- Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, Hefei, China
Background: Elizabethkingia meningoseptica is a bacterium causing potential nosocomial infections and is associated with a high mortality rate; however, the date of patients in the Hefei population who have been diagnosed with this infection is generally limited.
Purpose: The clinical and laboratory data of patients from a tertiary hospital in Hefei City who had E. meningoseptica infection were evaluated in this retrospective analysis.
Patients and methods: From May 2017 to November 2021, there were 24 patients infected with E. meningoseptica in the First Affiliated Hospital of Anhui Medical University. Data were gathered from the hospital's electronic medical records for all patients.
Results: The most prevalent symptom among the 24 patients was fever (83.3%), followed by edema (41.7%), cough (37.5%), altered consciousness (41.7%), and sputum (37.5%), and laboratory results presented with anemia (75%), hypoproteinemia (75%), elevated C-reactive protein (CRP) (66.7%), neutrophilia (54.2%), and leukocytosis (50.0%). Hepatic disease (1 vs. 7, P = 0.009) was the only significant risk factor for underlying diseases. The mean value of lymphocyte (LYMPH#) (1.4 vs. 0.83 × 109/L, P = 0.033) counts was higher in the survival group than death group, while both anemia (8 vs. 10, P = 0.024) and hypoproteinemia (8 vs. 10, P = 0.024) occurred more frequently in the death group compared with the survival one.
Conclusion: Fever was the most common symptom and the only significant factor of underlying diseases was hepatic disease (P = 0.009) that often occurred in death groups. In this investigation, the risk factors for death in patients were anemia, hypoproteinemia, and lymphocyte count. The susceptibility of some quinolones, piperacillin-tazobactam, and cotrimoxazole was relatively high, suggesting that they may be the preferred drugs for the treatment of E. meningoseptica infection. As E. meningoseptica can produce biofilm to pollute the hospital environment and cause infection in patients, the disinfection of the hospital environment should be strengthened and medical staff should pay attention to aseptic operations.
Introduction
Elizabethkingia meningoseptica, formerly known as Chryseobacterium meningosepticum or Flavobacterium meningosepticum, is a gram-negative rod that is aerobic, non-motile, non-fermenting, and does not generate spores (1). The bacteria are distributed widely in nature, including in water, fish, soils, insects, and frogs. They are also present in hospital settings, where they may contaminate medical equipment and flushing solutions (1, 2). Infections with E. meningoseptica are usually involved with indwelling devices and often influence immunocompromised patients, as well as neonates with neonatal meningitis and sepsis (1). It is an opportunistic pathogen that forms biofilms and can survive for extended periods in moist environments or water sources, including tap water (3). Currently, the genus Elizabethkingia contains six species: Elizabethkingia anophelis, E. meningoseptica, Elizabethkingia bruuniana, Elizabethkingia miricola, Elizabethkingia occulta, and Elizabethkingia ursingii (4). E. meningoseptica is the most virulent of the six known Elizabethkingia bacterial species (5). Furthermore, E. meningoseptica is a hospital pathogen, and correct identification of pathogens is essential for clinical diagnosis and treatment (6). Because of the intrinsic multidrug resistance of E. meningoseptica to commonly used antibiotics including aminoglycosides and β-lactams, the infection caused by this bacteria is difficult to cure and has a high mortality rate (1, 7). According to a study in Australia, antimicrobial resistance (AMR) genes, blaBlaB, blaGOB, and blaCME, were discovered in the genomes of all Elizabethkingia clinical isolates from Australia. Because of unique metallo-β-lactamases and unique extended-spectrum β-lactamases, Elizabethkingia species are considered to be resistant to most β-lactams (8).
In clinical microbiology laboratories, the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) system with an extended spectral database is extensively employed for microbial identification. It successfully identifies E. meningoseptica and E. anophelis but is unable to differentiate between the remaining species. Accurate species identification requires molecular approaches, such as whole genome sequencing and housekeeping gene sequencing (2, 9). 16S rRNA gene sequencing is regarded as an accurate approach for identifying Elizabethkingia species, according to the published studies (10). Some studies used sequencing of the 16S rRNA gene as a standard to verify the accuracy of the species identification system. The results of recognition of E. meningoseptica and E. anophelis were almost the same (6, 8, 10–12).
However, the clinical importance of isolating E. meningoseptica has always been questioned due to its poor pathogenicity. According to estimates from two medical centers in Taiwan, the annual incidence of E. meningoseptica infection has increased over the last decade (7). Studies have reported that almost all cases infected with E. meningoseptica occurred in the hospital environment, and the data on the incidence of E. meningoseptica infection mainly came from Taiwan. It is a new pathogen in hospitals, which is related to the high mortality in hospitals (13, 14). Notably, E. meningoseptica has intrinsic resistance to many antibiotics commonly used in intensive care settings, and patients in intensive care units (ICU) are increasingly at risk for E. meningoseptica (3, 13). With continuing advances in healthcare, clinicians are becoming increasingly aware of its clinical importance (7). Despite this, E. meningoseptica infection can be easily overlooked and misdiagnosed, resulting in further challenges in clinical practice with regard to timely diagnosis.
The majority of the reported cases originated in Taiwan, with only a few instances reported from Australia, India, the United States, and Europe (15). Determining whether E. meningoseptica is an emerging pathogenic bacterium is critical for clinical diagnosis and therapy (16). Particularly, there have only been a few studies reporting E. meningoseptica in Hefei City, and knowledge of how it infects and causes the disease is extremely limited. The clinical manifestations of infection with E. meningoseptica are diverse. Due to their inherent carbapenem resistance, the literature has paid less attention to their clinical features (17). Therefore, this study reviewed the clinical manifestations of patients infected with E. meningoseptica in the First Affiliated Hospital of Anhui Medical University, a tertiary hospital in Hefei, China, and further evaluated the laboratory findings, treatment history, and prognosis related to the bacteria. It is anticipated that this investigation may provide useful data for enhancing the detection and diagnosis of E. meningoseptica infection.
Patients and methods
Recruitment criteria and diagnosis of E. meningoseptica infection
All patients infected with E. meningoseptica in the First Affiliated Hospital of Anhui Medical University (Hefei, China) between May 2017 and November 2021 were included in this retrospective analysis. There were no widely accepted criteria for diagnosing E. meningoseptica infection. Therefore, in this investigation, a case was regarded as positive if E. meningoseptica was isolated from typically sterile sites, such as sputum or blood, submitted to the laboratory at the time of admission, and identified by the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; BioMérieux, France) in the clinical microbiology laboratory. We divided people infected with E. meningoseptica into survival and death groups according to previous reports from Taiwan (18). The survival group refers to the patient being cured or improved when discharged from the hospital, while the death group refers to the death of the patient while treated in the hospital. Overall, 14 patients were classified as survival group and 10 patients were classified as death group.
Data collection
Demographic information (gender, age, and occupation), laboratory, clinical, physical examination, comorbidities, basic diseases, treatment history, complications, and in-hospital outcomes (discharge clinical status and length of hospital stay) data were obtained from the hospital's electronic medical records.
Statistical analysis
The statistical analysis was performed using version 25.0 of SPSS. Continuous variables assuming a normal distribution were provided as the mean ± standard deviation (SD) and compared using the Student's t-test, whereas those without a normal distribution were provided as medians [interquartile range (IQR)] and compared using the Mann–Whitney U-test. For categorical variables, the data were presented as n (%) and compared using Fisher's exact test. At a two-tailed P-value of < 0.05, the results were declared statistically significant.
Results
Demographic and epidemiological characteristics
From May 2017 to November 2021, we reviewed 24 patients infected with E. meningoseptica in the First Affiliated Hospital of Anhui Medical University. Because the pathogen infection is associated with high mortality (19), we further divided the patients into survival group (14 cases) and death group (10 cases), with a mortality rate of 41.6%.
The 24 hospitalized patients were mainly from the department of intensive care medicine (9/24), and specimens were mainly sputum and blood. A total of 54.2% of patients were male. The age of the patients ranged from 6 to 74 years old, and 17 of them were over 45 years old. A total of 90% of patients in the death group were over 45 years old. Seventeen of the cases (70.8%) were infected with E. meningoseptica in summer and autumn. As shown in Table 1, there was no apparent statistical difference among patients in the survival group and death group, in terms of age, gender, mean hospitalization time, diagnosis season, and basic disease. The median duration from admission to discharge was 36.5 days (IQR: 19–58.8). The common underlying disease was brain disease (37.5%), hypertension (37.5%), and renal disease (33.3%). The only significant factor was hepatic disease, which was more common in the death group than in the survival group (60.0 vs. 7.1%, p=0.009). Invasive procedures, such as arterial or venous catheterization, tracheal intubation, lumbar cistern puncture, catheter, and mechanical ventilation, were used within 30 days prior to the isolation of E. meningoseptica. Of a total of 12 patients, there were seven in the survival group and five in the death group. All demographic and epidemiological characteristics are described in detail in Table 1.
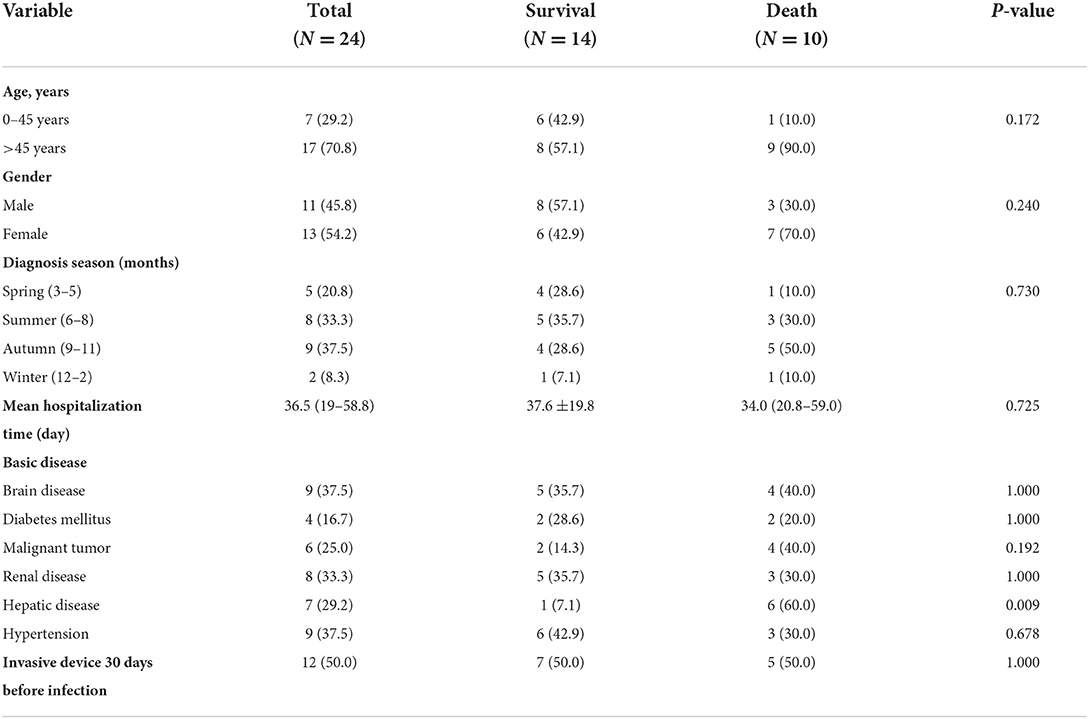
Table 1. Demographic and basic disease analysis of patients infected with E. meningoseptica (N = 24).
Clinical characteristics
The most common symptom observed in the 24 patients on presentation was fever (83.3%), followed by altered consciousness (41.7%), edema (41.7%), cough (37.5%), sputum (37.5%), abdominal distension (29.2%), chest discomfort (29.2%), chilly (25.0%), abdominal pain (20.8%), fatigue (20.8%), headache (16.6%), lethargy (12.5%), and asthma (12.5%). The mean maximal body temperature was 39.2 (SD, 0.9). Regarding complications, pneumonia was very common, which was observed in 12 of the cases (50.0%), while hydrothorax was observed in 11 cases (45.8%). However, the differences in clinical characteristics between the survival and death groups were not statistically significant (P > 0.05; Table 2).
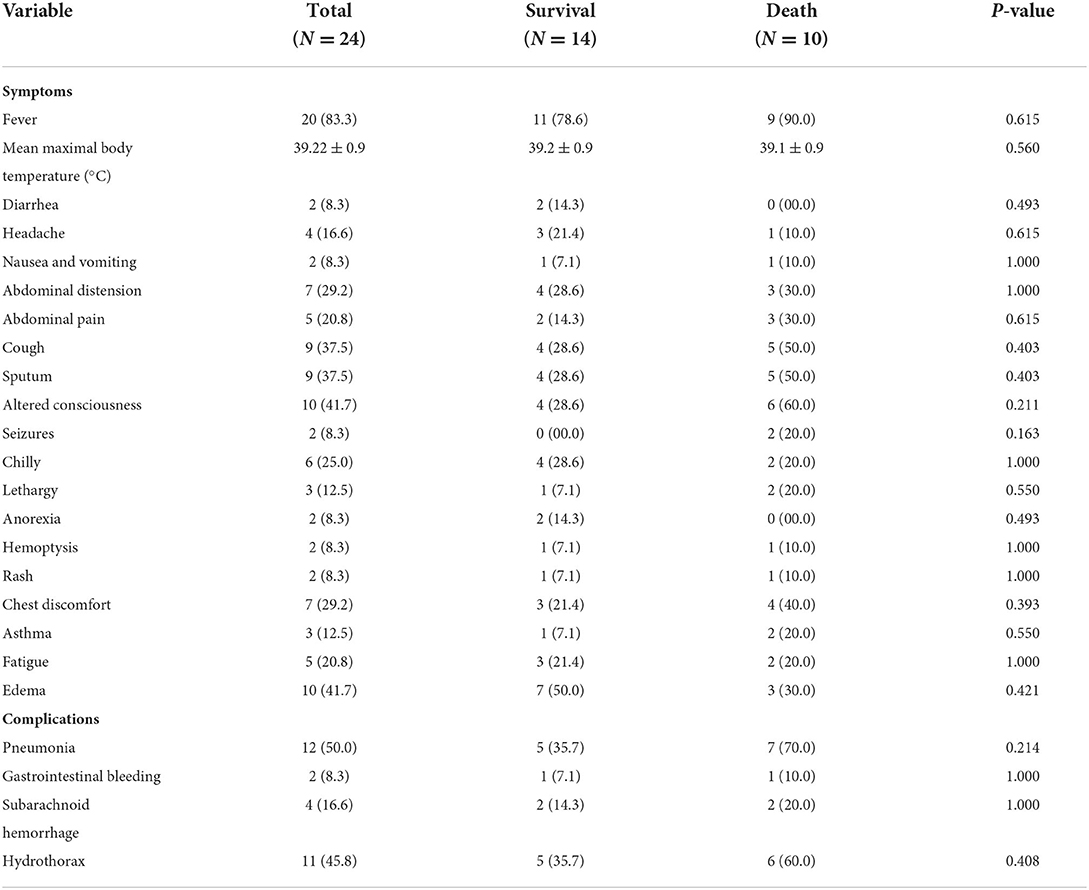
Table 2. Clinical symptoms and complications analysis of patients infected with E. meningoseptica (N = 24).
Laboratory findings
The laboratory data of 24 patients showed that anemia (75%) and hypoproteinemia (75%) were the most common diseases. Besides, hematological changes involved 16 cases with elevated C-reactive protein (CRP) levels (66.7%), 13 cases with neutrophilia (54.2%), and 12 cases with leukocytosis (50.0%). Twenty patients had different degrees of abnormal liver functions, with at least one of the following liver enzymes, namely alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), or lactate dehydrogenase (LDH) being above the normal range. Overall, the median levels of ALT, AST, ALP, GGT, and LDH were 30.5 (IQR: 17.5–74.8), 44.5 (IQR: 16.3–93.5), 81.0 (IQR: 62.0–124.0), 34.0 (IQR: 21.0–84.0), and 455.5 U/L (IQR: 249.0–873.5), respectively. The examination of hemostatic functions showed that the mean value of D-dimer (D–D) was 2.2 (IQR: 0.8–12.8) μg/ml, and it was significantly higher than the normal value (0.00–0.50 μg/ml). Moreover, the mean values of activated partial thromboplastin time (APTT) and prothrombin time (PT) were 40.5 (IQR, 37.4–49.2) and 14.5 (IQR, 13.9–16.4) s, respectively, while the mean glucose was 5.5 (IQR, 4.8–6.8) mmol/L.
Anemia occurred in eight cases (57.1%) in the survival group, as well as in 10 cases (100.0%) in the death group. There was a statistically significant difference in the incidence of Anemia between the two groups (P = 0.024). In these diseases, hypoproteinemia (P = 0.024) was also statistically significant in the two groups. In addition, lymphocyte counts (LYMPH#) (P = 0.033) were found to be statistically different between the two groups. In this case, the mean value of LYMPH# in the survival group was 1.4 (IQR: 1.0–1.8) × 109/L, and it was higher than the mean values of 0.83 (SD, 0.6) × 109/L in the death group. There was no statistically significant difference in other laboratory results between the survival group and the death group (Table 3).
Drug susceptibility results
The resistance rate of E. meningoseptica to cefazolin was 100.00%, and the resistance rate to amikacin, aztreonam, imipenem, and tobramycin was 95.8%. In the death group, ceftazidime, amikacin, aztreonam, cefazolin, cefepime, ceftriaxone, imipenem, and tobramycin were the most resistant antibiotics (100.0%). The most active antibiotic to E. meningoseptica was cotrimoxazole (87.5%), followed by levofloxacin (75.0%), piperacillin/tazobactam (75.0%), and ciprofloxacin (58.3%). The intermediate resistance to gentamycin and piperacillin/tazobactam was found in 20.8% and 16.7%. There was no statistical significance in susceptibility, intermediate, and resistance between the survival group and death group. The full antibiotic susceptibility profiles of isolates are shown in Table 4.
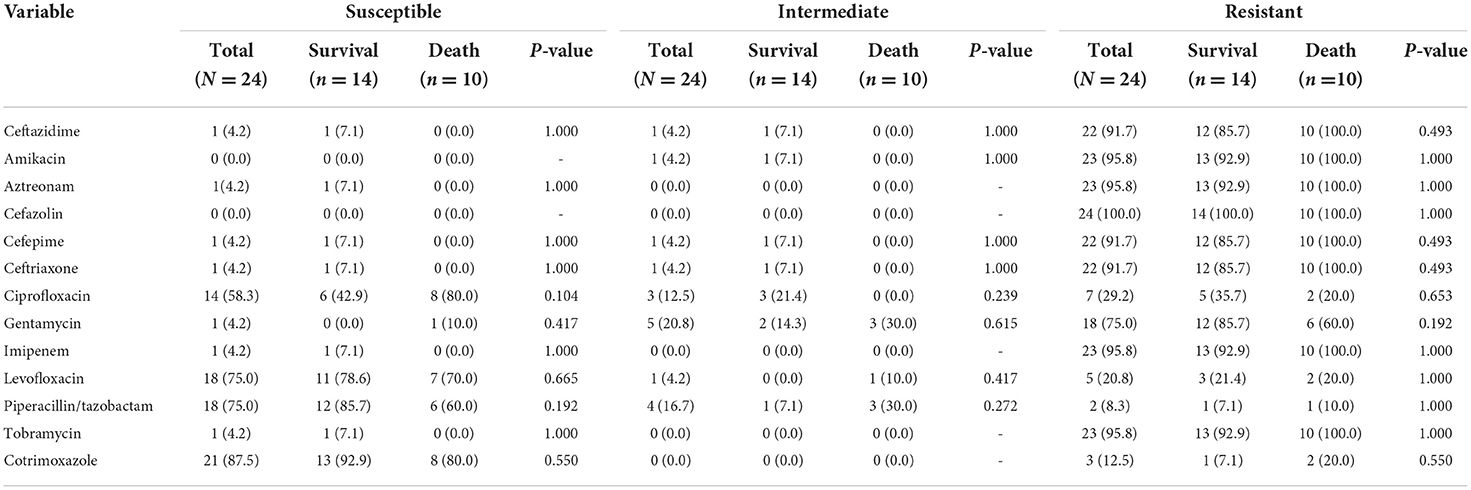
Table 4. In vitro of drug susceptibility results of patients infected with E. meningoseptica (N = 24).
Discussion
Elizabethkingia meningoseptica is most commonly isolated from freshwater, saltwater, and soil, as well as from moist and dry clinical environments, intravenous lipid solutions, equipment surfaces, and municipal water supplies, including those that are adequately chlorinated. It is an opportunistic pathogen, despite being nearly widespread. E. meningoseptica primarily infects elderly and immunocompromised patients in intensive care settings (20). Elizabeth O. King, a bacteriologist at the Centers for Disease Control in Atlanta, Georgia, United States, was the first person to report the bacteria in 1959 (21). Infections have been reported all over the world, including in the Central African Republic, Mauritius, Singapore, Taiwan, and the United States (8). According to a study conducted in Taiwan, China, E. meningoseptica was the third most frequent respiratory pathogen, next to Pseudomonas aeruginosa and the Acinetobacter calcoaceticus–Acinetobacter baumannii (ACB) complex, in a medical Center in southern Taiwan. It was also the fourth most frequent pathogen of carbapenem-resistant bacteremia there (17). With the increasing incidence of E. meningoseptica infections, the importance of being a hospital infection bacterium is increasingly recognized by human beings. However, information regarding this disease's incidence and treatment in the city of Hefei was insufficient. Thus, clinical data for this study was retrospectively collected from the hospital's electronic medical records of 24 patients who had E. meningoseptica infections. In addition, demographic information, clinical and laboratory features, treatment history, and infection outcomes were reported.
Among the 24 E. meningoseptica clinical isolates collected from the First Affiliated Hospital of Anhui Medical University, the major sources were sputum (33.3%) and blood (25.0%), respectively. Due to the high mortality rate, we divided the patients into survival group and death group. In this retrospective analysis, we found that patients over 45 had a mortality rate of 90% compared to those under 45 who had a mortality rate of 10%. Mortality rates varied from 23% to 52% in different studies (22). In our study, 41.6% of patients died, and most of the deaths were elderly patients with impaired immune function. Infections with E. meningoseptica throughout the year were most prevalent in summer and autumn (70.8%). In previous studies, neonatal patients, especially premature infants, had a high risk of E. meningoseptica infection. In this investigation, it was discovered that all isolates, except for one, were from adults, demonstrating the low rate of infectivity rate of this bacterium in children in the province of Anhui (18, 23). Univariate analysis of a prior study revealed that individuals with liver cirrhosis (P = 0.032) had a higher mortality rate (4). The only risk factor for underlying diseases in this study was hepatic disease (P = 0.009). According to our data, hypertension (37.5%) and brain disease (37.5%) were the most prevalent underlying diseases. However, a prior study revealed that diabetes mellitus (25%) and malignancy (36%) were the most prevalent underlying diseases (18). A study found that congestive heart failure (42.1%), chronic lung disease (63.2%), and hypertension (57.9%) were the most prevalent underlying diseases (24). These inconsistent results may be attributable to geographical disparities, variations in the size of the study sample, and changes in the study design. Noteworthy, invasive catheters, such as intravascular catheters or endotracheal intubation, are the significant factors in E. meningoseptica-related infections (25). The outbreak of E. meningoseptica infection was previously reported to be related to environmental pollution. The sources of environmental pollution included contaminated syringes in refrigerators, respiratory equipment, bottles, sink taps, sink drains, tube feeding, arterial catheter flushing fluid, pressure sensors, and disinfectants. However, it has also been reported that inadequate disinfection of nipple storage tanks in baby nurseries led to infant infections (26).
Few investigations have characterized the clinical manifestations of E. meningoseptica infection. Fever (83.3%) was the most prevalent clinical symptom in Hefei City. It was comparable to the 83.8% fever rate recorded by a Taiwan medical Center previously (18). Most patients presented with fever, suggesting infection. Bacterial infection often causes fever, and the clinical microbial culture and identification are needed to distinguish the infections of E. meningoseptica and other bacteria (21, 27). Lack of fever may delay the detection of infection or just indicate a poor general state (18). According to a study conducted in Taiwan, individuals with E. meningoseptic bacteremia had a greater prevalence of primary bacteremia, used fewer antibiotics, and experienced less shock when the condition first manifested than patients who did not have the infection (24). The clinical symptoms of the two groups in this investigation were not significantly different. Pneumonia, sepsis, and meningitis are the three main types of infections caused by E. meningoseptica, but there are rare reports of abdominal infection, endocarditis, eye infection, osteomyelitis, keratitis, urinary tract infection, septic arthritis, and skin or soft tissue infection (28–30). Among the complications in this study, there were 12 cases of pneumonia (50.0%) and 11 cases of hydrothorax (45.8%). Pneumonia was a major complication in patients infected by E. meningoseptica. There were 70% patients with pneumonia in the death group, as well as 35.7% patients with pneumonia in the survival group.
There was very little information available in the literature about the laboratory characteristics of E. meningoseptica, and most previous research only included a limited number of patients. Hypoproteinemia was identified as a risk factor for mortality in patients with E. meningoseptica infection in a prior investigation (22). In univariate analysis, lymphocyte count (P = 0.033), anemia (P = 0.024), and hypoproteinemia (P = 0.024) were the risk factors for death in our study. Specifically, anemia (75%) and hypoproteinemia (75%) were the most significant clinical findings in our investigation, as they were observed in the majority of the patients. As a result, we postulated that measuring albumin (ALB), total protein (TP), and hemoglobin (Hb) levels could aid in the diagnosis of E. meningoseptica infection. Comparable to usual bacterial infections, peripheral blood WBC and neutrophils counts, as well as the biochemical index CRP rose in the majority of E. meningoseptica-infected patients. A study by Arbune et al. (31) showed an infant's laboratory results were leukocytosis with neutrophilia, and anemia. At least one increased liver enzyme, including AST, ALT, ALP, LDH, and GGT, was observed in 24 patients. Consequently, the effect of E. meningoseptica infection on liver function was also worthy of consideration during the progression of the disease.
The Clinical and Laboratory Standards Institute had not developed interpretive breakpoints for E. meningoseptica minimum inhibitory concentrations (MICs) until 2013 (13). Antibiotics, as the main factors influencing the prognosis, are an essential component of the treatment. Elizabethkingia species infections are difficult to cure and have a high case-fatality rate, most likely as a result of their intrinsic antibiotic resistance (32). The bacteria are susceptible to ciprofloxacin, rifampicin, trimethoprim-sulfamethoxazole, and vancomycin, but resistant to β-lactam agents, aminoglycosides, and chloramphenicol (31, 33). In a case report from Sichuan, four isolates were isolated from urine, which were resistant to aminoglycosides (amikacin, tobramycin, and gentamicin), cephalosporins (ceftazidime, cefotaxime), cefoxitin, and aztreonam, and susceptible to trimethoprim-sulfamethoxazole (30). Our observations were consistent with previous findings. In this investigation, all isolates showed resistance to cefazolin, and the majority exhibited resistance to amikacin, aztreonam, imipenem, tobramycin, ceftazidime, cefepime, ceftriaxone, and gentamycin. According to earlier research conducted in Taiwan, the isolates of E. meningoseptica were more susceptible to the antibiotics piperacillin (15%), levofloxacin (30%), and minocycline (60%) (4). Our study showed that the most active antibiotic was cotrimoxazole (87.5%), followed by levofloxacin (75.0%), piperacillin/tazobactam (75.0%), and ciprofloxacin (58.3%). In a hospital in Beijing, 15 of 26 E. meningoseptica strains were resistant to sulfamethoxazole/ trimethoprim. A polymerase chain reaction was utilized to detect the resistance-determining genes for trimethoprim/sulfamethoxazole. Six isolates held the sulI gene and four isolates possessed the sulII gene; however, just one isolate contained the dfrA12 gene (34). In the investigation conducted by Jian et al., all E. meningoseptica isolates were resistant to ciprofloxacin, whereas 44% of them were resistant to levofloxacin. This resistance was mostly mediated by a single nucleotide mutation in the gyrA gene QRDR (35). The difference in drug susceptibility in different studies may be due to the regional drug resistance distribution and the size of the sample base. Chiu et al. (23) reported that vancomycin was successfully used for the treatment of E. meningoseptica infection. And genes related to tetracycline and vancomycin resistance were detected (36). However, in other studies, vancomycin was not suggested to be effective against this microorganism (37). As this was a retrospective analysis, data on vancomycin and minocycline were not available. An important aspect of infection management is the use of antibiotics in a timely manner. In a study, there was a contradiction between the genotype and phenotype of tetracyclines, sulfonamides, and quinolones. There were no quinolone resistance genes found, and 90% of the bacteria were ciprofloxacin-resistant (38). Nevertheless, in our study, 58.3% of strains were susceptible to ciprofloxacin. Twenty-one strains of Elizabethkingia isolated from five hospitals in Hong Kong were susceptible to ciprofloxacin, vancomycin, and cefoperazone-sulbactam (1). The main finding of Chen et al. (39) was that the microbial cure rate of piperacillin/tazobactam combined with methoxyprolin/sulfasalazine or fluoroquinolone seemed to be high, and the mortality rate was relatively low. Fifty-one percent of 100 E. meningoseptica isolates in a hospital in Shanghai had moderate antibacterial activity against sitafloxacin, suggesting that sitafloxacin may be a promising treatment for E. meningoseptica infection (40). In terms of our drug susceptibility results, the medication to consider first should be cotrimoxazole, followed by quinolones and piperacillin/tazobactam.
It is difficult to correctly identify E. meningoseptica by traditional microbiological methods, and it is common to misidentify E. anophelis and E. meningoseptica (6). However, delays in biological identification may lead to treatment failure. Different AST methods (viz., disc diffusion, broth-micro dilution, and E-test) have different drug susceptibility results, which further complicate the treatment. Literature shows that the paper diffusion method is not reliable, the broth-micro dilution method is the preferred method. While the automatic drug susceptibility testing system is more commonly used in clinical laboratories, there are still differences between the two (11, 41, 42). As a pathogen causing nosocomial infections, there is little information about its genome composition and related characteristics in the literature. A document indicated that the genomes of E. meningoseptica isolated from patients carried three β-lactamases. These strains were resistant to β-lactams and cephalosporins, which may be attributed to blaCME; resistance to carbapenems and penicillin–β-lactamase inhibitor combinations may be attributed to blaBlaB and blaGOB, which explains the molecular mechanism of drug resistance (8). A study in Hainan showed that clinical strains of E. meningoseptica had β-lactam, macrolide, tetracycline, quinolone, glycopeptide, and multidrug-resistance efflux pump genes. The clinical isolates contained antibiotic efflux pump genes, cmeB, adeF, and vanB, and glycopeptide resistance gene, vanW, in which vanB glycopeptide resistance gene vanW mutation was observed to be involved in the regulation of teicoplanin resistance (38). A literature in Wuhan showed that 23 and 32 new BlaBlaB and BlaGOB variants were found in Elizabethkingia spp., respectively. Some variations did not aggregate according to the species-specific branching, indicating that the MBL gene may be transmitted across species in Elizabethkingia species (43). In the study of Girdhar et al. (44), they identified 18 unique proteins related to the metabolic pathway of E. meningoseptica from 3,391 annotated proteins, which may be the starting point for drug design and development.
In addition, we used the MALDI-TOF MS database software and constructed the development tree for homology analysis of eight strains of the total 24 E. meningoseptica. The tree diagram based on correlation obtained by MALDI-TOF MS clustered the eight strains in the same group, showing that they had a strong genetic relationship. We found that three strains were highly similar in E. meningoseptica (~87%), suggesting common hospital sources. Eight strains were identified as E. meningoseptica by 16S rRNA gene sequencing, which was the same as the result of MALDI-TOF MS. Since there are few cases of patients collected at present and E. meningoseptica that have not accumulated large data, we will continue to collect cases for analysis in the later stage to provide help for clinical diagnosis and identification.
Various studies have shown that mortality is associated with inappropriate empirical antimicrobial therapy (4, 7, 15, 18). In addition, the tendency to form biofilms poses further challenges to the treatment of patients, especially when organisms have been established on indwelling devices, such as endotracheal tubes (7). In a study from Singapore, the identification of the gene for capsule biosynthesis, capD, and the gene for the AdeFGH efflux pump, adeG, in all Elizabethkingia species leads to the possibility of biofilm formation, which gives the bacteria the ability to remain on varied surfaces (6). The potential of E. meningoseptica to produce biofilm indicates that it has the ability to impede treatment. Numerous investigations involving E. meningoseptica infection or outbreaks caused by flume or faucet drainage pollution have demonstrated that once biofilm is developed, it is difficult to eliminate from the environment (5, 20, 45).
This study has a number of limitations. Because this is a retrospective single-center study, some data may be lost, incomplete, or improperly reported. Furthermore, because of the study's limited sample size, the explanation for our results may not be applicable in larger populations. Despite these limitations, this work offers an important potential for the investigation of E. meningoseptica infection in Hefei. It is also expected that this research would offer guidance and reference for clinicians to timely diagnose and treat E. meningoseptica infection.
Conclusion
In summary, the most common symptom in E. meningoseptica patients was fever, which was followed by altered consciousness, edema, cough, sputum, abdominal distension, chest discomfort, and chilly. The only significant factor of underlying diseases was that hepatic disease (P = 0.009) often occurred in death groups. A majority of patients experienced anemia and hypoproteinemia in the laboratory result. Our findings suggest that hypoproteinemia, unexplained fever, anemia, and lymphocyte count should be considered as E. meningoseptica selected diagnosis references for early treatment intervention. The susceptibility of some quinolones, piperacillin-tazobactam and cotrimoxazole was relatively high, suggesting that they may be the preferred drugs for the treatment of E. meningoseptica infection. Since such bacteria can form biofilms in a humid environment and spread with water or equipment relating to water as a source of transmission, it is necessary to strengthen the hand hygiene compliance of medical staff and control nosocomial infections to avoid the spread and spread of E. meningoseptica infection. This study analyzed 5 years of patient data from Anhui Province and could be used to improve strategies for preventing, diagnosing, and treating E. meningoseptica infections in other provinces and cities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University in accordance with the Declaration of Helsinki (ethical approval number: Quick-PJ 2022-10-31). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL and TLiu analyzed data and wrote original draft. CS, BW, TLi, YH, YX, and LT reviewed and edited the manuscript. YX and YL provided funding. All authors have read and agreed to publish the final manuscript.
Funding
This work was financially supported by Anhui Natural Science Foundation (grant number: 9021138201) and Scientific Research Project of Universities in Anhui Province (grant number: KJ2020A0170).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.964046/full#supplementary-material
References
1. Lau SK, Chow WN, Foo CH, Curreem SO, Lo GC, Teng JL, et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. (2016) 6:26045. doi: 10.1038/srep26045
2. Lin JN, Lai CH, Yang CH, Huang YH. Elizabethkingia infections in humans: from genomics to clinics. Microorganisms. (2019) 7:295. doi: 10.3390/microorganisms7090295
3. Balm MND, Salmon S, Jureen R, Teo C, Mahdi R, Seetoh T, et al. Bad design, bad practices, bad bugs: frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J Hosp Infect. (2013) 85:134–40. doi: 10.1016/j.jhin.2013.05.012
4. Lin J, Lai C, Yang C, Huang Y. Comparison of clinical manifestations, antimicrobial susceptibility patterns, and mutations of fluoroquinolone target genes between Elizabethkingia meningoseptica and Elizabethkingia anophelis isolated in Taiwan. J Clin Med. (2018) 7:538. doi: 10.3390/jcm7120538
5. Alyami AM, Kaabia NM, AlQasim MA, Al DF, Albehlal LB, Ahmed MA, et al. Chryseobacterium/Elizabethkingia species infections in Saudi Arabia. Saudi Med J. (2020) 41:309–13. doi: 10.15537/smj.2020.3.24985
6. Zajmi A, Teo J, Yeo CC. Epidemiology and characteristics of Elizabethkingia spp. infections in Southeast Asia. Microorganisms. (2022) 10:882. doi: 10.3390/microorganisms10050882
7. Jean S, Hsieh T, Ning Y, Hsueh P. Role of vancomycin in the treatment of bacteraemia and meningitis caused by Elizabethkingia meningoseptica. Int J Antimicrob Ag. (2017) 50:507–11. doi: 10.1016/j.ijantimicag.2017.06.021
8. Burnard D, Gore L, Henderson A, Ranasinghe A, Bergh H, Cottrell K, et al. Comparative genomics and antimicrobial resistance profiling of Elizabethkingia isolates reveal nosocomial transmission and in vitro susceptibility to fluoroquinolones, tetracyclines, and trimethoprim-sulfamethoxazole. J Clin Microbiol. (2020) 58:e00730–20. doi: 10.1128/JCM.00730-20
9. Nicholson AC, Gulvik CA, Whitney AM, Humrighouse BW, Graziano J, Emery B, et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. Nov. Antonie van Leeuwenhoek. (2018) 111:55–72. doi: 10.1007/s10482-017-0926-3
10. Chang Y, Lo H, Hsieh H, Chang S. Identification and epidemiological relatedness of clinical Elizabethkingia meningoseptica isolates from central Taiwan. J Microbiol Immunol Infect. (2014) 47:318–23. doi: 10.1016/j.jmii.2013.03.007
11. Govindaswamy A, Bajpai V, Trikha V, Mittal S, Malhotra R, Mathur P. Multidrug resistant Elizabethkingia meningoseptica bacteremia: experience from a level 1 trauma centre in India. Intract Rare Dis Res. (2018) 7:172–6. doi: 10.5582/irdr.2018.01077
12. Cheng YH, Perng CL, Jian MJ, Cheng YH, Lee SY, Sun JR, et al. Multicentre study evaluating matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of clinically isolated Elizabethkingia species and analysis of antimicrobial susceptibility. Clin Microbiol Infect. (2019) 25:340–5. doi: 10.1016/j.cmi.2018.04.015
13. Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing healthcare-associated infections. J Hosp Infect. (2014) 86:244–9. doi: 10.1016/j.jhin.2014.01.009
14. Huang Y, Lin Y, Wang F. Comparison of the therapeutic efficacy of fluoroquinolone and non-fluoroquinolone treatment in patients with Elizabethkingia meningoseptica bacteraemia. Int J Antimicrob Ag. (2018) 51:47–51. doi: 10.1016/j.ijantimicag.2017.05.018
15. Aldoghaim FS, Kaabia N, Alyami AM, Alqasim MA, Ahmed MA, Al Aidaroos A, et al. Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteraemia: a series of 12 cases at Prince Sultan Military Medical City KSA. New Microb New Infect. (2019) 32:100617. doi: 10.1016/j.nmni.2019.100617
16. Moore LSP, Owens DS, Jepson A, Turton JF, Ashworth S, Donaldson H, et al. Waterborne Elizabethkingia meningoseptica in adult critical care1. Emerg Infect Dis. (2016) 22:9–17. doi: 10.3201/eid2201.150139
17. Huang Y, Wu P, Lin Y, Wang F. Comparison of clinical characteristics of bacteremia from Elizabethkingia meningoseptica and other carbapenem-resistant, non-fermenting Gram-negative bacilli at a tertiary medical center. J Microbiol Immunol Infect. (2019) 52:304–11. doi: 10.1016/j.jmii.2018.06.007
18. Hsu MS, Liao CH, Huang YT, Liu CY, Yang CJ, Kao KL, et al. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical center in Taiwan, 1999–2006. Eur J Clin Microbiol. (2011) 30:1271–8. doi: 10.1007/s10096-011-1223-0
19. Raghavan S, Thomas B, Shastry BA. Elizabethkingia meningoseptica: emerging multidrug resistance in a nosocomial pathogen. BMJ Case Rep. (2017) 2017:221076. doi: 10.1136/bcr-2017-221076
20. Umair A, Nasir N. Clinical features and outcomes of critically ill patients with Elizabethkingia meningoseptica: an emerging pathogen. Acute Crit Care. (2021) 36:256–61. doi: 10.4266/acc.2020.01158
21. Barnawi AI, Kordy FN, Almuwallad OK, Kassarah KA. Early neonatal sepsis and meningitis caused byElizabethkingia meningoseptica in Saudi Arabia. Saudi Med J. (2020) 41:753–6. doi: 10.15537/smj.2020.7.25720
22. Pereira GH, Garcia DDO, Abboud CS, Barbosa VLDB, Da Silva PSL. Nosocomial infections caused by Elizabethkingia meningoseptica: an emergent pathogen. Brazil J Infect Dis. (2013) 17:606–9. doi: 10.1016/j.bjid.2013.02.011
23. Chiu CH, Waddingdon M, Greenberg D, Schreckenberger PC, Carnahan AM. Atypical Chryseobacterium meningosepticum and meningitis and sepsis in newborns and the immunocompromised, Taiwan. Emerg Infect Dis. (2000) 6:481–6. doi: 10.3201/eid0605.000506
24. Chen W, Chen Y, Ko H, Yu W, Yang K. Comparisons of clinical features and outcomes between Elizabethkingia meningoseptica and other glucose non-fermenting Gram-negative bacilli bacteremia in adult ICU patients. J Microbiol Immunol Infect. (2020) 53:344–50. doi: 10.1016/j.jmii.2018.08.016
25. Jean SS, Chang YC, Lin WC, Lee WS, Hsueh PR, Hsu CW. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J Clin Med. (2020) 9:275. doi: 10.3390/jcm9010275
26. Tai I, Liu T, Chen Y, Lien R, Lee C, Huang Y. Outbreak of Elizabethkingia meningoseptica sepsis with meningitis in a well-baby nursery. J Hosp Infect. (2017) 96:168–71. doi: 10.1016/j.jhin.2016.11.018
27. Teng L, Wang J, Lu H, Mao Y, Lai K, Tseng C, et al. Elizabethkingia intra-abdominal infection and related trimethoprim-sulfamethoxazole resistance: a clinical-genomic study. Antibiotics. (2021) 10:173. doi: 10.3390/antibiotics10020173
28. Ghafur A, Vidyalakshmi PR, Priyadarshini K, Easow JM, Raj R, Raja T. Elizabethkingia meningoseptica bacteremia in immunocompromised hosts: the first case series from India. South Asian J Cancer. (2020) 02:211–5. doi: 10.4103/2278-330X.119912
29. Abidi S, Tilouche L, Guedri Y, Karaborni S, Ghechir ZB, Ketata S, et al. Elizabethkingia meningoseptica: an uncommon cause of peritonitis in continuous ambulatory peritoneal dialysis: a case report and review of literature. Saudi J Kidney Dis Transpl. (2021) 32:855–60. doi: 10.4103/1319-2442.336783
30. Zong Z. Elizabethkingia meningoseptica as an unusual pathogen causing healthcare-associated bacteriuria. Int Med. (2014) 53:1877–9. doi: 10.2169/internalmedicine.53.2319
31. Arbune M, Fotea S, Nechita A, Stefanescu V. Emerging infection with Elizabethkingia meningoseptica in neonate. A case report. J Crit Care Med. (2018) 4:96–100. doi: 10.2478/jccm-2018-0013
32. Choi MH, Kim M, Jeong SJ, Choi JY, Lee I, Yong T, et al. Risk factors for Elizabethkingia acquisition and clinical characteristics of patients, South Korea. Emerg Infect Dis. (2019) 25:42–51. doi: 10.3201/eid2501.171985
33. Rastogi N, Mathur P, Bindra A, Goyal K, Sokhal N, Kumar S, et al. Infections due to Elizabethkingia meningoseptica in critically injured trauma patients: a 7-years study. J Hosp Infect. (2016) 92:30–2. doi: 10.1016/j.jhin.2015.07.008
34. Jiang X, Wang D, Wang Y, Yan H, Shi L, Zhou L. Occurrence of antimicrobial resistance genes sul and dfrA12 in hospital environmental isolates of Elizabethkingia meningoseptica. World J Microbiol Biotechnol. (2012) 28:3097–102. doi: 10.1007/s11274-012-1119-x
35. Jian M, Cheng Y, Perng C, Shang H. Molecular typing and profiling of topoisomerase mutations causing resistance to ciprofloxacin and levofloxacin in Elizabethkingia species. PeerJ. (2018) 6:e5608. doi: 10.7717/peerj.5608
36. Chen S, Soehnlen M, Blom J, Terrapon N, Henrissat B, Walker ED. Comparative genomic analyses reveal diverse virulence factors and antimicrobial resistance mechanisms in clinical Elizabethkingia meningoseptica strains. PLoS ONE. (2019) 14:e222648. doi: 10.1101/668061
37. Lee S, Tsai C, Lee B. Chryseobacterium meningosepticum sepsis complicated with retroperitoneal hematoma and pleural effusion in a diabetic patient. J Chin Med Assoc. (2008) 71:473–6. doi: 10.1016/S1726-4901(08)70151-5
38. Yang C, Liu Z, Yu S, Ye K, Li X, Shen D. Comparison of three species of Elizabethkingia genus by whole-genome sequence analysis. Fems Microbiol Lett. (2021) 368:fnab018. doi: 10.1093/femsle/fnab018
39. Chan JC, Chong CY, Thoon KC, Tee NWS, Maiwald M, Lam JCM, et al. Invasive paediatric Elizabethkingia meningoseptica infections are best treated with a combination of piperacillin/tazobactam and trimethoprim/sulfamethoxazole or fluoroquinolone. J Med Microbiol. (2019) 68:1167–72. doi: 10.1099/jmm.0.001021
40. Zhou X, Wang L, Wu S, Yang Y, Guo Y, Shen S, et al. Antimicrobial activities of sitafloxacin and comparators against the clinical isolates of less common nonfermenting gram-negative bacteria. J Glob Antimicrob Res. (2022) 30:123–6. doi: 10.1016/j.jgar.2022.06.003
41. Kuo S, Tan M, Huang W, Wu H, Chen F, Liao Y, et al. Susceptibility of Elizabethkingia spp. to commonly tested and novel antibiotics and concordance between broth microdilution and automated testing methods. J Antimicrob Chemoth. (2021) 76:653–8. doi: 10.1093/jac/dkaa499
42. Patro P, Das P, Padhi P. Intrinsically resistant bacteria as looming disaster: a rare case report of Elizabethkingia meningoseptica meningitis in a neonate. J Lab Phys. (2021) 13:70–3. doi: 10.1055/s-0041-1724234
43. Hu R, Zhang Q, Gu Z. Molecular diversity of chromosomal metallo-β-lactamase genes in Elizabethkingia genus. Int J Antimicrob Ag. (2020) 56:105978. doi: 10.1016/j.ijantimicag.2020.105978
44. Girdhar N, Kumari N, Krishnamachari A. Computational characterization and analysis of molecular sequence data of Elizabethkingia meningoseptica. BMC Res Notes. (2022) 15:1–8. doi: 10.1186/s13104-022-06011-5
Keywords: Elizabethkingia meningoseptica, infection, clinical and laboratory features, fever, hepatic disease
Citation: Li Y, Liu T, Shi C, Wang B, Li T, Huang Y, Xu Y and Tang L (2022) Epidemiological, clinical, and laboratory features of patients infected with Elizabethkingia meningoseptica at a tertiary hospital in Hefei City, China. Front. Public Health 10:964046. doi: 10.3389/fpubh.2022.964046
Received: 09 June 2022; Accepted: 22 August 2022;
Published: 20 September 2022.
Edited by:
Jozsef Soki, University of Szeged, HungaryReviewed by:
Guiqin Sun, Zhejiang Chinese Medical University, ChinaEdward D. Walker, Michigan State University, United States
Copyright © 2022 Li, Liu, Shi, Wang, Li, Huang, Xu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanhong Xu, eHlob25nMTk2NEAxNjMuY29t; Ling Tang, dGxpbmdAbWFpbC51c3RjLmVkdS5jbg==
†These authors have contributed equally to this work
 Yajuan Li
Yajuan Li Tingting Liu
Tingting Liu Cuixiao Shi
Cuixiao Shi Bo Wang
Bo Wang Tingting Li
Tingting Li Ying Huang
Ying Huang Yuanhong Xu
Yuanhong Xu Ling Tang
Ling Tang