- 1Department of Pulmonary Diseases, Yancheng Traditional Chinese Medicine Hospital, Yancheng, China
- 2Department of Gastroenterology, Yancheng Third People's Hospital, Yancheng, China
- 3School of Medicine, Affiliated Yancheng Hospital, Southeast University, Yancheng, China
- 4Zhejiang University School of Medicine, Hangzhou, China
- 5Chu Kochen Honors College of Zhejiang University, Hangzhou, China
Eosinophils are differentiated by bone marrow multipotent progenitor cells and are further released into peripheral blood after maturation. Human eosinophils can exhibit unique multi-leaf nuclear morphology, which are filled with cytoplasmic granules that contain cytotoxicity and immune regulatory proteins. In recent years, many studies focused on the origin, differentiation and development process of eosinophils. It has been discovered that the eosinophils have the regulatory functions of innate and adaptive immunity, and can also function in several diseases, including asthma, chronic obstructive pulmonary diseases, acute respiratory distress syndrome, malignant tumors and so on. Hence, the role and effects of eosinophils in various diseases are emphasized. In this review, we comprehensively summarized the development and differentiation process of eosinophils, the research progress of their related cytokines, diseases and current clinical treatment options, and discussed the potential drug target, aiming to provide a theoretical and practical basis for the clinical prevention and treatment of eosinophil-related diseases, especially respiratory diseases. To conclude, the guiding significance of future disease treatment is proposed based on the recent updated understandings into the cell functions of eosinophils.
Introduction
Eosinophils are differentiated by bone marrow multipotent progenitor cells and are further released into peripheral blood after maturation under the actions of interleukin-5 (IL-5) and interleukin-33 (IL-33). After a short stay in peripheral blood, eosinophils migrate to the lungs, thymus and gastrointestinal tract (1, 2).
During the occurrence and development of related diseases, eosinophils are recruited to the disease lesions and exert their cellular functions under the influence of the local microenvironment. It has been reported that eosinophils play an effective role in allergic diseases and anti-parasitic infections (3). Recently, studies have explored inflammatory cytokines and biomarkers in several diseases (4, 5). The further discovery of different eosinophil subtypes and related cytokines and media has enriched the cognition of their cellular functions, including anti-tumor effects, regulation of hematopoietic stem cell homeostasis (6, 7).
In this review, we summarized the development and differentiation process of eosinophils, the research progress of their related cytokines, diseases and current clinical treatment options, and discussed the potential drug target, aiming to provide a theoretical and practical basis for the clinical prevention and treatment of eosinophil-related diseases, especially respiratory diseases.
Origin and differentiation of eosinophils
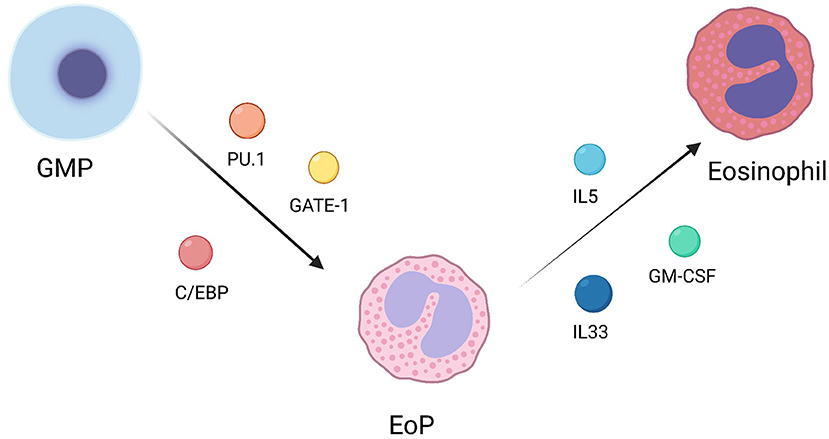
Eosinophils, along with neutrophils and basophils, are major members of granulocytes. Granulocyte/monocyte progenitor (GMP) is their common progenitor. GMP could differentiate into eosinophile lineage-committed progenitor (EoP) under the regulation of transcription factors, including PU.1, C/EBP, and GATA-1. Later, EoP continues to differentiate into mature eosinophils under the regulation of GM-CSF, IL-5 and IL-33 (Figure 1) (8). In addition, it has been reported that some regulatory factors could effectively regulate eosinophil differentiation. For instance, eosinophil differentiation can be inhibited by rapamycin through a pathway independent of the IL-5 signaling pathway in mice (9). Xia et al. demonstrated that protein tyrosine phosphatase SHP2 could regulate IL-5 levels through Erk signaling pathway to regulate the differentiation of eosinophils (10). Exogenous interleukin-17A (IL-17A) could also inhibit the differentiation of eosinophils (11).
Eosinophil-related molecules
Particulate proteins from eosinophil-derived granules
Eosinophil-derived granule proteins (EDGPS) mainly consist of main basic protein (MBP), eosinophil cation protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophilic peroxidase (EPO). MBP is located at the center of the granules and exists in the form of two homologous proteins, MBP-1 and MBP-2. MBP is small in size and consists of a single chain of 117 amino acid residues, which is highly alkaline that is toxic to both parasites and bacteria (12). ECP and EDN are initially found to be acidophilic granulocyte-related RNA enzymes that are associated with neurotoxicity. They can exhibit RNA hydrolyze capabilities, and their homologous gene sequences only exist in primate genomes (13). ECP is a single-stranded cation protein that is homologous to lychee RNA, which exhibit toxicity to worms and can bind to bacterial cell wall components such as lipid polysaccharides (14). In addition, ECP can also affect the proliferation of T lymphocytes and B lymphocytes, thereby promoting the degranulation of mast cells, and regulate the classical activation pathway of complements (15). EDN is a single-stranded peptide with antiviral properties and the ability to degrade single-stranded RNA, which can also serve as a biomarker for the activation and degranulation of eosinophils in patients with asthma (16, 17). EPO is a hemoglobin-containing halogen peroxidase that catalyzes the reactions of halides present in plasma and pseudohalides, which promote asthma-related characteristic phenotypes through post-translational modification of proteins in the airway of asthma using carbonylation (6, 18).
Cytokines associated with eosinophils
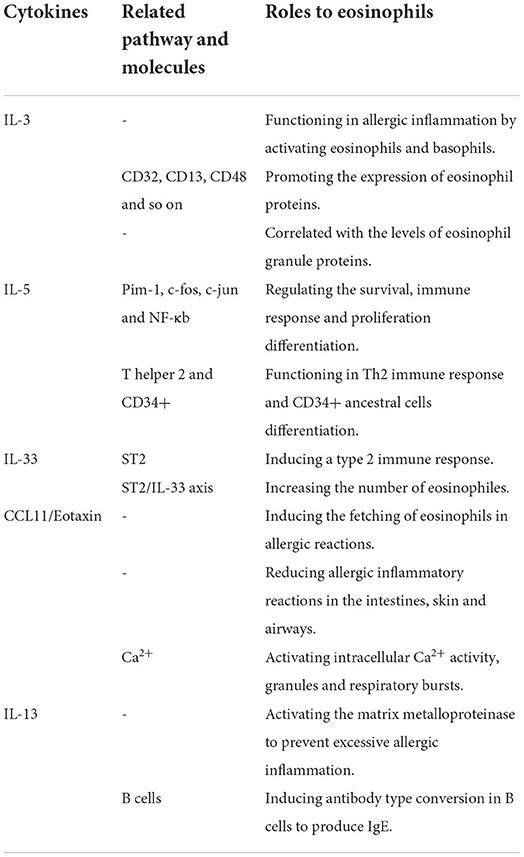
Eosinophils can synthesize, store and secrete various cytokines. In eosinophils, many cytokines, chemokines and growth factors are stored preformed in particles, ready for immediate release, unlike other granulocytes. Specific particles are rich in cytokines, including IL-2, IL-3, IL-4, IL-5, IL-6, IL-13, IL-33, interferon-γ (IFN-γ), GM-CSF, and tumor necrosis factor-α (TNF-α). Convergence factors include RANTES, Eotaxin, and MIP-1 alpha, while growth factors include stem cell factors and transforming growth factors (TGF) (Table 1).
IL-3
IL-3 can promote the production of not only eosinophils, basophils and mast cells, but also granulocytes, monocytes, and macrophages. For the ability to drive the entire myelopoiesis spectrum, IL-3 was firstly known as multi-CSF (19). T cells and mast cells are the main cellular sources of IL-3 (20). IL-3 takes a great important part in allergic inflammation by activating eosinophils and basophils (21). Eosinophils are strongly correlated with IL-3, it is known that IL-3 and GM-CSF play essential roles in the early stage of eosinophil differentiation, and IL-5 plays a role in the end stage of eosinophil maturation (22, 23). Compared to IL-5 and GM-CSF, several studies have shown that IL-3 is more able to promote the expression of eosinophil proteins like CD32, CD13, CD48 and so on (24). In addition, a research has demonstrated that the amounts of sputum IL-3 are strongly correlated with levels of eosinophil granule proteins, and decreased lung function (25).
IL-5
IL-5 is an important cytokine and has exhibit many physiological functions, including prolongation of survival, induction, activation, and degranulation of eosinophils. Eosinophils could express IL-5Rα at high level on their surface and release a large amount of IL-5 (26). When IL-5 is engaged with IL-5R, a range of proteins are induced phosphorylation, which further activating Pim-1, c-fos, c-jun and NF-κb that could regulate the survival, immune response, and proliferation differentiation of eosinophils (27–29). However, it has been shown that a systemic increase in IL-5 does not necessarily lead to pathological conditions that are mediated by eosinophils (30). Besides, IL-5 plays an important role in the development of the Th2 immune response and is essential for the differentiation of CD34+ ancestral cells into eosinophils (31, 32).
IL-33
IL-33 belongs to the IL-1 cytokine family and is a ligand of the transmembrane protein ST2 encoded by the IL-1rl1 gene (33). In a stable state, IL-33 is located in nuclei and is associated with chromoplast by chromoplast binding matrix sequence, which promotes cell stability by serving as a transcription inhibitor (34). Since IL-33 does not contain a signal sequence, the secretion manner of IL-33 is different from conventional cytokines (35). After mechanical damage, necrosis cell death, and the activation of cells through the ATP signaling pathway without cell death, IL-33 would be released into extracellular space (36, 37). By activating immune cells that express ST2 in the mucosa organs throughout the body, IL-33 could induce a type 2 immune response, which promotes the growth of blood and tissue eosinophils by IL-5-mediated pathway (33, 38–41). Johnston et al. revealed that the ST2/IL33 axis is the best way for proliferation of eosinophils, and this can be achieved by raising the surface IL-5Rα of eosinophils (41). IL-33 expression is closely related to the increase of peripheral hemophilic granulocytes. Smith et al. demonstrated that IL-33 gene sequence led to a decrease of IL-33 protein levels. In addition, a decrease in the number of peripheral blood eosinophils in mice was also observed (42).
CCL11/Eotaxin
CCL11/Eotaxin is an important acidophil-specific chemokine, which is involved in the chemotaxis of eosinophils to tissue, and could serve as an efficient activator inducing the fetching of eosinophils in allergic reactions (43). The expression of CCL11/Eotaxin in eosinophils is associated with intracellular particles (44). knocking out the CCL11/Eotaxin gene could significantly reduce the accumulation of eosinophils in tissues, thus reducing allergic inflammatory reactions in the intestines, skin, and airways (45). Similar to CCL5/RANTES, CCL11/Eotaxin activates intracellular Ca2+ activity, granules, and respiratory bursts in eosinophils, indicating that it processed in a self-secreting manner (46, 47).
IL-13
IL-13 is another important inflammatory factor released by eosinophils, which is stored in particle crystallization as a pre-formed medium. IL-13 has an impact on the development of asthma airway disease and pulmonary fibrosis, and it could also activate the matrix metalloproteinase in the airways to prevent excessive allergic inflammation (48). IL-13 can also induce antibody type conversion in B cells to produce IgE, which plays an important role in allergic inflammation (49, 50). In addition, parasites such as worms are also dependent on IL-13 for intestinal discharge from mice (51).
Eosinophil-related diseases
Asthma
Asthma is a common chronic airway inflammatory disease, in which allergen-induced asthma occupies the majority. The pathological characteristics of asthma are the accumulation and activation of eosinophils in the airways. The clinical characteristics mainly include chronic inflammation, reversible airflow restriction, high mucus secretion, high bronchial reactive accompanied by cough, sputum, wheezing, and other clinical symptoms (52). It was found that the levels of eosinophils in peripheral blood in asthma patients are closely related to the severity of asthma (53, 54).
Elevated levels of eosinophils in peripheral blood commonly represent severe asthma, also known as T helper 2 (Th2) asthma (55). Since the granulocyte proteins of eosinophils could coordinate the immune response to worms in the Th2 cytokine cascade response, eosinophils are primarily related to parasitic infection (56). The cascade starts with the reaction of IgE and antigens. Although antigens could pose a threat to the host in worm infection, the targets of IgE in patients with asthma are relatively harmless, such as tree pollen and animal fur. However, IgE can activate hypertrophic cells, macrophages, and basophils, which in turn leads to the production of histamines and other inflammatory cytokines secretion. The airway inflammatory microenvironment of asthma has a strong chemoattraction to mature CD4+T cells and eosinophils, which leads to severe type Th2 asthma and is accompanied with high levels of eosinophils in blood and sputum (57). In recent years, it was also found that patients with classic asthma, cough-mutant asthma, and chest tightness variant asthma usually have the common clinical feature of eosinophilic airway inflammation. The recruitment of mature eosinophils from peripheral circulation may be the main mechanism for the growth of pulmonary eosinophils (58). Additionally, circulating progenitor cells were also proved to accumulate in the inflammatory site and differentiate into mature immune cells to promote tissue inflammation (59–61).
Acute lung injury and acute respiratory distress syndrome
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are characterized by increased pulmonary vascular permeability, severe inflammation, secondary pulmonary edema, and refraction of hypoxia (62, 63). During the progression of ALI and ARDS, the accumulation of inflammatory cells and cytokines leads to the destruction of the capillary endothelium and alveolar epithelial barrier, which subsequently promote the development of pulmonary edema and hypoxia (64). Willetts et al. concluded that patients with a good prognosis of ALI have an increased number of eosinophils in the lung compared to patients with a poor prognosis (65). Zhu et al. revealed that early-induced and period-stable eosinophils of ALI are located in the lung parenchyma, and these eosinophils recruited from peripheral blood are derived from the bone marrow. Notably, the study found that CD101 can serve as a marker for distinguishing between different eosinophil subtypes in lipopolysaccharide (LPS)-induced ALI animal models. And that under normal circumstances, CD101-negative eosinophils are predominant. In addition, CD101-negative and CD101-positive eosinophils exert different cellular effects on the inflammatory response. CD101-negative eosinophils could reduce LPS-induced early white blood cell aggregation and cytokine production, while CD101-positive eosinophils may increase LPS-induced early white blood cell aggregation and cytokine production (66).
Eosinophil pneumonia
Eosinophil pneumonia can be divided into acute and chronic eosinophil pneumonia. Acute eosinophilic pneumonia (AEP) is commonly considered to be secondary to allergic reactions to irritants and drugs, which is rarely thought to be caused by parasitic infection (67, 68). Patients with AEP can recover on their own without external intervention, and thus eliminating adverse environmental exposure is the main treatment (68). The distinctive feature of chronic eosinophilic pneumonia (CEP) is eosinophils infiltration into the alveolar cavity and pulmonary interstitium.
Tumor occurrence and metastasis
The relationship between eosinophils and tumors can be traced back to 1893 (69). An increase in tumor-related eosinophils was found in tumor tissues. Several studies showed an increase in tumor-associated tissue eosinophilia (TATE), mainly in colon tumors, esophageal squamous cell carcinoma, nasopharyngeal cancer, penile cancer, laryngeal cancer, lung adenocarcinoma, bladder cancer, and prostate cancer (70–74). Eosinophils are associated with necrotic regions, and there is evidence that eosinophils have cytotoxic effects on tumor cells both in vivo and in vitro. Through the observation of granule protein near the tumor, Caruso et al. demonstrated that eosinophils produce anti-tumor cytotoxic reactions through degranulation, but the tumor-killing mechanism of eosinophils remains unclear. A mouse tumor model with increased peripheral eosinophils was accompanied with inhibition of tumor development (75). In contrast, mice lacking eosinophils showed increased tumor progression, which was associated with a decrease in the number of tumor eosinophils. In humans, an increase in the number of eosinophils is often observed after immunotherapy such as IL-2, IL-4, GM-CSF, and tumor vaccine (76–80).
Therapeutic drugs targeting eosinophils
Glucocorticoids
Glucocorticoids (GC) are important therapeutic drugs for acidophil-related diseases such as allergies, asthma, acidophil gastrointestinal diseases (81, 82). Glucocorticoid receptors (GR) come from the NR3C1-nuclear receptor sub-family 3, and the GC diffuses through the cell membrane, enters the cell, and binds to its receptor GR to induce the activation of the GR signaling pathway. Once activated, GR is transferred to the nucleus and interacts with transcription factors to inhibit the expression of inflammatory genes and enhance the expression of anti-inflammatory genes (83, 84). GR mainly exists in cells in the airways in the form of GRα. The existence of GR explains the presence of GC in the airways of most asthma patients, as well as the significant effects of GC on inflammatory cells and therapeutic effects on asthma (85, 86). Glucocorticoids could promote the clearance of eosinophils by directly inducing apoptosis and suppressing survival signals of eosinophils via cytokines such as IL-3, IL-5, and GM-CSF (87, 88). Of note, Shen et al. demonstrated that corticosteroids not only play an anti-inflammatory role by regulating the release of IL-5 and pulmonary eosinophils but also inhibit bone marrow eosinophil production (89). IL-5 in eosinophils was also proved to protect against glucocorticoid-induced apoptosis (90). Additionally, Wu et al. concluded that GC can also act in synergy with iron death inducers to induce the death of eosinophils in airway inflammation (91).
Eosinophil regulation mediated by cytokines
Monoclonal antibodies targeting IL-5 and IL-5R
IL-5 is considered to be a key regulator of disease-related eosinophils and has an impact on the developmental stages of multiple eosinophil lineages (92). The specific receptor of IL-5, IL-5R is expressed at all developmental stages of the eosinophil lineage. Therefore, IL-5 affects all stages of eosinophil maturation, from the proliferation and differentiation of EoP to survival and activation of mature eosinophils (93, 94).
Several studies showed that treatment targeting IL-5 could significantly reduce levels of mature eosinophils, but have little effect on the levels of EoP (95, 96). To date, two anti-IL5 drugs, meperizumab and relizumab have been approved by FDA. The two monoclonal antibodies are recombinant humanized monoclonal antibodies that could inhibit the binding of IL-5 and IL-5Rα. Biologics targeting IL-5 is designed to reduce the survival of acidophils in tissues. Although the two monoclonal antibodies are effective in reducing peripheral eosinophils, they are less effective in eliminating tissue eosinophils (97, 98). Because of the decrease in eosinophils, mepolizumab and reslizumab improve the symptoms of severe asthma, they provide significant and clinically relevant improvements in exacerbation rate and oral corticosteroid (OCS) reduction, thus greatly improve the clinical treatment of asthma (99).
For IL-5Rα, FDA has approved a biologic agent targeting IL-5 receptors called benazizumab. Benazizumab is also a humanized monoclonal antibody that could reduce eosinophils through a cell-mediated cytotoxic pathway (100). Therefore, benazizumab can directly kill eosinophils, which was shown to significantly inhibit the increase of eosinophils in tissue and thereby improve clinical manifestations (101, 102).
Intriguingly,Both meperizumab and benralizumab can significantly reduce the counts of peripheral eosinophils in eosinophilic asthma, a recent study has demonstrated that compared to meperizumab, benralizumab is able to decrease peripheral eosinophil counts in more patients (103).
IL-33/ST2 axis regulator
IL-33 plays an important role in airway diseases (37, 39). The levels of IL-33 are closely associated with the severity of asthma (104, 105). The regulation of the IL-33/ST2 axis is a representative treatment strategy for immune disorders associated with cytokine signaling disorders. In the past two decades, the treatment strategies blocking the IL-33/ST2 axis have been widely used in animal model diseases. Blocking the IL33/ST2 axis is protective in allergic diseases, especially in the respiratory system. There are three main therapeutic strategies for directly blocking the binding of IL-33 to ST2, including IL-33 neutralizing antibodies, soluble decoy receptors, and anti-ST2 receptor antibodies.
Several neutralizing antibodies against IL-33 have been developed, which have been used in clinical trials for the treatment of allergic diseases. Soluble receptor antagonists were also developed to bind free IL-33. So far, at least two IL-33 bait receptors have been developed, including a form of soluble ST2 (sST2) and the fusion protein IL-33 Trap, formed by sST2 and the secondary protein IL-1RAcP. Anti-ST2 could be used to treat chronic obstructive pneumonia (106). In addition to blocking the IL-33/ST2 signaling pathway, activating the IL-33 signaling pathway by recombinant IL-33 recombinant also has certain therapeutic effects in some disease models (107).
Current drug development directions and potential targets
For eosinophil-related diseases, targeted therapies have gradually become a hot topic. Many drugs are in the process of research and put into clinical trials.
A recent study showed that exogenous IL-17A can significantly reduce ovalbumin-induced allergic inflammation. It was found that the down-regulated expression of CC chemokine receptors 3 (CCR3), GATA binding proteins 1 (GATA-1), and GATA binding proteins 2 (GATA-2) could inhibit the differentiation of eosinophils both in vivo and in vitro, suggesting that exogenous IL-17 is most likely to prevent allergic airway inflammation by inhibiting eosinophil differentiation in the bone marrow (11). This study highlights the importance of targeted inhibition of eosinophil differentiation in allergic inflammation, which may be a new therapeutic target for asthma.
It was also found that the elevated levels of Bcl-2 protein are responsible for the persistence of eosinophils and neutrophils during allergic airway inflammation Furthermore, the Bcl-2 inhibitor ABT-737 and ABT-199 may inhibit allergic airway inflammation by promoting the death of inflammatory cells, especially in asthma dominated by neutrophils and insensitive to corticosteroids (108). Notably, nano Bcl-2 inhibitor, Nf-ABT-199 could deliver ABT-199 specifically to mitochondria of bronchitis cells, which was proved to significantly reduce airway inflammation and inhibit inflammatory cell infiltration and mucus excessive secretion by effectively inducing eosinophil apoptosis. In addition, Nf-ABT-199 had no significant effect on cell vitality, airway epithelial barrier, and liver function, indicating non-toxicity and good biocompatibility (109).
Iron death inducers were explored to induce iron death of eosinophils in mice, thereby alleviating eosinophils airway inflammation. Interestingly, iron death inducers could act in synergy with dexamethasone to induce the death of eosinophils (91). The synergistic effect of iron death inducers and glucocorticoids has advantages in the treatment of allergic diseases, showing that iron death inducer may be a promising treatment strategy for acidophilic airway inflammation.
Moreover, CCL6 expression was significantly increased in asthma patients. The OVA model of asthma was constructed using the CCL6 knockout mouse model, which significantly reduced airway inflammation in mice. The CCL6-CCR1 axis was involved in the process of differentiation from HSC to eosinophils, and the use of specific CCR1 antagonist BX471 can significantly inhibit eosinophil differentiation in both in vitro and in vivo experiments (110). In addition, there exists evidence that mCCL6 could activate CCR1 downstream of the Gαi protein and related phosphorylated signaling proteins, thereby promoting HSC differentiation, which provides sufficient evidence for the involvement of the CCL6-CCR1 axis. Recently, several CCR1 antagonists have been developed in the context of inflammatory diseases, which have shown potential therapeutic effects in clinical trials.
Conclusions and prospects
Nowadays, research on the origin, differentiation and development process of eosinophils has been relatively mature. The role and effects of eosinophils in various diseases have been explored. Eosinophils play an important pathophysiological role in the body due to its unique degranulation and related secreted cytokines, and when it functions, it can cause the body to produce corresponding symptoms. In recent years, the research on different cytokines in diseases has made great progress (111, 112).
The diseases most associated with eosinophils include asthma, acute lung injury/acute respiratory distress syndrome, eosinophil pneumonia, parasitic infection and esophagitis. Due to the universality of respiratory diseases, more attention is paid to the exploration of respiratory diseases and the development of corresponding drugs in clinical practice. In terms of drugs for controlling eosinophils, they could be divided into traditional glucocorticoids and cytokine regulators. Glucocorticoids focus on inducing eosinophil apoptosis, while cytokine regulators focus on blocking and interference of classical cytokines and related pathways. Most future drug development directions and targets related to eosinophils are derived from the abnormally expressed cytokines and proteins in eosinophil-related diseases, and also from the study of cell death mechanisms such as iron death.
Generally speaking, in order to develop more eosinophil-related drugs in the future, the first step is to deepen the research on the origin, development and differentiation of eosinophils. Combined with the development of single cell sequencing technology, the upstream progenitor cells of eosinophils can be grouped to further explore the origin of eosinophils. Secondly, we should study the cytokines related to eosinophils more extensively, and more research on cytokines can better provide a wide range of targets for eosinophils. Furthermore, it is also a key point to promote the research of eosinophil-related drugs by further accelerating clinical trials and allowing more drugs to show their curative effects and give feedback as soon as possible, so as to provide reference value and development direction for more similar drugs.
Author contributions
ZT and HZ had the idea for the article. JZ and ZH performed the literature search and data analysis. ZX and TH drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Steinbach KH, Schick P, Trepel F, Raffler H, DHrmann J, Heilgeist G, et al. Estimation of kinetic parameters of neutrophilic, eosinophilic, and basophilic granulocytes in human blood. Blut. (1979) 39:27–38. doi: 10.1007/BF01008072
2. Lamous'E-Smith ESN, Furuta GT. Eosinophils in the gastrointestinal tract. Curr Gastroenterol Rep. (2006) 8:390–395. doi: 10.1007/s11894-006-0024-6
3. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. (2012) 18:716–25. doi: 10.1038/nm.2678
4. Wu J, Lu WY, Cui LL. Clinical significance of STAT3 and MAPK phosphorylation, and the protein expression of cyclin D1 in skin squamous cell carcinoma tissues. Mol Med Rep. (2015) 12:8129–34. doi: 10.3892/mmr.2015.4460
5. Wang L, Wu J, Song S, Chen H, Hu Y, Xu B, et al. Plasma exosome-derived sentrin SUMO-specific protease 1: a prognostic biomarker in patients with osteosarcoma. Front Oncol. (2021) 11:296. doi: 10.3389/fonc.2021.625109
6. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: Biological properties and role in health and disease. Clinical and Experimental Allergy. (2008) 38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x
7. Zhang C, Yi W, Li F, Du X, Wang H, Wu P, et al. Eosinophil-derived CCL-6 impairs hematopoietic stem cell homeostasis. Cell Res. (2018) 28:323–35. doi: 10.1038/cr.2018.2
8. Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage - committed progenitors in the murine bone marrow. J Exp Med. (2005) 201:1891–7. doi: 10.1084/jem.20050548
9. Hua W, Liu H, Xia L-X, Tian B-P, Huang H-Q, Chen Z-Y, et al. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology. (2015) 20:1055–65. doi: 10.1111/resp.12554
10. Xia Lx, Hua W, Jin Y, Tian Bp, Qiu Zw, Zhang C, et al. Eosinophil differentiation in the bone marrow is promoted by protein tyrosine phosphatase SHP2. Cell Death Dis. (2016). 7. doi: 10.1038/cddis.2016.74
11. Tian B-p, Hua W, Xia L-x, Jin Y, Lan F, Lee JJ, et al. Exogenous interleukin-17A inhibits eosinophil differentiation alleviates allergic airway inflammation. Am J Respirat Cell Molecular Biol. (2015). 52(4):459-470. doi: 10.1165/rcmb.2014-0097OC
12. Barker RL, Gleich GJ, Pease LR. Acidic precursor revealed in human eosinophil granule major basic-protein CDNA. J Exp Med. (1988) 168:1493–8. doi: 10.1084/jem.168.4.1493
13. Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukocyte Biol. (2008) 83:1079–87. doi: 10.1189/jlb.1107725
14. Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein - a clue to the function of the eosinophil granulocyte. Respir Res. (2011) 14:12. doi: 10.1186/1465-9921-12-10
15. Rosenberg HF. The eosinophil ribonucleases. Cell Mol Life Sci. (1998) 54:795–803. doi: 10.1007/s000180050208
16. Rosenberg HF, Domachowske JB. Eosinophil-derived neurotoxin. In: Nicholson AW, editor. Ribonucleases. (2001). p. 273–286. doi: 10.1016/S0076-6879(01)41158-X
17. Rosenberg HF. Eosinophil-derived neurotoxin (EDN/RNase 2) and the mouse eosinophil-associated rnases (mears): expanding roles in promoting host defense. Int J Mol Sci. (2015) 16:15442–55. doi: 10.3390/ijms160715442
18. Wang Z, DiDonato JA, Buffa J, Comhair SA, Aronica MA, Dweik RA, et al. Eosinophil peroxidase catalyzed protein carbamylation participates in asthma. J Biol Chem. (2016) 291:22118–35. doi: 10.1074/jbc.M116.750034
19. Dougan M, Dranoff G, Dougan SK. IGM-CSF, L-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. (2019) 50:796–811. doi: 10.1016/j.immuni.2019.03.022
20. Stoeckle C, Simon HU. CD8+ T cells producing IL-3 and IL-5 in non-I g E-mediated eosinophilic diseases. Allergy. (2013) 68:1622–5. doi: 10.1111/all.12311
21. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. (2013) 132:72–80. e12. doi: 10.1016/j.jaci.2013.03.044
22. Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, et al. CD34+/interleukin-5R α messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. (1999) 20:9–13. doi: 10.1165/ajrcmb.20.1.3449
23. Panousis C, Dhagat U, Edwards KM, Rayzman V, Hardy MP, Braley H, et al. CSL311, a Novel, Potent, Therapeutic Monoclonal Antibody For The Treatment Of Diseases Mediated by the Common β Chain of the IL-3, GM-CSF and IL-5 Receptors. MAbs;. Taylor & Francis. p. 436–453. (2016). doi: 10.1080/19420862.2015.1119352
24. Esnault S, Kelly EA, Johansson MW, Liu LY, Han S-T, Akhtar M, et al. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clini Immunol. (2014) 150:90–100. doi: 10.1016/j.clim.2013.11.009
25. Koller DY, Wojnarowski C, Herkner KR, Weinländer G, Raderer M, Eichler I, et al. High levels of eosinophil cationic protein in wheezing infants predict the development of asthma. J Allergy Clin Immunol. (1997) 99:752–6. doi: 10.1016/S0091-6749(97)80007-3
26. Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. (2016) 16:186–200. doi: 10.1097/ACI.0000000000000251
27. Kouro T, Kikuchi Y, Kanazawa H, Hirokawa K, Harada N, Shiiba M, et al. Critical proline residues of the cytoplasmic domain of the IL-5 receptor alpha chain and its function in IL-5-mediated activation of JAK kinase and STAT5. Int Immunol. (1996) 8:237–45. doi: 10.1093/intimm/8.2.237
28. Pazdrak K, Adachi T, Alam R. Src homology 2 protein tyrosine phosphatase (SHPTP2) Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival. J Exp Med. (1997) 186:561–8. doi: 10.1084/jem.186.4.561
29. Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, et al. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SHS-containing proteins and activation of bruton tyrosine and janus-2 kinases. J Exp Med. (1994) 180:2101–11. doi: 10.1084/jem.180.6.2101
30. Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin-5. J Exp Med. (1990) 172:1425–31. doi: 10.1084/jem.172.5.1425
31. Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, et al. Purified interleukin-5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. (1988) 167:43-56. doi: 10.1084/jem.167.1.43
32. Shalit M, Sekhsaria S, Malech HL. Modulation of growth and differentiation of eosinophils from human peripheral-blood. cd34(+) cells by il5 and other growth-factors. Cell Immunol. (1995) 160:50–7. doi: 10.1016/0008-8749(95)80008-7
33. Schmitz J, Owyang A, Oldham E, Song YL, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23:479–90. doi: 10.1016/j.immuni.2005.09.015
34. Carriere V, Roussel L, Ortega N, Lacorre D-A, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci. U.S.A. (2007) 104:282–7. doi: 10.1073/pnas.0606854104
35. Keller M, Rueegg A, Werner S, Beer H-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. (2008) 132:818–31. doi: 10.1016/j.cell.2007.12.040
36. Cayrol C, Girard J-P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci. U.S.A. (2009) 106:9021–6. doi: 10.1073/pnas.0812690106
37. Cayrol C, Girard J-P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. (2014) 31:31–7. doi: 10.1016/j.coi.2014.09.004
38. Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Reviews Immunol. (2016) 16:676–89. doi: 10.1038/nri.2016.95
39. Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity. (2015) 42:1005–19. doi: 10.1016/j.immuni.2015.06.006
40. Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. (2016) 71:977–88. doi: 10.1111/all.12861
41. Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, et al. IL-33 Precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. (2016) 197:3445–53. doi: 10.4049/jimmunol.1600611
42. Smith D, Helgason H, Sulem P, Bjornsdottir US, Lim AC, Sveinbjornsson G, et al. A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. Plos Genetics. (2017) 13. doi: 10.1371/journal.pgen.1006659
43. Jose PJ, Griffithsjohnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. Eotaxin - a potent eosinophil chemoattractant cytokine detected in a guinea-pig model of allergic airways inflammation. J Exp Med. (1994) 179:881–7. doi: 10.1084/jem.179.3.881
44. Yokoyama A, Kohno N, Ito M, Abe M, Hiwada K, Yamada H, et al. Eotaxin levels in pleural effusions: comparison with monocyte chemoattractant protein-1 and IL-8. Internal Medicine. (2000) 39:547–52. doi: 10.2169/internalmedicine.39.547
45. Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. (1997) 185:785–90. doi: 10.1084/jem.185.4.785
46. Elsner J, Hochstetter R, Kimmig D, Kapp A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur J Immunol. (1996) 26:1919–25. doi: 10.1002/eji.1830260837
47. El-Shazly A, Masuyama K, Nakano K, Eura M, Samejima Y, Ishikawa T. Human eotaxin induces eosinophil-derived neurotoxin release from normal human eosinophils. Int Arch Allergy Immunol. (1998) 117:55–8. doi: 10.1159/000053573
48. Zheng T, Zhu Z, Wang ZD, Homer RJ, Ma B, Riese RJ, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase-and cathepsin-dependent emphysema. J Clini Investigat. (2000) 106:1081–93. doi: 10.1172/JCI10458
49. Punnonen J, Aversa G, Cocks BG, McKenzie ANJ, Menon S, Zurawski G, et al. Interleukin-13 Induces Interleukin-4-Independent Igg4 And Ige Synthesis And Cd23 Expression By Human B-Cells. Proc Natl Acad Sci U S A. (1993) 90:3730–4. doi: 10.1073/pnas.90.8.3730
50. Wills-Karp M, Luyimbazi J, Xu XY, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. (1998) 282:2258–61. doi: 10.1126/science.282.5397.2258
51. Urban JF, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al. IL-13, IL-4R alpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. (1998) 8:255–64. doi: 10.1016/S1074-7613(00)80477-X
52. Morosco G, Kiley J. National asthma education and prevention program - Expert panel report 3: Guidelines for the diagnosis and management of asthma summary report (2007). J Allergy Clin Immunol. (2007) 120:S93–S93. doi: 10.1016/j.jaci.2007.09.043
53. Louis R, Lau LCK, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. (2000) 161:9–16. doi: 10.1164/ajrccm.161.1.9802048
54. Woodruff PG, Khashayar R, Lazarus SC, Janson S, Avila P, Boushey HA, et al. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. (2001) 108:753–8. doi: 10.1067/mai.2001.119411
55. Pavlidis S, Takahashi K, Kwong FNK, Xie J, Hoda U, Sun K, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respirat J. (2019) 53. doi: 10.1183/13993003.00938-2018
56. Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. (2004) 113:30–7. doi: 10.1016/j.jaci.2003.10.050
57. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma - from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. (2000) 161:1720–45. doi: 10.1164/ajrccm.161.5.9903102
58. Cao C, Li W, Hua W, Yan F, Zhang H, Huang H, et al. Proteomic analysis of sputum reveals novel biomarkers for various presentations of asthma. J Transl Med. (2017) 4:15. doi: 10.1186/s12967-017-1264-y
59. Blanchet M-R, McNagny KM. Stem cells, inflammation and allergy. Allergy Asthma Clin Immunol. (2009) 2009 5:13–13. doi: 10.1186/1710-1492-5-13
60. Hui CCK, McNagny KM, Denburg JA, Siracusa MC. In situ hematopoiesis: a regulator of T(H)2 cytokine-mediated immunity and inflammation at mucosal surfaces. Mucosal Immunol. (2015) 8:701–11. doi: 10.1038/mi.2015.17
61. Salter BM, Sehmi R. Hematopoietic processes in eosinophilic asthma. Chest. (2017) 152:410–6. doi: 10.1016/j.chest.2017.01.021
62. Weiss CH, McSparron JI, Chatterjee RS, Herman D, Fan E, Wilson KC, et al. Summary for clinicians: mechanical ventilation in adult patients with acute respiratory distress syndrome clinical practice guideline. Ann Am Thorac Soc. (2017) 14:1235–8. doi: 10.1513/AnnalsATS.201704-332CME
63. Butt Y, Kurdowska A, Allen TC. Acute lung injury a clinical and molecular review. Arch Pathol Lab Med. (2016) 140:345–50. doi: 10.5858/arpa.2015-0519-RA
64. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
65. Willetts L, Parker K, Wesselius LJ, Protheroe CA, Jaben E, Graziano P, et al. Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir Res. (2011) 26:12. doi: 10.1186/1465-9921-12-116
66. Zhu C, Weng Q-Y, Zhou L-R, Cao C, Li F, Wu Y-F, et al. Homeostatic and early-recruited CD101(-) eosinophils suppress endotoxin-induced acute lung injury. Eur Respirat J. (2020) 56. doi: 10.1183/13993003.02354-2019
67. Eng SS, DeFelice ML. The role and immunobiology of eosinophils in the respiratory system: a comprehensive review. Clin Rev Allergy Immunol. (2016) 50:140–58. doi: 10.1007/s12016-015-8526-3
68. Philit F, Etienne-Mastroianni B, Parrot A, Guerin C, Robert D, Cordier JF, et al. Idiopathic acute eosinophilic pneumonia - a study of 22 patients. Am J Respir Crit Care Med. (2002) Nov 1;166(9):1235-1239. doi: 10.1164/rccm.2112056
69. Wendlinger S, Wohlfarth J, Kreft S, Siedel C, Kilian T, Dischinger U, et al. Blood eosinophils are associated with efficacy of targeted therapy in patients with advanced melanoma. Cancers. (2022) 14:2294. doi: 10.3390/cancers14092294
70. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. (2004) 113:11–28. doi: 10.1016/j.jaci.2003.10.047
71. Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx. (2002) 29:175–81. doi: 10.1016/S0385-8146(01)00135-3
72. Ono Y, Ozawa M, Tamura Y, Suzuki T, Suzuki K, Kurokawa K, et al. Tumor-associated tissue eosinophilia of penile cancer. Int J Urology. (2002) 9:82–7. doi: 10.1046/j.1442-2042.2002.00424.x
73. Costello R, O'Callaghan T, Sebahoun G. Eosinophils and antitumour response. Revue De Medecine Interne. (2005) 26:479–84. doi: 10.1016/j.revmed.2005.02.013
74. LunaMore S, Florez P, Ayala A, Diaz F, Santos A. Neutral and acid mucins and eosinophil and argyrophil crystalloids in carcinoma and atypical adenomatous hyperplasia of the prostate. Pathol Res Pract. (1997) 193:291–8. doi: 10.1016/S0344-0338(97)80006-4
75. Caruso RA, Parisi A, Quattrocchi E, Scardigno M, Branca G, Parisi C, et al. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct Pathol. (2011) 35:145–9. doi: 10.3109/01913123.2011.578233
76. Huland E, Huland H. Tumor-associated eosinophilia in. interleukin-2-treated patients - evidence of toxic eosinophil degranulation on bladder-cancer cells. J Cancer Res Clin Oncol. (1992) 118:463–7. doi: 10.1007/BF01629431
77. Simon HU, Plotz S, Simon D, Seitzer U, Braathen LR, Menz G, et al. Interleukin-2 primes eosinophil degranulation in hypereosinophilia and Wells' syndrome. Eur J Immunol. (2003) 33:834–9. doi: 10.1002/eji.200323727
78. Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of. interleukin-4. Science. (1992) 257:548–51. doi: 10.1126/science.1636093
79. Bristol JA, Zhu MZ Ji H, Mina M, Xie YF, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Molecular Therapy. (2003) 7:755–64. doi: 10.1016/S1525-0016(03)00103-5
80. Schaefer JT, Patterson JW, Deacon DH, Smolkin ME, Petroni GR, Jackson EM, et al. Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. J Transl Med. (2010) 20:8. doi: 10.1186/1479-5876-8-79
81. Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematol-Am Soc Hemat. (2015) 92–7. doi: 10.1182/asheducation-2015.1.92
82. Uppal V, Kreiger P, Kutsch E. Eosinophilic gastroenteritis and colitis: a comprehensive review. Clin Rev Allergy Immunol. (2016) 50:175–88. doi: 10.1007/s12016-015-8489-4
83. Beck IME, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. (2009) 30:830–82. doi: 10.1210/er.2009-0013
84. Xavier AM, Olimpio Anunciato AK, Rosenstock TR, Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol. (2016) 19:7. doi: 10.3389/fendo.2016.00031
85. Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generatetranscription specificity. Trends Cell Biol. (2006) 16:301–7. doi: 10.1016/j.tcb.2006.04.005
86. Adcock IM, Gilbey T, Gelder CM, Chung KF, Barnes PJ. Glucocorticoid receptor localization in normal and asthmatic lung. Am J Respir Crit Care Med. (1996) 154:771–82. doi: 10.1164/ajrccm.154.3.8810618
87. Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. (2003) 8:481–95. doi: 10.1023/A:1025590308147
88. Her E, Frazer J, Austen KF, Owen WF. Eosinophil hematopoietins antagonize the programmed cell-death of eosinophils. - cytokine and glucocorticoid effects on eosinophils maintained by endothelial-cell conditioned medium. J Clini Investigat. (1991) 88:1982–7. doi: 10.1172/JCI115524
89. Shen HH, O'Byrne PM, Ellis R, Wattie J, Tang CB, Inman MD. The effects of intranasal budesonide on allergen-induced production of interleukin-5 and eotaxin, airways, blood, and bone marrow eosinophilia, and eosinophil progenitor expansion in sensitized mice. Am J Respir Crit Care Med. (2002) 166:146–53. doi: 10.1164/rccm.2008161
90. Pazdrak K, Moon Y, Straub C, Stafford S, Kurosky A. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. (2016) 21:421–31. doi: 10.1007/s10495-016-1226-5
91. Wu Y, Chen H, Xuan N, Zhou L, Wu Y, Zhu C, et al. Induction of ferroptosis-like cell death of eosinophils exerts synergistic effects with glucocorticoids in allergic airway inflammation. Thorax. (2020) 75:918–27. doi: 10.1136/thoraxjnl-2020-214764
92. Kouro T, Takatsu K. IL-5-and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. (2009) 21:1303–9. doi: 10.1093/intimm/dxp102
93. Lu TX, Lim E-J, Besse JA, Itskovich S, Plassard AJ, Fulkerson PC, et al. miR-223 deficiency increases eosinophil progenitor proliferation. J Immunol. (2013) 190:1576–82. doi: 10.4049/jimmunol.1202897
94. Wechsler ME, Fulkerson PC, Bochner BS, Gauvreau GM, Gleich GJ, Henkel T, et al. Novel targeted therapies for eosinophilic disorders. J Allergy Clin Immunol. (2012) 130:563–71. doi: 10.1016/j.jaci.2012.07.027
95. Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. (2003) 111:714–9. doi: 10.1067/mai.2003.1382
96. Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet LP, Lemiere C, et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clini Exp Aller. (2016) 46:793–802. doi: 10.1111/cea.12695
97. Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol-in Practice. (2015) 3:167–74. doi: 10.1016/j.jaip.2015.01.013
98. Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clini Exp Aller. (2012) 42:712–37. doi: 10.1111/j.1365-2222.2011.03854.x
99. Henriksen DP, Bodtger U, Sidenius K, Maltbaek N, Pedersen L, Madsen H, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma–a systematic review and meta-analysis. Eur Clin Respir J. (2018) 5:1536097. doi: 10.1080/20018525.2018.1536097
100. Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. (2010) 125:1237–44. doi: 10.1016/j.jaci.2010.04.005
101. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta 2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. (2016) 388:2115–27. doi: 10.1016/S0140-6736(16)31324-1
102. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor a monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2016) 388:2128–41. doi: 10.1016/S0140-6736(16)31322-8
103. Ghassemian A, Park JJ, Tsoulis MW, Kim H. Targeting the IL-5 pathway in eosinophilic asthma: a comparison of mepolizumab to benralizumab in the reduction of peripheral eosinophil counts. Allergy Asthma Clin Immunol. (2021) 17:1–7. doi: 10.1186/s13223-020-00507-0
104. Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. (2009) 183:5094–103. doi: 10.4049/jimmunol.0802387
105. Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. (2009) 183:6469–77. doi: 10.4049/jimmunol.0901575
106. Xi H, Katschke KJ Jr., Li Y, Tom T, Lee WP, et al. IL-33 amplifies an innate immune response in the degenerating retina. J Exp Med. (2016) 213:189–207. doi: 10.1084/jem.20150894
107. Lv R, Zhao J, Lei M, Xiao D, Yu Y, Xie J. IL-33 Attenuates Sepsis by Inhibiting IL-17 Receptor Signaling through Upregulation of SOCS3. Cellular Physiology and Biochemistry. (2017) 42:1961–72. doi: 10.1159/000479836
108. Tian B-p, Xia L-x, Bao Z-q, Zhang H, Xu Z-w, Mao Y-y, et al. Bcl-2 inhibitors reduce steroid-insensitive airway inflammation. J Allergy Clin Immunol. (2017) 140:418–30. doi: 10.1016/j.jaci.2016.11.027
109. Tian B-P, Li F, Li R, Hu X, Lai T-W, Lu J, et al. Nanoformulated ABT-199 to effectively target Bcl-2 at mitochondrial membrane alleviates airway inflammation by inducing apoptosis. Biomaterials. (2019) 192:429–39. doi: 10.1016/j.biomaterials.2018.06.020
110. Du X, Li F, Zhang C, Li N, Huang H, Shao Z, et al. Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation. Signal Transduct Target Ther. (2021) 6:91. doi: 10.1038/s41392-021-00482-x
111. Wu J, Guo Y, Lu X, Huang F, Lv F, Wei D, et al. Th1/Th2 cells and associated cytokines in acute hepatitis E and related acute liver failure. J Immunol Res. (2020) 2020. doi: 10.1155/2020/6027361
Keywords: eosinophils, cellular functions, molecular mechanisms, disease pathology, therapeutic drugs
Citation: Tao Z, Zhu H, Zhang J, Huang Z, Xiang Z and Hong T (2022) Recent advances of eosinophils and its correlated diseases. Front. Public Health 10:954721. doi: 10.3389/fpubh.2022.954721
Received: 27 May 2022; Accepted: 04 July 2022;
Published: 25 July 2022.
Edited by:
Zongxin Ling, Zhejiang University, ChinaReviewed by:
Haicheng Tang, Fudan University, ChinaYuzhu Dai, The 903th Hospital of the People's Liberation Army, China
Copyright © 2022 Tao, Zhu, Zhang, Huang, Xiang and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tu Hong, aG9uZ3R1QHpqdS5lZHUuY24=; Ze Xiang, MzE5MDEwMjM3OEB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Zhang Tao
Zhang Tao Hua Zhu2,3†
Hua Zhu2,3† Ze Xiang
Ze Xiang Tu Hong
Tu Hong