
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 28 September 2022
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.949170
This article is part of the Research Topic What, How, and Where to Eat is More than an Individual Choice: New Ways to Achieve Healthy Eating View all 11 articles
 Huanyu Wu1†
Huanyu Wu1† Jianing Wang2†
Jianing Wang2† Hongyan Jiang1†
Hongyan Jiang1† Xin Liu1
Xin Liu1 Xinyi Sun1
Xinyi Sun1 Yunyan Chen1
Yunyan Chen1 Cong Hu1
Cong Hu1 Zheng Wang1
Zheng Wang1 Tianshu Han1
Tianshu Han1 Changhao Sun1*
Changhao Sun1* Wei Wei1*
Wei Wei1* Wenbo Jiang1*
Wenbo Jiang1*Background: Current studies on the protective effects of dietary spermidine (SPD) on cardiovascular disease (CVD) are mainly limited to animal studies, and the relationship between dietary SPD and CVD mortality remains inconclusive.
Objective: This study aims to evaluate the association between dietary SPD intake and CVD and all-cause mortality.
Methods: A total of 23,894 people enrolled in the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2014 were recruited for this study. The dietary intake of SPD from 11 specific food origins and total SPD was categorized into tertiles or quartiles. Cox proportional hazard regression models were developed to evaluate the association of SPD intake with CVD and all-cause mortalities.
Results: Among the 23,894 participants, 2,365 deaths, including 736 deaths due to CVD, were documented. After adjustment for potential confounders, compared with participants in the lowest quartile, participants in the highest quartile of total SPD had a significantly lower risk of CVD mortality (HR = 0.68, 95% CI: 0.51–0.91) and all-cause mortality (HR = 0.70, 95% CI: 0.60–0.82); participants in the highest tertiles or quartiles of vegetable-derived SPD, cereal-derived SPD, legume-derived SPD, nut-derived SPD, and cheese-derived SPD had a lower risk of CVD mortality (HR vegetable − derivedSPD = 0.68, 95% CI: 0.54–0.86; HR cereal − derivedSPD = 0.75, 95% CI: 0.57–0.97; HR legume − derivedSPD = 0.68, 95% CI: 0.52–0.88; HR nut − derivedSPD = 0.66, 95% CI: 0.53–0.80; HR cheese − derivedSPD = 0.68, 95% CI: 0.52–0.88) and all-cause mortality (HR vegetable − derivedSPD = 0.73, 95% CI: 0.64–0.84; HR cereal − derivedSPD = 0.80, 95% CI: 0.69–0.93; HR legume − derivedSPD = 0.70, 95% CI: 0.60–0.80;HR nut − derivedSPD = 0.72, 95% CI: 0.64–0.81; HR cheese − derivedSPD = 0.70, 95% CI: 0.61–0.81) than those in the lowest tertiles or quartiles. Moreover, subgroup analysis showed consistent associations among the people with hypertension and hyperlipidemia.
Conclusion: Higher intake of dietary SPD is associated with decreased risk of CVD and all-cause mortality, and among specific food origin SPD, SPD derived from vegetables, cereals, legumes, nuts, and cheese was associated with reduced CVD and all-cause mortality.
The rising prevalence of cardiovascular disease (CVD) has become the leading cause of morbidity and mortality worldwide that reduces human life expectancy and causes a heavy burden on the healthcare system (1). Increasing evidence establishes that nutritional factors are strongly associated with the development, treatment, and prevention of CVD (2, 3). Spermidine (SPD), as a naturally occurring endogenous polyamine, is widely available in foods of both animal and plant origins and plays a crucial role in the growth and development of eukaryotic cells (4). Increasing epidemiologic research has demonstrated that SPD has a wide range of beneficial effects, such as cardiovascular protection, immune system regulation, and neuroprotective effects (5–7).
Numerous mechanism studies have indicated that SPD could exhibit remarkable cardiac and vascular protection in a variety of model organisms by stimulating autophagy as well as anti-inflammatory and anti-oxidative stress pathways. For example, feeding SPD reduced myocardial hypertrophy and improved cardiomyocyte elasticity in aged mice via enhancing arterial expression of autophagy markers (8); significantly decreased infarct size and alleviated myocardial hypertrophy in SD rats by increasing autophagic flux (9); alleviated atherosclerosis by reducing epithelial fat accumulation in APOE model mice (10); and downregulated endoplasmic reticulum stress signaling components in mice with kidney injury (11).
With increasing mechanism evidence of the beneficial effects of SPD, greater attention has been paid to its life span-extending effects, which have been well demonstrated across species including yeast, nematodes, flies, mice, and rats (12–14). However, we cannot conclude that its beneficial effects are applicable to the general population because research on the relationship between SPD and survival time has been mostly limited to animal models with no epidemiological validation. Therefore, we proposed a hypothesis that higher dietary SPD is associated with increased survival time in human beings. In this study, we examined the association between dietary SPD intake and all-cause and disease-specific mortality in the U.S. population recruited in the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2014.
NHANES is a stratified, multistage study using a nationally representative sample of the non-institutionalized civilian population of the U.S. Detailed NHANES has been provided elsewhere (15). After excluding participants with missing information on dietary SPD, all-cause and CVD mortality, and other covariates, 23,894 adults (age ≥ 18 years) with the data of interviews and examinations who participated in NHANES from 2003 to 2014, including 10,942 men and 12,952 women, were selected for this study. The NHANES protocol was approved by the National Health Statistics Research Ethics Committee, and written informed consent was obtained before data collection.
A 24-h dietary recall survey was used to obtain food intake on 2 non-consecutive days. The first 24-h dietary recall was conducted in-person, and the second 24-h dietary recall was conducted by telephone 3 to 10 days later. Dietary energy and nutrient intakes were estimated using the USDA Dietary Study Food and Nutrient Database. Dietary intake components were integrated into 37 MyPyramid major groups and subgroups according to the USDA MyPyramid Equivalency Database 2.0 (MPED 2.0) User's Guide for Survey Foods. The mean values of nutrient intake on the first and the second day of the 24-h dietary recall were calculated in the analysis. Dietary supplement use was obtained through a dietary supplement questionnaire.
We calculated the amount of total dietary SPD and the amount of SPD in foods of animal origin including fresh meat, cooked meat, dairy products, eggs, cheese, and seafood, and in foods of plant origin including vegetables, fruits, cereals, legumes, and nuts. The average SPD content (nmol/g) in various foods was determined based on previous research (16). The daily intake of SPD (nmol/d) was based on the average SPD content (nmol/g) and the daily intake of various food components (mg/d).
The main exposures in our study were the accounts of total dietary SPD intake and SPD intake from foods of plant origin including vegetables, fruits, cereals, legumes, and nuts, and foods of animal origin including fresh meat, cooked meat, dairy products, eggs, cheese, and seafood. The outcome was the status of mortality as determined by the National Death Index (NDI). The NDI is a considerably reliable and widely used death identification resource. The ICD-10 was used to determine disease-specific death. ICD-10 codes I00–I09, I11, I13, I20–I51, or I60–I69 were assigned to death due to CVD. In summary, a total of 736 deaths due to CVD and 2,365 deaths due to all-cause were documented.
The covariates in our study included age (years), sex (male/female), race/ethnicity (non-Hispanic white/Mexican American/non-Hispanic black/other), Alternative Healthy Eating Index (AHEI), current smoker (yes or no, a current smoker was defined as someone who had smoked 100 cigarettes in his or her lifetime and reported currently smoking), current drinker (having at least 12 alcohol drinks per year or not), education level (less than high school education, high school, or above), annual household income (<$20 000, ≥$20 000 and <$45 000, ≥$45 000 and <$75 000, ≥$75 000 and <$100 000, or ≥$100 000), total energy intake from the 24-h dietary recall (kcal/d), body mass index (BMI, kg/m2), regular exercise (having engaged in recreational moderate and vigorous physical activity (MVPA) in the past 30 days or no), and a history of hypertension, diabetes, or hyperlipidemia defined as a physician diagnosis of self-reported hypertension, diabetes, or hyperlipidemia.
Demographic, dietary nutrient intake, and anthropometric characteristics were presented using the mean and standard deviation for the continuous variables, and number and percentage for categorical variables. The baseline characteristics were analyzed using chi-square tests and generalized linear models adjusted for age, and gender. All statistical analyses were performed by R 4.1.2 software, and the two-sided P < 0.05 was regarded as statistically significant.
CPH models were used to calculate hazard ratios (HRs) and 95% CI for all-cause and CVD mortality. The time scale in the Cox model used the follow-up time obtained by person-months from the date of the interview to their death, or the end of 2015. The dietary SPD was categorized into quartiles and tertiles, and the lowest quartile and tertile are regarded as the reference group. The confounders in the CPH model included age, sex, race, smoking status, drinking status, exercise, total energy intake, education level, energy intake, annual household income, BMI, AHEI, diabetes, hypertension, and hyperlipidemia. We performed a log transformation of all non-normal continuous variables. The gender interaction in the Cox proportional hazard (CPH) model was conducted.
A total of five sets of sensitivity analyses were performed in this study. In sets 1 to set 2, we analyzed the relationship between dietary SPD and all-cause and CVD mortality in the hypertension population and hyperlipidemia population, respectively, to identify the robustness of our results. In set 3 and set 4, we analyzed the relationship between dietary SPD and all-cause and CVD mortality in male and female populations separately. The participants who had a follow-up time <5 years were analyzed in set 5.
The demographic and nutritional characteristics of the participants are presented in Table 1. Compared with survivors, the participants with CVD and all-cause mortality were more likely to be male, older, and non-Hispanic whites; have a higher prevalence of hypertension, diabetes, and hyperlipidemia; have lower BMI, household income, education level, and total energy intake; and more prone to have a higher intake of dairy-derived SPD and a lower intake of total SPD, vegetable-derived SPD, legume-derived SPD, fresh meat-derived SPD, nut-derived SPD, and cheese-derived SPD (all P < 0.05).
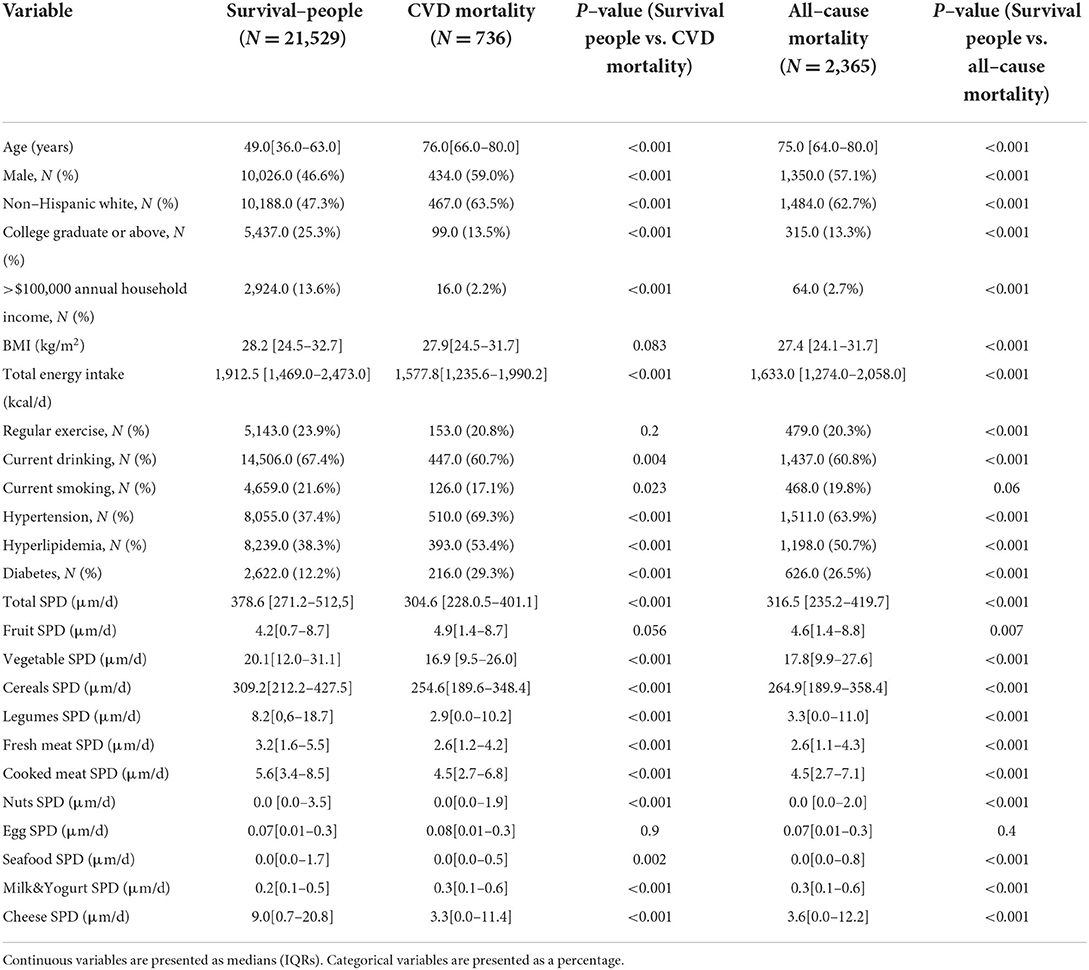
Table 1. Baseline characteristics of variables in survived people, CVD mortality, and all-cause mortality status.
The associations between dietary total SPD and specific food-derived SPD intake with all-cause and CVD mortality in the total population are presented in Figures 1, 2. As indicated by HR and 95% CI, the participants in the highest quartiles (quartile 4) of total SPD had a lower risk of CVD mortality (HR = 0.68, 95% CI: 0.51–0.91) and all-cause mortality (HR = 0.70, 95% CI: 0.60–0.82) than those in the lowest quartile (quartile 1). Also, the intake of SPD from four specific food sources (vegetables, cereals, legumes, nuts, and cheese) was significantly associated with mortality outcomes.
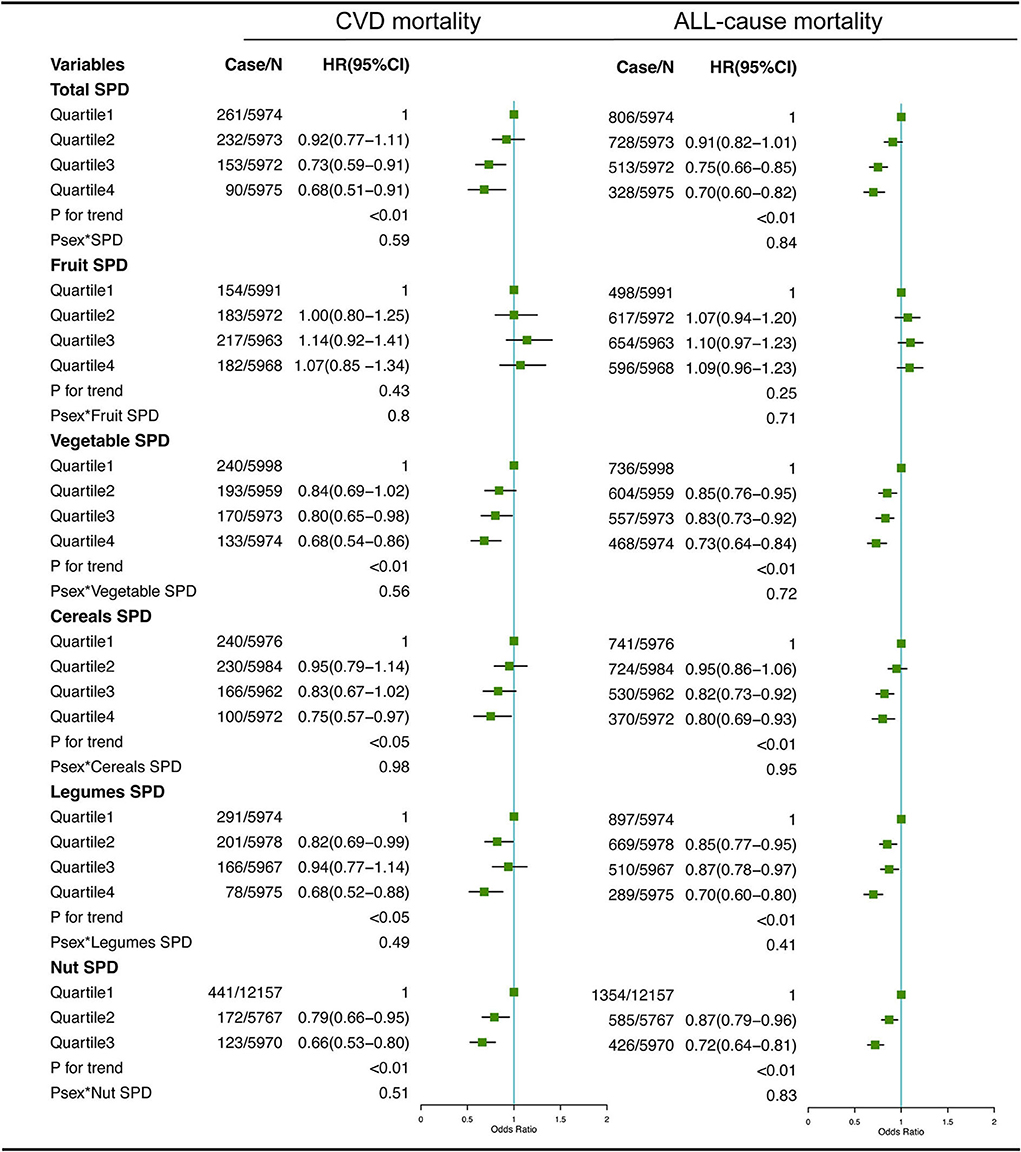
Figure 1. Multivariate adjusted hazard ratios (HRs) of the dietary total SPD, fruit-derived SPD, vegetable-derived SPD, cereal-derived SPD, legume-derived SPD, and nut-derived SPD with CVD and all-cause mortality. A logarithmic transformation was performed for non-normal continuous variables. Adjusting factors included age, gender, race, income, education level, regular exercise, smoking, alcohol consumption, BMI, body mass index; total energy intake, AHEI, Alternative Healthy Eating Index; diabetes, hypertension, and hyperlipidemia.
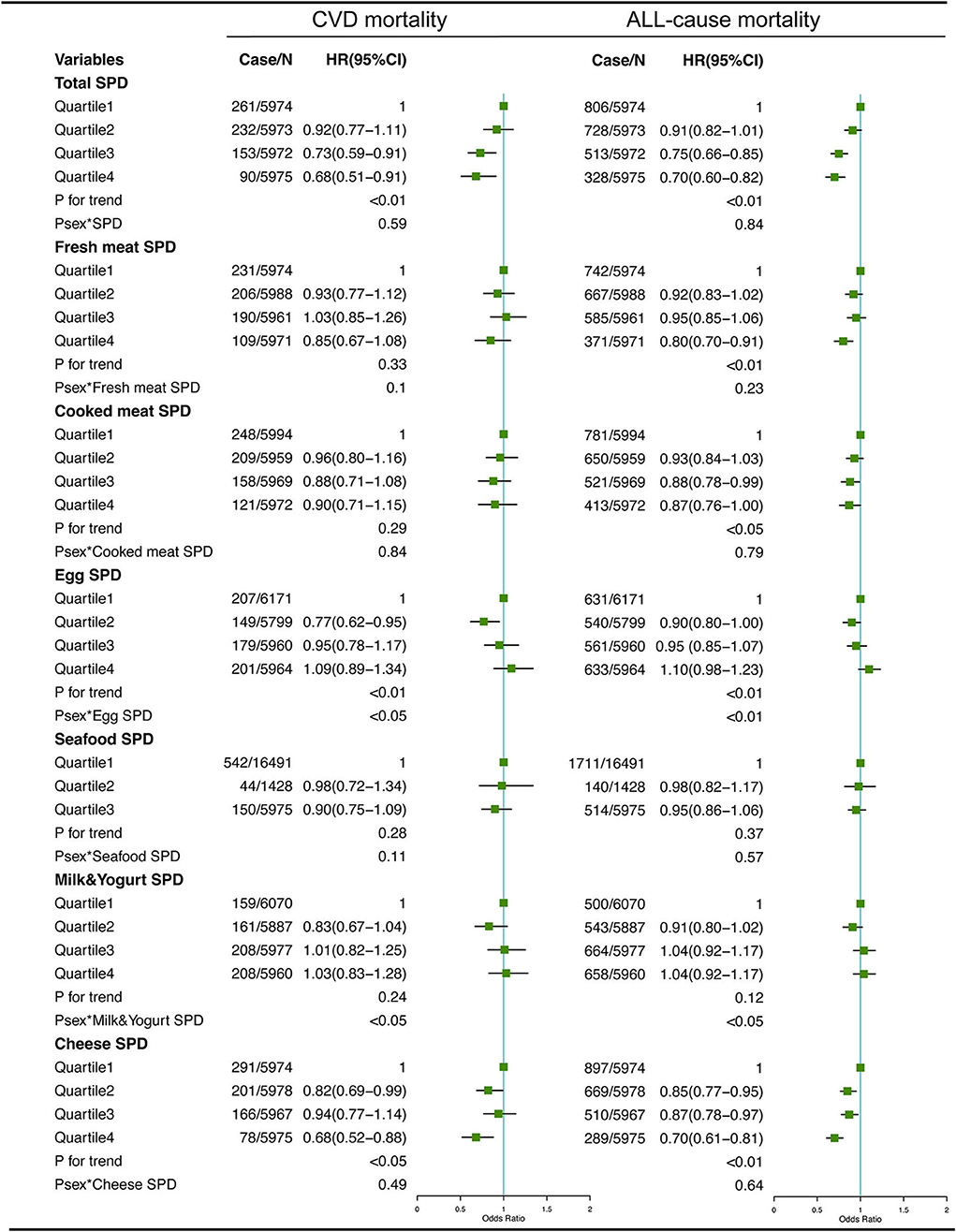
Figure 2. Multivariate adjusted hazard ratios (HRs) of the dietary total SPD, fresh meat-derived SPD, cooked meat-derived SPD, egg-derived SPD, seafood-derived SPD, milk & yogurt-derived SPD, and cheese-derived SPD with CVD and all-cause mortality. A logarithmic transformation was performed for non-normal continuous variables. Adjusting factors included age, gender, race, income, education level, regular exercise, smoking, alcohol consumption, BMI, body mass index; total energy intake, AHEI, Alternative Healthy Eating Index; diabetes, hypertension, and hyperlipidemia.
For different dietary sources of SPD, the participants in the highest quartiles or tertiles had a lower risk of CVD mortality (HR = 0.68, 95% CI: 0.54–0.86 for vegetable-derived SPD; HR = 0.75, 95% CI: 0.57–0.97 for cereal-derived SPD; HR = 0.68, 95% CI: 0.52–0.88 for legume-derived SPD; HR = 0.66, 95% CI: 0.53–0.80 for nut-derived SPD; HR = 0.68, 95% CI: 0.52–0.88 for cheese-derived SPD) and all-cause mortality (HR = 0.73, 95% CI: 0.64–0.84 for vegetable-derived SPD; HR = 0.80, 95% CI: 0.69–0.93 for cereal-derived SPD; HR = 0.70, 95% CI: 0.60–0.80 for legume-derived SPD; HR = 0.72, 95% CI: 0.64–0.81 for nut-derived SPD; HR = 0.70, 95% CI: 0.61–0.81 for cheese-derived SPD). Sex was not a significant effect modifier of the aforementioned association (P effect modification with sex > 0.05).
Consistent with the results of the total sample, analysis of subgroups among people with hypertension (Supplemental Figures 1, 2) and hyperlipidemia (Supplemental Figures 3, 4), as well as men (Supplemental Figures 5, 6), women (Supplemental Figures 7, 8), and those with less than 5 years of follow-up (Supplemental Figures 9, 10), also showed negative associations between the total and specific food source SPD intake and CVD and all-cause mortality, which indicated our results were relatively robust.
To the best of our knowledge, this study is the first epidemiologic study to assess the association between dietary SPD intake and all-cause and CVD mortality among the U.S. adult population. The results of this study showed that the consumption of total dietary SPD, vegetable-derived SPD, cereal-derived SPD, legume-derived SPD, nut-derived SPD, and cheese-derived SPD was associated with decreased risk of all-cause and CVD mortality. In addition, these associations were relatively robust, which could be consistently observed among different subgroups.
Currently, increasing experimental evidence has demonstrated the autophagy, anti-inflammatory, and anti-oxidative stress effects of SPD treatment. In addition, previous studies have indicated that SPD could prolong the life span of different species, from yeast to rodents, and promote the manifestation of age-related diseases via the induction of protective autophagy (12). However, very few epidemiological studies have found that dietary SPD may increase the survival time in the adult population, which is the most important finding of this study. Several studies have established an inverse association between dietary SPD and the risk of cancer-specific mortality, which could partially support our findings. In line with our epidemiological analysis finding, a prospective, population-based cohort study showed that dietary SPD intake was negatively linked to the prevalence of CVD. Moreover, animal studies also corroborated the cardiovascular protective effect of SPD. An aging mice model has revealed that SPD feeding could reduce myocardial hypertrophy and improve aging-related cardiomyocyte elasticity (17). Another animal study showed that SPD significantly reduced the infarct size of myocardial infarction in SD rats by increasing autophagic flux through the AMPK/mTOR signaling pathway and alleviated myocardial hypertrophy (9), and SPD-induced autophilia could prevent atherosclerosis by reducing epithelial fat accumulation in vascular smooth muscle cells (VSMCs) of the APOE model mice (10), alleviate vascular calcification in kidney injury (11), reverse aging-related vascular calcification, and improve aging-reduced aortic elasticity (8). These studies argued for autophagy as a pivotal mechanism underlying SPD-induced cardioprotection.
Autophagy is a complex degradation/recycling system in charge of non-apoptotic cell death and intracellular degradation of misfolded or aggregated proteins and dysfunctional organelles such as mitochondria, which is essential for maintaining cellular renovation and homeostasis (7, 18, 19). The role of SPD-induced autophagy has been widely demonstrated to be essential not only for cardiovascular system protection but also for alleviation of age-related cognitive impairment, and life span extension (7, 20, 21). Interestingly, autophagy and serum SPD in humans and multiple model organisms showed a significant age-related decrease (8). However, the prevalence of CVD dramatically increases with age (22). This has led to the conjecture that the autophagic capacity decreased with age, leading to a large accumulation of damaged cells and dysfunctional intracellular organelles in the cardiovascular system, thus causing CVD. However, intake of more dietary SPD and SPD-induced autophagy reversed this process, resulting in cardioprotective effects. This mechanism evidence may strongly support the negative association of dietary SPD with the reduced risk of CVD mortality.
In addition to the induction of autophagy, several potential mechanisms for the cardioprotective effects of SPD may exist as follows. As known, oxidative damage and inflammation are widely reported as the main factors leading to atherosclerosis, which is the most typical and major pathological change of CVD. The accumulation of oxidized LDL in the vascular endothelium is the most important factor during early plaque formation in atherosclerosis (23, 24), and the aggregation of pro-inflammatory factors may contribute to the acceleration of vascular calcification. As the most important member of the polyamine family, SPD is synthesized from putrescine and serves as a precursor of spermine (25). Numerous in vitro and in vivo experiments have shown that SPD and spermine may act as scavengers of ROS and then protect the cardiovascular system from oxidative damage (26). SPD has been linked to increased titin phosphorylation, which inhibits downstream inflammation and thus increases cardiomyocyte elasticity (17). In addition, due to its polycationic nature, SPD can readily bind negatively charged biological macromolecules, including DNA, RNA, proteins, and phospholipids, and can modulate the function of these macromolecules in many cases. It has been shown that SPD can enhance the stability and flexibility of DNA (26), which may be an underlying mechanism for the cardioprotective effect of SPD. Moreover, arginine, the raw material for SPD synthesis in vivo, is a substrate for the synthesis of nitric oxide (NO), which is a recognized cardiovascular dilator (27). NO also showed an age-related decrease (28, 29). It has been shown that SPD could improve the bioavailability of arginine for NO synthesis, implying that relatively sufficient SPD can lead to greater conversion of arginine to NO, thereby protecting the cardiovascular system (11, 12). This is another potential mechanism for the cardioprotective effect of SPD. In our study, these beneficial effects were found to be relatively robust, which was significant across many subgroups, including different genders and disease states, suggesting that a higher intake of dietary SPD contributes to a cardiovascular protective effect. Furthermore, significant sex-mediated effects were not found, except for the egg-derived SPD.
Another key finding of this study was the negative association between SPD from a specific food origin and CVD mortality. Our results suggest that vegetables, cereals, legumes, nuts, and cheese would be better sources of SPD to protect the cardiovascular system. SPD is widely found in all foods containing nucleic acids and is abundant in coarse cereals, wheat germ, vegetables, and fermented foods containing bacteria and fungi such as stinky cheese and natto (16, 30). We found that dietary intake of SPD from vegetables, cereals, legumes, nuts, and cheese sources could significantly reduce the risk of CVD mortality. These dietary SPD sources with cardioprotective effects coincided with the Mediterranean dietary pattern, which is a recognized dietary pattern with proven cardiovascular protective effects (31). Although the negative relationships between the consumption of vegetables, cereals, legumes, nuts, and cheese and CVD mortality have been well established (32–35), the association between dietary intake of SPD from the aforementioned foods and CVD mortality was still significant after adjusting for AHEI, which indicates the protective role of dietary SPD was independent of other beneficial ingredients in food. Therefore, vegetables, cereals, legumes, nuts, and cheese should be consumed in greater quantities as an ideal food source of cardiovascular protective SPD. In addition, SPD is a highly absorbable polyamine that could be taken up in the small intestine and utilized by multiple systems without secondary degradation in the circulatory system, which allows dietary SPD to significantly contribute to elevated SPD concentrations in the cardiovascular system (36, 37). That is also an underlying biological basis for the cardioprotective effects of dietary SPD.
This study has several strengths. First, this is the first epidemiologic study to evaluate the relationship between dietary SPD and CVD mortality in a representative population sample of U.S. adults. Second, we identified specific dietary sources of SPD with cardio protective effects, which provide guidance for dietary supplementation of SPD. Third, the association reported in this study was relatively robust that it was significant in a multitude of subgroups so that we could provide dietary supplementation strategies for SPD in gender-specific populations, as well as in populations with multiple chronic diseases. We are also aware that this study has certain limitations. First, although 24-h dietary recall is the most valid and universal method of investigating dietary information in observational studies, measurement error arising from day-to-day variation in food intake still exists. Second, we were unable to control for variables that were not measured in the observational study. Third, this study lacked an internal exposure evaluation of dietary SPD, which needs to be refined for future research.
In conclusion, a higher intake of dietary SPD is associated with decreased risk of CVD and all-cause mortality, and among specific food origin SPD, vegetable-, cereal-, legume-, nut-, and cheese-derived SPD was associated with reduced CVD and all-cause mortality.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
CS, TH, and WJ conceived the idea. TH and WW drafted the manuscript. HW, WJ, and HJ conducted data interpretation. CH, JW, and ZW conducted the first analysis. YC, WJ, XL, and XS conducted the second analysis for verification. All authors critically assessed, reviewed and approved the manuscript.
This research was supported by funds from HMU Marshal Initiative Funding (HMUMIF-21011 to WJ; HMUMIF-21013 to WW).
We thank all participants in our study for their cooperation and participation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.949170/full#supplementary-material
SPD, spermidine; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; HR, hazard ratio; NDI, National Death Index; BMI, body mass index; AHEI, Alternative Healthy Eating Index; CPH, Cox proportional hazards; NO, nitric oxide.
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Preventive Services Task US, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US preventive services task force recommendation statement. JAMA. (2020) 324:2069–75. doi: 10.1001/jama.2020.21749
3. Petermann-Rocha F, Parra-Soto S, Gray S, Anderson J, Welsh P, Gill J, et al. Vegetarians, fish, poultry, and meat-eaters: who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur Heart J. (2021) 42:1136–43. doi: 10.1093/eurheartj/ehaa939
4. Tong D, Hill JA. Spermidine promotes cardioprotective autophagy. Circ Res. (2017) 120:1229–31. doi: 10.1161/CIRCRESAHA.117.310603
5. Yang Q, Zheng C, Cao J, Cao G, Shou P, Lin L, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. (2016) 23:1850–61. doi: 10.1038/cdd.2016.71
6. Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. (2013) 16:1453–60. doi: 10.1038/nn.3512
7. Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. (2016) 30:147–60. doi: 10.1016/j.ccell.2016.05.016
8. LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. (2013) 134:314–20. doi: 10.1016/j.mad.2013.04.004
9. Yan J, Yan J-Y, Wang Y-X, Ling Y-N, Song X-D, Wang S-Y, et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br J Pharmacol. (2019) 176:3126–42. doi: 10.1111/bph.14706
10. Michiels CF, Kurdi A, Timmermans J-P, De Meyer GRY, Martinet W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis. (2016) 251:319–27. doi: 10.1016/j.atherosclerosis.2016.07.899
11. Liu X, Chen A, Liang Q, Yang X, Dong Q, Fu M, et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway. Aging Cell. (2021) 20:e13377. doi: 10.1111/acel.13377
12. Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. (2018) 359:eaan2788. doi: 10.1126/science.aan2788
13. Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. (2009) 11:1305–14. doi: 10.1038/ncb1975
14. Schroeder S, Hofer SJ, Zimmermann A, Pechlaner R, Dammbrueck C, Pendl T, et al. Dietary spermidine improves cognitive function. Cell Rep. (2021) 35:108985. doi: 10.1016/j.celrep.2021.108985
15. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. (2019) 322:1178–87. doi: 10.1001/jama.2019.13771
16. Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, Toro-Funes N, Veciana-Nogués MT, Vidal-Carou MC. Polyamines in Food. Front Nutr. (2019) 6:108. doi: 10.3389/fnut.2019.00108
17. Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. (2016) 22:1428–38. doi: 10.1038/nm.4222
18. Wang I-F, Guo B-S, Liu Y-C, Wu C-C, Yang C-H, Tsai K-J, et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A. (2012) 109:15024–9. doi: 10.1073/pnas.1206362109
19. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. (2011) 147:728–41. doi: 10.1016/j.cell.2011.10.026
20. Xu T-T, Li H, Dai Z, Lau GK Li B-Y, Zhu W-L, Liu X-Q, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY). (2020) 12:6401–14. doi: 10.18632/aging.103035
21. Ghosh I, Sankhe R, Mudgal J, Arora D, Nampoothiri M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides. (2020) 83:102083. doi: 10.1016/j.npep.2020.102083
22. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. (2020) 75:2921–30. doi: 10.1016/j.jacc.2020.04.038
23. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. (2011) 12:204–212. doi: 10.1038/ni.2001
24. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. (2006) 47:C7–12. doi: 10.1016/j.jacc.2005.09.068
25. Fan J, Feng Z, Chen N. Spermidine as a target for cancer therapy. Pharmacol Res. (2020) 159:104943. doi: 10.1016/j.phrs.2020.104943
26. Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. (1998) 95:11140–5. doi: 10.1073/pnas.95.19.11140
27. Patel KP, Schultz HD. Angiotensin peptides and nitric oxide in cardiovascular disease. Antioxid Redox Signal. (2013) 19:1121–32. doi: 10.1089/ars.2012.4614
28. Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. (2005) 66:286–94. doi: 10.1016/j.cardiores.2004.12.027
29. Eisenberg T, Abdellatif M, Zimmermann A, Schroeder S, Pendl T, Harger A, et al. Dietary spermidine for lowering high blood pressure. Autophagy. (2017) 13:767–9. doi: 10.1080/15548627.2017.1280225
30. Kalač P. Health effects and occurrence of dietary polyamines: a review for the period 2005-mid 2013. Food Chem. (2014) 161:27–39. doi: 10.1016/j.foodchem.2014.03.102
31. Fernández-García JC, Martínez-Sánchez MA, Bernal-López MR, Muñoz-Garach A, Martínez-González MA, Fitó M, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on the serum polyamine metabolome in individuals at high cardiovascular disease risk: a randomized clinical trial. Am J Clin Nutr. (2020) 111:975–82. doi: 10.1093/ajcn/nqaa064
32. Li H, Li J, Shen Y, Wang J, Zhou D. Legume Consumption and All-Cause and Cardiovascular Disease Mortality. Biomed Res Int. (2017) 2017:8450618. doi: 10.1155/2017/8450618
33. Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E, et al. Benefits of the mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis. (2015) 58:50–60. doi: 10.1016/j.pcad.2015.04.003
34. Chen G-C, Tong X, Xu J-Y, Han S-F, Wan Z-X, Qin J-B, et al. Whole-grain intake and total, cardiovascular, and cancer mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. (2016) 104:164–72. doi: 10.3945/ajcn.115.122432
35. De Pergola G, D'Alessandro A. Influence of mediterranean diet on blood pressure. Nutrients. (2018) 10:E1700. doi: 10.3390/nu10111700
36. Porter CW, Bergeron RJ. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. (1983) 219:1083–5. doi: 10.1126/science.6823570
Keywords: SPD, CVD mortality, all-cause mortality, NHANES, autophagy
Citation: Wu H, Wang J, Jiang H, Liu X, Sun X, Chen Y, Hu C, Wang Z, Han T, Sun C, Wei W and Jiang W (2022) The association of dietary spermidine with all-cause mortality and CVD mortality: The U.S. National Health and Nutrition Examination Survey, 2003 to 2014. Front. Public Health 10:949170. doi: 10.3389/fpubh.2022.949170
Received: 20 May 2022; Accepted: 25 August 2022;
Published: 28 September 2022.
Edited by:
Yutang Wang, Federation University Australia, AustraliaReviewed by:
Rui Pu, Zhejiang University, ChinaCopyright © 2022 Wu, Wang, Jiang, Liu, Sun, Chen, Hu, Wang, Han, Sun, Wei and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbo Jiang, MTAyNTkzQGhyYm11LmVkdS5jbg==; Wei Wei, d2Vpd2VpYnViYmxlMTk5NEAxNjMuY29t; Changhao Sun, Y2hhbmdoYW9zdW4yMDAyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.