- 1First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Cardiology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 3Department of Intensive Care Unit, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Introduction: Exposure to air pollution has been linked to the mortality of heart failure. In this study, we sought to update the existing systematic review and meta-analysis, published in 2013, to further assess the association between air pollution and acute decompensated heart failure, including hospitalization and heart failure mortality.
Methods: PubMed, Web of Science, EMBASE, and OVID databases were systematically searched till April 2022. We enrolled the studies regarding air pollution exposure and heart failure and extracted the original data to combine and obtain an overall risk estimate for each pollutant.
Results: We analyzed 51 studies and 7,555,442 patients. Our results indicated that heart failure hospitalization or death was associated with increases in carbon monoxide (3.46% per 1 part per million; 95% CI 1.0233–1.046, P < 0.001), sulfur dioxide (2.20% per 10 parts per billion; 95% CI 1.0106–1.0335, P < 0.001), nitrogen dioxide (2.07% per 10 parts per billion; 95% CI 1.0106–1.0335, P < 0.001), and ozone (0.95% per 10 parts per billion; 95% CI 1.0024–1.0166, P < 0.001) concentrations. Increases in particulate matter concentration were related to heart failure hospitalization or death (PM2.5 1.29% per 10 μg/m3, 95% CI 1.0093–1.0165, P < 0.001; PM10 1.30% per 10 μg/m3, 95% CI 1.0102–1.0157, P < 0.001).
Conclusion: The increase in the concentration of all pollutants, including gases (carbon monoxide, sulfur dioxide, nitrogen dioxide, ozone) and particulate matter [(PM2.5), (PM10)], is positively correlated with hospitalization rates and mortality of heart failure.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42021256241.
Introduction
Heart failure (HF) is a group of clinical syndromes characterized by dyspnea or fatigue caused by ventricular filling or ejection disorders or both (1), which is the ultimate destination of all cardiovascular diseases and is the leading cause of global morbidity and mortality (2). The incidence of HF is rising steadily worldwide, but the prognosis is still poor (3). More than 64 million people are suffering from HF in the world, with an estimated prevalence of 1–2% among adults in developed countries (4).
Air pollution is a worldwide problem affecting human health (5). It has been recognized as an independently detrimental factor to respiratory and cardiovascular diseases (6). According to the report of the World Health Organization, almost 7 million people die due to air pollution every year, indicating air pollution to be the world's largest environmental health risk (7). Particulate matters (PM) and gases are the main components of air pollution that have been reported to induce stroke (8), myocardial infarction (9), HF, atrial fibrillation, and sudden cardiac death (10) in multiple populations. Air pollution-induced cardiovascular events have received more attention than other related toxicity, such as pulmonary diseases (11). Epidemiological studies suggest an association between short-term fluctuations in ambient air pollution and the risk of hospitalization for acute cardiovascular events, including acute decompensated HF (12). In 2013, Shah et al. (13) reviewed and meta-analyzed the studies on air pollution and HF published from 1948 to 2012. The results showed that the increase in the concentration of all pollutants, except ozone, is positively related to the incidence and mortality of HF patients. However, all studies, but one in this meta-analysis, were conducted in developed countries. Moreover, over 22 studies, including 4 million participants and 14 studies in developing countries, focusing on the relationship between air pollution and HF have been published after Shah's study (13).
Thus, we updated the systematic review and meta-analysis to reassess the relationship between air pollutants and HF outcomes (hospitalization and death). This systematic review and meta-analysis were performed according to the guidelines of the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) criteria (Supplementary Table S1). This meta-analysis has been registered with PROSPERO (ID: CRD42021256241).
Methods
Search strategy
Literature was searched in four databases, including PubMed, Web of Science, EMBASE, and OVID databases, from their inception until 30 April 2022. The following keywords were included for the search: “heart failure,” “air pollution,” “particulate matter,” “ozone,” “carbon monoxide,” “sulfur dioxide,” “nitrogen dioxide,” and “carbon dioxide.”
First, we performed a preliminary screening of the titles and abstracts. Then, we further evaluated the full texts of potentially eligible studies. We manually searched the reference lists of all the included studies. Literature selection and study quality assessment were completed by two independent authors (Y.Y. and Y.G.), and conflicts between the two authors were resolved after a discussion with an arbitrator (Y.P.).
Inclusion and exclusion criteria
Articles that met the following criteria were included: (1) original human epidemiological studies reported associations between air pollution exposure and HF hospitalization rate or mortality up to and including lag (day) 7, confidence interval (95%CI); (2) case-crossover, time series (both assessed by generalized linear regression models); (3) focused on exposure of outdoor (ambient) air pollution, but not of indoor air pollution; and (4) published in English. Studies were excluded if they were (1) animal or experimental studies; (2) case reports, comments, or reviews; or (3) studies that reported an unclear increment of air pollutant concentrations.
Data extraction
Data were extracted from all the selected studies, including (1) study characteristics (first author, published year, study location, and period); (2) study populations (sample size and range of age); (3) outcomes [hospitalization rates and mortality, lag (days)]; (4) air pollution measurement method and increment of air pollution used in effect evaluation, including per interquartile range (IQR), standard deviation (SD), or per 10 μg/m3; and (5) effect estimates of the association between air pollution and HF (OR, RR, with 95% CI). The effect estimates of the single-pollutant model were extracted.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale (NOS) checklist was applied to assess the quality of the studies enrolled in this study. Two authors (Y.Y. and Y.G.) worked independently, and inconsistencies in the quality assessment were resolved through discussion with an arbitrator (Y.P.). The score ranges from 0 to 9 points. A higher score indicates a higher study quality. A study with a score of ≥7 was regarded as high quality; otherwise, the study was considered low quality (14).
Data synthesis
We synthesized the data extracted from the included studies by the following formula. The relative risk (RR) is combined into a standardized pollutant concentration increment before the meta-analysis as follows: 10 μg/m3 for PM2.5 and PM10; 10 parts per billion for nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone (O3); and 1 part per million for carbon monoxide (CO).
Many studies provide multiple estimates of a single lag, such as lag 0 or lag 1, which are merged separately. The shortest lag is used to evaluate the overall risk estimate. A few studies provided only cumulative lag (e.g., lag 0–1 or lag 0–2), which is not suitable for merging in a single lag analysis but is used to determine the overall risk estimates.
The original study data were further stratified by study design (case-crossover vs. time series), age (all ages vs. 60 years), and outcome (hospitalization vs. mortality). Assuming the prevalence of air pollution exposure is 100%, we used our overall risk estimate and formula to calculate the population-attributable risk for each pollutant as follows:
Statistical analysis
Stata version 15.0 (StataCorp., College Station, TX, USA) software was used to calculate the impact of each pollutant on the HF hospitalization rate or mortality and the 95% CI. The significance of the pooled OR and RR was determined by the Z test (15), and a p-value of < 0.05 was considered statistically significant.
The heterogeneity test was performed by the standard I2 test. If I2 ≥ 50% (P ≤ 0.10), it was considered that there was heterogeneity among the studies, and then, the Dersimonian–Laird random-effects model was used to combine the values. If I2 < 50% (P > 0.10), the research was regarded as homogeneous, and then, the Mantel–Haenszel fixed-effects model was performed to merge values. We expected that the heterogeneity between studies was due to different study designs, analysis methods, different lagging exposures, and geographic and population differences.
The sensitivity analysis was performed by eliminating individual studies one by one to evaluate the stability of the results. The funnel plot and Egger linear regression were used to test for publication bias, and if there was publication bias, the cut-and-compensation method was used to correct the bias.
Results
Search process and study characteristics
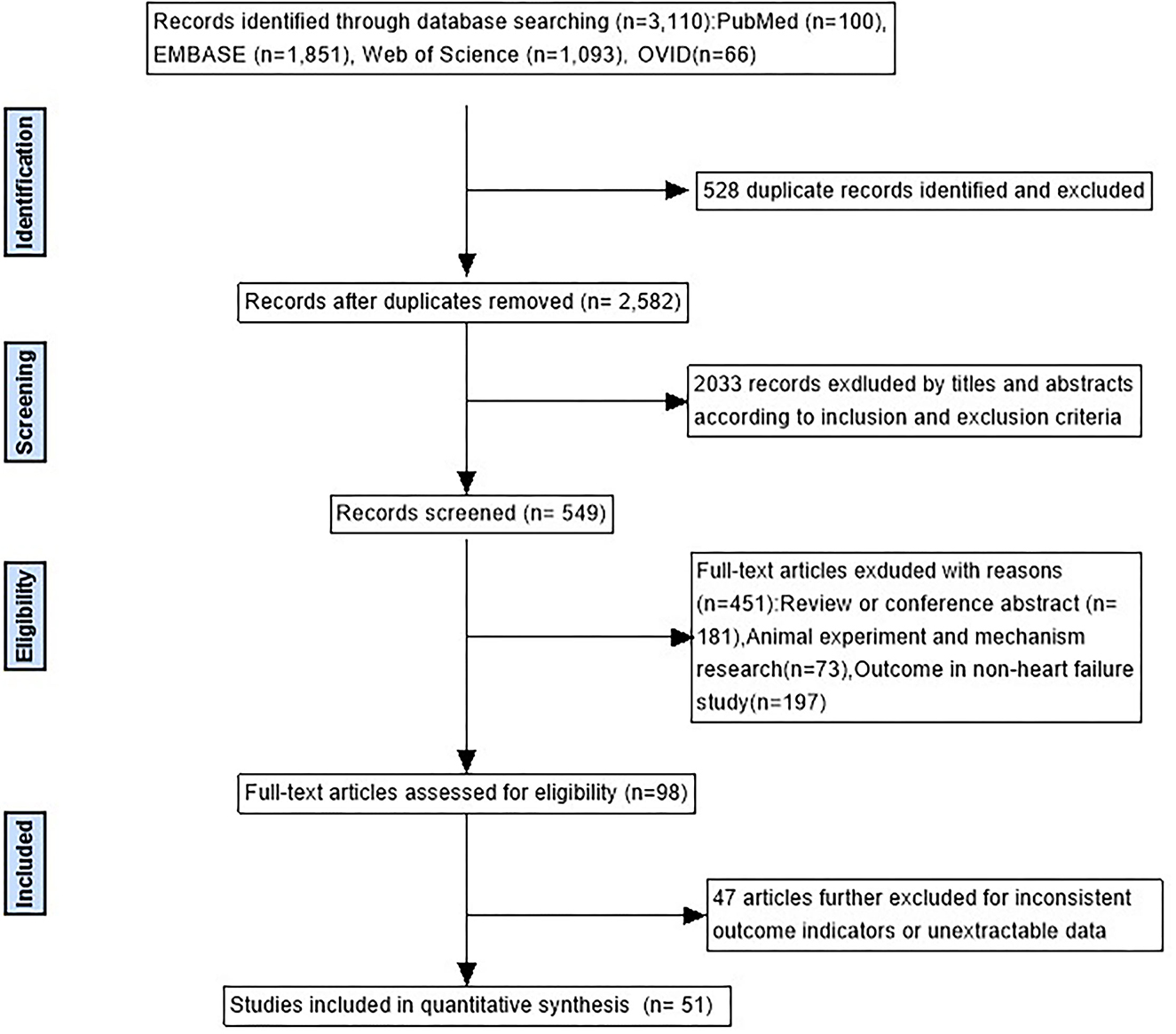
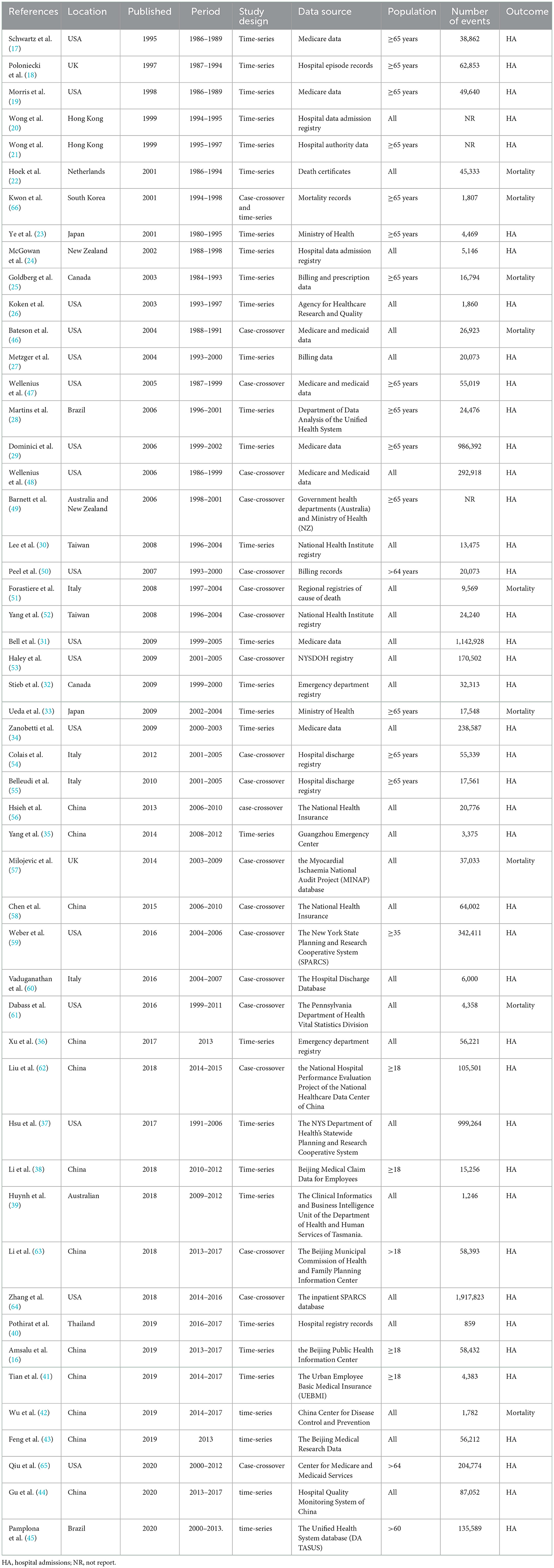
As can be seen in Figure 1, there were 3,110 studies enrolled in the initial search. After removing duplicates and screening titles, 549 studies underwent in-depth review, and 51 studies matched the inclusion criteria. In all, 30 studies used a time series design (16–45), 20 studies used a case-crossover design (46–65), and 1 used both study designs (66). Of the 51 studies, 19 were from developing countries and 32 from developed countries. Specifically, 21 are in the Americas (17 in the United States, 2 in Canada, and 2 in Brazil), 20 in Asia (16 in China, 1 in Thailand, 1 in South Korea, and 2 in Japan), 7 in Europe (4 in Italy, 2 in the United Kingdom, and 1 in the Netherlands), and 3 in Oceania (2 in Australia and 1 in New Zealand). The general characteristics of the studies included in the meta-analysis and details of the quality assessment are displayed in Table 1 and Supplementary Table S2, respectively.
Exposure to air pollution and the rate of HF hospitalization or mortality
As can be seen in Figure 2, HF hospitalization or mortality was positively correlated with all air pollution, which was consistent with the results of Shah's (13) meta-analysis, except O3. HF hospitalization or mortality was increased by 3.46% (95%CI 1.0233–1.046, P < 0.001) per increase of 1 part per million of CO. Each 10 ppb increase in SO2, O3, and NO2, respectively, was associated with 2.20% (95%CI 1.0106–1.0335, P < 0.001), 0.24% (95% CI 1.0024–1.0166, P < 0.001), and 2.07% (95% CI 1.0106–1.0335, P < 0.001) increases in the risk of HF-related hospitalization or mortality. PM2.5 (1.29%, 95%CI 1.0093–1.0165, P < 0.001) and PM10 (1.30%, 95%CI 1.0102–1.0157, P < 0.001) were found to be positively associated with HF hospitalization or mortality (Supplementary Figures S1–S6). In addition, we conducted a subgroup analysis based on study design and age (Figure 3). There was no change in effect direction across all pollutants in these analyses (Supplementary Table S4).
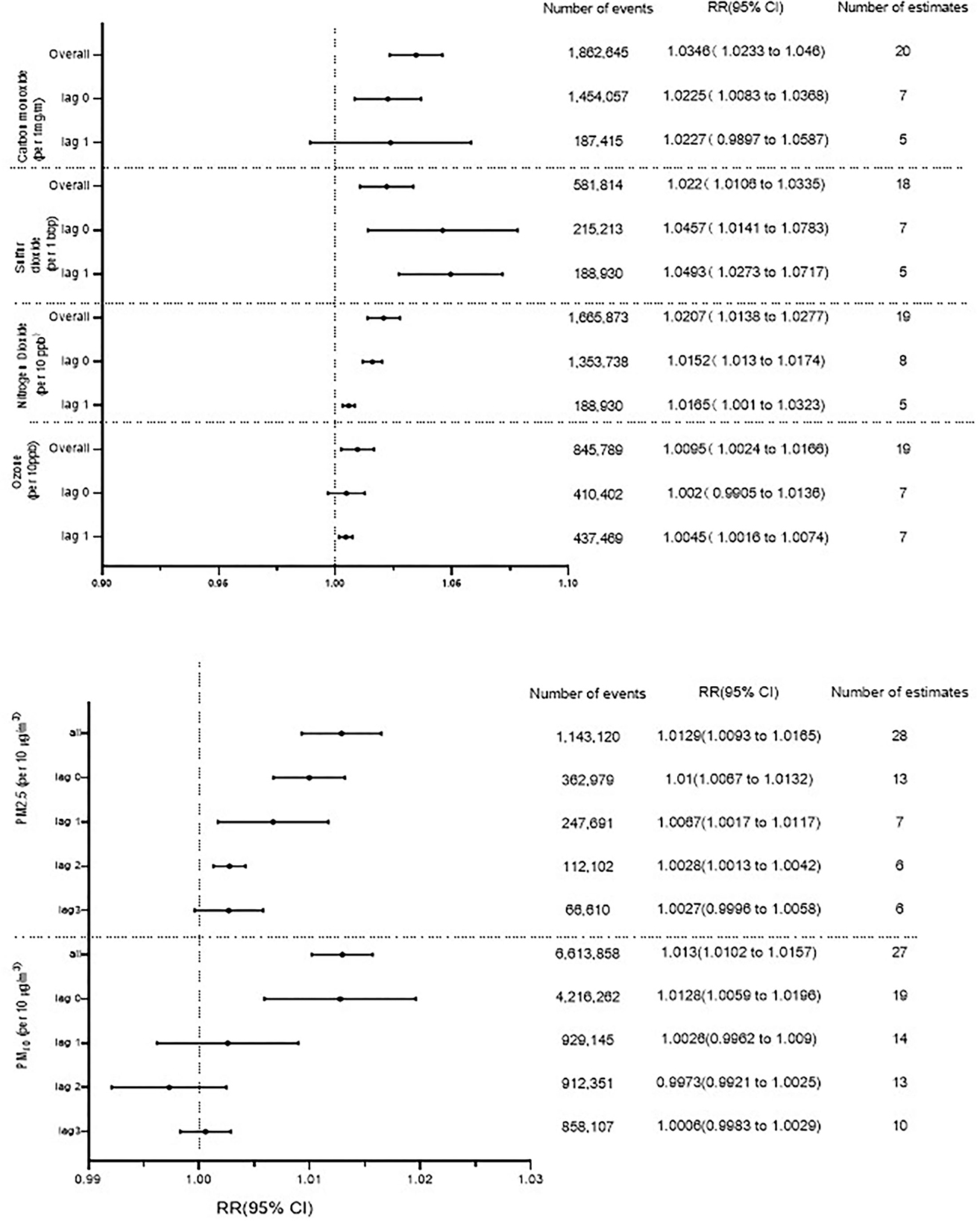
Figure 2. Association between gaseous and particulate air pollutants and heart failure hospitalization or heart failure mortality. ppm, parts per million; ppb, parts per billion.
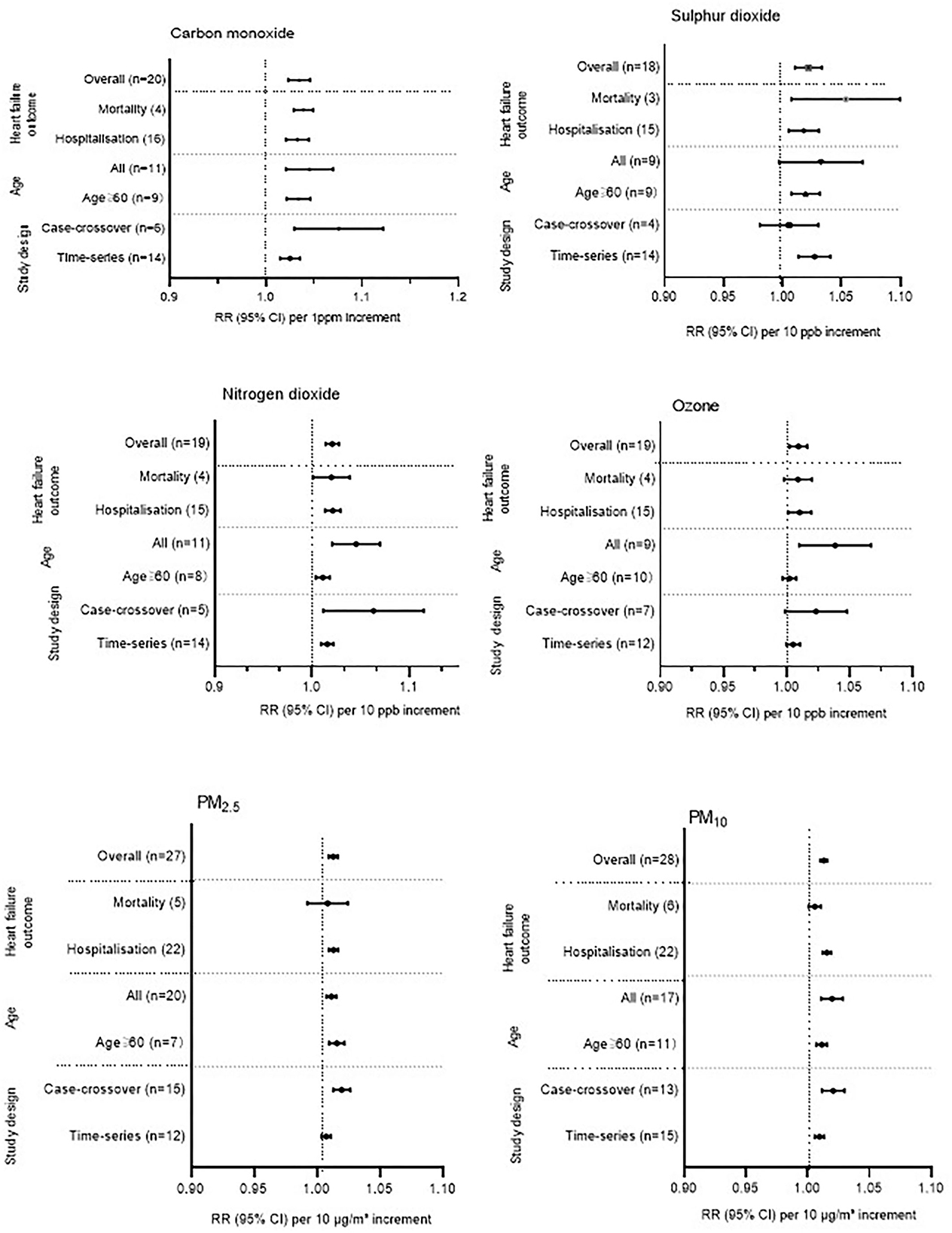
Figure 3. Additional analysis across all gaseous and particulate air pollutants. Some studies provided separate estimates for all age groups and for people older than 65 years. This study, therefore, appears two times in the additional analysis when stratified by age. For the overall analysis, we have used the estimates provided for all age groups. Some studies provided separate estimates stratified by study design and, therefore, appear twice in the additional analysis. For the overall analysis, we used the estimates provided for the time-series study design. ppm, parts per million; ppb, parts per billion.
Sensitivity analysis and publication bias
Sensitivity analysis showed that the relationship between PM2.5 exposure and HF hospitalization or mortality was influenced by Amsalu et al. (16) (Supplementary Figure S13). The pooled standardized RR was changed to 1.0142 (95% CI: 1.0102–1.0182) after removing Amsalu's study. The association between PM10 exposure and HF hospitalization or mortality was affected by Morris et al. (19) (Supplementary Figure S14). After excluding, the pooled RR was changed to 1.0186 (1.0133–1.024). The sensitivity bias was also found in CO [Bell et al.'s (31), Supplementary Figure S16] and NO2 [Ye et al.'s (23), Supplementary Figure S15]. We recalculated the pooled RR and 95% CI after removing those studies (Supplementary Table S3). The sensitivity analysis of SO2 and O3 did not change by excluding each study, suggesting that the results were stable (Supplementary Figures S17, S18).
There was publication bias on studies of PM2.5, CO, and NO2 (Supplementary Figures S7, S10, S11), since the p-value of Begg's test was < 0.05. Other pollutants have no substantial publication bias (Egger's test, p > 0.05, Supplementary Figures 8, 9, 12). After using trimming and filling methods to adjust asymmetry, the effect value was slightly lower than that before correction, but the direction of effect estimation does not change (Table 2).
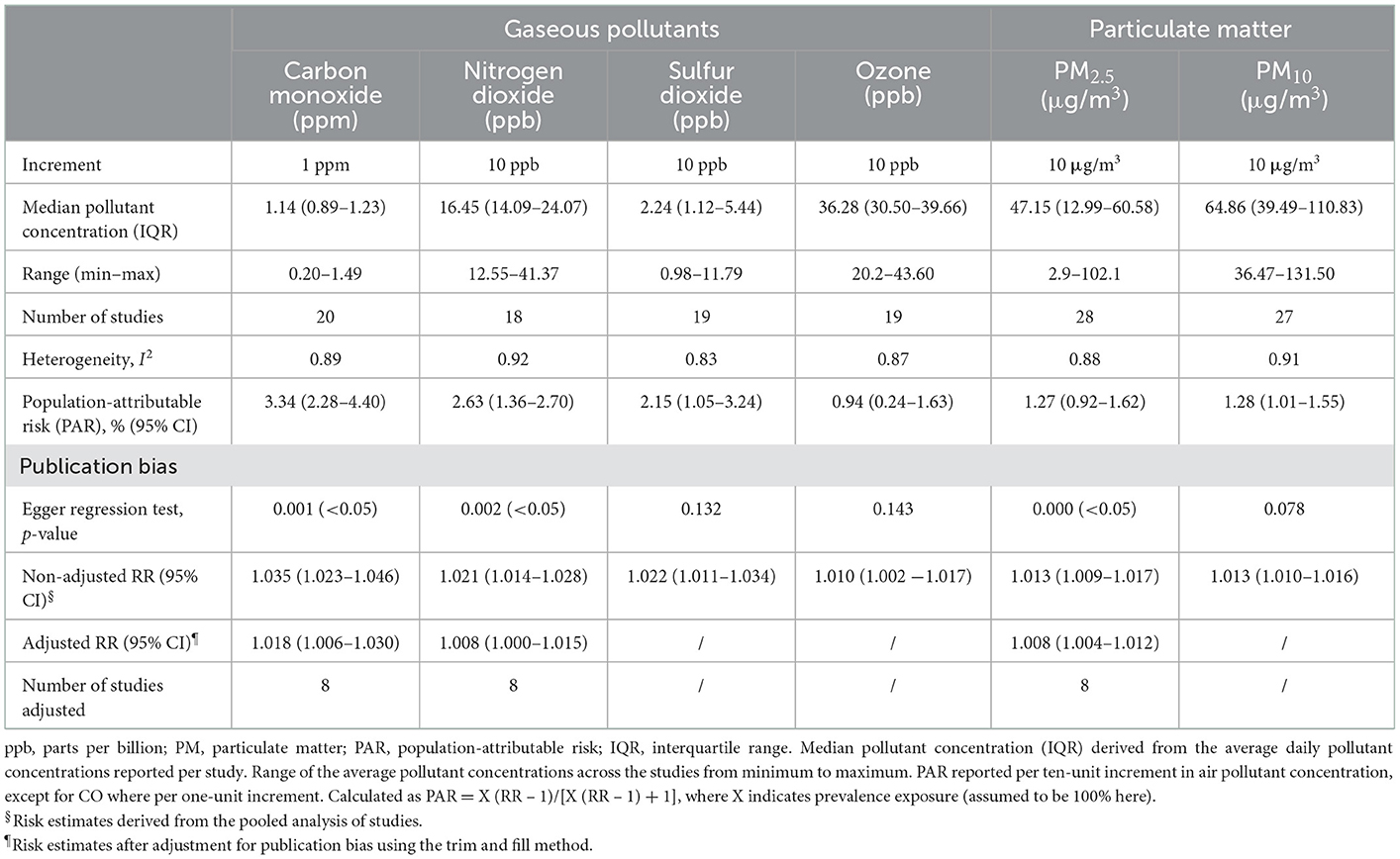
Table 2. Heterogeneity, population-attributable risk, and assessment for publication bias stratified by gaseous and particulate air pollutants.
Discussion
This updated systematic review and meta-analysis enrolled 51 human epidemiological studies conducted in 11 countries. We found that the increase in four gas pollutants (CO, NO2, SO2, and O3) and two particulate matters (PM2.5 and PM10) was positively correlated with the HF hospitalization or mortality rate, regardless of the overall effect or lag effect. These results suggest that air pollution exposure was the risk factor for hospitalization or death in patients with HF. Among all the included pollutants, CO exposure had the greatest impact on the risk of hospitalization or death in patients with HF, while O3 exposure seemed to be the weakest one.
In 1995, Schwartz et al. (17) conducted the first human epidemiological study on air pollution exposure and the HF hospitalization rate in the United States and found that PM10 and CO, but not SO2 and O3, were positively correlated with HF. Since then, many studies explored the relationship between air pollution and HF. These studies showed inconsistent results. In 2013, a meta-analysis by Shah (13) showed that air pollution had a close temporal association with HF hospitalization and mortality. However, only one of the 35 studies was conducted in developing countries, making it difficult to assess the impact in developing countries. In Shah's study, exposures to PM2.5, PM10, SO2, NO2, and CO were positively correlated with HF hospitalization and mortality. However, exposure to ozone did not present this impact. Moreover, there were few studies on air pollution and HF in developing countries at that time. Only five studies in Shah's meta-analysis were from developing countries. In our meta-analysis, we updated the literature to 30 April 2022 and added 22 studies, including 14 studies from developing countries. We enrolled 7,555,442 cases, which was much more than the 3,374,700 participants in Shah's study (13). These made our evidentiary weight stronger.
Our results showed a high degree of heterogeneity, which might be due to the different pollutant detection methods and ethnicity and populations in different studies. Therefore, we aggregated estimates of effects based on standardized increments, which could help reduce heterogeneity. In a sensitivity analysis, we found that the direction remained unchanged and the correlation was positive after removing some studies.
Studies showed that PM2.5 is a critical factor for overall HF progression by regulating lung oxidative stress, inflammation, and RV remodeling (67). PM2.5 exposure triggered oxidative stress in the heart and systemic inflammation (68) and inhibited vascular endothelial repair capacity (69). It was reported that air pollution aggravated aortic endothelial dysfunction in HF rats (69). In addition, CO aggressively binds to hemoglobin with an affinity 200 times greater than that of oxygen, resulting in a reduced fraction of oxygenated hemoglobin in the bloodstream (70). This weakens the blood's capacity to deliver oxygen to the tissues (71). Thus, chronic exposure to high levels of CO will induce tissue hypoxia. Some studies indicated that exposure to SO2 was sufficient to disrupt excitatory synaptic inputs to cardiac vagal neurons, the reflexive control part of heart rate, and induce tachycardia (72). Taken together, many studies reported the relationship between air pollution and HF. However, the molecular mechanism is still ambiguous.
There were several limitations to our study. First, part of the data came from routine administrative sources, which might introduce bias due to coding errors and misclassification. Second, there were three kinds of pollutants (PM2.5, SO2, and NO2) that had publication bias despite the direction of the adjusted overall effect remaining unchanged. Third, we found significant heterogeneity across all pollutants, which indicated the differences in population demographics, sample size, and patient characteristics.
Heart failure is a common and fatal disease. We found that exposure to all pollutants, including ozone, was positively associated with HF hospitalization and mortality. However, due to the limited number of studies on short-term effects, caution should still be taken in interpreting our results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
X-hC, PY, and Y-hP designed the study, coordinated the study, and directed its implementation. Y-sY and Y-yG collected data and conducted the follow-up work. Y-sY and J-fZ wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China under grant no. 81700243, the subject of Jiangsu Province Hospital of Traditional Chinese Medicine under grant no. Y2020CX42, and the graduate training innovation project of Jiangsu Province (grant nos. SJCX21_0780 and KYCX21_1714).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.948765/full#supplementary-material
References
1. Murphy SP, Ibrahim NE, Januzzi JJ. Heart failure with reduced ejection fraction: a review. JAMA. (2020) 324:488–504. doi: 10.1001/jama.2020.10262
2. Ruan G, Ren H, Zhang C, Zhu X, Xu C, Wang L. Cardioprotective effects of QiShenYiQi dripping pills on transverse aortic Constriction-Induced heart failure in mice. Front Physiol. (2018) 9:324. doi: 10.3389/fphys.2018.00324
3. Billingsley HE, Hummel SL, Carbone S. The role of diet and nutrition in heart failure: a state-of-the-art narrative review. Prog Cardiovasc Dis. (2020) 63:538–51. doi: 10.1016/j.pcad.2020.08.004
4. Bueno H, Moura B, Lancellotti P, Bauersachs J. The year in cardiovascular medicine 2020: heart failure and cardiomyopathies. Eur Heart J. (2021) 42:657–70. doi: 10.1093/eurheartj/ehaa1061
5. Wu YF, Li ZY, Dong LL, Li WJ, Wu YP, Wang J, et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy. (2020) 16:435–50. doi: 10.1080/15548627.2019.1628536
6. Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. (2010) 33:2196–201. doi: 10.2337/dc10-0698
7. Giovanis E, Ozdamar O. Health status, mental health and air quality: evidence from pensioners in Europe. Environ Sci Pollut Res Int. (2018) 25:14206–25. doi: 10.1007/s11356-018-1534-0
8. Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): a longitudinal cohort study. Lancet. (2016) 388:696–704. doi: 10.1016/S0140-6736(16)00378-0
9. Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, et al. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc. (2013) 2:e4309. doi: 10.1161/JAHA.112.004309
10. Burroughs PM, Uwamungu JC, Bulka CM, Swett K, Perreira KM, Kansal MM, et al. Occupational exposures and cardiac structure and function: ECHO-SOL (echocardiographic study of latinos). J Am Heart Assoc. (2020) 9:e16122. doi: 10.1161/JAHA.120.016122
11. Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc. (2017) 6:e007170. doi: 10.1161/JAHA.117.007170
12. Lokken RP, Wellenius GA, Coull BA, Burger MR, Schlaug G, Suh HH, et al. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology. (2009) 20:137–42. doi: 10.1097/EDE.0b013e31818ef34a
13. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. (2013) 382:1039–48. doi: 10.1016/S0140-6736(13)60898-3
14. Chen X, Liu F, Niu Z, Mao S, Tang H, Li N, et al. The association between short-term exposure to ambient air pollution and fractional exhaled nitric oxide level: a systematic review and meta-analysis of panel studies. Environ Pollut. (2020) 265:114833. doi: 10.1016/j.envpol.2020.114833
15. Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, et al. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res. (2020) 185:109471. doi: 10.1016/j.envres.2020.109471
16. Amsalu E, Wang T, Li H, Liu Y, Wang A, Liu X, et al. Acute effects of fine particulate matter (PM2.5) on hospital admissions for cardiovascular disease in Beijing, China: a time-series study. Environ Health-Glob. (2019) 18. doi: 10.1186/s12940-019-0506-2
17. Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. (1995) 142:23–35. doi: 10.1093/oxfordjournals.aje.a117541
18. Poloniecki JD, Atkinson RW, de Leon AP, Anderson HR. Daily time series for cardiovascular hospital admissions and previous day's air pollution in London, UK. Occup Environ Med. (1997) 54:535–40. doi: 10.1136/oem.54.8.535
19. Morris RD, Naumova EN. Carbon monoxide and hospital admissions for congestive heart failure: evidence of an increased effect at low temperatures. Environ Health Persp. (1998) 106:649–53. doi: 10.1289/ehp.98106649
20. Wong TW, Lau TS, Yu TS, Neller A, Wong SL, Tam W, et al. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med. (1999) 56:679–83. doi: 10.1136/oem.56.10.679
21. Wong CM, Ma S, Hedley AJ, Lam TH. Does ozone have any effect on daily hospital admissions for circulatory diseases? J Epidemiol Community Health. (1999) 53:580–1. doi: 10.1136/jech.53.9.580
22. Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. (2001) 12:355–7. doi: 10.1097/00001648-200105000-00017
23. Ye F, Piver WT, Ando M, Portier CJ. Effects of temperature and air pollutants on cardiovascular and respiratory diseases for males and females older than 65 years of age in Tokyo, July and August 1980–1995. Environ Health Persp. (2001) 109:355–9. doi: 10.1289/ehp.01109355
24. McGowan JA, Hider RN, Chacko E, Town GI. Particulate air pollution and hospital admissions in Christchurch, New Zealand. Aust N Z J Public Health. (2002) 26:23–9. doi: 10.1111/j.1467-842X.2002.tb00266.x
25. Goldberg MS, Burnett RT, Valois M-F, Flegel K, Bailar JC III, Brook J, et al. Associations between ambient air pollution and daily mortality among persons with congestive heart failure. Environ Res. (2003) 91:8–20. doi: 10.1016/S0013-9351(02)00022-1
26. Koken PJM, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in denver. Environ Health Persp. (2003) 111:1312–7. doi: 10.1289/ehp.5957
27. Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd K, et al. Ambient air pollution and cardiovascular emergency department visits. Epidemiology. (2004) 15:46–56. doi: 10.1097/01.EDE.0000101748.28283.97
28. Martins LC, Pereira LA, Lin CA, Santos UP, Prioli G, Luiz OC, et al. The effects of air pollution on cardiovascular diseases: lag structures. Rev Saude Publica. (2006) 40:677–83. doi: 10.1590/S0034-89102006000500018
29. Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. (2006) 295:1127. doi: 10.1001/jama.295.10.1127
30. Lee I, Tsai S, Ho C, Chiu H, Yang CY. Air pollution and hospital admissions for congestive heart failure in a tropical city: Kaohsiung, taiwan. Inhal Toxicol. (2008) 19:899–904. doi: 10.1080/08958370701479406
31. Bell ML, Peng RD, Dominici F, Samet JM. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide. Circulation. (2009) 120:949–55. doi: 10.1161/CIRCULATIONAHA.109.851113
32. Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health-Glob. (2009) 8:25. doi: 10.1186/1476-069X-8-25
33. Ueda K, Nitta H, Ono M. Effects of fine particulate matter on daily mortality for specific heart diseases in Japan. Circ J. (2009) 73:1248–54. doi: 10.1253/circj.CJ-08-1149
34. Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. (2009) 8:58. doi: 10.1186/1476-069X-8-58
35. Yang C, Chen A, Chen R, Qi Y, Ye J, Li S, et al. Acute effect of ambient air pollution on heart failure in Guangzhou, China. Int J Cardiol. (2014) 177:436–41. doi: 10.1016/j.ijcard.2014.09.003
36. Xu Q, Wang S, Guo Y, Wang C, Huang F, Li X, et al. Acute exposure to fine particulate matter and cardiovascular hospital emergency room visits in Beijing, China. Environ Pollut. (2017) 220:317–27. doi: 10.1016/j.envpol.2016.09.065
37. Hsu WH, Hwang SA, Kinney PL, Lin S. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci Total Environ. (2017) 578:626–32. doi: 10.1016/j.scitotenv.2016.11.008
38. Li M, Wu Y, Tian Y, Cao Y, Song J, Huang Z, et al. Association between PM2.5 and daily hospital admissions for heart failure: a Time-Series analysis in beijing. Int J Env Res Pub He. (2018) 15:2217. doi: 10.3390/ijerph15102217
39. Huynh QL, Blizzard CL, Marwick TH, Negishi K. Association of ambient particulate matter with heart failure incidence and all-cause readmissions in Tasmania: an observational study. Bmj Open. (2018) 8:e021798. doi: 10.1136/bmjopen-2018-021798
40. Pothirat C, Chaiwong W, Liwsrisakun C, Bumroongkit C, Deesomchok A, Theerakittikul T, et al. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J Thorac Dis. (2019) 11:3070–83. doi: 10.21037/jtd.2019.07.37
41. Tian Y, Liu H, Wu Y, Si Y, Song J, Cao Y, et al. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: time series study in 184 major Chinese cities. BMJ. (2019) 367:l6572. doi: 10.1136/bmj.l6572
42. Wu T, Ma Y, Wu X, Bai M, Peng Y, Cai W, et al. Association between particulate matter air pollution and cardiovascular disease mortality in Lanzhou, China. Environ Sci Pollut R. (2019) 26:15262–72. doi: 10.1007/s11356-019-04742-w
43. Feng W, Li H, Wang S, Van Halm-Lutterodt N, An J, Liu Y, et al. Short-term PM10 and emergency department admissions for selective cardiovascular and respiratory diseases in Beijing, China. Sci Total Environ. (2019) 657:213–21. doi: 10.1016/j.scitotenv.2018.12.066
44. Gu J, Shi Y, Zhu Y, Chen N, Wang H, Zhang Z, et al. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med. (2020) 17:e1003188. doi: 10.1371/journal.pmed.1003188
45. Pontes Pamplona YDA, Arbex MA, Ferreira Braga AL, Amador Pereira LA, Martins LC. Relationship between air pollution and hospitalizations for congestive heart failure in elderly people in the city of São Paulo. Environ Sci Pollut R. (2020) 27:18208–20. doi: 10.1007/s11356-020-08216-2
46. Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? Epidemiology. (2004) 15:143–9. doi: 10.1097/01.ede.0000112210.68754.fa
47. Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. (2005) 161:1030–6. doi: 10.1093/aje/kwi135
48. Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven united states cities. Am J Cardiol. (2006) 97:404–8. doi: 10.1016/j.amjcard.2005.08.061
49. Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. (2006) 114:1018–23. doi: 10.1289/ehp.8674
50. Peel JL, Metzger KB, Klein M, Flanders WD, Mulholland JA, Tolbert PE. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am J Epidemiol. (2007) 165:625–33. doi: 10.1093/aje/kwk051
51. Forastiere F, Stafoggia M, Berti G, Bisanti L, Cernigliaro A, Chiusolo M, et al. Particulate matter and daily mortality. Epidemiology. (2008) 19:571–80. doi: 10.1097/EDE.0b013e3181761f8a
52. Yang CY. Air pollution and hospital admissions for congestive heart failure in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. (2008) 71:1085–90. doi: 10.1080/15287390802114428
53. Haley VB, Talbot TO, Felton HD. Surveillance of the short-term impact of fine particle air pollution on cardiovascular disease hospitalizations in New York State. Environ Health. (2009) 8:42. doi: 10.1186/1476-069X-8-42
54. Colais P, Faustini A, Stafoggia M, Berti G, Bisanti L, Cadum E, et al. Particulate air pollution and hospital admissions for cardiac diseases in potentially sensitive subgroups. Epidemiology. (2012) 23:473–81. doi: 10.1097/EDE.0b013e31824d5a85
55. Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. (2010) 21:414–23. doi: 10.1097/EDE.0b013e3181d5c021
56. Hsieh Y, Tsai S, Yang C. Fine particulate air pollution and hospital admissions for congestive heart failure: a case-crossover study in Taipei. Inhal Toxicol. (2013) 25:455–60. doi: 10.3109/08958378.2013.804609
57. Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart. (2014) 100:1093–8. doi: 10.1136/heartjnl-2013-304963
58. Chen Y, Weng Y, Chiu Y, Yang C. Short-term effects of coarse particulate matter on hospital admissions for cardiovascular diseases: a case-crossover study in a tropical city. J Toxicol Env Heal a. (2015) 78:1241–53. doi: 10.1080/15287394.2015.1083520
59. Weber SA, Insaf TZ, Hall ES, Talbot TO, Huff AK. Assessing the impact of fine particulate matter (PM2.5) on respiratory cardiovascular chronic diseases in the New York City Metropolitan area using Hierarchical Bayesian Model estimates. Environ Res. (2016) 151:399–409. doi: 10.1016/j.envres.2016.07.012
60. Vaduganathan M, De Palma G, Manerba A, Goldoni M, Triggiani M, Apostoli P, et al. Risk of cardiovascular, hospitalizations from exposure to coarse particulate matter (PM10) below the european union safety threshold. Am J Cardiol. (2016) 117:1231–5. doi: 10.1016/j.amjcard.2016.01.041
61. Dabass A, Talbott EO, Bilonick RA, Rager JR, Venkat A, Marsh GM, et al. Using spatio-temporal modeling for exposure assessment in an investigation of fine particulate air pollution and cardiovascular mortality. Environ Res. (2016) 151:564–72. doi: 10.1016/j.envres.2016.08.024
62. Liu H, Tian Y, Song J, Cao Y, Xiang X, Huang C, et al. Effect of ambient air pollution on hospitalization for heart failure in 26 of China's largest cities. Am J Cardiol. (2018) 121:628–33. doi: 10.1016/j.amjcard.2017.11.039
63. Li H, Wu J, Wang A, Li X, Chen S, Wang T, et al. Effects of ambient carbon monoxide on daily hospitalizations for cardiovascular disease: a time-stratified case-crossover study of 460,938 cases in Beijing, China from 2013 to 2017. Environ Health-Glob. (2018) 17:82. doi: 10.1186/s12940-018-0429-3
64. Zhang W, Lin S, Hopke PK, Thurston SW, van Wijngaarden E, Croft D, et al. Triggering of cardiovascular hospital admissions by fine particle concentrations in New York state: before, during, and after implementation of multiple environmental policies and a recession. Environ Pollut. (2018) 242:1404–16. doi: 10.1016/j.envpol.2018.08.030
65. Qiu X, Wei Y, Wang Y, Di Q, Sofer T, Abu Awad Y, et al. Inverse probability weighted distributed lag effects of short-term exposure to PM2.5 and ozone on CVD hospitalizations in New England Medicare participants - exploring the causal effects. Environ Res. (2020) 182:231–9. doi: 10.1016/j.envres.2019.109095
66. Ho-Jang Kwon SCFN. Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. (2001) Epidemiology. (2001) 12:413–9. doi: 10.1097/00001648-200107000-00011
67. Yue W, Tong L, Liu X, Weng X, Chen X, Wang D, et al. Short term Pm2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox Biol. (2019) 22:101161. doi: 10.1016/j.redox.2019.101161
68. Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. (2008) 116:937–42. doi: 10.1289/ehp.11027
69. Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, et al. Environment:Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. (2011) 68:224–30. doi: 10.1136/oem.2009.053991
70. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. (2010) 89:219–29. doi: 10.1177/0022034509359125
71. Babu D, Motterlini R, Lefebvre RA. CO and CO-releasing molecules (CO-RMs) in acute gastrointestinal inflammation. Br J Pharmacol. (2015) 172:1557–73. doi: 10.1111/bph.12632
Keywords: heart failure, air pollution, particulate matter, gas pollutant, meta-analysis
Citation: Yang Y-s, Pei Y-h, Gu Y-y, Zhu J-f, Yu P and Chen X-h (2023) Association between short-term exposure to ambient air pollution and heart failure: An updated systematic review and meta-analysis of more than 7 million participants. Front. Public Health 10:948765. doi: 10.3389/fpubh.2022.948765
Received: 20 May 2022; Accepted: 29 December 2022;
Published: 23 January 2023.
Edited by:
Mariana Matera Veras, University of São Paulo, BrazilReviewed by:
Asaad Sharhani, Ahvaz Jundishapur University of Medical Sciences, IranPeng Yin, Chinese Center for Disease Control and Prevention, China
Copyright © 2023 Yang, Pei, Gu, Zhu, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Yu,  yupengdoctor@126.com; Xiao-hu Chen,
yupengdoctor@126.com; Xiao-hu Chen,  chenxhdoctor1962@163.com
chenxhdoctor1962@163.com
†These authors have contributed equally to this work
 Yu-shan Yang
Yu-shan Yang