
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 26 October 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.973125
This article is part of the Research TopicGlobal Excellence in Children and Health: Asia and AustralasiaView all 7 articles
Background: Biliary atresia (BA) is a severe inflammatory obliterative cholangiopathy of infancy that requires early diagnosis and prompt surgical intervention. In this study, we aimed to obtain comprehensive evidence on the diagnostic performance of liver stiffness measurement by ultrasound elastography in the detection of BA through a meta-analysis.
Methods: The PubMed, EMBASE, Cochrane Library, and Web of Science databases were searched for studies that investigated the diagnostic performance of ultrasound elastography in the detection of BA up to January 10, 2022. In this study, in order to summarize the diagnostic performance of ultrasound elastography, the summary receiver operating characteristic (SROC) modeling was constructed. Heterogeneity was estimated with the I2 statistic. Multiple subgroup analyses were also performed.
Results: Fourteen studies from eleven articles, including 774 BA patients, 850 non-BA patients, and 173 controls were included in the present meta-analysis. The summary sensitivity and specificity of ultrasound elastography for liver stiffness were 85% [95% confidence interval (CI): 79–89%] and 82% (95% CI: 73–88%) with the I2 value of 82.90 and 84.33%, respectively. The area under the SROC curve (AUROC) using ultrasound elastography for diagnosing BA was 0.90 (95% CI: 0.87–0.92). In addition, a subgroup analysis of 9 two-dimensional shear wave elastography studies was also performed. Subgroup analysis revealed that the summary sensitivity and specificity were 85% (95% CI: 77–91%) and 79% (95% CI: 71–86%), respectively, and the summary AUROC was 0.89 (95% CI: 0.86–0.92).
Conclusions: Ultrasound elastography exhibits good diagnostic accuracy for BA and can be served as a non-invasive tool to facilitate the differential diagnosis of BA.
Biliary atresia (BA) is a severe inflammatory obliterative cholangiopathy of infancy (1, 2). This disease is a global problem, and its incidence rate varies markedly across different regions (3–7). If not treated timely, BA would eventually progress into end-stage liver disease, and finally leading to death in the first 2 years of life (8). A surgical procedure called Kasai portoenterostomy (KPE) is the current treatment option (1, 9). In fact, the success of KPE surgery for BA depends in large part on the age at which it is performed (8). Therefore, early diagnosis plays a vital role.
However, in infants with cholestasis, early identification of BA remains challenging (10, 11). Currently, several modalities have been chosen to evaluate the potential anomaly of biliary system, such as conventional ultrasonography, hepatobiliary scintigraphy, and magnetic resonance cholangiopancreatography (MRCP) (12, 13). Unfortunately, both hepatobiliary scintigraphy and MRCP provided a relatively low specificity for diagnosis of BA (14–16). The ultrasonographic features of gallbladder abnormalities are suggestive of BA, but require an analyst with expertise (10, 17). Although liver biopsy or intraoperative cholangiography (IOC) has traditionally been regarded as a relatively accurate test for the diagnosis of BA, it is an invasive procedure (12, 18).
Recently, ultrasound elastography has been developed as a novel quantitative sonographic technique to assist in this effort through the non-invasive measurement of liver stiffness (2). Transient elastography (TE) is the most widely validated shear wave-based elastography technique (19, 20). Nevertheless, it has certain drawbacks (20–23). Several studies using this technique have shown that it has more technical failures in young children (24, 25). Point shear wave elastography (p-SWE) and two-dimensional shear wave elastography (2D-SWE) are recently developed techniques (19). Importantly, 2D-SWE is able to directly visualize the elasticity measurements by displaying a color coding elastographic map on a gray-scale ultrasound image in real time (21, 26). Previous research has already demonstrated that 2D-SWE is an efficient tool for the diagnosis of liver fibrosis, comparing favorably to TE and p-SWE (27).
To date, there are accumulating reports on the value of ultrasound elastography in the diagnosis of BA; however, they utilized relatively small samples. Moreover, there is variability in the diagnostic performance presented in previous studies. In this study, therefore, we aimed to obtain comprehensive evidence on the diagnostic performance of ultrasound elastography in BA through a meta-analysis.
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (28). Within this paper, we focus on analyzing the diagnostic performance of ultrasound elastography for BA.
A computerized search was conducted using the PubMed, EMBASE, Cochrane Library, and Web of Science databases to identify studies, which evaluated the diagnostic value of ultrasound elastography for BA up to January 10, 2022. The search terms included biliary atresia, atresia, extrahepatic biliary atresia, biliary, elastography and stiffness. In addition, we also examined the references of the initially identified articles to identify additional relevant publications. All initial records were exported to Endnote (version X9).
Studies were included if they fulfilled following criteria: (1) the study evaluated the accuracy of liver stiffness measurement by ultrasound elastography for the diagnosis of BA; (2) the study enrolled more than 10 infants with BA; and (3) the study had sufficient data, thus allowing us to construct 2 × 2 contingency tables for test performance for further analysis. Studies meeting any of the following criteria were excluded: (1) studies were not relevant to ultrasound elastography diagnosis; (2) non-original research articles, including reviews, conference abstracts, letters, protocols, guidelines and commentary; (3) data incomplete; and (4) studies published in non-English language.
Using a standardized form, the following data were extracted from the selected eligible studies: (1) study characteristics, such as first author, year of publication, and region; (2) demographic and clinical characteristics, such as age, male/female ratio, and reference standard; and (3) technical characteristics of ultrasound elastography, such as ultrasound elastography system, type of ultrasound elastography, and probe. In addition, a 2 × 2 contingency table was also builded using the data retrieved from each study. If one article has evaluated more than one type of ultrasound elastography methods, we considered each type of ultrasound elastography method as an independent study.
The revised Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess the quality of the studies included in this analysis (29). Two researchers independently searched the databases, read and selected the articles to include in the present meta-analysis, extracted the required information from the included studies, and assessed the quality of the included studies. If there were disagreements between the two authors, a third review author would be consulted.
To assess the diagnostic performance of ultrasound elastography for the detection of BA, the summary sensitivity, specificity and the corresponding 95% confidence intervals (CIs) were calculated using a random-effect model in the present meta-analysis. In addition, using the data from different studies, we also simultaneously constructed the summary receiver operating characteristic (SROC) curves of ultrasound elastography in diagnosing BA. Three methods, including the summary sensitivities and specificities, the summary diagnostic odds ratios (DORs) and area under the SROC curve (AUROC), were used to examine the accuracy of ultrasound elastography for the detection of BA.
Heterogeneity was assessed using the Cochrane-Q test and the inconsistency index I2 statistic (30). I2 value > 50% is suggestive of substantial heterogeneity. Spearman correlation coefficient was also calculated to evaluate the threshold effect. Subgroup analysis and meta-regression analysis were performed to explore the potential source of heterogeneity. In this meta-analysis, a subgroup analysis was also conducted to assess the performance of the relatively new type of ultrasound elastography (2D-SWE) for the diagnosis of BA.
We used the Deeks' funnel plot asymmetry test to evaluate possible publication bias (31). A P < 0.05 was considered to indicate a significant bias. In the present meta-analysis, the analyses were conducted using Stata version 15.0 (Stata Corp.).
Figure 1 displays the flow diagram. Initially, our search strategy retrieved 979 records. A total of 596 records were then retained after removing duplications. Furthermore, 585 studies were excluded, including reviews, conference abstracts, protocols, studies not relevant to ultrasound elastography diagnosis, or studies with insufficient information and data, etc. Finally, 11 studies (12, 32–41) were included in this meta-analysis.
Basic characteristics of the included studies are summarized in Table 1. Totally, 14 studies (nine 2D-SWE studies, three p-SWE studies, and two TE studies) from 11 articles were identified for evaluation. All studies were published between 2016 and 2022, of which, six studies were published between 2020 and 2022. Most of the studies included in the present meta-analysis were from China. Overall, 1,797 subjects, including 774 BA patients, 850 non-BA (i.e., other causes of cholestasis) patients, and 173 controls, were included in this meta-analysis. Patients in the BA group had a mean age of 50.2 days, and ~47.0% were male (range: 30.8–60.0%). Methodological quality of included studies according to QUADAS-2 is displayed in Supplementary material.
Technical characteristics of ultrasound elastography in the included studies are given in Table 2. For the measurement of liver stiffness, a total of three types of ultrasound elastography techniques (i.e., TE, p-SWE, and 2D-SWE) were used in the included studies. Specifically, two studies were performed with TE, three studies involved both p-SWE and 2D-SWE, and six studies were performed with 2D-SWE only. Among the included studies, the devices used to perform ultrasound elastography varied, including Acuson in six studies, Aixplorer in five studies, FibroScan 502 Touch in two studies, and TUS-Aplio 500 in one study. On the other hand, based on the technique, ultrasound elastography can be categorized as TE (FibroScan 502 Touch; Echosens), p-SWE (Virtual Touch Quantification; Siemens Healthineers), and 2D-SWE, including ShearWave Elastography (SuperSonic Imagine), Virtual Touch Tissue and Imaging Quantification (Siemens Healthineers) and Acoustic Structure Quantification (Toshiba Medical Systems). With regard to the measure of liver stiffness, eight ultrasound elastography studies (two TE studies and six 2D-SWE studies) used elasticity (in kilopascals) and six studies (three p-SWE studies and three 2D-SWE studies) used shear wave speed (in meters per second). We summarized the liver stiffness measurement by ultrasound elastography of infants in different groups in the included studies, as illustrated in Table 3.
Fourteen studies from eleven articles provided information regarding the performance of liver stiffness measurement by ultrasound elastography for diagnosing BA.
We extracted the raw data for diagnostic test accuracy, and then these were used to examine the accuracy of ultrasound elastography for the diagnosis of BA (Table 4). Among the 14 included studies, the sensitivity and specificity ranged from 71 to 97.4% and 64 to 100%, respectively. Based on the combined results, the summary sensitivity and specificity of liver stiffness measurement by ultrasound elastography for the prediction of BA were 85% (95% CI: 79–89%) and 82% (95% CI: 73–88%) with the I2 value of 82.90 and 84.33%, respectively (Figure 2). The cut-off values from each study are displayed in Table 4. Liver stiffness was measured using a unit of elasticity (in kilopascals) in the two TE studies with a cut-off value of 7.7 kPa for each study, and in the six 2D-SWE studies with a mean cut-off value of 9.3 kPa (range: 7.10–12.35 kPa). In addition, the three p-SWE studies and the other three 2D-SWE studies used a unit of shear wave speed (in meters per second), with mean cut-off values of 1.6 m/s (range: 1.53–1.77 m/s) and 1.92 m/s (range: 1.84–2.0 m/s), respectively.
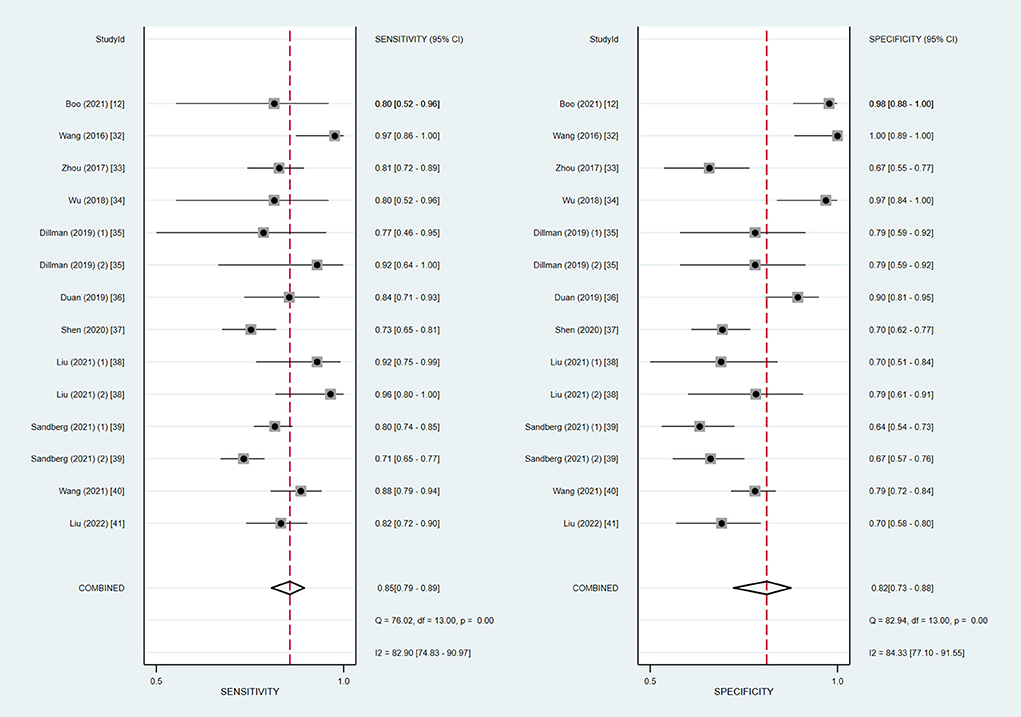
Figure 2. Coupled forest plots of the summary sensitivity and specificity of ultrasound elastography for the diagnosis of biliary atresia (BA).
As is shown in Figure 3, the summary AUROC value was 0.90 (95% CI: 0.87–0.92) when ultrasound elastography was used for diagnosing BA. The summary DOR value was 24 (95% CI: 11–51). A Deeks' funnel plot asymmetry test was used to evaluate possible publication bias (Figure 4). When ultrasound elastography was used to diagnose BA, a significant publication bias was present (P < 0.05).
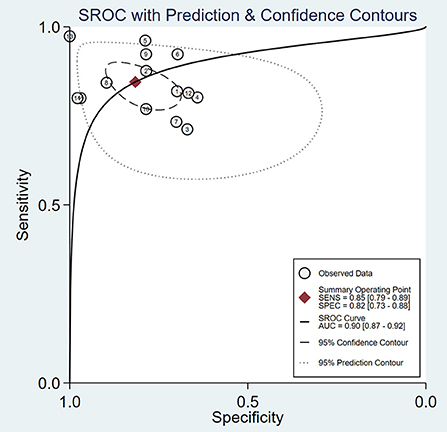
Figure 3. Summary receiver operating characteristic (SROC) curve of the diagnostic performance of ultrasound elastography for the diagnosis of biliary atresia (BA).
In addition, we also summarized the relevant studies using the 2D-SWE technique and evaluated the diagnostic accuracy of 2D-SWE in diagnosing BA. In our meta-analysis, a subgroup analysis of the nine studies using 2D-SWE indicated pooled sensitivity and specificity of 85% (95% CI: 77–91%) and 79% (95% CI: 71–86%) with the I2 value of 85.82 and 81.72%, respectively (Figure 5). The summary AUROC value of 2D-SWE was 0.89 (95% CI: 0.86–0.92), for diagnosing BA. Moreover, the summary DOR value was 22 (95% CI: 9–57).
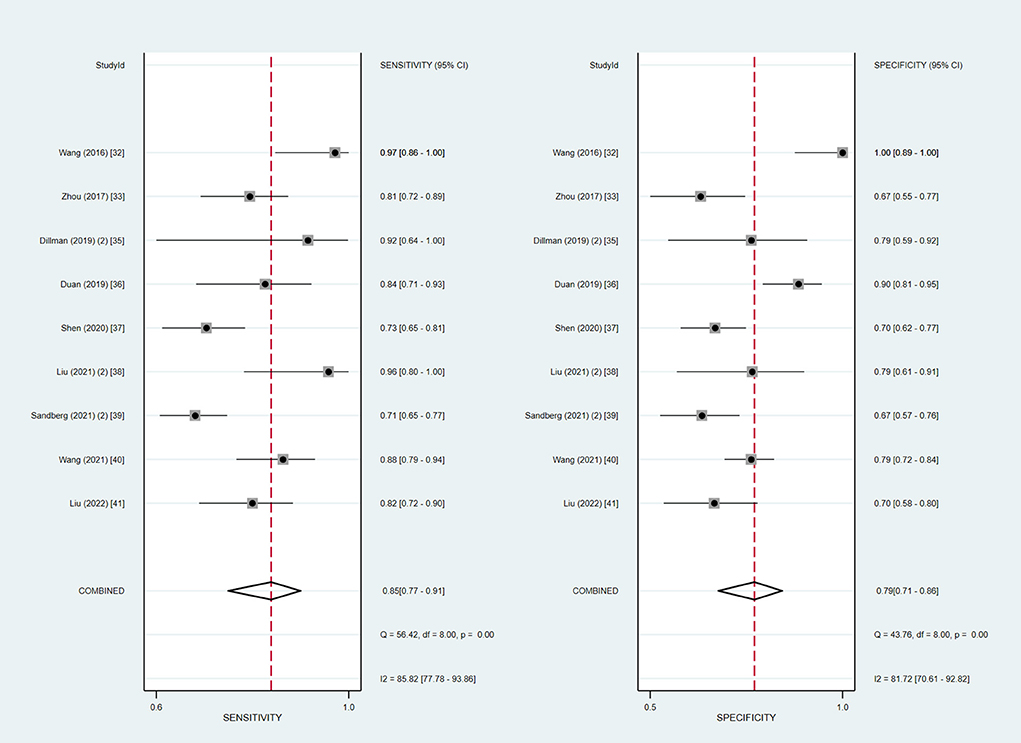
Figure 5. Coupled forest plots of the summary sensitivity and specificity of the liver stiffness measurement in the two-dimensional shear wave elastography (2D-SWE) subgroup.
The Spearman correlation coefficient was −0.275 (P = 0.342), confirming that the threshold effect was not significant in ultrasound elastography studies and, therefore, a non-threshold heterogeneity was examined by I2 statistics. The inconsistency index I2 statistic revealed substantial heterogeneity with regard to the summary sensitivity and specificity among studies (I2 = 82.90 and 84.33%, respectively) (Figure 2).
To explore the origin of the heterogeneity, we further performed subgroup analysis and meta-regression analysis. The meta-regression analysis showed that publication year, method of processing measurement, ultrasound elastography systems, and ultrasound elastography techniques could be the reasons of the heterogeneity. The subgroup analysis based on ultrasound elastography technique showed that 2D-SWE had higher sensitivity (85 vs. 81%) and AUROC (0.89 vs. 0.82) but lower specificity (79 vs. 87%) than other ultrasound elastography techniques in diagnosing BA. More detailed information regarding the subgroup analysis is described in Table 5.
Measuring liver stiffness with ultrasound elastography in cholestatic infants might be helpful in the differential diagnosis of BA (2, 34). To this end, based on data acquired from published studies, we conducted the present meta-analysis to provide evidence-based insight regarding the diagnostic performance of ultrasound elastography for BA.
In this meta-analysis, we identified 14 studies from 11 articles with 1,797 subjects (including 774 BA patients, 850 non-BA patients, and 173 controls) for evaluation, and found that ultrasound elastography provided a summary sensitivity of 85% (95% CI: 79–89%), specificity of 82% (95% CI: 73–88%), and AUROC of 0.90 (95% CI: 0.87–0.92). Our findings indicated that liver stiffness measurement by ultrasound elastography had a good diagnostic accuracy for the diagnosis of BA.
BA is a challenging liver disease in infancy (12). It remains the leading indication for pediatric liver transplantation throughout the world, despite surgical treatment (8, 42). Of note, time-to-treatment is a critical factor in determining outcome (43). For timely diagnosis of BA, therefore, a non-invasive and accurate diagnostic tool is required (12). Several quantitative ultrasound elastography techniques such as TE, p-SWE and 2D-SWE have been widely used to evaluate pediatric liver diseases such as fibrosis and BA by measuring liver stiffness (20, 44). In recent years, a good diagnostic performance for ultrasound elastography has been extensively reported in pediatric patients, especially with liver diseases. Of interest, in a recent meta-analysis of 12 studies including 550 pediatric patients, the summary sensitivity and specificity of ultrasound shear wave elastography for predicting liver significant fibrosis were 81% (95% CI: 71–88%) and 91% (95% CI: 83–96%), respectively (45). Hwang et al. (46) in a meta-analysis reported a summary sensitivity of 95% (95% CI: 74–99%) and a specificity of 90% (95% CI: 81–95%) for TE for the evaluation of significant liver fibrosis in children with an AUROC of 0.96 (95% CI: 0.94–0.98). Another previous meta-analysis performed by Kim et al. (21) found that, for evaluation of portal hypertension in children, the summary sensitivity and specificity of ultrasound elastography were 90% (95% CI: 83%–94%) and 79% (95% CI: 73–84%), respectively, and the summary AUROC was 0.92 (95% CI: 0.90–0.94). This suggests that ultrasound elastography is a promising tool for assessment of liver diseases in the pediatric population (44).
Nevertheless, in a real clinical practice, some factors specific to pediatric patients that may affect liver stiffness measurement with ultrasound elastography need to be considered, such as age, probe choice, a small and thin body size, and ability to lay still and cooperate (20, 25, 45, 46). For example, even under ideal conditions, the success rate of TE for the measurement of liver stiffness in children younger than 24 months was lower (25).
This meta-analysis demonstrated that, for ultrasound elastography, the summary sensitivity and specificity for the diagnosis of BA were 85% (95% CI: 79–89%) and 82% (95% CI: 73–88%), respectively. Moreover, a subgroup analysis of nine 2D-SWE studies was also performed and was shown to have similar diagnostic performance. The summary sensitivity and specificity of liver stiffness measured by 2D-SWE for diagnosing BA were 85% (95% CI: 77%–91%) and 79% (95% CI: 71–86%), respectively. The summary AUROC was 0.89 (95% CI: 0.86–0.92). Therefore, ultrasound elastography, as a promising novel imaging modality, could aid in accurate diagnosis of BA.
It is worth noting that, in several previous studies (33, 36, 39), the diagnostic performance of ultrasound elastography for BA was reported to be inferior to that of conventional ultrasound. In Zhou's study (33), for identifying BA, the performance of liver stiffness measurement was not exceed that of gray-scale ultrasound findings, including fibrotic cord thickness and gallbladder classification (AUROC: 0.790 vs. 0.868–0.922). In the original study of Sandberg et al. (39), triangular cord sign was reported to be the strongest predictor for BA among the individual gray-scale imaging findings (sensitivity: 88%; specificity: 96%), which was superior to the p-SWE (sensitivity: 80%; specificity: 64%) and 2D-SWE (sensitivity: 71%; specificity: 67%). Nevertheless, the use of gray-scale ultrasound findings including triangular cord sign to identify BA requires an analyst with expertise (10, 17). It is somewhat subjective and operator dependent. Notably, a previous study found that in up to 83% of BA patients within 30 days and 44% of BA patients over 30 days, the triangular cord sign was absent (47).
Due to some factors such as different types of ultrasound elastography systems and techniques, and different age groups and serum biochemical index levels in infants with cholestasis, the optimal cut-off values proposed for BA diagnosis varied across the studies included in our meta-analysis. It should be noted that different vendors and different probes could be expected to have different stiffness values (44). Hence, it is important in clinical practice to recognize the differences between the various ultrasound elastography systems and techniques used to measure liver stiffness (19, 20).
A major strength of the present meta-analysis is that we comprehensively investigated the diagnostic performance of liver stiffness, measured by different ultrasound elastography systems and techniques including TE, p-SWE and 2D-SWE. We also conducted a subgroup analysis and assessed the performance of 2D-SWE for the diagnosis of BA. In contrast to TE and p-SWE, 2D-SWE is a relatively novel ultrasound elastography technique (19, 48). Finally, through subgroup analysis and meta-regression analysis, we could explore the origin of heterogeneity.
Some notable limitations of this study should be acknowledged. First, substantial heterogeneity was observed across the studies included in this meta-analysis. Also, a significant publication bias was also present among studies. This might be attributable to several reasons, such as the types of ultrasound elastography technique from different vendors, study population, a variable distribution of severity of the disease, and study design, etc. Additionally, most included studies were small in size. Thus, any interpretation of the results of our present meta-analysis should be mindful of the heterogeneity and publication bias. Second, due to the limited number of articles, our meta-analysis included studies that used different ultrasound elastography systems and techniques. Nevertheless, we conducted subgroup analysis based on ultrasound elastography system and technique. Further studies are required to investigate the diagnostic performance of specific ultrasound elastography systems/techniques for BA. Third, we only included studies published in English, leading to a linguistic bias. In addition, most of the included studies were from China. Due to this, caution is necessary in interpreting our findings.
In conclusion, ultrasound elastography exhibits good performance in the diagnosis of BA and can be served as a non-invasive tool to facilitate the differential diagnosis of BA from other neonatal cholestasis.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
BD, XY, and GL contributed to the study design and literature search. BD, ZW, XY, and HW completed the data analysis. XY and HW generated and improved the figures and tables. BD completed the manuscript. BD, ZW, and GL proofread the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Collaborative Innovation Center for Maternal and Infant Health Service Application Technology from Quanzhou Medical College (grant no. XJM1802).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.973125/full#supplementary-material
1. Lendahl U, Lui VCH, Chung PHY, Tam PKH. Biliary Atresia-emerging diagnostic and therapy opportunities. EBioMedicine. (2021) 74:103689. doi: 10.1016/j.ebiom.2021.103689
2. Zhou W, Zhou L. Ultrasound for the diagnosis of biliary atresia: from conventional ultrasound to artificial intelligence. Diagnostics. (2021) 12:51. doi: 10.3390/diagnostics12010051
3. Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: a national study 1986-96. J Hepatol. (1999) 31:1006–13. doi: 10.1016/S0168-8278(99)80312-2
4. McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. (2000) 355:25–9. doi: 10.1016/S0140-6736(99)03492-3
5. Hsiao CH, Chang MH, Chen HL, Lee HC, Wu TC, Lin CC, et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. (2008) 47:1233–40. doi: 10.1002/hep.22182
6. Chung PHY, Zheng S, Tam PKH. Biliary atresia: east versus west. Semin Pediatr Surg. (2020) 29:150950. doi: 10.1016/j.sempedsurg.2020.150950
7. Tiao MM, Tsai SS, Kuo HW, Chen CL, Yang CY. Epidemiological features of biliary atresia in Taiwan, a national study 1996-2003. J Gastroenterol Hepatol. (2008) 23:62–6. doi: 10.1111/j.1440-1746.2007.05114.x
8. Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. (2018) 68:1163–73. doi: 10.1002/hep.29905
9. Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. (2006) 148:467–74. doi: 10.1016/j.jpeds.2005.12.054
10. Zhou W, Yang Y, Yu C, Liu J, Duan X, Weng Z, et al. Ensembled deep learning model outperforms human experts in diagnosing biliary atresia from sonographic gallbladder images. Nat Commun. (2021) 12:1259. doi: 10.1038/s41467-021-21466-z
11. Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. (2004) 39:115–28. doi: 10.1097/00005176-200408000-00001
12. Boo YA, Chang MH, Jeng YM, Peng SF, Hsu WM, Lin WH, et al. diagnostic performance of transient elastography in biliary atresia among infants with cholestasis. Hepatol Commun. (2021) 5:882–90. doi: 10.1002/hep4.1672
13. Napolitano M, Franchi-Abella S, Damasio BM, Augdal TA, Avni FE, Bruno C, et al. Practical approach for the diagnosis of biliary atresia on imaging, part 2: magnetic resonance cholecystopancreatography, hepatobiliary scintigraphy, percutaneous cholecysto-cholangiography, endoscopic retrograde cholangiopancreatography, percutaneous liver biopsy, risk scores and decisional flowchart. Pediatr Radiol. (2021) 51:1545–54. doi: 10.1007/s00247-021-05034-7
14. Kianifar HR, Tehranian S, Shojaei P, Adinehpoor Z, Sadeghi R, Kakhki VR, et al. Accuracy of hepatobiliary scintigraphy for differentiation of neonatal hepatitis from biliary atresia: systematic review and meta-analysis of the literature. Pediatr Radiol. (2013) 43:905–19. doi: 10.1007/s00247-013-2623-3
15. Liu B, Cai J, Xu Y, Peng X, Zheng H, Huang K, et al. Three-dimensional magnetic resonance cholangiopancreatography for the diagnosis of biliary atresia in infants and neonates. PLoS ONE. (2014) 9:e88268. doi: 10.1371/journal.pone.0088268
16. Norton KI, Glass RB, Kogan D, Lee JS, Emre S, Shneider BL, et al. cholangiography in the evaluation of neonatal cholestasis: initial results. Radiology. (2002) 222:687–91. doi: 10.1148/radiol.2223010969
17. Zhou L, Shan Q, Tian W, Wang Z, Liang J, Xie X. Ultrasound for the diagnosis of biliary atresia: a meta-analysis. Am J Roentgenol. (2016) 206:W73–82. doi: 10.2214/AJR.15.15336
18. Nakamura H, Yamataka A. Non-invasive and accurate diagnostic system for biliary atresia. EBioMedicine. (2018) 36:16–7. doi: 10.1016/j.ebiom.2018.09.032
19. Barr RG. Shear wave liver elastography. Abdom Radiol. (2018) 43:800–7. doi: 10.1007/s00261-017-1375-1
20. Banc-Husu AM, Bass LM. Transient elastography in pediatric liver disease. J Pediatr Gastroenterol Nutr. (2021) 73:141–4. doi: 10.1097/MPG.0000000000003168
21. Kim DW, Yoon HM, Jung AY, Lee JS, Oh SH, Kim KM, et al. Diagnostic performance of ultrasound elastography for evaluating portal hypertension in children: a systematic review and meta-analysis. J Ultrasound Med. (2019) 38:747–59. doi: 10.1002/jum.14764
22. Park SH, Kim SY, Suh CH, Lee SS, Kim KW, Lee SJ, et al. What we need to know when performing and interpreting US elastography. Clin Mol Hepatol. (2016) 22:406–14. doi: 10.3350/cmh.2016.0106
23. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. (2017) 7:1303–29. doi: 10.7150/thno.18650
24. Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, et al. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. (2012) 171:353–60. doi: 10.1007/s00431-012-1778-5
25. Goldschmidt I, Streckenbach C, Dingemann C, Pfister ED, di Nanni A, Zapf A, et al. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. (2013) 57:109–13. doi: 10.1097/MPG.0b013e31829206a0
26. Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, et al. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology. (2015) 276:845–61. doi: 10.1148/radiol.2015150619
27. Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J Hepatol. (2014) 61:550–7. doi: 10.1016/j.jhep.2014.04.044
28. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
29. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
31. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
32. Wang X, Qian L, Jia L, Bellah R, Wang N, Xin Y, et al. Utility of shear wave elastography for differentiating biliary atresia from infantile hepatitis syndrome. J Ultrasound Med. (2016) 35:1475–9. doi: 10.7863/ultra.15.08031
33. Zhou LY, Jiang H, Shan QY, Chen D, Lin XN, Liu BX, et al. Liver stiffness measurements with supersonic shear wave elastography in the diagnosis of biliary atresia: a comparative study with grey-scale US. Eur Radiol. (2017) 27:3474–84. doi: 10.1007/s00330-016-4710-y
34. Wu JF, Lee CS, Lin WH, Jeng YM, Chen HL, Ni YH, et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology. (2018) 68:616–24. doi: 10.1002/hep.29856
35. Dillman JR, DiPaola FW, Smith SJ, Barth RA, Asai A, Lam S, et al. Prospective assessment of ultrasound shear wave elastography for discriminating biliary atresia from other causes of neonatal cholestasis. J Pediatr. (2019) 212:60–5.e3. doi: 10.1016/j.jpeds.2019.05.048
36. Duan X, Peng Y, Liu W, Yang L, Zhang J. Does supersonic shear wave elastography help differentiate biliary atresia from other causes of cholestatic hepatitis in infants less than 90 days old? Compared with Grey-Scale US. Biomed Res Int. (2019) 2019:9036362. doi: 10.1155/2019/9036362
37. Shen Q, Tan SS, Wang Z, Cai S, Pang W, Peng C, et al. Combination of gamma-glutamyl transferase and liver stiffness measurement for biliary atresia screening at different ages: a retrospective analysis of 282 infants. BMC Pediatr. (2020) 20:276. doi: 10.1186/s12887-020-02172-z
38. Liu YF Ni XW, Pan Y, Luo HX. Comparison of the diagnostic value of virtual touch tissue quantification and virtual touch tissue imaging quantification in infants with biliary atresia. Int J Clin Pract. (2021) 75:e13860. doi: 10.1111/ijcp.13860
39. Sandberg JK, Sun Y, Ju Z, Liu S, Jiang J, Koci M, et al. Ultrasound shear wave elastography: does it add value to gray-scale ultrasound imaging in differentiating biliary atresia from other causes of neonatal jaundice? Pediatr Radiol. (2021) 51:1654–66. doi: 10.1007/s00247-021-05024-9
40. Wang Y, Jia LQ, Hu YX, Xin Y, Yang X, Wang XM. Development and validation of a nomogram incorporating ultrasonic and elastic findings for the preoperative diagnosis of biliary atresia. Acad Radiol. (2021) 28(Suppl. 1):S55–63. doi: 10.1016/j.acra.2020.08.035
41. Liu Y, Peng C, Wang K, Wu D, Yan J, Tu W, et al. The utility of shear wave elastography and serum biomarkers for diagnosing biliary atresia and predicting clinical outcomes. Eur J Pediatr. (2022) 181:73–82. doi: 10.1007/s00431-021-04176-y
42. Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl. (2017) 23:96–109. doi: 10.1002/lt.24640
43. Durkin N, Deheragoda M, Davenport M. Prematurity and biliary atresia: a 30-year observational study. Pediatr Surg Int. (2017) 33:1355–61. doi: 10.1007/s00383-017-4193-1
44. Thumar V, Squires JH, Spicer PJ, Robinson AL, Chan SS. Ultrasound elastography applications in pediatrics. Ultrasound Q. (2018) 34:199–205. doi: 10.1097/RUQ.0000000000000379
45. Kim JR, Suh CH, Yoon HM, Lee JS, Cho YA, Jung AY. The diagnostic performance of shear-wave elastography for liver fibrosis in children and adolescents: a systematic review and diagnostic meta-analysis. Eur Radiol. (2018) 28:1175–86. doi: 10.1007/s00330-017-5078-3
46. Hwang JY, Yoon HM, Kim JR, Lee JS, Jung AY, Kim KM, et al. Diagnostic performance of transient elastography for liver fibrosis in children: a systematic review and meta-analysis. Am J Roentgenol. (2018) 211:W257–66. doi: 10.2214/AJR.18.19535
47. Hwang SM, Jeon TY, Yoo SY, Choe YH, Lee SK, Kim JH. Early US findings of biliary atresia in infants younger than 30 days. Eur Radiol. (2018) 28:1771–7. doi: 10.1007/s00330-017-5092-5
Keywords: ultrasound elastography, biliary atresia, diagnosis, stiffness, meta-analysis
Citation: Dong B, Weng Z, Lyu G, Yang X and Wang H (2022) The diagnostic performance of ultrasound elastography for biliary atresia: A meta-analysis. Front. Public Health 10:973125. doi: 10.3389/fpubh.2022.973125
Received: 19 June 2022; Accepted: 07 October 2022;
Published: 26 October 2022.
Edited by:
Ho Cheung William Li, The Chinese University of Hong Kong, ChinaReviewed by:
Linda Beenet, University of California, Los Angeles, United StatesCopyright © 2022 Dong, Weng, Lyu, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guorong Lyu, bGdyX2ZldXNAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.