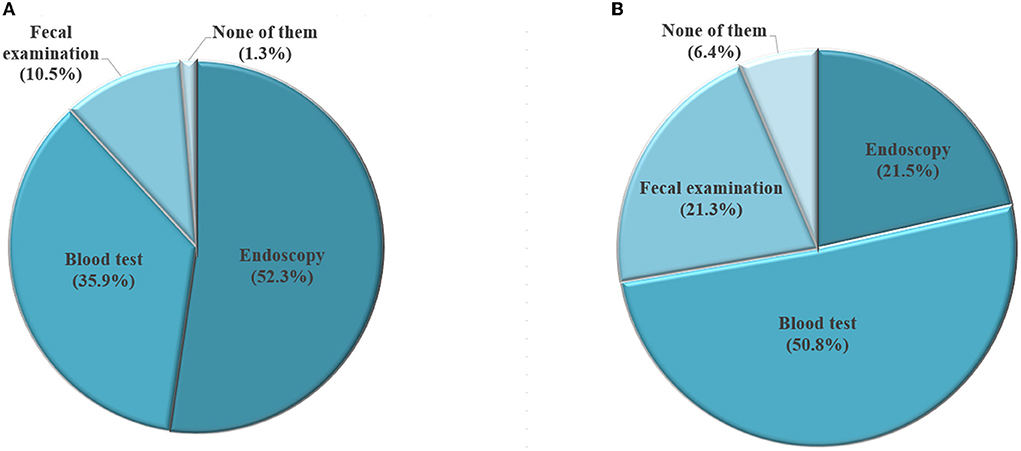
Figure 4. Selection of screening method among participants who have had gastric cancer screening (A, N = 1,086) and those who haven't had gastric cancer screening (B, N = 1,461).
To our knowledge, the current study is the first investigation aimed to explore the knowledge level, attitudes to GC screening in high-risk populations in China. In general, the study participants reported a moderate level of knowledge in GC risk factors and warning symptoms. Majority of participants intended to take GC screening in the next 5 years. Significant factors influencing intention to screen were income level, previous history of GC screening or chronic gastric diseases, perceived overall health, total knowledge score, and HBM components (perceived benefit, perceived severity, perceived barriers, cues to action). “Endoscopy is uncomfortable,” “worry about screening results,” and “no symptoms” were the domain reasons for not willing to take screening.
Adequate knowledge about risk factors and warning symptoms of GC play an important role in cancer screening and early diagnosis. Poor knowledge about GC has been considered a barrier of GC screening (35). Result of our study also found that participants with high score of knowledge had a 50% increased intention to take GC screening. In 2015, China government implemented a Nationwide Three-Year Cancer Prevention Plan (2015–2017), announcing an ambitious goal to have the public awareness rate of essential cancer knowledge reach 60% (36). Our current study population in Southeastern China has shown a moderate level of knowledge. However, recent studies from other regions of China, including Central and Northeastern China, reported that people still have poor knowledge about GC (21, 37). More importantly, knowledge level varied among different types of risk factors. Specifically, participants were more familiar with life-style related risk factors, such as irregular diet habits, alcohol drinking, consumption of pickled/smoked foods, hot/spicy food, which is in line with a previous study (21). However, some imperative risk factors, such as male gender, family history of GC among first degree relatives were relatively rarely known. Finding from other previous study also identified these were the two least known risk factors (21). It seems that people tend to be more sensitive to those modifiable risk factors, but easily neglect unmodifiable factors such as age and heredity. Future health education program may need to particularly address high-risk populations under exposure to inherent risk factors. On the other hand, the need to improve knowledge about warning symptoms of GC is also clearly shown in the results of this study. In particular, findings indicate that a considerable proportion of surveyed participants lack knowledge of important symptoms such as early satiety and iron-deficiency anemia (IDA). IDA of gastrointestinal cancer origin is particularly common and longstanding due to bleeding. In the preoperative setting, a retrospective review by Jung et al. reported anemia in 43.6% (99/227) of GC patients. Of those, 24.2% (24/99) developed IDA (38). Recognition of warning signs was associated with anticipating faster help-seeking for potential symptoms of cancer (27). Knowing potential warning symptoms of GC may facilitate patients' treatment-seeking behavior.
Insight about demographic factors that facilitate or impede the intention to conduct GC screening may also be critical to promote health-behavior change. Multivariate analysis result of the current study implies that income level was the most robust factor associated with screening intention. High cost of endoscopy was also reported by surveyed participants as one of the major barriers toward screening. Similarly, Shin and Lee in a cross-sectional study reported that as the level of income increases, and the tendency to uptake screening also increases (OR = 1.36, 95% CI: 1.06–1.73) (29). Undoubtedly, affordability plays an important role in screening behavior. In China, endoscopy is conducted via opportunistic screening and individual own self is responsible for the related medical cost (39). Japan and Korea are the only two countries in the world that offer nationwide population-based GC screening (40). A Korean study shows that people were likely to intend to receive GC screening if it were offered free of charge or for a copayment (24). Our study also found participants were more likely to take GC screening if it is free of charge. Indeed, the screening rate in Korea has increased from 40.0% in 2005 to 74.8% in 2015 after the introduction of the National Cancer Screening Program which offer free or co-payment screening (41). Establishment of a population-based screening program to guarantee free access to endoscopy, particularly for high-risk populations, would be extremely critical for China and other high-risk regions to increase the early diagnosis rate of GC and consequently reduce the mortality rate.
Analysis results of HBM indicate that the following five components were significantly associated with screening intention, including perceived susceptibility (risk of getting GC is high), severity (afraid of getting GC), perceived benefit (GC screening is effective in saving life), perceived barriers (is uncomfortable), and cues-to-action (only take screening when it is free of charge). The finding of HBM could be utilized as a theoretical fundamental to design future health promotion program. In particular, discomfort related to endoscopy has been regarded as the most important reason for not being willing to take screening. Meanwhile, the majority of respondents without previous experience with endoscopic screening prioritized blood test for their future screening plan. These results implied that many people fear physical discomfort from the invasive endoscopy procedure. Although China government launched endoscopic screening program since 2005 in more than 110 high-risk areas throughout the country, the compliance rate (33.5%) was found to be low (20). To reduce the public's fear of endoscopy, recognition of its effectiveness for early detection of GC should be emphasized, and more efforts should be addressed to minimize the discomfort associated with the screening procedure. Alternative screening methods other than endoscopy could also be developed and implemented in order to improve the public's willingness to be screened. Furthermore, as Helicobacter pylori (a group I carcinogen) has been confirmed to have an important role in gastric carcinogenesis (42), people over 40 years old can be further stratified by Helicobacter pylori infection in order to find the most target population for endoscopic screening.
This study has several limitations that should be considered. The first pertains to the use of convenience sampling, in which the selection bias could not be eliminated, and its cross-sectional nature. It cannot, therefore, be used to infer causality. Second, data were collected from participants' self-reports; thus, these may be subjected to socially desirable responses. Third, it should be noted that the intention to take screening does not necessarily result in actual receipt of screening; therefore, results should be interpreted with caution. Fourth, the assessment of knowledge was done prior to screening intention, thus may potentially influence participants' responses to screening intentions. A final limitation of this study is that the study population was recruited from five major cities in Fujian province, which may limit generalizability. Despite these limitations, the study data contribute tremendously to the understanding of the influencing factors of GC screening intention in high-risk populations in China.
The present study showed high intention to be screened for GC among high-risk populations aged 40 years and above in China, which is of great importance for a country with low GC screening coverage but high GC burden. Our results imply that economic factor might be the most robust indicator driving respondent's screening intention. To some degree, previous history of gastric diseases and GC screening, perceived overall health status, knowledge level related to GC risk factors and symptoms, and HBM components all contribute to decisions related to future screening intention. Population-based screening program is urgently needed to provide free access to screening, particularly for those high-risk populations. Additionally, continuous education campaigns are needed to improve knowledge of GC risk factors and symptoms in China and to promote the benefits of early cancer diagnosis by screening. Finally, more alternative screening methods other than endoscopy could also be encouraged to improve the general public's willingness to be screened.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by Medical Ethics Committee at the Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
ZHua: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, writing—original draft, and writing—review and editing. WL: data curation, investigation, and writing—review and editing. RM: writing—original draft and writing—review and editing. ZHu and LW: conceptualization, supervision, writing—original draft, and writing—review and editing. YL: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, visualization, funding acquisition, writing—original draft, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No.72004025). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The researcher would like to thank all participants involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.974923/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
5. Liu MM, Wen L, Liu YJ, Cai Q, Li LT, Cai YM. Application of data mining methods to improve screening for the risk of early gastric cancer. BMC Med Inform Decision Mak. (2018) 18 (Suppl. 5):121. doi: 10.1186/s12911-018-0689-4
6. Du YQ, Cai QC, Liao Z, Fang J, Zhu CP. Expert consensus on early gastric cancer screening procedures in China(draft)(2017, Shanghai). Chin J Gastroenterol. (2018) 23:92–7.
7. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. (2018) 6:e555–e67. doi: 10.1016/S2214-109X(18)30127-X
8. Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Comprehensive Cancer Network. (2013) 11:531–46. doi: 10.6004/jnccn.2013.0070
9. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. (2011) 14:301–16. doi: 10.1007/s10120-011-0085-6
10. Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol. (2006) 4:709–16. doi: 10.1016/j.cgh.2006.03.025
11. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. (2011) 12:725–30.
12. Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol. (2007) 13:4316–20. doi: 10.3748/wjg.v13.i32.4316
13. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. (2015) 385:977–1010. doi: 10.1016/S0140-6736(14)62038-9
14. Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. (2016) 388:2606. doi: 10.1016/S0140-6736(16)32226-7
15. Lee HY, Park EC, Jun JK, Hahm MI, Jung KW, Kim Y, et al. Trends in socioeconomic disparities in organized and opportunistic gastric cancer screening in Korea (2005-2009). Cancer Epidemiol Biomarkers Prev. (2010) 19:1919–26. doi: 10.1158/1055-9965.EPI-09-1308
16. Suh M, Song S, Cho HN, Park B, Jun JK, Choi E, et al. Trends in participation rates for the national cancer screening program in Korea, 2002-2012. Cancer Res Treat. (2017) 49:798–806. doi: 10.4143/crt.2016.186
17. Choi KS, Jun JK, Lee HY, Park S, Jung KW, Han MA, et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. (2011) 102:1559–64. doi: 10.1111/j.1349-7006.2011.01982.x
18. Shen L, Zhou C, Liu L, Zhang L, Lu D, Cai J, et al. Application of oral contrast trans-abdominal ultrasonography for initial screening of gastric cancer in rural areas of China. Dig Liver Dis. (2017) 49:918–23. doi: 10.1016/j.dld.2017.04.008
19. Kim BJ, Heo C, Kim BK, Kim JY, Kim JG. Effectiveness of gastric cancer screening programs in South Korea: organized vs opportunistic models. World J Gastroenterol. (2013) 19:736–41. doi: 10.3748/wjg.v19.i5.736
20. Chen R, Liu Y, Song G, Li B, Zhao D, Hua Z, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut. (2021) 70:251–60. doi: 10.1136/gutjnl-2019-320200
21. Liu Q, Zeng X, Wang W, Huang RL, Huang YJ, Liu S, et al. Awareness of risk factors and warning symptoms and attitude towards gastric cancer screening among the general public in China: a cross-sectional study. BMJ Open. (2019) 9:e029638. doi: 10.1136/bmjopen-2019-029638
22. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. (2006) 12:354–62. doi: 10.3748/wjg.v12.i3.354
23. Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. (2008) 23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x
24. Hahm MI, Choi KS, Park EC, Kwak MS, Lee HY, Hwang SS. Personal background and cognitive factors as predictors of the intention to be screened for stomach cancer. Cancer Epidemiol Biomarkers Prev. (2008) 17:2473–9. doi: 10.1158/1055-9965.EPI-08-0027
25. Hay JL, Orom H, Kiviniemi MT, Waters EA. “I don't know” my cancer risk: exploring deficits in cancer knowledge and information-seeking skills to explain an often-overlooked participant response. Med Decis Making. (2015) 35:436–45. doi: 10.1177/0272989X15572827
26. McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. (2003) 36:525–35. doi: 10.1016/S0091-7435(03)00016-1
27. Quaife SL, Forbes LJ, Ramirez AJ, Brain KE, Donnelly C, Simon AE, et al. Recognition of cancer warning signs and anticipated delay in help-seeking in a population sample of adults in the UK. Br J Cancer. (2014) 110:12–8. doi: 10.1038/bjc.2013.684
28. Bai Y, Li ZS, Zou DW, Wu RP, Yao YZ, Jin ZD, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. (2010) 59:722–8. doi: 10.1136/gut.2009.192401
29. Shin JY, Lee DH. Factors associated with the use of gastric cancer screening services in Korea: the Fourth Korea National Health and Nutrition Examination Survey 2008 (KNHANES IV). Asian Pac J Cancer Prev. (2012) 13:3773–9. doi: 10.7314/APJCP.2012.13.8.3773
31. Li D, Wu C, Cai Y, Liu B. Association of NFKB1 and NFKBIA gene polymorphisms with susceptibility of gastric cancer. Tumour Biol. (2017) 39:1010428317717107. doi: 10.1177/1010428317717107
32. Fujian Provincial Bureau of Statistics. Fujian Province Bulletin of the 7th National Census (No.2). (2021). Available online at: https://tjj.fujian.gov.cn/xxgk/tjgb/202105/t20210520_5598804.htm (accessed July 3, 2022).
33. Becker MH. The health belief model and personal health behavior. Health Edu Mono. (1974) 2:324–508. doi: 10.1177/109019817400200407
35. Hatamian S, Etesam S, Mazidimoradi A, Momenimovahed Z, Salehiniya H. The barriers and facilitators of gastric cancer screening: a systematic review. J Gastrointest Cancer. (2021) 52:839–45. doi: 10.1007/s12029-021-00652-8
36. China nationwide three-year cancer prevention plan (2015-2017). Chin J Clin Oncol Rehabilit. (2016) 23:177.
37. Aerdak N, Zhou J. An analysis of relevant cancer knowledge cognition and its factors among high risk group of upper gastrointestinal cancer in some parts of Urumqi. China Cancer. (2017) 26:268–71.
38. Jung M, Kim H, Yoon HY, Kim CB. Pre- and post-gastrectomy anemia in gastric cancer patients. Korean J Clin Oncol. (2011) 7:88–95. doi: 10.14216/kjco.11024
39. Di L, Wu H, Zhu R, Li Y, Wu X, Xie R, et al. Multi-disciplinary team for early gastric cancer diagnosis improves the detection rate of early gastric cancer. BMC Gastroenterol. (2017) 17:147. doi: 10.1186/s12876-017-0711-9
40. Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. (2015) 29:895–905. doi: 10.1016/j.bpg.2015.09.013
41. Lee EY, Lee YY, Suh M, Choi E, Mai TTX, Cho H, et al. Socioeconomic inequalities in stomach cancer screening in Korea, 2005-2015: after the introduction of the national cancer screening program. Yonsei Med J. (2018) 59:923–9. doi: 10.3349/ymj.2018.59.8.923
Keywords: knowledge, attitude, intention, cancer screening, stomach cancer
Citation: Huang Z, Liu W, Marzo RR, Hu Z, Wong LP and Lin Y (2022) High-risk population's knowledge of risk factors and warning symptoms and their intention toward gastric cancer screening in Southeastern China. Front. Public Health 10:974923. doi: 10.3389/fpubh.2022.974923
Received: 21 June 2022; Accepted: 21 July 2022;
Published: 11 August 2022.
Edited by:
Bijaya Kumar Padhi, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Qi Zhong, Anhui Medical University, ChinaCopyright © 2022 Huang, Liu, Marzo, Hu, Wong and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulan Lin, eXVsYW5saW5AZmptdS5lZHUuY24=; Roy Rillera Marzo, cnJtdGV4YXNAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.