- 1The Second Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 2Yangjiang Hospital of Traditional Chinese Medicine, Yangjiang, China
- 3Medical College of Jiaying University, Meizhou, China
- 4The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Taishan Hospital of Traditional Chinese Medicine, Jiangmen, China
- 6State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, China
Objective: This paper evaluates the application value of the STOP-Bang questionnaire combined with the Epworth Sleepiness Scale (ESS) in screening for obstructive sleep apnea (OSA) in the population.
Method: Thousand-six hundred seventy-one patients with suspected OSA who visited the Sleep Medicine Center of the First Affiliated Hospital of Guangzhou Medical University from August 2017 to August 2020 were monitored by overnight polysomnography (PSG) after completing the ESS scale and STOP-Bang questionnaire. The sensitivity, specificity, positive predictive value, negative predictive value and receiver operating characteristic (ROC) curves of the two scales were calculated, and the accuracy in predicting OSA of the STOP-Bang questionnaire combined with ESS was analyzed.
Results: With Apnea Hypopnea Index (AHI) cutoffs of ≥5, ≥15 and ≥30 events/h, the areas under the ROC curve scored by STOP-Bang were 0.724, 0.703 and 0.712, and those of ESS were 0.632, 0.634 and 0.695; the diagnostic odds ratio (DOR) values of STOP-Bang for OSA, moderate to severe OSA, and severe OSA were 3.349, 2.651 and 3.189, and those of ESS were 2.665, 2.279 and 3.289. The STOP-Bang score of three was used as the cut-off point for OSA diagnosis with higher sensitivity and lower specificity, while ESS had higher specificity. STOP-Bang (≥3) combined with ESS significantly improved its specificity for predicting OSA.
Conclusion: The STOP-Bang questionnaire is a simple and effective new tool for screening patients for OSA, while a STOP-Bang score of ≥3 combined with ESS can further improve its specificity. Thus, we suggest further screening with ESS after a STOP-Bang score of ≥3 in suspected patients.
Introduction
Obstructive sleep apnea (OSA) is a recurring narrowing or partial or complete collapse of the airway, snoring/apnea and low ventilation during sleep, leading to frequent hypoxemia, hypercapnia and common sleep-related breathing disorders. And as we know the Editorial Board of The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (AASM Scoring Manual) would like to notify the membership and the sleep community that an update for the AASM Scoring Manual (Version 2.4) was released April 1, 2017 (1). The prevalence of OSA is about 1–5% in children, 9% in adult women and 24% in adult males (2, 3). OSA is harmful, can even lead to death and is associated with the increased mortality of patients (4, 5). Although the connection remains debated, several mechanisms such as intermittent hypoxemia, sleep deprivation, hypercapnia disruption of the hypothalamic-pituitary-adrenal axis have been associated with poor neurocognitive performance. Different treatments have been proposed to treat OSAS patients as continuous positive airway pressure (CPAP), mandibular advancement devices (MAD), surgery; however, the effect on neurocognitive functions is still debated. CPAP treatment seems to improve cognitive defects associated with OSA. Limited studies have evaluated the effects of the other therapies on cognitive function as oral appliance or barbed surgery (6). The scary thing is that a lot of OSA in the population goes undiagnosed (7, 8). As we all know, polysomnography (PSG) is the gold standard for the diagnosis of OSA, but it is difficult to apply PSG widely in primary hospitals as sleep rooms and professional and technical personnel are required, while PSG examination is expensive, complicated and time-consuming. The STOP-Bang questionnaire is a simple and effective screening tool for the risk assessment of suspected sleep disordered breathing (9–11) which includes eight indicators: snoring (S), tiredness (T), observed apnea during sleep (O), blood pressure (P), BMI, age, neck circumference and gender; scores ≥3 are 93% sensitive and 43% specific for moderate OSA, and 100% sensitive and 37% specific for severe OSA (12). It is not difficult to see that although the STOP-Bang questionnaire has good sensitivity, its specificity is poor. A meta-analysis confirms the high performance of the STOP-Bang questionnaire in the sleep clinic and surgical population for screening of OSA. The higher the STOP-Bang score, the greater is the probability of moderate-to-severe OSA (13). It was recently found that STOP-Bang combined with serum bicarbonate can significantly improve the diagnostic value of STOP-Bang for OSA patients (14). Considering that blood drawing tests are troublesome and time-consuming, while the Epworth Sleepiness Scale (ESS) is relatively easy to perform, this study evaluated STOP-Bang and ESS for suspected OSA patients, and then the statistical analysis of the PSG data was completed to further evaluate the application value of STOP-Bang combined with ESS for screening for OSA in the population. We hypothesized that the combination of ESS would significantly improve the specificity of STOP-Bang in predicting OSA.
Materials and methods
Study subjects
All participants were recruited from the Sleep Medical Center of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, from August 2017 to August 2020. From a total of 1,861 patients, 1,671 were eventually included: 1,300 males and 371 females; mean age of 47.45 ± 13.90 years; average neck circumference of 38.36 ± 3.93 cm; and mean BMI of 26.49 ± 4.20 kg/m2. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University with Ethical Approval No. 05, 2017, and all patients gave and signed their informed consent. The inclusion criteria were: (1) older than 18 years; (2) total sleep time of >4 h; (3) autonomous behavior and cognitive ability; and (4) able to answer the questionnaire. The exclusion criteria were: (1) history of various mental and psychological diseases; (2) brain tumors or epilepsy; (3) long-term or current use of benzodiazepines, barbiturates or other sedative and sleeping drugs; (4) severe organ failure leading to an inability to complete the examination; (5) previously diagnosed or treated; (6) did not complete the questionnaire; (7) total sleep time of < 4 h; and (8) OSA dominated by central or mixed events.
Methods
In our study, we collected the basic data of the 1,671 suspected patients: (1) basic anthropological data; (2) basic demographics (e.g., gender, age, occupation); (3) anthropometric parameters (height, weight, neck circumference, waist circumference, etc.); (4) previous history (history of hypertension, diabetes, cardiovascular and cerebrovascular diseases, and other related diseases); (5) personal history (smoking and drinking); and (6) sleep-related breathing events (e.g., snoring, apnea, sleep suppression). Patients were asked to complete ESS and STOP-Bang 1 h before the PSG examination. According to the PSG monitoring results, the patients were divided into the normal group [AHI < 5 events/h (n = 470)], mild OSA group (AHI ≥5 and < 15 events/h [n = 378)], moderate OSA group [AHI ≥ 15 and < 30 events/h (n = 320)] and severe OSAHS group [AHI ≥30 events/h (n = 503)].
Questionnaire
The STOP-Bang questionnaire has eight questions on snoring, tiredness, observed apnea, hypertension, body mass index >35 kg/m2, age >50, neck circumference >40 cm and male that are answered with “yes” or “no.” It adds one point for “yes” and zero points for “no.” When the total points are >3, this indicates that the patient is at high risk for OSA. ESS, which includes 8 questions, asks respondents to rate their sleepiness from zero to three in eight daily situations. For each question, a score of zero indicates no lethargy, and one, two and three indicate light, moderate and heavy lethargy respectively. The highest score of ESS is 24 (the most excessive daytime sleepiness), with a threshold for daytime sleepiness of 10 points or more.
Polysomnography
All patients were synchronously monitored with an Alice 5 PSG (Philips Wellcome, USA) for at least 7 h, and the use of alcohol, coffee, sedatives and hypnotics was prohibited on the same day. The monitoring indicators included electro-encephalogram, electromyography, blood oxygen saturation, electro-oculogram, electrocardiogram, snoring, mouth airflow, nasal airflow, chest breathing and body position. The raw data was automatically read by the instrument, then manually analyzed by trained sleep professionals for parameters such as sleep duration and sleep breathing events based on the Manual for the Scoring of Sleep and Associated Events published by the American Academy of Sleep Medicine (AASM) in 2012, and finally corrected by the same physicians (15). According to the guidelines for the diagnosis and treatment of OSA, patients were defined as having OSA when their obstructive apnea was dominated by respiratory events and their Apnea Hypopnea Index (AHI) was not below five events/h. Patients with suspected OSA were classified into four groups based on AHI: AHI < 5 events/h, AHI ≥ 5 and < 15 events/h, AHI ≥ 15 and < 30 events/h and AHI ≥ 30 events/h.
Sample size calculation
We take all the data into study and the sample size was not calculated.
Reduce the potential for bias
Firstly, it was not the same person who conducted the questionnaire and the PSG and they don't know the patient's condition. Secondly, the sample size is very huge in our study. Lastly, there are fewer interference factors in our study because both the questionnaire and PSG data were obtained from the same patient.
Statistical analysis
Statistical analysis was performed using SPSS v16.0. One-Way ANOVA was adopted for the normal distribution of data. Post-hoc analysis was conducted for comparison between the two groups. The chi-square test was used for comparison between count data groups. The diagnostic results of each scale and PSG were calculated as the sensitivity, specificity, positive predictive value and negative predictive value of each scale in a four-grid scale form. The diagnostic results of PSG and each scale were analyzed in a four-fold table, and the sensitivity, specificity, positive predictive value and negative predictive value of each amount was calculated. The ROC curves were used to analyze the OSA diagnostic performance of STOP-Bang combined with ESS.
Results
General data
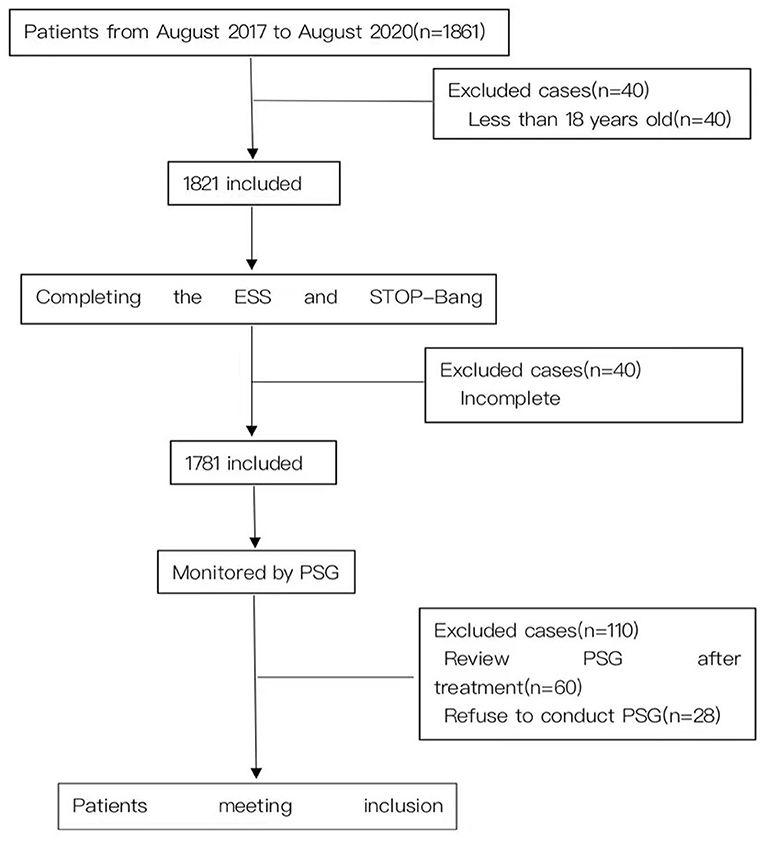
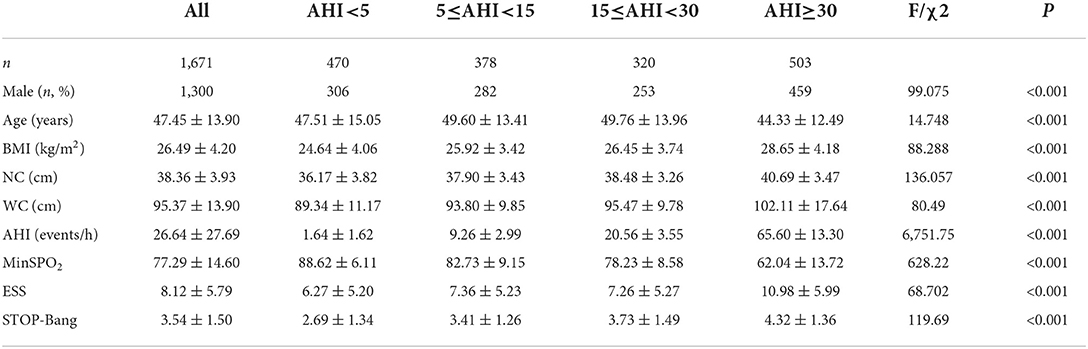
A total of 1,671 suspected patients (including 1,300 males) were recruited for this study (Figure 1). The mean age of the subjects was 47.45 ± 13.90 years old, the mean BMI was 26.49 ± 4.20 kg/m2 and the mean neck and waistline circumferences were 38.36 ± 3.93 and 95.37 ± 13.90 cm respectively. The mean AHI of the subjects was 26.64 ± 27.69 events/h, and the mean lowest oxygen saturation (LSpO2) was 77.29 ± 14.60%. The mean ESS and STOP-Bang scores were 8.12 ± 5.79 and 3.54 ± 1.50 respectively. There were no statistically significant differences in age and ESS scores between the mild and moderate OSA groups. In addition, there were statistically significant differences in other items among the four groups. The proportion of males in the mild group was higher than in the normal group, higher in the moderate group than the mild group, and higher in the severe group than the moderate group, and the differences were statistically significant (P < 0.05). Similarly, these differences were reflected in the indicators of BMI, neck circumference, waist circumference, AHI, ESS and STOP-Bang (Table 1).
Area under ROC curve
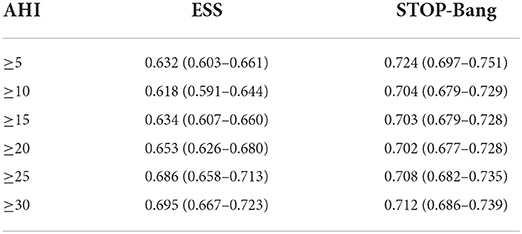
The area under the curve (AUC) of the two scales was compared using AHI cutoffs of 5, 10, 15, 20, 25, and 30 events/h, respectively: STOP-Bang was 0.724, 0.704, 0.703, 0.702, 0.708, and 0.712, and ESS was 0.632, 0.618, 0.634, 0.653, 0.686, and 0.695. It was found that the AUC of STOP-Bang was higher than that of ESS (Table 2; Figures 2–4).
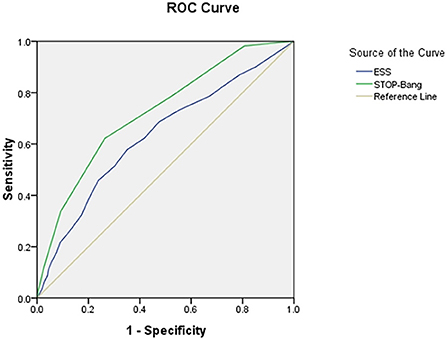
Figure 2. ROC curve of the ESS and STOP-Bang at AHI cutoff of ≥5 events/h (At an AHI cutoff of ≥5 events/h, the diagnostic performance of the STOP-Bang is better than the ESS. AHI, apnea-hypopnea index; ESS, Epworth Sleepiness Scale; STOP-Bang, STOP-Bang questionnaire; ROC, receiver operating characteristic).
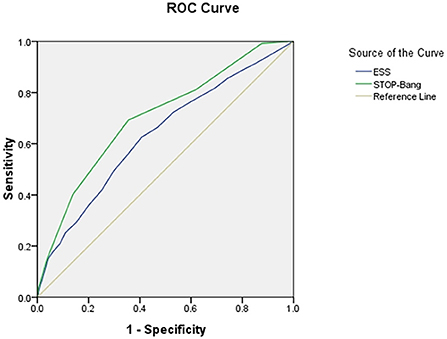
Figure 3. ROC curve of the ESS and STOP-Bang at AHI cutoff of ≥15 events/h (At an AHI cutoff of ≥15 events/h, the diagnostic performance of the STOP-Bang is better than the ESS. AHI, apnea-hypopnea index; ESS, Epworth Sleepiness Scale; STOP-Bang, STOP-Bang questionnaire; ROC, receiver operating characteristic).
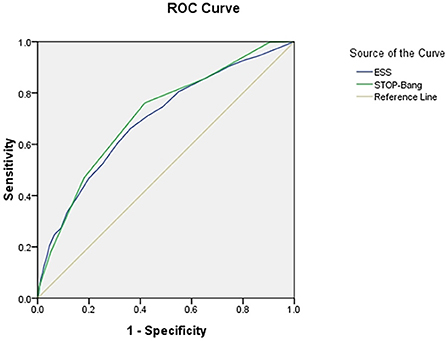
Figure 4. ROC curve of the ESS and STOP-Bang at AHI cutoff of ≥30 events/h (At an AHI cutoff of ≥30 events/h, the diagnostic performance of the STOP-Bang is better than the ESS. AHI, apnea-hypopnea index; ESS, Epworth Sleepiness Scale; STOP-Bang, STOP-Bang questionnaire; ROC, receiver operating characteristic).
Sensitivity and specificity of STOP-Bang
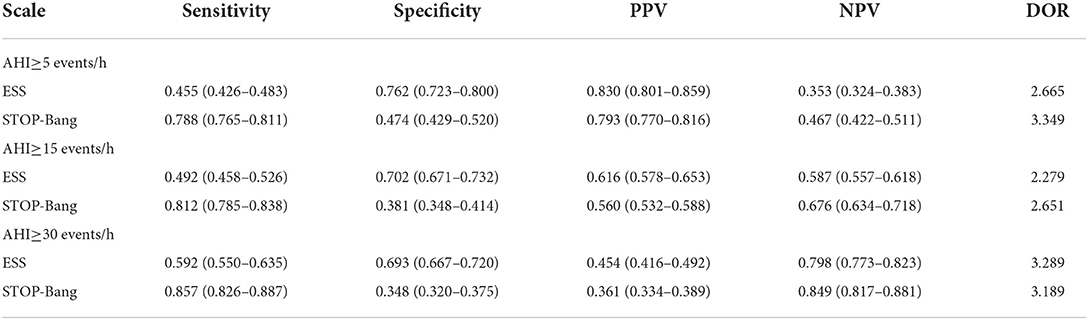
Using the STOP-Bang score of three as the cutoff, the sensitivity and specificity of STOP-Bang for OSA, moderate to severe OSA, and severe OSA were 0.788, 0.812, and 0.857, and 0.474, 0.381 and 0.348, respectively (Table 3).
Specificity of STOP-Bang combined with ESS
The specificity of STOP-Bang (3) for OSA, moderate to severe OSA, and severe OSA was 0.474 (0.429–0.520), 0.381 (0.348–0.414) and 0.348 (0.320–0.375). When combined with ESS, the specificity increased to 0.668 (0.609–0.727), 0.598 (0.556–0.640) and 0.592 (0.557–0.627) (Table 4).
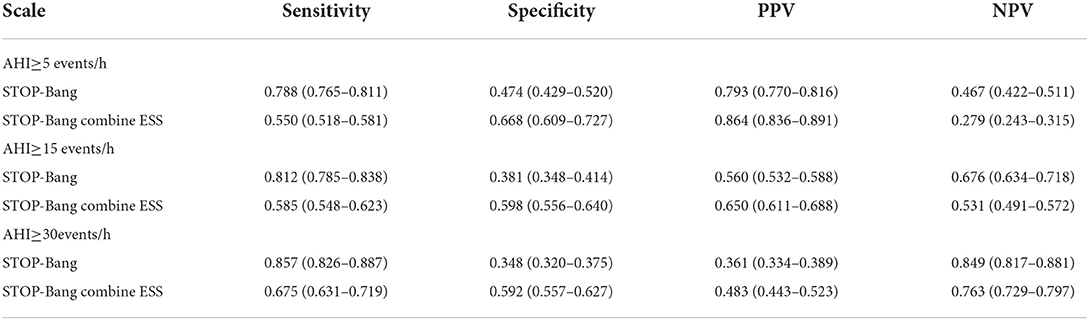
Table 4. Compare STOP-Bang combine ESS with STOP-Bang the scale predictors of each group patients [percentage (95%CI)].
Two-step screening procedure screens for OSA risk
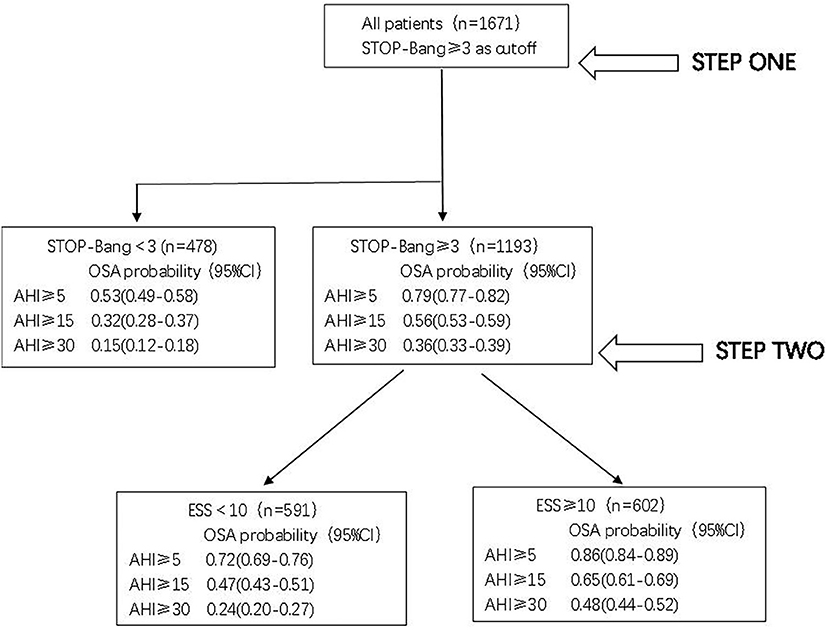
According to the above analysis, a two-step screening method can be formed. In the first step, STOP-Bang was used to screen all 1,671 patients; the risk of OSA, moderate to severe OSA, and severe OSA in patients with scores lower than 3 was 0.53 (0.49–0.58), 0.32 (0.28–0.37) and 0.15 (0.12–0.18), indicating that these patients were less likely to have OSA.
For 1,193 patients (STOP-Bang score ≥3), the risk of OSA was further evaluated by ESS. For patients with an ESS score ≥10, the risk of OSA, moderate to severe OSA, and severe OSA was 0.86 (0.84–0.89), 0.65 (0.61–0.69) and 0.48 (0.44–0.52). The risk was significantly higher than that of patients with ESS < 10; in particular, the risk of severe OSA was as much as 2.0 times higher (Figure 5).
Discussion
In this study, 1,201 of the 1,671 patients suspected of OSA were confirmed, and the proportion of men was far higher than that of women, in line with the epidemiological characteristics of OSAHS. Scholars all over the world have developed a variety of OSA screening tools, among which a large number of scales are complex, requiring the use of computers and complicated mathematical calculations, making it difficult to promote the use of such tools in clinical practice (16–18). STOP-Bang is a relatively new questionnaire used for screening OSA. In this study, the STOP-Bang questionnaire showed an increasing trend with the aggravation of OSA, the comparison between the normal group and mild, moderate and severe OSA groups was statistically significant, and differences among OSA groups were also statistically significant. Similarly, these differences were reflected in ESS. However, there was no statistical difference between the mild and moderate OSA groups, suggesting that STOP-Bang is better than ESS at distinguishing severe OSA. As can be seen from the AUC, STOP-Bang was higher than ESS for OSA, moderate to severe OSA, and severe OSA, showing good predictive value for OSA patients. The results of this study are similar to those of other studies (19, 20), which show that the STOP-Bang questionnaire is a simple, effective and easy tool for risk assessment in patients with suspected OSA.
Diagnostic odds ratio (DOR) was applied in the meta-analysis to compare the accuracy of various prediction models and questionnaires for sleep-disordered breathing (21). In this study, the DOR values of STOP-Bang for OSA, moderate to severe OSA, and severe OSA were 3.349, 2.651, and 3.189 respectively, and the DOR values of ESS for OSA, moderate to severe OSA, and severe OSA were 2.665, 2.279, and 3.289 respectively. It can be seen that the DOR values of STOP-Bang in diagnosing OSA and moderate severe OSA are higher than those of ESS, while the DOR value of STOP-Bang in diagnosing severe OSA is similar to that of ESS. Therefore, STOP-Bang has better predictive value for OSA than ESS.
The lack of awareness of OSA among the public and health professionals leads to a failure in the timely diagnosis of OSA patients, and studies have found that the vast majority (>80%) of patients with moderate to severe OSA remain undiagnosed (22). Untreated OSA patients are at increased risk for metabolic syndrome, cardiovascular disease and impaired neurocognitive function and mental health (23–26), and the disease significantly reduces the quality of life of patients (27, 28) and even contributes to their premature death (29–31). The adverse health effects of OSA can significantly increase economic costs (32, 33), while the treatment of OSA can bring economic benefits (34). Although PSG is the gold standard for diagnosing OSA, it is time-consuming and expensive, and has long waiting times, making timely diagnosis difficult to achieve. Tools for screening patients at high risk for OSA are becoming increasingly important in order to identify patients with OSA early and schedule further diagnosis and treatment.
The results of this study provide a simple and effective program for screening suspected OSA patients. We recommend a two-step screening. The first step is preliminary screening with the STOP-Bang questionnaire. Those with scores lower than three have a low risk of OSA, but its low specificity can easily cause a high false positive rate, so it is necessary to conduct the second step screening for those with scores of three or above.
The ESS score is mainly based on the patients' own cognitive score, which is completely subjective, while the STOP-Bang questionnaire is mainly based on objective factors. The combination of the two scales can complement each other to improve the predictive value of OSA. In this study, when combined with ESS, the specificity of the STOP-Bang questionnaire in predicting OSA, moderate to severe OSA, and severe OSA in patients was 0.668, 0.598, and 0.592. It can be seen that the specificity of the STOP-Bang questionnaire combined with ESS in predicting OSA patients can be significantly improved. Therefore, we suggest that the second step of screening should be carried out in combination with ESS for people with a STOP-Bang questionnaire of three or more. For patients at high risk for OSA, we suggest a PSG examination followed by stratified management according to the examination results, including behavior adjustment, weight loss, drugs, continuous positive airway pressure ventilation, oral appliances, surgery and other individualized treatments. With the deepening of public understanding of OSA and the formation and efforts of multidisciplinary teams, the layered management of OSA is becoming increasingly important, and the rational diagnosis and treatment of OSA can be expected in the future.
Like many studies, this study has several shortcomings. The single-center cohort study mainly includes subjects from Guangzhou, but as the National Respiratory Medicine Center, our unit accepts a large number of research subjects from all over the country and should represent the Chinese population to a certain extent; and the members of the normal and severe OSAHS groups were younger than those of the mild and moderate groups, which may have had a certain impact on the results.
In conclusion, combining ESS with the STOP-Bang score improves its specificity at the cost of reducing its sensitivity in predicting OSA. As such, we recommend a two-step screening process for suspected OSA patients: the initial screening using the highly sensitive STOP-Bang score (three points), and then combining it with ESS to improve the specificity. This screening approach can assist doctors in conducting stratified management according to the OSA risk levels of patients, identifying high-risk patients and having them undergo PSG examination as soon as possible, and carrying out early intervention for patients with a definite diagnosis, thereby minimizing the harm caused by OSA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ, ZZh, CL, MC, and CW are the guarantor of the manuscript and take responsibility for the content of this manuscript. YZ, ZZh, RC, and XC contributed to the design of the study. JZ, JL, and XO were involved in the data analysis. ZZo, XO, and RC contributed to the acquisition of primary data. JL and RC wrote the initial draft of the manuscript. ZZh, RC, JD, and XC contributed significantly to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Natural Science Foundation of Guangdong Province (2021A1515011373).
Acknowledgments
We would like to thank Professor Nanshan Zhong from State Key Laboratory of Respiratory Disease for the constructive advice he gave.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. (2017) 13:665–6. doi: 10.5664/jcsm.6576
2. Bin-Hasan S, Katz S, Nugent Z, Nehme J, Lu Z, Khayat A, et al. Prevalence of obstructive sleep apnea among obese toddlers and preschool children. Sleep Breath. (2018) 22:511–5. doi: 10.1007/s11325-017-1576-4
3. Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. (2020) 22:6. doi: 10.1007/s11886-020-1257-y
4. Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. (2011) 34:435–42. doi: 10.1093/sleep/34.4.435
5. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. (2009) 6:e1000132. doi: 10.1371/journal.pmed.1000132
6. Pollicina I, Maniaci A, Lechien JR, Iannella G, Vicini C, Cammaroto G, et al. Neurocognitive performance improvement after obstructive sleep apnea treatment: state of the art. Behav Sci. (2021) 11:180. doi: 10.3390/bs11120180
7. Torres G, Sánchez-de-la-Torre M, Barbé F. Relationship between OSA and hypertension. Chest. (2015) 148:824–32. doi: 10.1378/chest.15-0136
8. Zeng L, Chen R, Hu L, Wang D, Chen M, Lai Y, et al. Concern about sleep disorders in underresourced settings is imminent. J Clin Sleep Med. (2021) 17:2339–40. doi: 10.5664/jcsm.9590
9. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. (2008) 108:812–21. doi: 10.1097/ALN.0b013e31816d83e4
10. Nurgul Y. A simple and validated test for detecting patients with OSA: STOP-BANG questionnaire. Ann Card Anaesth. (2021) 24:313–4. doi: 10.4103/aca.ACA_205_20
11. Zheng Z, Sun X, Chen R, Lei W, Peng M, Li X, et al. Comparison of six assessment tools to screen for obstructive sleep apnea in patients with hypertension. Clin Cardiol. (2021) 44:1526–34. doi: 10.1002/clc.23714
12. Mason M, Hernández-Sánchez J, Vuylsteke A, Smith I. Usefulness of the STOP-Bang questionnaire in a cardiac surgical population. J Cardiothorac Vasc Anesth. (2018) 32:2694–9. doi: 10.1053/j.jvca.2018.04.049
13. Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK. Memtsoudis S, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0143697. doi: 10.1371/journal.pone.0143697
14. Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. (2013) 143:1284–93. doi: 10.1378/chest.12-1132
15. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. (2012) 8:597–619. doi: 10.5664/jcsm.2172
16. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. (2010) 57:423–38. doi: 10.1007/s12630-010-9280-x
17. Sun X, Zheng Z, Liang J, Chen R, Huang H, Yao X, et al. Development and validation of a simple clinical nomogram for predicting obstructive sleep apnea. (2022) e13546. doi: 10.1111/jsr.13546
18. Luo M, Zheng HY, Zhang Y, Feng Y, Li DQ Li XL, et al. A nomogram for predicting the likelihood of obstructive sleep apnea to reduce the unnecessary polysomnography examinations. Chin Med J. (2015) 128:2134–40. doi: 10.4103/0366-6999.162514
19. Hong C, Chen R, Qing S, Kuang A, Yang H, Su X, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med. (2018) 14:191–7. doi: 10.5664/jcsm.6930
20. Qing SM, Chen RK, Liu H, Zhang S, Kuang AL. Su XF, et al. [Comparison of the NoSAS score with four different questionnaires as screening tools for obstructive sleep apnea-hypopnea syndrome]. Zhonghua Jie He He Hu Xi Za Zhi. (2018) 41:213–9. doi: 10.3760/cma.j.issn.1001-0939.2018.03.013
21. Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. (2009) 110:928–39. doi: 10.1097/ALN.0b013e31819c47b6
22. Swanson LM, Arnedt JT, Rosekind MR, Belenky G, Balkin TJ, Drake C. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation sleep in America poll. J Sleep Res. (2011) 20:487–94. doi: 10.1111/j.1365-2869.2010.00890.x
23. Hirotsu C, Haba-Rubio J, Togeiro SM, Marques-Vidal P, Drager LF, Vollenweider P, et al. Obstructive sleep apnea as a risk factor for incident metabolic syndrome: a joined Episono and HypnoLaus prospective cohorts study. Eur Respir J. (2018) 52:1801150. doi: 10.1183/13993003.01150-2018
24. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G. Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. (2017) 136:1840–50. doi: 10.1161/CIRCULATIONAHA.117.029400
25. Davies CR, Harrington JJ. Impact of obstructive sleep apnea on neurocognitive function and impact of continuous positive air pressure. Sleep Med Clin. (2016) 11:287–98. doi: 10.1016/j.jsmc.2016.04.006
26. Lang CJ, Appleton SL, Vakulin A, McEvoy RD, Vincent AD, Wittert GA, et al. Associations of undiagnosed obstructive sleep apnea and excessive daytime sleepiness with depression: an Australian population study. J Clin Sleep Med. (2017) 13:575–82. doi: 10.5664/jcsm.6546
27. Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin sleep cohort study. Sleep. (1998) 21:701–6.
28. Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S, et al. The association of sleep-disordered breathing and sleep symptoms with quality of life in the sleep heart health study. Sleep. (2001) 24:96–105. doi: 10.1093/sleep/24.1.96
29. Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. (2015) 1:17–27. doi: 10.1016/j.wjorl.2015.08.001
30. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. (2005) 353:2034–41. doi: 10.1056/NEJMoa043104
31. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. J Clin Sleep Med. (2014) 10:355–62. doi: 10.5664/jcsm.3600
32. Leger D, Bayon V, Laaban JP, Philip P. Impact of sleep apnea on economics. Sleep Med Rev. (2012) 16:455–62. doi: 10.1016/j.smrv.2011.10.001
33. Roche J, Rae DE, Redman KN, Knutson KL, von Schantz M, Gómez-Olivé FX, et al. Impact of obstructive sleep apnea on cardiometabolic health in a random sample of older adults in rural South Africa: building the case for the treatment of sleep disorders in underresourced settings. J Clin Sleep Med. (2021) 17:1423–34. doi: 10.5664/jcsm.9214
Keywords: obstructive sleep apnea, Epworth Sleepiness Scale, STOP-Bang questionnaire, diagnostic, polysomnography
Citation: Zheng Z, Zhang Y, Chen M, Chen X, Li C, Wang C, Zhu J, Lin J, Ou X, Zou Z, Wang Z, Deng J and Chen R (2022) Application value of joint STOP-Bang questionnaire and Epworth Sleepiness Scale in screening for obstructive sleep apnea. Front. Public Health 10:950585. doi: 10.3389/fpubh.2022.950585
Received: 23 May 2022; Accepted: 01 August 2022;
Published: 29 September 2022.
Edited by:
Mihajlo Jakovljevic, Hosei University, JapanReviewed by:
Antonino Maniaci, University of Catania, ItalyBaran Balcan, Koç University Hospital, Turkey
Copyright © 2022 Zheng, Zhang, Chen, Chen, Li, Wang, Zhu, Lin, Ou, Zou, Wang, Deng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junzhong Deng, ZGVuZ2p1bnpob25nMDc1OUAxNjMuY29t; Riken Chen, Y2hlbnJpa2VuQDEyNi5jb20=
†These authors have contributed equally to this work
 Zhenzhen Zheng
Zhenzhen Zheng Yitao Zhang2†
Yitao Zhang2† Junyan Lin
Junyan Lin Zhiwei Wang
Zhiwei Wang Riken Chen
Riken Chen