
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 24 August 2022
Sec. Aging and Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.964609
This article is part of the Research TopicWomen in Science: Aging and Public Health 2022View all 23 articles
Background: The past decade has witnessed an improvement in survival rates for breast cancer, with significant inroads achieved in diagnosis and treatment approaches. Even though chemotherapy is effective for this patient population, cardiotoxicity remains a major challenge, especially in older people. It has been established that cardiovascular events are a major cause of death in older female primary breast cancer patients that underwent chemotherapy. In the present study, the independent prognostic factors were identified to develop a novel nomogram for predicting long-term heart disease-specific survival (HDSS) and improving patient management.
Method: Older female primary breast cancer patients that underwent chemotherapy from 2010 to 2015 were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database and randomly assigned to a training cohort and a validation cohort at a ratio of 7:3. HDSS was the primary endpoint of this study. Univariate and multivariate Cox regression analyses were conducted on the training cohort to identify independent prognostic factors of HDSS and construct a nomogram to predict the 5- and 8-year HDSS. The performance of the constructed nomogram was evaluated by calibration curve, receiver operating characteristic (ROC) curve, and decision curve analyses. Finally, a risk classification system was constructed to assist in patient management.
Result: A total of 16,340 patients were included in this study. Multivariate Cox regression analysis identified six independent prognostic factors: age, race, tumor stage, marital status, surgery, and radiotherapy. A nomogram based on these six factors yielded excellent performance, with areas under the curve of the ROC for 5- and 8-year HDSS of 0.759 and 0.727 in the training cohort and 0.718 and 0.747 in the validation cohort. Moreover, the established risk classification system could effectively identify patients at low-, middle-, and high- risk of heart disease-associated death and achieve targeted management.
Conclusion: Independent prognostic factors of HDSS in older female primary breast cancer patients that underwent chemotherapy were determined in this study. A novel nomogram for predicting 5- and 8-year HDSS in this patient population was also established and validated to help physicians during clinical decision-making and screen high-risk patients to improve outcomes.
Cancer is the second most common cause of death in the US, behind heart disease (1). According to the latest data released by the American Cancer Society, the expected number of female breast cancer cases will increase by 287,850 in the US in 2022, leading to an estimated 43,250 deaths. Interestingly, it has been reported that since the 1950s, the incidence of breast cancer has increased by 0.5% per year. However, with early detection of breast cancer through screening, increased sensitization, and improved treatments, breast cancer mortality has fallen by 42% over the past 30 years (2). Increasing age and female gender are reportedly significant risk factors for breast cancer. The risk of developing invasive breast cancer in women under 49, 50–59, 60–69, and older than 70 years old has been reported to be 2.1, 2.4, 3.5, and 7%, respectively (2).
Increasing age is a natural driver of cardiovascular morbidity and mortality in the general population; cardiovascular diseases have been documented to be a significant risk factor for mortality in older females with breast cancer. Abdel-Qadir et al. showed that among breast cancer women aged 66 years or older with no cardiovascular disease, the 10-year risk of breast cancer- and cardiovascular disease-associated death were 11.9 and 7.6%, respectively. Interestingly, among patients with pre-existing cardiovascular disease, the risk of death from cardiovascular disease and breast cancer was comparable for the first 5 years. However, the risk of death from cardiovascular disease exceeded breast cancer over time, with a 10-year cumulative mortality rate of 16.9 and 14.6%, respectively (3). Over the years, anthracycline-based chemotherapy has exhibited high efficacy in treating breast cancer. However, it has been shown that cardiotoxicity and heart failure risks increase with cumulative doses of anthracyclines (4, 5). Accordingly, mortality caused by cardiovascular disease in older breast cancer patients that underwent chemotherapy accounts for poor long-term heart disease specific survival (HDSS).
Although risk factors associated with HDSS in breast cancer have been identified, there is currently no universally accepted scoring system to predict long-term HDSS in this subpopulation. Given that different clinical-pathological variables can affect the patient prognosis, a new approach that integrates key prognostic predictors is warranted to help during treatment selection and improve patient quality of life. Nomograms are nowadays widely accepted as a simple multivariate visualization tool for predicting individual patient survival outcomes, especially in oncology (6, 7). Compared with the tumor-node-metastasis staging system, nomograms can more accurately estimate the survival of individual patients by integrating key variables to aid in clinical decision-making and facilitate the development of precision medicine (6). To our knowledge, no nomogram has been documented in the literature for predicting HDSS in this subpopulation. More in-depth analysis of this subpopulation is necessary to identify prognostic factors associated with HDSS and develop scientifically appropriate cardiovascular mortality prevention measures to improve survival outcomes. Therefore, this study aimed to identify independent prognostic factors associated with HDSS in this subpopulation by analyzing relevant data from the Surveillance, Epidemiology, and End Results (SEER) database and to develop a novel nomogram for predicting the 5- and 8-year HDSS.
The SEER database (https://seer.cancer.gov/seerstat/) collects data from 18 separate cancer registries covering ~30% of the US population. It was used in this retrospective cohort study (8). SEER Stat software v8.3.9.2 was used to identify the relevant data of this subpopulation in the SEER database from 2000 to 2018 with the reference number 16336-Nov2020 [Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018)]. Since SEER is a publicly available database, and the acquired data does not include personal information, no ethics approval and informed consent are required. This research was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (9).
The inclusion criteria consisted of (i) patients with the following site-specific codes for cancer that originated in the breast: C50.0 (Nipple), C50.1 (Central portion of the breast), C50.2 (Upper-inner quadrant of the breast), C50.3 (Lower-inner quadrant of the breast), C50.4 (Upper-outer quadrant of the breast), C50.5 (Lower-outer quadrant of the breast), C50.6 (Axillary tail of the breast), C50.8 (Overlapping lesion of the breast), and C50.9 (Breast, NOS) (10); (ii) older female (age ≥65) (11–14); (iii) patient underwent chemotherapy; (iv) “diseases of the heart” and “alive” were used to classify patient death classification according to “COD to site rec KM”; (v) primary tumor; and (6) complete follow-up data available. Patients were excluded for the following reasons: (i) male; (ii) no chemotherapy; (iii) age <65; (iv) breast cancer is not the primary tumor; (v) demographic and clinical data, including age, race, marital status, Breast-Adjusted AJCC 6th Stage (tumor stage), tumor grade, surgery, radiotherapy, and tumor size, were not available; (vi) survival time < 1 month. Finally, 16,340 patients were included in this study and randomly divided into training (n = 11,440) and validation cohorts (n = 4,900) according to a ratio of 7:3. The former was used to identify HDSS-related independent prognostic factors and establish a prognostic nomogram and risk classification system for this subpopulation. The latter was used to verify the constructed nomogram and risk classification system.
Patient demographic characteristics (age, race, and marital status), tumor factors (tumor size, tumor grade, and tumor stage), disease characteristics (primary site, breast subtype, ER status, PR status, HER2 status, and distant (bone, brain, liver, and lung) metastasis, and treatment information (surgery and radiotherapy) were analyzed in this study. The optimal cut-off values for age and tumor size in the training and validation cohorts determined by the X-tile software were 71 and 76 years old and 22 and 36 mm, respectively (Supplementary File 1) (15). Patients were categorized into white, black, and others (American Indian/AK Native, Asian/Pacific Islander) based on race. Marital status was divided into “married” and “single/other”. Surgery and radiotherapy were categorized into “Yes” and “No” groups. Distant (bone, brain, liver, and lung) metastasis was divided into “Present” and “Absent”. Tumor grades were divided into grades I, II, III, and IV, and clinical tumor stages were classified as stages I, II, III, and IV. The breast subtypes were divided into HR-/HER2- (Triple Negative), HR-/HER2+ (HER2 enriched), HR+/HER2- (Luminal A), and HR+/HER2+ (Luminal B). Moreover, the HER2, PR, and ER statuses were divided into “Positive” or “Negative”. The HDSS, defined as the time interval from the date of diagnosis until death due to heart disease, was the primary endpoint of this study.
All data were analyzed using SPSS (version 22.0) and R (version 4.0.3) software. A p-value <0.05 was statistically significant. First, values were assigned to each variable included in this study. The statistical difference between the enrolled variables was identified using the Kaplan-Meier method and univariate Cox regression analysis. Then, variables with a p-value <0.05 were incorporated into a multivariate Cox regression analysis to eliminate confounding effects and identify HDSS-related independent prognostic factors in this subpopulation. HDSS-related independent prognostic factors were then used to construct a nomogram to predict 5- and 8-year HDSS. The corresponding scores of the independent prognostic factors in the HDSS nomogram were obtained. Then, the bootstrap-corrected concordance index (C-index) and calibration curves were constructed to verify the prediction and discrimination performance of the nomogram, and a decision curve analysis (DCA) was constructed to demonstrate the clinical utility value of the nomogram. The discriminative power of the nomogram was assessed by constructing the receiver operating characteristic (ROC) curves for the 5- and 8-year HDSS based on the area under the curve (AUC) values of the corresponding variables. In addition, the total score was calculated as the sum of the scores corresponding to the HDSS-related independent prognostic factors, and the optimal cut-off value for the total score was obtained using the X-tile software. Then, a risk classification system was established to stratify the cardiovascular mortality risk of this subpopulation into low-, middle-, and high-risk subgroups. Finally, Kaplan-Meier method was used to identify the differences between the three risk subgroups.
16,340 older female primary breast cancer patients that underwent chemotherapy retrieved from the SEER database were randomly divided into training (n = 11,440, 70%) and validation (n = 4,900, 30%) cohorts. The majority of patients were aged between 65 and 70 years old (n = 10,013, 61.28%), white (n = 13,225, 80.94%), and married (n = 9,309, 56.97%). No significant difference was found between low-grade (grade I–II) and high-grade (grade III–IV) tumors. Moreover, low-stage (stage I–II) tumors occupied a higher proportion (78.34%) of cases than high-stage (stage III–IV) tumors. The size of most tumors was <22 mm, while C50.4, C50.8, and C50.2 represented the top three primary sites, accounting for 69.38%. The incidence of distant metastases was relatively low. Besides, luminal A was the most common molecular subtype, accounting for 51.51%. Most patients were classified as ER-positive (n = 11,393, 69.72%), PR-positive (n = 9,085, 55.60%), and HER2-negative (n = 11,643, 71.25%). As for the treatment, 97.3 and 62.93% of patients underwent surgery and radiotherapy, respectively (Table 1).
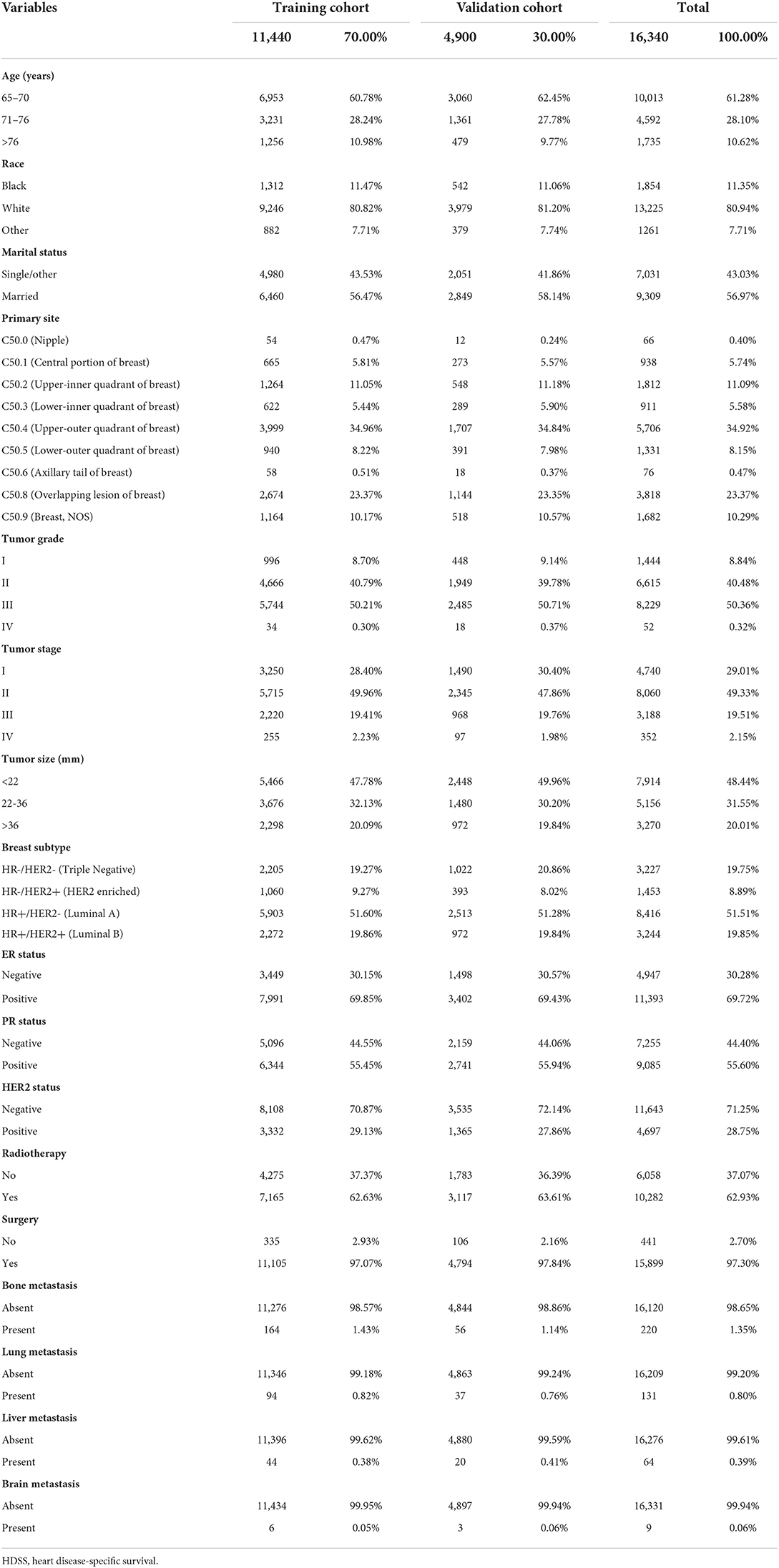
Table 1. The baseline demographic and clinicopathologic characteristics of the HDSS-related variables of older female primary breast cancer patients that underwent chemotherapy.
According to the results of univariate Cox regression analysis and Kaplan–Meier curves, age, race, marital status, primary site, tumor grade, tumor stage, tumor size, breast subtype, PR status, surgery, radiotherapy, and distant (bone, liver, and lung) metastasis were significantly associated with HDSS (p < 0.05). In contrast, no significant difference in ER status, HER2 status, and brain metastasis were found (Figure 1). Then, HDSS-related variables with a p-value<0.05 during univariate Cox regression analysis were used to perform multivariate Cox regression analysis to eliminate the effects of confounding variables. The results showed that age, race, marital status, tumor stage, surgery, and radiotherapy were independent prognostic factors of HDSS in this subpopulation (Table 2).
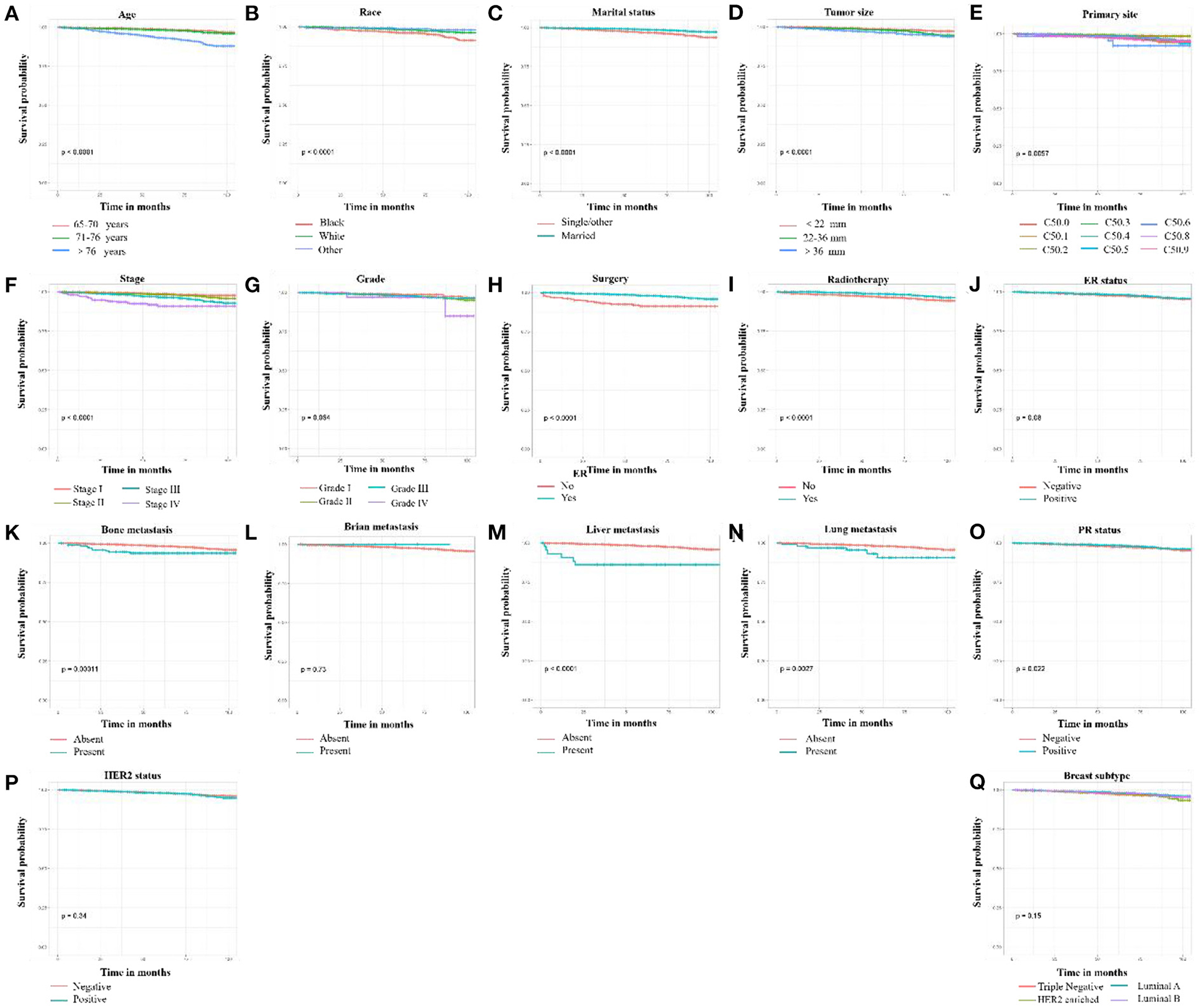
Figure 1. Kaplan-Meier curves of HDSS-related variables in older female primary breast cancer patients that underwent chemotherapy. (A) age, (B) race, (C) marital status, (D) tumor size, (E) primary site, (F) tumor stage, (G) tumor grade, (H) surgery, (I) radiotherapy, (J) ER status, (K) bone metastasis, (L) brain metastasis, (M) liver metastasis, (N) lung metastasis, (O) PR status, (P) HER2 status, and (Q) breast subtype.
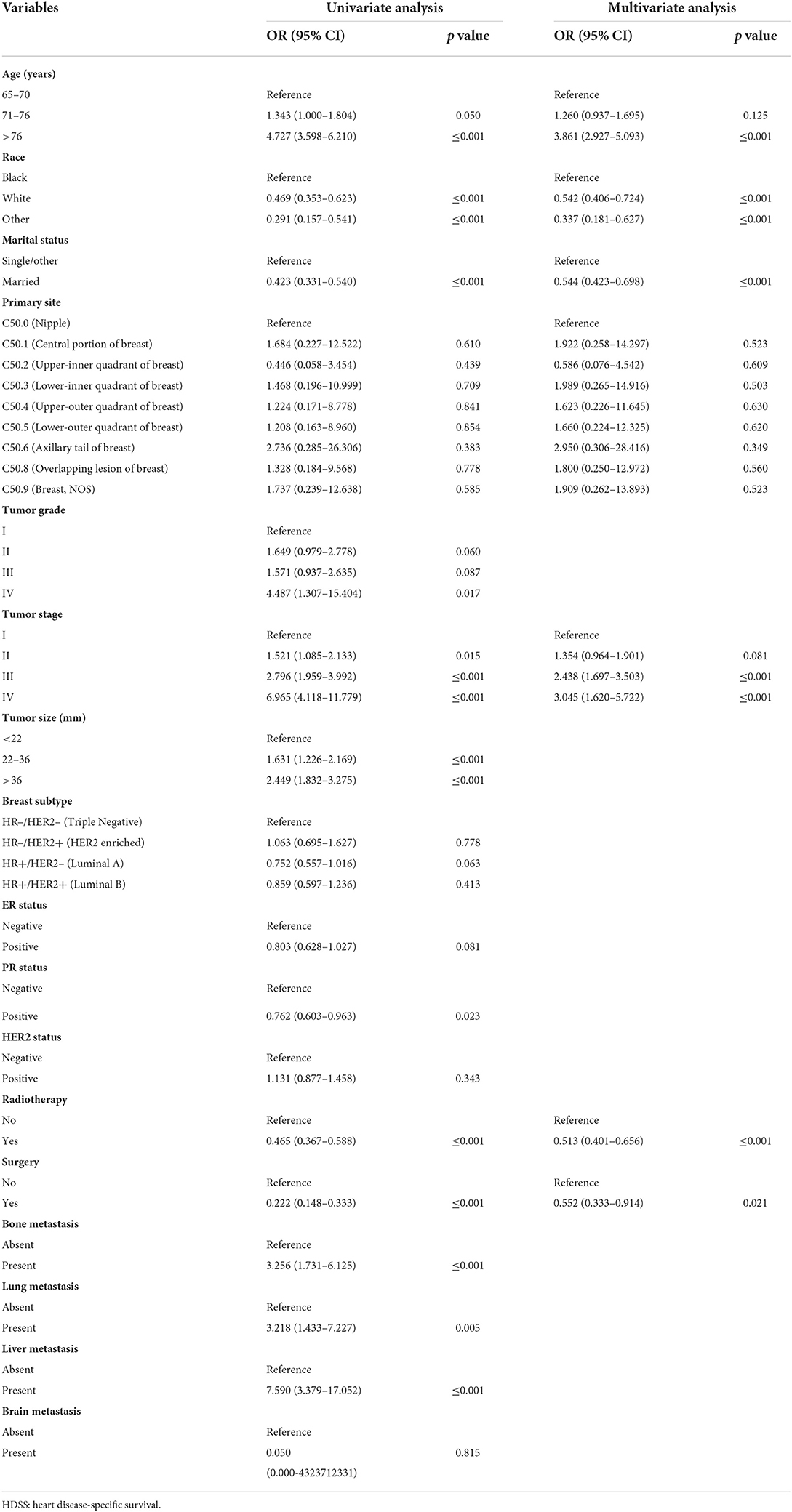
Table 2. The univariate and multivariate Cox regression analyses of the HDSS-related variables of older female primary breast cancer patients that underwent chemotherapy.
The six aforementioned HDSS-related independent prognostic factors were used to establish a prognostic nomogram for predicting long-term HDSS in older female primary breast cancer patients that underwent chemotherapy (Figure 2). As shown in Figure 2, the corresponding point value of the independent prognostic factors in the HDSS nomogram were obtained by drawing a straight line to the top point row and then were summed to get the total point. The 5- and 8-year HDSS were obtained by drawing vertical lines from the total point row to the bottom timeline. A good prognosis was found for 65–70 years old married patients of other races (American Indian/AK Native, Asian/Pacific Islander) and lower tumor stage (stage I) that underwent surgery and radiotherapy. The calibration curves for 5- and 8-year survival showed good agreement between actual and predicted outcomes based on the constructed nomogram in this subpopulation (Figure 3). The bootstrap-corrected C-index was 0.757 (95% CI: 0.694–0.820) and 0.730 (95% CI: 0.634–0.826) in the training cohort and validation cohort. The AUCs for the 5-year HDSS in the training and validation cohorts were 0.759 and 0.718, respectively. Consistently, the AUCs for the 8-year HDSS in the training and validation cohorts were 0.718 and 0.747, respectively (Figure 4). These findings suggested that the constructed nomogram had good discriminatory power (Figure 4). Moreover, we compared the predictive accuracy between individual independent prognostic factors and the constructed nomogram (Figure 5). The results showed that the AUC of the constructed nomogram was higher than each factor at 5- and 8-years in the training and validation cohorts, indicating that the nomogram yielded a more accurate predictive performance for HDSS in this subpopulation. In addition, DCA showed that the constructed nomogram had high prospects for clinical application (Figure 6).
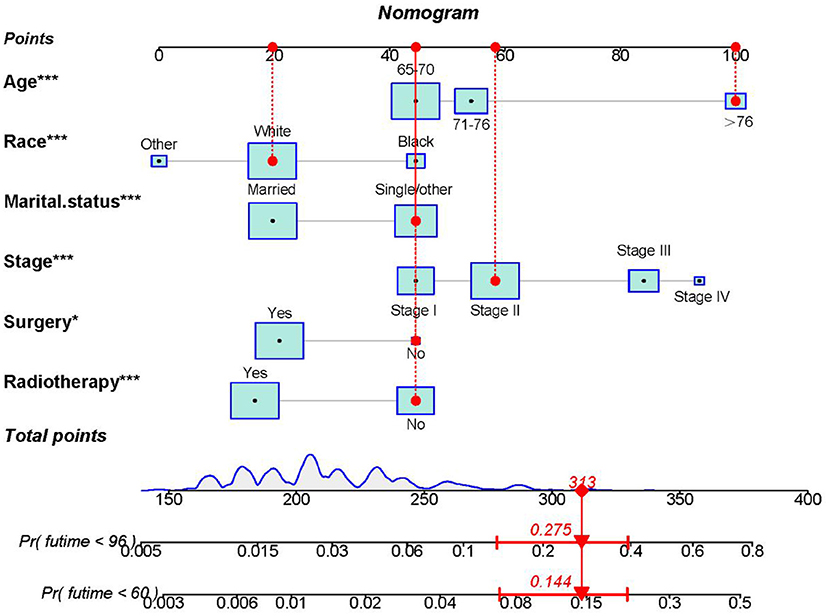
Figure 2. The nomogram was constructed to predict the 5- and 8-year HDSS in older female primary breast cancer patients that underwent chemotherapy. To calculate the HDSS of an individual patient, point values for each prognostic predictor were obtained by drawing a straight line to the top point row. Next, the corresponding point values were summed to get the total score below. The 5- and 8-year HDSS were obtained by drawing vertical lines from the total score row to the bottom timeline. For example, for an 80-year-old unmarried white race female patient with stage II disease that did not undergo surgery or radiotherapy, the total score is 100 (80 years old) +20 (white race) +45 (single/other) +58 (stage II) +45 (no surgery) +45 (no radiotherapy) = 313, and the corresponding risk of heart disease-associated death at 5- and 8-year are 0.144 and 0.275, while the corresponding HDSS of the patient at 5- and 8-year are 0.856 and 0.725.
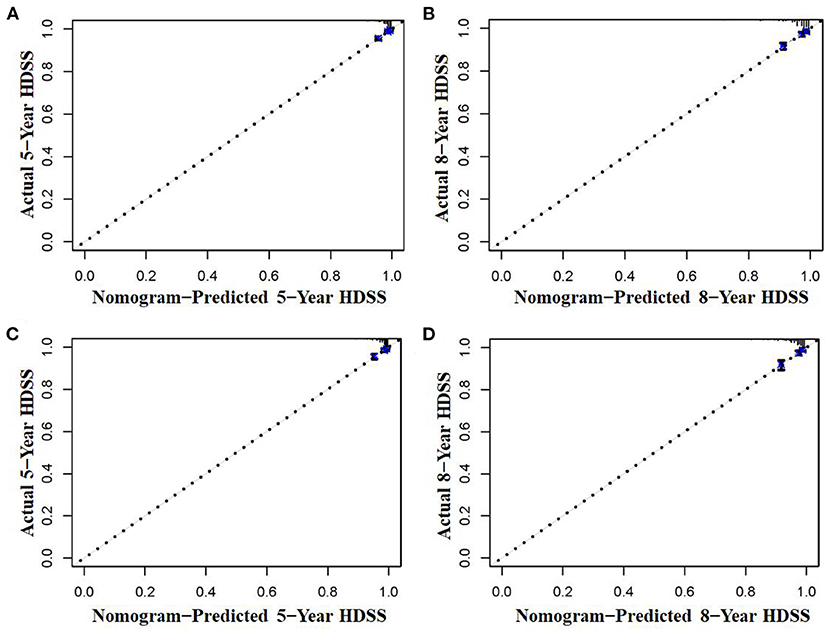
Figure 3. The calibration curves of the nomogram were used to predict the 5- and 8-year HDSS in older female primary breast cancer patients that underwent chemotherapy in the training (A,B) and validation cohorts (C,D).
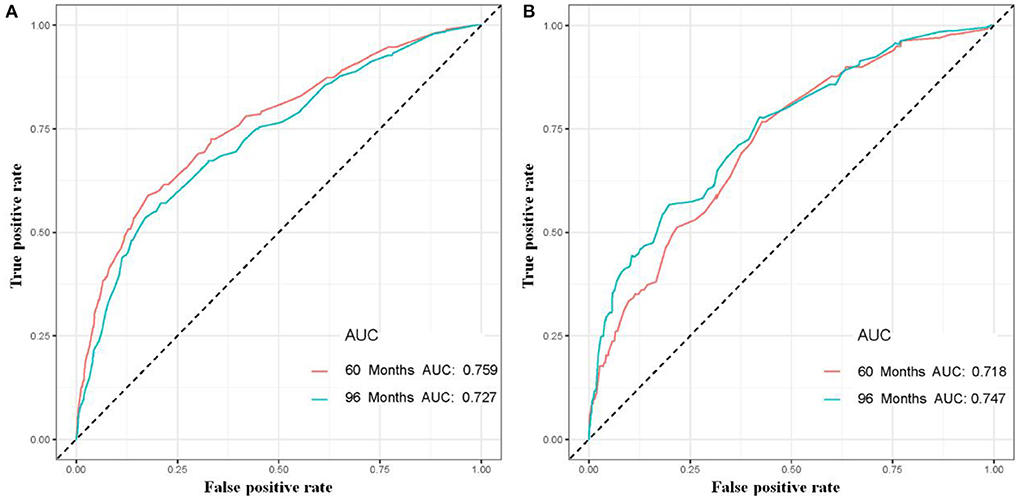
Figure 4. The 5- and 8-year receiver operating characteristic curves of older female primary breast cancer patients that underwent chemotherapy in the training (A) and validation (B) cohorts.
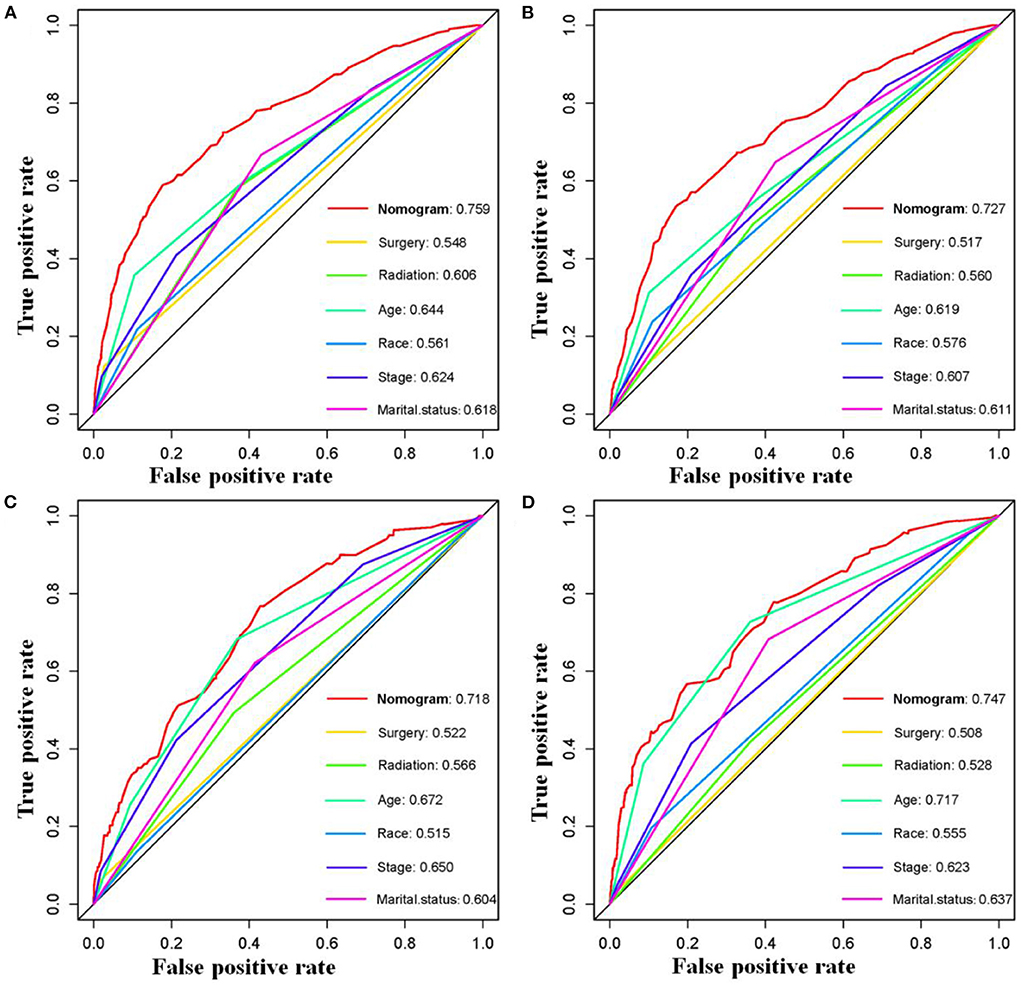
Figure 5. Comparison of prediction accuracy between the constructed novel nomogram and each HDSS-related independent prognostic factors in older female primary breast cancer patients that underwent chemotherapy at 5-(A) and 8-(B) year in the training cohort and 5-(C) and 8-(D) year in the validation cohort, respectively.
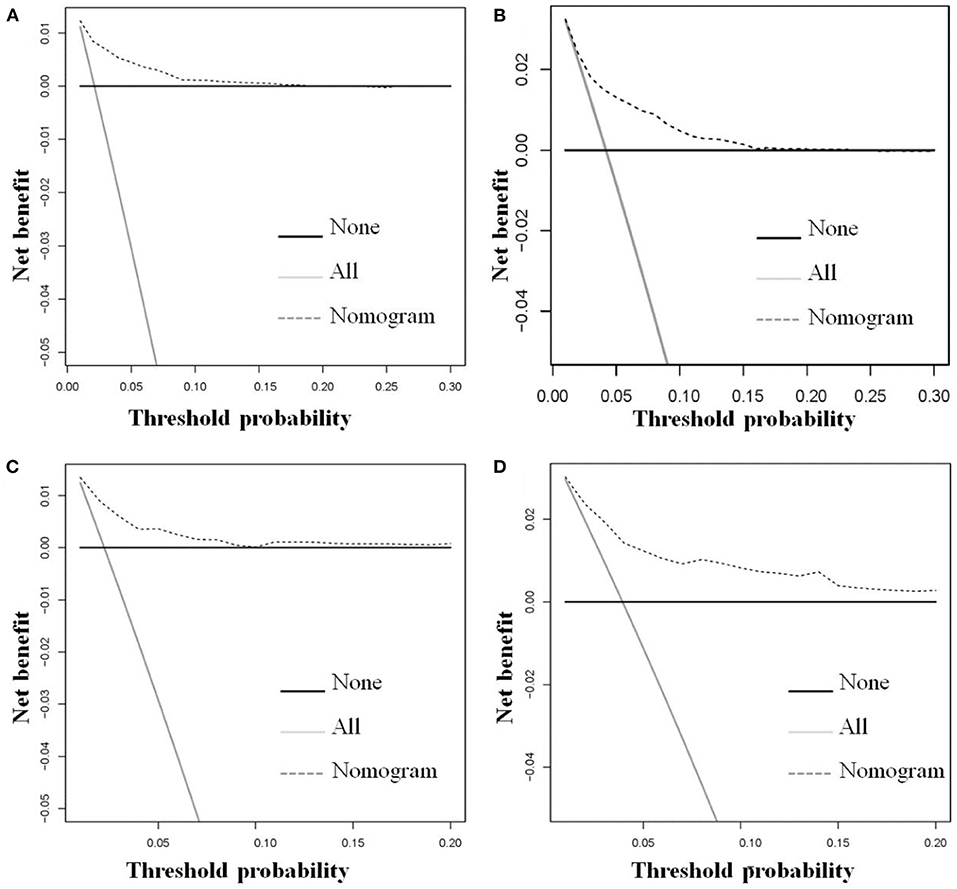
Figure 6. The decision curve analysis of the constructed novel nomogram was used to predict the 5-(A) and 8-(B) year HDSS in the training cohort and the 5-(C) and 8-(D) year HDSS in the validation cohort for older female primary breast cancer patients that underwent chemotherapy.
In addition to predicting patient HDSS, it is essential to classify patients based on their cardiovascular mortality risk for individualized management. A cardiovascular mortality risk classification system was constructed using the six HDSS-related independent prognostic factors. Specifically, the total points of all patients were obtained by summing the assigned point values for each independent prognostic factor. The optimal cut-off values for the total point were 223 and 260, according to the results of the X-tile software (Supplementary File 1). Accordingly, patients were further divided into three cardiovascular mortality risk subgroups: low- (<223), middle- (223–260), and high- (>260), and a Kaplan-Meier survival curve was generated (Figure 7). As shown in Figure 7, the risk classification system could effectively classify older female primary breast cancer patients that underwent chemotherapy into three subgroups, indicating that the HDSS nomogram could classify patients based on the cardiovascular mortality risk to improve patient management.
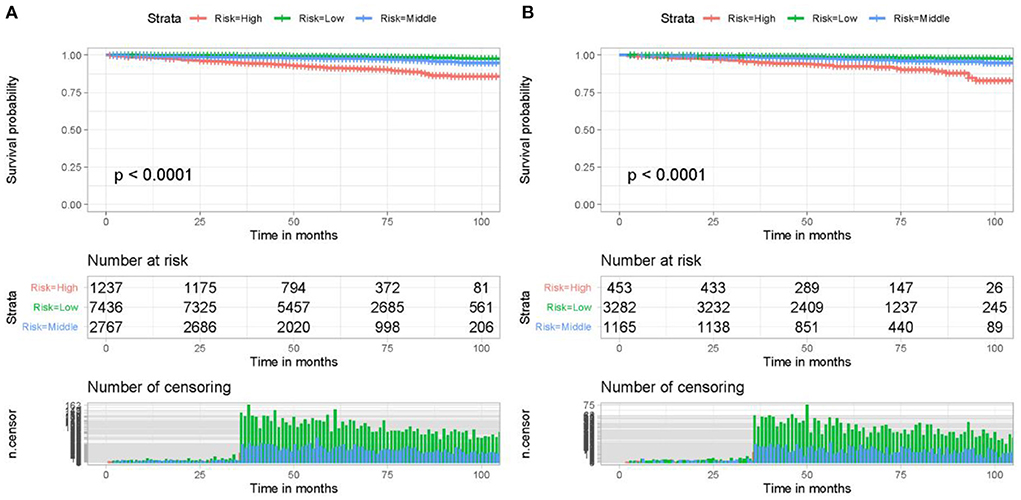
Figure 7. The risk classification system could effectively classify older female primary breast cancer patients that underwent chemotherapy in the training cohort (A) and validation cohort (B) into three risk subgroups with significant differences. Kaplan-Meier survival curves demonstrated that older female primary breast cancer patients that underwent chemotherapy in the high-risk subgroup had a worse prognosis than those in the low-risk subgroup.
Depending on the tumor stage, subtype, and gene expression results, treatment modalities for breast cancer mainly involve local therapy, including surgery and radiotherapy, and systemic therapy, encompassing chemotherapy, hormone therapy, targeted therapy, and immunotherapy. Among these, chemotherapy is well-established as an effective treatment for breast cancer. Anthracycline-based treatment regimens have been used to treat breast cancer since the 1970s. Nonetheless, its use can reportedly cause cardiac side effects, including cardiomyopathy, ischemia, arrhythmias, and myocardial necrosis, resulting in severe and irreversible left ventricular dysfunction (16, 17). Two main mechanisms can explain this cardiotoxicity: (i) anthracyclines cause myocyte DNA damage, bind to topoisomerase IIβ and disrupt replication (18, 19); (ii) anthracyclines form complexes with intracellular iron, which in turn generate reactive oxygen species that damage DNA, proteins, and lipids, including mitochondrial membranes, and accelerate myocyte death (20, 21). In this regard, Howard et al. showed that doxorubicin-based adjuvant chemotherapy for breast cancer could cause arrhythmias and conduction abnormalities in 2.6% of patients compared to 1% of patients who did not receive doxorubicin (4). Consistently, Guglin et al. showed that anthracyclines could cause atrial fibrillation in 2–10% of patients during or after chemotherapy (22). In addition, cardiotoxicity caused by chemotherapeutic drugs is usually progressive and irreversible. Cardinale et al. showed that recovery of left ventricular function and reduced cardiac events was feasible with early detection and prompt treatment. However, complete left ventricular ejection fraction (LVEF) recovery was not observed in patients treated with chemotherapy over 6 months. On average, LVEF decreases moderately but consistently by ~4% after 3 years of anthracycline exposure (23, 24). Based on these findings, McGowan et al. hypothesized that in the new era of targeted therapy, most breast cancer patients treated with anthracyclines might become the heart disease patients of tomorrow (18).
In addition to anthracycline-based chemotherapy, age is another major risk factor for heart disease. Interestingly, Jeon et al. showed that patients aged ≥50 years old sustained a significant increase in the risk of heart disease compared with those aged <50 years old (16). The incidence of breast cancer increases with age, doubling approximately every 10 years until menopause, where breast cancer growth slows (25). The incidence of heart disease increases steadily with age, but the rate of increase becomes steeper at menopause (26). Gernaat et al. showed that heart disease-related mortality in breast cancer patients ranged from 1.6 to 10.4% (27). In addition, older patients are widely thought to have a poorer prognosis, associated with reduced physical function, cognitive impairment, and comorbidities, such as hypertension, hyperlipidemia, and diabetes. In such circumstances, aggressive treatment is not indicated, and the course of treatment may be shortened, thus affecting the treatment outcome (28, 29). Therefore, there is an urgent need for research on survival and risk factors associated with HDSS in this subpopulation.
In this study, a large-scale population-based data analysis was conducted on 16,340 older female primary breast cancer patients that underwent chemotherapy from the SEER database. Age, race, marital status, tumor stage, surgery, and radiotherapy were identified as independent prognostic factors of HDSS and used to establish a nomogram to predict the HDSS at 5 and 8 years in this subpopulation. The nomogram constructed could provide a quantitative method for HDSS prediction for individual patients in this subpopulation. Importantly, we also used this nomogram to develop a cardiovascular mortality risk classification system that could classify these patients into three risk subgroups: high, middle, and low, allowing clinicians to assess various parameters more objectively and accurately, leading to better patient management.
Herein, we found that race was an independent prognostic factors of HDSS in this subpopulation. Our study showed that black women had a poorer prognosis than white women. Consistently, Berkman et al. showed that among women diagnosed with breast cancer between 1990 and 2010, the heart disease-associated mortality in black women was 6.43 times higher than white women, which may be explained by a lack of regular screening and poor access to health care resources and surgical treatment than whites (30–33). Besides, a shortage of educational resources could contribute to the lack of early recognition and intervention of risk factors associated with cardiovascular disease. Last but not least, lack of exercise, smoking, and shortage of healthy food have been documented to contribute to racial disparities in cardiovascular mortality (33, 34). Indeed, surgery remains the mainstay of breast cancer treatment, allowing effective tumor resection and improving survival. An increasing body of evidence suggests that older female patients with stage IV breast cancer who undergo surgery have better overall survival and cancer-specific survival than those who do not, even in patients with bone metastases (35–38). The similar conclusions were reached in our study, where patients who underwent surgery had significantly higher HDSS than those who did not. In addition, radiotherapy was also a protective factor for HDSS in this subpopulation. Radiotherapy can reduce the tumor size and allow control of distant metastases, reducing the burden of the primary tumor on the body and improving the body's ability to cope with the risk of heart disease.
Interestingly, our study showed that marital status was an independent prognostic factor for HDSS in this patient population. The 5-and 8-year HDSS of married patients was higher than that of divorced, widowed, and single patients regardless of age, race, and tumor grade. Previous studies have shown that married patients, who receive help and encouragement from their spouses, exhibit better compliance with prescribed treatment regimens, and married patients with greater financial resources are more likely to have access to early screening facilities and medical assistance (39–41). Moreover, we observed that the HDSS of patients with stage III/IV disease was lower than those with stage I/II, providing compelling evidence of the importance of improving early diagnosis rates.
Although this study constructed a novel nomogram with good performance for predicting HDSS, some limitations were present. Given the retrospective nature of clinical studies, selection bias was inevitable in our study. Moreover, much uncertainty surrounded the specific cardiovascular causes of death due to the coding system used in the SEER database. Besides, there were missing records for treatment data, such as patient chemotherapy regimen and duration and the presence of coexisting cardiovascular disease at diagnosis. Indeed, further studies in other centers or databases are essential to validate our nomogram.
Extra caution should be taken by clinicians when treating older female primary breast cancer patients with chemotherapy, given the risk of cardiac disease. Our study showed that unmarried patients with old age, black race, and higher tumor stage with no surgery or radiotherapy had a poor HDSS. Management of heart disease in this patient population should be strengthened, and prompt interventions should be taken to improve outcomes. Our established nomogram and risk classification system for predicting the HDSS at 5 and 8 years could assist physicians in clinical decision-making and managing this subpopulation.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
MY designed and supervised the study. CH and ZD undertook the study, performed the literature review, extracted the data, and analyzed the pooled data. ZD and HL drew the figures and organized the tables. MY and ZZ provided critical comments and revised the manuscript. All authors read and approved the final manuscript.
This research was funded by the Regional Innovation and Cooperation Program of Science and Technology Department of Sichuan Province (Grant Number: 2021YFQ0028), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant Number: ZYJC18039).
We are grateful to SEER database and the 18 registries that provide cancer research data, and all colleagues for their contributions to the study. The work was performed in West China Hospital, Sichuan University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.964609/full#supplementary-material
HDSS, heart disease-specific survival; SEER, Surveillance, Epidemiology and End Results; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; ROC, receiver operating characteristic; DCA, decision curve analysis; AUC, area under the curve; LVEF, left ventricular ejection fraction.
1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000491
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
3. Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. (2017) 2:88–93. doi: 10.1001/jamacardio.2016.3841
4. Hochster H, Wasserheit C, Speyer J. Cardiotoxicity and cardioprotection during chemotherapy. Curr Opin Oncol. (1995) 7:304–9. doi: 10.1097/00001622-199507000-00002
5. Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. (2016) 27:6–13. doi: 10.1097/EDE.0000000000000394
6. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
7. Tong Y, Cui Y, Jiang L, Pi Y, Gong Y, Zhao D. Clinical characteristics, prognostic factor and a novel dynamic prediction model for overall survival of elderly patients with chondrosarcoma: a population-based study. Front Public Health. (2022) 10:901680. doi: 10.3389/fpubh.2022.901680
8. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. (2002) 40:IV-3-18. doi: 10.1097/00005650-200208001-00002
9. Ghaferi AA, Schwartz TA, Pawlik TM. STROBE reporting guidelines for observational studies. JAMA Surg. (2021) 156:577–8. doi: 10.1001/jamasurg.2021.0528
10. Weberpals J, Jansen L, Muller OJ, Brenner H. Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. Eur Heart J. (2018) 39:3896–903. doi: 10.1093/eurheartj/ehy167
11. Lamont EB, Schilsky RL, He Y, Muss H, Cohen HJ, Hurria A, et al. Generalizability of trial results to elderly Medicare patients with advanced solid tumors (Alliance 70802). J Natl Cancer Inst. (2015) 107:336. doi: 10.1093/jnci/dju336
12. Boero IJ, Paravati AJ, Hou J, Gillespie EF, Schoenbrunner A, Unkart J, et al. The impact of surgeons on the likelihood of mastectomy in breast cancer. Ann Surg. (2019) 269:951–8. doi: 10.1097/SLA.0000000000002698
13. Sammon JD, Abdollah F, Reznor G, Pucheril D, Choueiri TK, Hu JC, et al. Patterns of declining use and the adverse effect of primary androgen deprivation on all-cause mortality in elderly men with prostate cancer. Eur Urol. (2015) 68:32–9. doi: 10.1016/j.eururo.2014.10.018
14. Lamba N, Kearney RB, Catalano PJ, Hassett MJ, Wen PY, Haas-Kogan DA, et al. Population-based estimates of survival among elderly patients with brain metastases. Neuro Oncol. (2021) 23:661–76. doi: 10.1093/neuonc/noaa233
15. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
16. Jeon YW, Bang HW, Suh YJ, Kim G. The long-term effect of age on cardiovascular disease in patients with breast cancer who received chemotherapy. Breast Cancer Res Treat. (2020) 180:665–74. doi: 10.1007/s10549-020-05568-8
17. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement From the American Heart Association. Circulation. (2018) 137:e30–66. doi: 10.1161/CIR.0000000000000556
18. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. (2017) 31:63–75. doi: 10.1007/s10557-016-6711-0
19. Morita M, Shimomura A, Tokuda E, Horimoto Y, Kawamura Y, Ishizuka Y, et al. Is adjuvant chemotherapy necessary in older patients with breast cancer? Breast Cancer. (2022) 29:498–506. doi: 10.1007/s12282-021-01329-7
20. Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. (2002) 62:4592–8.
21. Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. (2014) 64:938–45. doi: 10.1016/j.jacc.2014.06.1167
22. Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. (2009) 11:1579–86. doi: 10.1093/europace/eup300
23. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. (2010) 55:213–20. doi: 10.1016/j.jacc.2009.03.095
24. Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, et al. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. (2017) 135:1397–412. doi: 10.1161/CIRCULATIONAHA.116.023463
25. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. (2000) 321:624–8. doi: 10.1136/bmj.321.7261.624
26. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. (1986) 111:383–90. doi: 10.1016/0002-8703(86)90155-9
27. Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. (2017) 164:537–55. doi: 10.1007/s10549-017-4282-9
28. Burdett N, Vincent AD, O'Callaghan M, Kichenadasse G. Competing risks in older patients with cancer: a systematic review of geriatric oncology trials. J Natl Cancer Inst. (2018) 110:825–30. doi: 10.1093/jnci/djy111
29. Foster JA, Salinas GD, Mansell D, Williamson JC, Casebeer LL. How does older age influence oncologists' cancer management? Oncologist. (2010) 15:584–92. doi: 10.1634/theoncologist.2009-0198
30. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. (2001) 116:404–16. doi: 10.1016/S0033-3549(04)50068-7
31. Finkelstein EA, Khavjou OA, Mobley LR, Haney DM, Will JC. Racial/ethnic disparities in coronary heart disease risk factors among WISEWOMAN enrollees. J Womens Health (Larchmt). (2004) 13:503–18. doi: 10.1089/1540999041280963
32. Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. (2001) 135:352–66. doi: 10.7326/0003-4819-135-5-200109040-00012
33. Berkman A. B FC, Ades PA, Dickey S, Higgins ST, Trentham-Dietz A, Sprague BL, Lakoski SG. Racial differences in breast cancer, cardiovascular disease, and all-cause mortality among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. (2014) 148:407–13. doi: 10.1007/s10549-014-3168-3
34. Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA. (1998) 280:356–62. doi: 10.1001/jama.280.4.356
35. Peng P, Chen JY, Han YT, Chen X, Li HY, Hu CH, et al. Impact of surgery on survival in breast cancer with bone metastases only: a SEER database retrospective analysis. BMC Surg. (2021) 21:378. doi: 10.1186/s12893-021-01378-x
36. Chen YQ, Xu JW, Xu XF, Wang XL, Huo LQ, Wang L, et al. Predicting the survival benefit of local surgery in patients aged 70 years or older with stage IV breast cancer: a population-based analysis. Breast. (2021) 59:124–34. doi: 10.1016/j.breast.2021.06.007
37. Obeng-Gyasi S, Asad S, Fisher JL, Rahurkar S, Stover DG. Socioeconomic and surgical disparities are associated with rapid relapse in patients with triple-negative breast cancer. Ann Surg Oncol. (2021) 28:6500–9. doi: 10.1245/s10434-021-09688-3
38. Cheng R, Wang Z, Kong X, Wang J, Fang Y, Qi L. Factors associated with chemotherapy benefit in breast cancer patients with midrange Oncotype DX breast recurrence scores. Cancer Lett. (2021) 503:213–9. doi: 10.1016/j.canlet.2021.01.016
39. Haley WE. Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. J Support Oncol. (2003) 1:25–9.
40. Baine M, Sahak F, Lin C, Chakraborty S, Lyden E, Batra SK. Marital status and survival in pancreatic cancer patients: a SEER based analysis. PLoS ONE. (2011) 6:e21052. doi: 10.1371/journal.pone.0021052
Keywords: breast cancer, heart disease-specific survival, female, chemotherapy, nomogram, risk classification system, SEER
Citation: Huang C, Ding Z, Li H, Zhou Z and Yu M (2022) A novel nomogram for predicting long-term heart-disease specific survival among older female primary breast cancer patients that underwent chemotherapy: A real-world data retrospective cohort study. Front. Public Health 10:964609. doi: 10.3389/fpubh.2022.964609
Received: 08 June 2022; Accepted: 10 August 2022;
Published: 24 August 2022.
Edited by:
Colette Joy Browning, Federation University Australia, AustraliaReviewed by:
Yingying Xu, The First Affiliated Hospital of China Medical University, ChinaCopyright © 2022 Huang, Ding, Li, Zhou and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongke Zhou, emhvdXpvbmdrZTE5NjhAMTI2LmNvbQ==; Min Yu, MTUxMjMzMDMyMTJAMTM5LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.