- 1Department of Epidemiology and Biostatistics, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
- 2Guangzhou Health Care Promotion Center for Primary and Middle Schools, Guangzhou, China
Objective: An ongoing debate surrounds the relationship between body composition and pubertal timing, in particular for boys. This cross-sectional study aimed to investigate the association of body composition with pubertal timing among children and adolescents.
Methods: A total of 1,493 boys and 1,261 girls who entered puberty were enrolled in Guangzhou, China. Tanner stages were evaluated by examination of breast development for girls and testicular volume for boys. Fat mass (FM) and fat-free mass (FFM) were determined by bioelectrical impedance analysis. Parameters for body composition were transformed into age-and gender-specific Z-scores. The association of body composition with pubertal timing was examined using multinomial logistic regression with inverse probability weighting (IPW) based on the propensity score.
Results: For boys, IPW analysis showed Z-scores of body fat percentage (BF%) and FM index (FMI) were negatively associated with early puberty (OR = 0.75, 95% CI = 0.64–0.87; OR = 0.74, 95% CI = 0.63–0.88). As for girls, in contrast to boys, positive associations were seen between BF% and FMI with early puberty (OR = 1.39, 95% CI = 1.19–1.64; OR = 1.59, 95% CI = 1.33–1.90). With respect to appendicular skeletal muscle mass index (ASMI), there was a positive association with early puberty and a negative one with late puberty in boys (OR = 1.26, 95% CI = 1.07–1.49; OR = 0.82, 95% CI = 0.69–0.99).
Conclusion: There is a positive association of FM with early puberty for girls while negative for boys. FFM yields a positive association with early puberty and a negative one with late puberty in boys, but not in girls. Our findings highlight the gender differences in the connection between body composition and pubertal onset.
Introduction
Puberty is an important transition phase marked by rapid physical and psychological changes from childhood to adulthood. The first landmark of pubertal events is the onset of puberty, of which the earliest external sign is the appearance of breast buds in girls and testicular enlargement in boys (1). The features of pubertal development are in a similar sequence for all children. However, age and pubertal status are partly dissociable, with a wide between-individual variation in the timing of pubertal onset. Girls normally enter puberty at ages ranging from 8 to 13 years, while boys occur ~1 year later (2). Since the late 19th century, a worldwide secular trend toward an earlier onset of puberty has been observed among both boys and girls (3–5). Beyond immediate impacts on physical and physiologic development (6–8), early onset of puberty has been broadly connected with adverse long-term adult health consequences, including increased risks of obesity, type 2 diabetes, cardiovascular diseases, and cancers (9–13). On the other hand, late puberty has a potentially negative effect on psychosocial stress and could also raise the risks of conditions, such as coronary heart disease for females, asthma for males, and poor overall health for both genders (9, 14–16). The onset of puberty is a complex and largely unknown process governed by interactions between genes and the environment (17). Identification of modifiable risk factors and underlying mechanisms for variation in puberty timing could provide useful insights to inform preventive measures to reduce the hazard.
In the 1970s, the “critical weight” hypothesis proposed by Frisch suggested increment in fat accretion is essential for triggering the puberty spurt (18). The relationship between obesity and pubertal onset has received substantial focus in recent decades (19–23). Association between obesity and early puberty has been well-established in girls (19–23), but less clear in boys. While some studies found obesity was linked to earlier puberty in boys (22, 24, 25), others reported a null association (19, 26), and yet others observed a positive association with late puberty (21, 23, 27).
The inconsistent findings in boys across studies could be due to the following reasons. (1) Reliable measure of pubertal events was challenging in boys, with different measures leading to diverse conclusions. Most studies applied the self-reported puberty questionnaires for voice break, pubic hair, growth spurt, or genital development (21, 28–30), which were susceptible to reporting bias. By contrast, examination of testicular volume is a more objective and stable assessment of the pubertal stage (31). (2) The limitation of body mass index (BMI) as a proxy of adiposity in most research might have also accounted for the inconsistency. BMI is actually a crude measure of body weight status that does not discriminate between fat mass (FM) and fat-free mass (FFM) (32). Especially in boys, weight gain is mainly structured by the increment of FFM, and thus using BMI might increase bias in the link of obesity to puberty. As BMI does not reflect the true picture of obesity, increasing attention has been attracted to the measure of body composition. Direct measurement of body fat could be obtained using bioelectrical impedance analysis (BIA), which determines the electrical impedance through the entire body and calculates estimates of FM and FFM. BIA technology has evolved remarkably, making it the most cost-effective method of measuring body composition scans (33). (3) In addition to FM, FFM might also have an impact on pubertal timing (34, 35), but with insufficient evidence on this issue. FFM and FM confer different health outcomes. Exploring the potential associations of FFM with puberty timing might be helpful to better understand the mechanism of pubertal onset. (4) Furthermore, confounding bias might constitute the main source of the inconsistencies across studies. Multiple factors, such as social and economic factors, physical activity, sleep, and dietary habit, might obscure the relationship between obesity and puberty timing (36–39). To overcome the problem of confounding bias, the propensity score-based inverse probability weighting (IPW) approach has been proposed recently (40). As an alternative method of covariate adjustment, propensity score-based IPW creates a pseudo-population in which covariate distribution is balanced between groups and mimics a randomization procedure, consequently controlling confounders in observational data (41). The propensity score-based IPW method has been reported to offer an advantage over traditional methods in minimizing the likelihood of confounding by a number of authors (42–44).
This cross-sectional study was designed to systematically investigate the association of FM and FFM with pubertal timing. In 2,754 children and adolescents (1,493 boys and 1261 girls) from Guangzhou, China, we used the segmental multi-frequency BIA to obtain body composition measures, evaluated tanner stage using testicular volume for boys and breast development for girls, and then introduced IPW based on propensity score to balance covariates and reduce confounding bias in effect estimation of body composition on pubertal timing.
Materials and methods
Study population
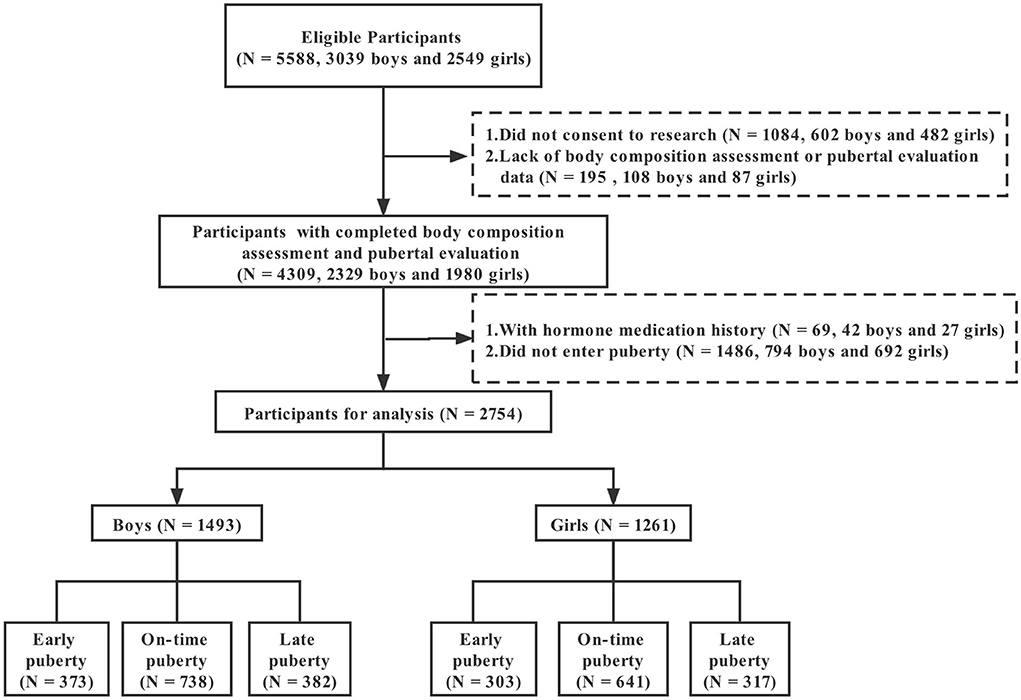
This cross-sectional study was conducted from September 2019 to May 2020 in Guangzhou, China, with a multistage stratified cluster sampling method. The 11 administrative districts in Guangzhou were classified into three stratums according to the Gross Domestic Product (GDP) per capita in 2018. Within each stratum, one district was randomly selected. One elementary school and one middle school were then chosen randomly from each district. All students in grades 2–4 of primary schools and grades 7–8 of secondary schools were recruited with an invitation letter detailing the research objectives and potential benefits and risks of participation. Figure 1 presents the flow of study participants. A total of 5,588 students, including 3,039 boys and 2,549 girls, were invited to participate, and 2,329 boys and 1,980 girls attended and completed body composition assessment and pubertal evaluation, with response rates of 76.64 and 77.68% for boys and girls, respectively. After excluding the students with a history of hormone therapy (42 boys and 27 girls) or without entering puberty (794 boys and 692 girls), a total of 1,493 boys and 1,261 girls were finally included. All participants and their guardians provided written informed consent. The study was approved by the Ethics Review Committee of Guangdong Pharmaceutical University, China (Medical Ethics Review No.: 2018-27).
Measurements
Anthropometry and body composition
All measurements were obtained by trained researchers. Anthropometric measures were taken on standing participants wearing light clothes and without shoes. Height was measured with a portable stadiometer (Seca-213, SECA, Hamburg, Germany) to the nearest 0.1 cm. Body composition was assessed by segmental multi-frequency BIA using the octopolar Inbody 570 device (Inbody Co., Ltd., Korea). Participants, wearing light clothing, stood barefoot on the metal footplates and held the hand electrodes. Outputs from the device were body impedance, body weight, and predicted measurements via the manufacturer's internal equations, including regional and total body FFM, FM, muscle mass (MM), etc. Weight, FM, FFM, and MM were recorded to the nearest 0.1 kg.
BF% and FFM percentage (FFM%) were calculated as (FM/weight) × 100% and (FFM/weight) × 100%, respectively. Appendicular skeletal muscle mass was calculated as the sum of muscle mass of the arms and legs. FM index (FMI), FFM index (FFMI), and appendicular skeletal muscle mass index (ASMI) were calculated as corresponding body composition parameters (kg) divided by the square of the height (m2). To account for differences in body composition by age and gender, we transformed age- and gender-specific Z-scores for BF%, FMI, FFM%, FFMI, and ASMI.
Timing of puberty
Pubertal stage was assessed by Tanner criteria of breast development (B1-B5) in girls and genital development (G1-G5) in boys (45). Two trained researchers, gender-matched to the participant, conducted the assessment under standardized conditions. Breast development was measured by visual inspection and palpation in girls, and testicular volume using a set of orchidometer beads in boys. Pubertal onset in girls was defined as progression to breast Tanner stage 2 (B2) and in boys as progression to a testicular volume of 4 ml (G2) (46, 47). After excluding prepubertal participants (Tanner stage 1), pubertal participants were classified into early (≤25th percentile of age for each stage), on-time (25th−75th percentile of age for each stage), and late pubertal timing (>75th percentile of age for each stage) (48).
Other covariates
A self-developed, standard questionnaire was applied to collect information about demographic characteristics (age, gender, family income, and parental education level), life behavior (physical activity, sedentary time, and sleep duration), dietary habits (intake frequency of meat, aquatic products, vegetables, bean products, fruits, egg, and milk), and history of disease and therapy. Parents completed the questionnaire of students in second–third grades, while students in higher grades completed the questionnaire themselves.
Statistical analyses
All statistical analyses were conducted for girls and boys separately in R software (version 4.0.2). Normally distributed variables were described as mean ± standard deviation (SD), non-normally distributed continuous variables as the median and interquartile range (IQR), and categorical variables as frequency and percentage. Further analyses were performed on age- and gender-specific Z-score data of body composition. One-way ANOVA analysis was used to determine the differences in single body composition variables among on-time, early, and late puberty groups. P-values for pairwise comparisons among three pubertal timing groups were adjusted using a Bonferroni correction. A multinomial logistic regression model was used to examine the association between body composition and pubertal timing, with on-time puberty as a reference. The IPW method based on propensity score was implemented to control for potential confounding. Firstly, propensity score estimations of body composition variables were calculated by linear regression model based on least-square estimation. Body composition variables were regressed in the propensity estimation model on the covariates that have been reported as potential confounding factors, including age, family income, parental education level, physical activity, sedentary time, sleep duration, intake frequencies for meat, aquatic products, vegetables, bean products, fruits, egg and milk, and FM (when analyzing of FFM variables) or FFM (when analyzing of FM variables). Then, the weight of 1/propensity score was adopted for the sample weighting process. The correlation coefficient was calculated to evaluate the quality of balancing performance after the weighting process. After that, the association between body composition and puberty timing was investigated by a multinomial logistic regression model within the weighted sample. Differences between ORs were compared by the Z test. To assess the sensitivity of results for the choice of statistical models and acquire robust effect estimations, traditional covariate adjustment regression analysis was performed, and the corresponding results were compared with IPW regression results. All reported P-values were two-sided, with P < 0.05 as statistically significant.
Results
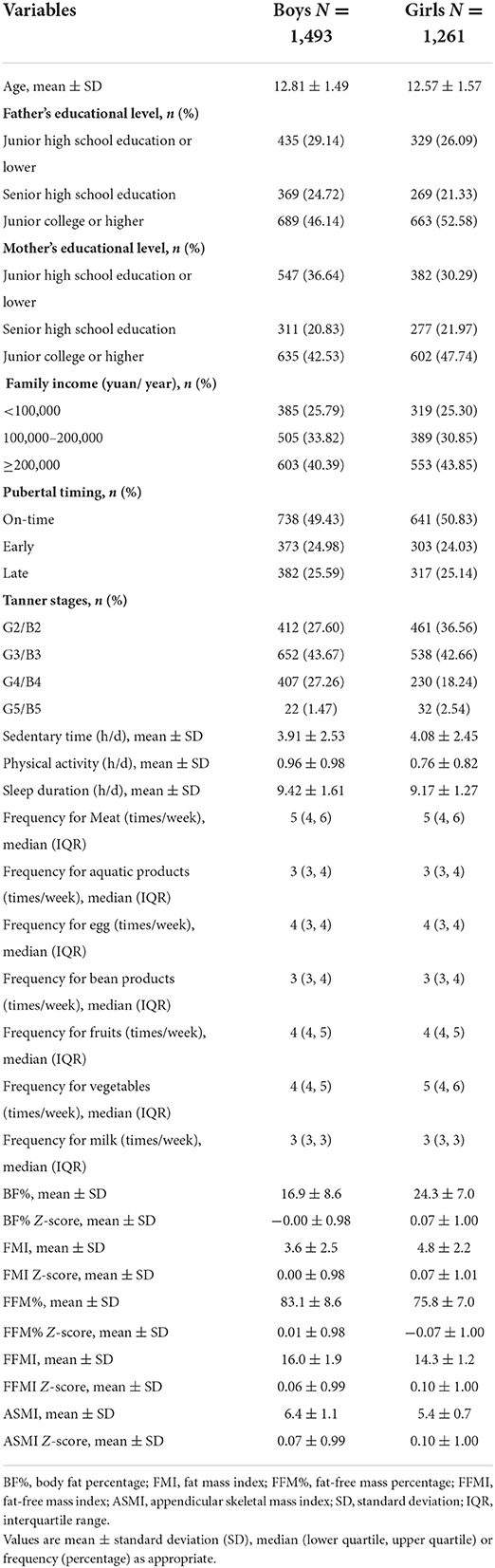
A total of 1,493 boys and 1,261 girls who had entered puberty were finally included in the analysis. Table 1 shows the characteristics of participants by gender. Boys had an average age of 12.81 (standard deviation 1.49) years and girls of 12.57 (standard deviation 1.57) years. According to the quantiles of age of each Tanner stage, 373 (24.98%) boys and 303 (24.03%) girls were assigned to early puberty, 738 (49.43%) boys and 641 (50.83%) girls to on-time puberty, and 382 (25.59%) boys and 317 (25.14%) girls to late puberty.
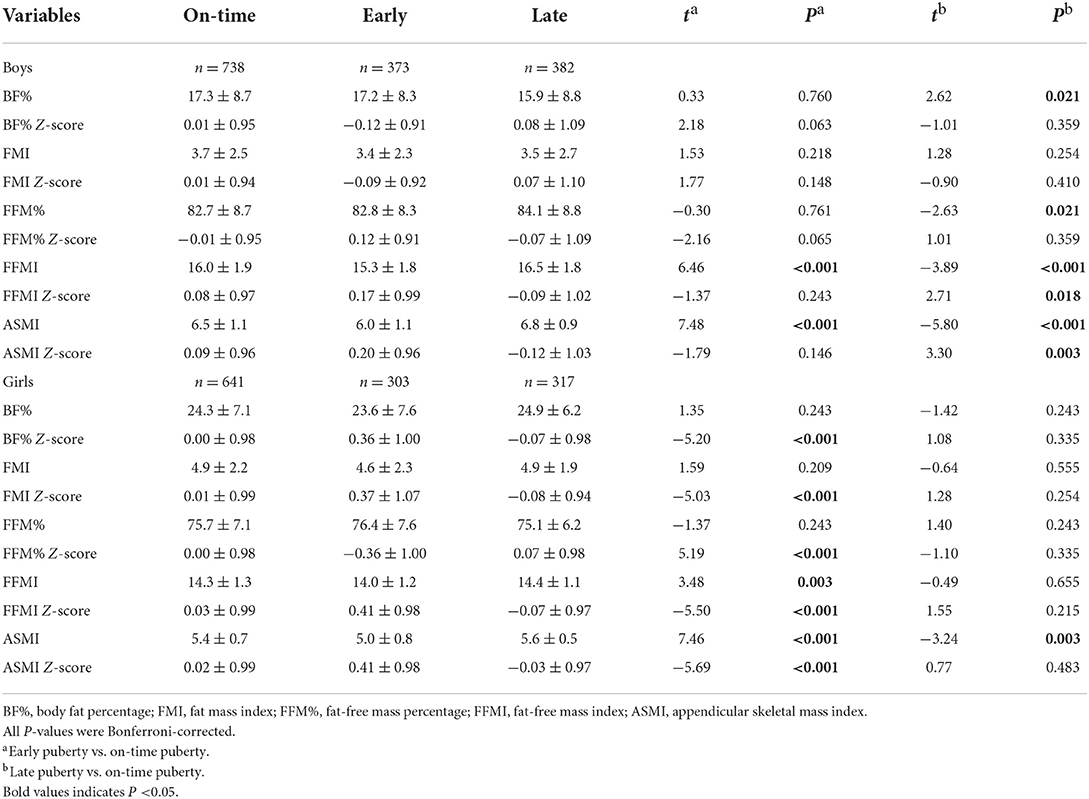
As compared to those with on-time puberty, boys with late puberty had lower Z-scores of FFMI, and ASMI (P for Bonferroni = 0.018, and 0.003, respectively), and boys with early puberty appeared to have lower BF% Z-score and higher FFM% Z-score (P for Bonferroni = 0.063 and 0.065, respectively). Non-significant differences were observed in other body composition Z-scores between boys with on-time and late puberty or early puberty. For girls, when compared to on-time pubertal peers, those with early puberty had higher Z-scores of BF%, FMI, FFMI, and ASMI but lower Z-score of FFM% (all P for Bonferroni < 0.001, Table 2), while girls in late puberty showed no different Z-scores of body composition (all P > 0.05).
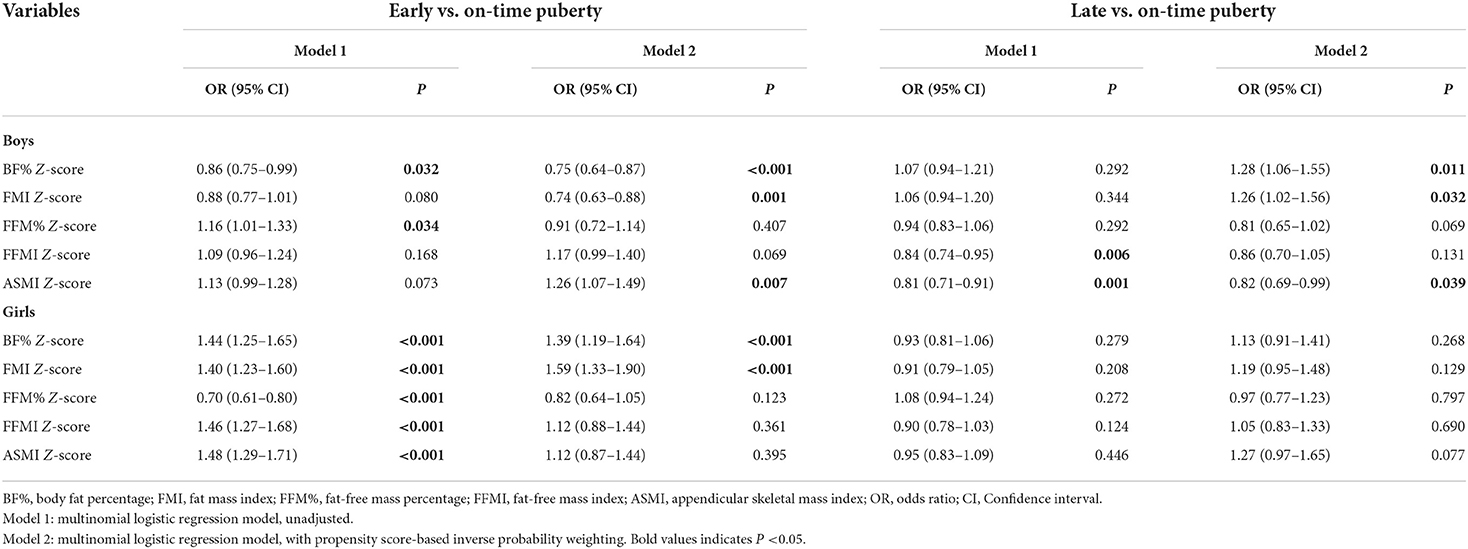
After weighting the participants with inverse probability based on the propensity score, all covariates showed weak correlation coefficients with single body composition variables both in boys and girls (Supplementary Tables 1, 2), suggesting a good balance of participants between groups. In IPW regression analysis of boys, higher Z-scores of BF% and FMI were both significantly associated with the decreased risk of early puberty (OR = 0.75, 95% CI = 0.64–0.87, P < 0.001; OR = 0.74, 95% CI = 0.63–0.88, P = 0.001) but with the increased risk of late puberty (OR = 1.28, 95% CI = 1.06–1.55, P = 0.011; OR = 1.26, 95% CI = 1.02–1.56, P = 0.032). For the ASMI, increasing Z-score yielded significant associations with greater early puberty risk (OR = 1.26, 95% CI = 1.07–1.49, P = 0.007) but lower risk of late puberty (OR = 0.82, 95% CI = 0.69–0.99, P = 0.039). As for girls, in contrast to boys, higher Z-scores of BF% and FMI significantly increased the risk of early puberty onset (OR = 1.39, 95% CI = 1.19–1.64, P < 0.001; OR = 1.59, 95% CI = 1.33–1.90, P < 0.001, Table 3). Nevertheless, the associations of Z-scores of FFM variables with early puberty did not reach statistical significance, as well as FM and FFM Z-scores with late puberty. Additionally, significant differences in ORs for the association of FM with early puberty were seen between genders (Z = −5.45 and −6.24 for BF% and FMI, respectively; both P < 0.05). In sensitivity analysis, we examined the robustness of the body composition-puberty associations by traditional covariate adjustment regression. The traditional adjustment model retained the significant associations shown by the IPW regression analysis, but with wider confidence intervals for ORs (Supplementary Table 3).
Discussion
This cross-sectional study, consisting of 1,493 boys and 1,261 girls who have entered puberty, investigated the relationship between body composition and pubertal timing using the propensity score-based IPW method, which could produce less-biased effect estimates compared to the traditional adjustment method (40). We found that FM was closely associated with pubertal timing in both boys and girls, but the association differed. There was a positive association of increased FM with a high risk of early puberty in girls, but an inverse one in boys. Besides, FFM seemed to promote boys to early onset of puberty. Our findings can contribute to a better understanding of the relationship between body composition and pubertal timing and shed light on some important gender differences conferring pubertal development.
The connection between adiposity and early puberty has been already consistently reported in girls (35, 49–52), whichever measure of pubertal timing was used. We found a similarity to previous works in all our regression models. Puberty is regulated by the hypothalamic-pituitary-gonadal axis. Several biological mechanisms have been hypothesized to explain adiposity-promoting gonadal axis initiation. One of the hypotheses is that increased adiposity could enhance aromatase activity and stimulate the conversion of androgens into estrogen, thereby triggering earlier pubertal onset (53). Similarly, insulin resistance induced by adiposity is linked to a decreased level of sex-hormone-binding globulin, which increases sex steroid bioavailability and precipitates early breast development (54). Besides, over-expressed leptin due to increased FM may also drive the central pulsatile release of gonadotrophin and accelerate the initiation of puberty (55). Nevertheless, the data linking adiposity to puberty in boys is even more limited and reveals a lack of concurrence. In this study, both traditional adjustment and IPW analyses that showed increased BF% and FMI were inversely associated with early puberty risk but positively associated with late puberty risk, consistent with the previous work on NHANES III data (56). Our findings add to the small body of literature that the pattern in the relationship of FM with pubertal timing differed by gender. Although the behind mechanism is unexplained, some lines of evidence now support our findings. Increased aromatization of androgens to estrogens driven by high adiposity can subsequently in turn inhibit gonadotropin secretion, which is related to a form of hypogonadotropic hypogonadism in males (57). An additional explanation may be related to gender divergence in leptin, an initial contributor to facilitate puberty onset. With the pubertal onset, leptin levels increased in girls but decreased in boys (58). Between leptin and FM, there was a positive association in girls but a non-significant one in boys (59). Meanwhile, leptin is obviously associated with early puberty in girls, but not in boys, or even possibly associated with delayed puberty in boys. One relevant observation is the positive correlation of leptin level with estradiol in girls but a negative one with testosterone in boys (60). On balance, there seemed to be biological plausibility with respect to the reverse relationship between FM and pubertal timing for boys in contrast to girls.
It is worth noting that a negative relationship between leptin level with FFM has been observed in boys, but a positive one in girls (59). Data related to gender differences in the accumulation of body composition have shown that boys gained more FFM while girls gained more FM (32). Intriguingly, we found a positive association of ASMI with early puberty and a negative association with late puberty in boys but a null association in girls in IPW analysis. Based on the aforementioned evidence, the gender differences in leptin involved in puberty may provide an explanation as to why high FFM appeared to advance the onset of puberty in boys. However, to our knowledge, only two of the previous studies have explored the relationship between FFM and pubertal timing in boys, one cross-sectional study in adolescents of Chongqing, China, and the other, a longitudinal study in children of Taiwan, China (35, 49). The former indicated a null association, while the latter suggested a positive association. More research will be needed to better understand the relationship of body composition with boys' pubertal timing and the underlying mechanism.
This study has several key strengths. A unique strength is that we utilized the IPW method based on propensity score to equate the confounding between pubertal groups and thereby can acquire robust results. In the analytical process, we further implemented the traditional adjustment model as sensitivity analysis, which reported a similar pattern of results to IPW analysis. Additionally, the results from the IPW method showed narrower 95% confidence intervals of effect estimates compared to the traditional adjustment method, further confirming the robustness of our findings. Furthermore, direct examination of breast development and testicular volume by the same well-trained operators allowed us to avoid a recall and reporting bias for assessing the pubertal stage.
Our findings should be interpreted in the context of the following limitations. One limitation concerns the cross-sectional nature, and thereby this study can only establish an association, but not causation. Increasing evidence appears to suggest a bidirectional causal relationship between adiposity and pubertal onset in girls (49, 61, 62). For this consideration, pubertal timing may also influence FFM accumulation. The causal direction between body composition and pubertal timing remains uncertain. Second, although we have controlled for known and measured confounders using the IPW approach, confounding by unmeasured or unknown variables can never be ruled out. Third, although BIA showed good consistency with DXA for measuring FFM, it might underestimate FM (63). Accordingly, the strength of FM-puberty associations might be underestimated in our present study. Nevertheless, the direction of the association should be reliable. Additionally, the study populations were only based in the Guangzhou region, China. Our findings may not be generalizable to other regions of China or other countries, due to ethnicity sensitivity concerning body composition and pubertal timing.
In sum, this study provides some novel contributions to the debate on the relationship between body composition and pubertal timing via the innovative methodology. Propensity score-based IPW analysis suggests gender dimorphisms in the connection of body composition with pubertal timing. While girls have a positive association of FM with early puberty, in boys the association is negative with early puberty and positive with late puberty. By contrast, FFM appears to be positively associated with early puberty and negatively associated with late puberty in boys, but not in girls. Future longitudinal studies or bidirectional Mendelian randomization analyses with large sample sizes are still needed to clarify the causal direction in the connection of body composition with pubertal onset.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Guangdong Pharmaceutical University, China (Medical Ethics Review No.: 2018-27). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
YZ: methodology, investigation, data curation, and writing—original draft. JL: investigation and resources. DZ, LY, SL, and WY: investigation and data curation. WT: resources and supervision. YY: conceptualization, resources, supervision, and project administration. LL: conceptualization, methodology, investigation, resources, writing—review and editing, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81874271).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.943886/full#supplementary-material
References
1. Biro FM. Puberty. Adolesc Med State Art Rev. (2007) 18:425–33. doi: 10.1542/9781581104080-puberty
2. Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. (2012) 77:137–45. doi: 10.1159/000336325
3. Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. (2020) 174:e195881. doi: 10.1001/jamapediatrics.2019.5881
5. Deng YS, Liang JP, Zong YN Yu P, Xie RS, Guo YF, et al. Timing of spermarche and menarche among urban students in Guangzhou, China: trends from 2005 to 2012 and association with Obesity. Sci Rep. (2018) 8:1–8. doi: 10.1038/s41598-017-18423-6
6. Hoyt LT, Niu L, Pachucki MC, Chaku N. Timing of puberty in boys and girls: Implications for population health. SSM Popul Health. (2020) 10:100549. doi: 10.1016/j.ssmph.2020.100549
7. Shen Y, Varma DS, Zheng Y, Boc J, Hu H. Age at menarche and depression: results from the NHANES 2005-2016. PeerJ. (2019) 7:e7150. doi: 10.7717/peerj.7150
8. Cheng HL, Harris SR, Sritharan M, Behan MJ, Medlow SD, Steinbeck KS. The tempo of puberty and its relationship to adolescent health and well-being: A systematic review. Acta Paediatr. (2020) 109:900–13. doi: 10.1111/apa.15092
9. Day FR, Elks CE, Murray A, Ong KK, Perry JRB. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. (2015) 5:11208. doi: 10.1038/srep11208
10. Chang CJ, Lai MM, Lin CC, Liu CS, Li TC, Li CI, et al. Age at menarche and its association with the metabolic syndrome in Taiwan. Obes Res Clin Pract. (2016) 10(Suppl. 1):s26–34. doi: 10.1016/j.orcp.2015.10.003
11. Farahmand M, Tehrani FR, Gandevani SB, Azizi F. Is there any association between age at menarche and risk of metabolic syndrome? The Tehran Lipid & Glucose Study. Arch Iran Med. (2019) 22:495–500. Available online at: http://www.aimjournal.ir/Article/aim-3914
12. Heys M, Schooling CM, Jiang C, Cowling BJ, Lao X, Zhang W, et al. Age of menarche and the metabolic syndrome in China. Epidemiology. (2007) 18:740–6. doi: 10.1097/EDE.0b013e3181567faf
13. Cheng TS, Day FR, Lakshman R, Ong KK. Association of puberty timing with type 2 diabetes: a systematic review and meta-analysis. PLoS Med. (2020) 17:e1003017. doi: 10.1371/journal.pmed.1003017
14. Dwyer AA. Psychosexual effects resulting from delayed, incomplete, or absent puberty. Curr Opin Endocr Metab Res. (2020) 14:15–21. doi: 10.1016/j.coemr.2020.04.003
15. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. (2015) 131:237–44. doi: 10.1161/CIRCULATIONAHA.114.010070
16. Loomba-Albrecht LA, Styne DM. The physiology of puberty and its disorders. Pediatr Ann. (2012) 41:e1–9. doi: 10.3928/00904481-20120307-08
17. Ebling F. Puberty: mind and body. J Neuroendocrinol. (2003) 15:323–4. doi: 10.1046/j.1365-2826.2003.01018.x
18. Frisch RE. Body fat, menarche, fitness and fertility. Hum Reprod. (1987) 2:521–33. doi: 10.1093/oxfordjournals.humrep.a136582
19. Abou El Ella SS, Barseem NF, Tawfik MA, Ahmed FA. BMI relationship to the onset of puberty: assessment of growth parameters and sexual maturity changes in Egyptian children and adolescents of both sexes. J Pediatr Endocrinol Metab. (2020) 33:121–8. doi: 10.1515/jpem-2019-0119
20. Lian QG, Mao YY, Luo S, Zhang XW, Tu XW, Zuo XY, et al. Puberty timing associated with obesity and central obesity in Chinese Han girls. BMC Pediatr. (2019) 19:1. doi: 10.1186/s12887-018-1376-4
21. Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. (2014) 99:e1519–29. doi: 10.1210/jc.2014-1384
22. Adami F, Benedet J, Takahashi LAR, da Silva Lopes A, da Silva Paiva L, de Vasconcelos FAG. Association between pubertal development stages and body adiposity in children and adolescents. Health Qual Life Outcomes. (2020) 18:93. doi: 10.1186/s12955-020-01342-y
23. Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. (2009) 20:237–42. doi: 10.1016/j.tem.2009.02.004
24. Dai YL, Fu JF, Liang L, Gong CX, Xiong F, Luo FH, et al. Association between obesity and sexual maturation in Chinese children: a muticenter study. Int J Obes. (2014) 38:1312–6. doi: 10.1038/ijo.2014.116
25. Surana V, Dabas A, Khadgawat R, Marwaha RK, Sreenivas V, Ganie MA, et al. Pubertal onset in apparently healthy Indian boys and impact of obesity. Indian J Endocrinol Metab. (2017) 21:434–8. doi: 10.4103/ijem.IJEM_18_17
26. De Simone M, Danubio ME, Amicone E, Verrotti A, Gruppioni G, Vecchi F. Age of onset of pubertal characteristics in boys aged 6-14 years of the Province of L'Aquila (Abruzzo, Italy). Ann Hum Biol. (2004) 31:488–93. doi: 10.1080/03014460410001705013
27. Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc Med. (2010) 164:139–44. doi: 10.1001/archpediatrics.2009.258
28. Bygdell M, Kindblom JM, Celind J, Nethander M, Ohlsson C. Childhood BMI is inversely associated with pubertal timing in normal-weight but not overweight boys. Am J Clin Nutr. (2018) 108:1259–63. doi: 10.1093/ajcn/nqy201
29. Ernst A, Brix N, Lunddorf LLH, Olsen J, Ramlau-Hansen CH. Placental weight Z-score and pubertal timing: a population-based cohort study. Paediatr Perinat Epidemiol. (2020) 35:206–16. doi: 10.1111/ppe.12712
30. Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol. (2020) 49:834–44. doi: 10.1093/ije/dyaa056
31. Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. (2002) 25:535–50. doi: 10.1006/jado.2002.0494
32. Chen FF, Liu JT, Huang GM, Mi J. Developmental characteristics on body composition in Chinese urban children and adolescents aged 3-17years old. Chin J Epidemiol. (2020) 41:213−9. doi: 10.3760/cma.j.issn.0254-6450.2020.02.014
33. Lemos T, Gallagher D. Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obes. (2017) 24:310–4. doi: 10.1097/MED.0000000000000360
34. Bandini LG, Must A, Naumova EN, Anderson S, Caprio S, Spadano-Gasbarro JI, et al. Change in leptin, body composition and other hormones around menarche–a visual representation. Acta Paediatr. (2008) 97:1454–9. doi: 10.1111/j.1651-2227.2008.00948.x
35. Guan PY, Wang H, Guo J, Wang LY, Jiang JJ. Correlation of pubertal timing, obesity, and body composition of children and adolescents in Chongqing City. J Hyg Res. (2016) 45:568–73.
36. Kanerva N, Kontto J, Erkkola M, Nevalainen J, Männistö S. Suitability of random forest analysis for epidemiological research: exploring sociodemographic and lifestyle-related risk factors of overweight in a cross-sectional design. Scand J Public Health. (2018) 46:557–64. doi: 10.1177/1403494817736944
37. Villamor E, Jansen EC. Nutritional determinants of the timing of puberty. Annu Rev Public Health. (2016) 37:33–46. doi: 10.1146/annurev-publhealth-031914-122606
38. Wang J, Kwok MK, Au Yeung SL, Zhao J, Li AM, Lam HS, et al. Age of puberty and sleep duration: observational and mendelian randomization study. Sci Rep. (2020) 10:3202. doi: 10.1038/s41598-020-59811-9
39. Stumper A, Mac Giollabhui N, Abramson LY, Alloy LB. Early pubertal timing mediates the association between low socioeconomic status and poor attention and executive functioning in a diverse community sample of adolescents. J Youth Adolesc. (2020) 49:1420–32. doi: 10.1007/s10964-020-01198-x
40. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
41. Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. (2011) 32:1704–8. doi: 10.1093/eurheartj/ehr031
42. Park DW, Seung KB, Kim YH, Lee JY, Kim WJ, Kang SJ, et al. Long-term safety and efficacy of stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 5-year results from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) registry. J Am Coll Cardiol. (2010) 56:117–24. doi: 10.1016/j.jacc.2010.04.004
43. Sudan R, Jain-Spangler K. Tailoring bariatric surgery: sleeve gastrectomy, roux-en-y gastric bypass and biliopancreatic diversion with duodenal switch. J Laparoendosc Adv Surg Tech A. (2018) 28:956–61. doi: 10.1089/lap.2018.0397
44. Scherrer JF, Salas J, Friedman MJ, Cohen BE, Schneider FD, Lustman PJ, et al. Clinically meaningful posttraumatic stress disorder (PTSD) improvement and incident hypertension, hyperlipidemia, and weight loss. Health Psychol. (2020) 39:403–12. doi: 10.1037/hea0000855
45. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. (1976) 51:170–9. doi: 10.1136/adc.51.3.170
46. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. (1969) 44:291–303. doi: 10.1136/adc.44.235.291
47. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. (1970) 45:13–23. doi: 10.1136/adc.45.239.13
48. Mendle J, Beltz A, Carter R, Dorn L. Understanding puberty and its measurement: ideas for research in a new generation. J Res Adolesc. (2019) 29:82–95. doi: 10.1111/jora.12371
49. Chen YC, Fan HY, Yang C, Hsieh RH, Pan WH, Lee YL. Assessing causality between childhood adiposity and early puberty: a bidirectional Mendelian randomization and longitudinal study. Metabolism. (2019) 100:153961. doi: 10.1016/j.metabol.2019.153961
50. Li WY, Liu Q, Deng X, Chen YW, Liu SD, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. (2017) 14:1266. doi: 10.3390/ijerph14101266
51. Fan HY, Huang YT, Hsieh RH, Chao JC, Tung YC, Lee YL, et al. Birthweight, time-varying adiposity growth and early menarche in girls: a Mendelian randomisation and mediation analysis. Obes Res Clin Pract. (2018) 12:445–51. doi: 10.1016/j.orcp.2018.07.008
52. Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. (2009) 94:1527–32. doi: 10.1210/jc.2008-2489
53. Bélanger C, Luu-The Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. (2002) 34:737–45. doi: 10.1055/s-2002-38265
54. Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci. (2002) 102:151–66. doi: 10.1042/CS20010197
55. Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci. (2008) 1135:265–79. doi: 10.1196/annals.1429.009
56. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. (2002) 110:903–10. doi: 10.1542/peds.110.5.903
57. Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. (2006) 27:619–26. doi: 10.2164/jandrol.106.000125
58. Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab. (2000) 85:2509–18. doi: 10.1210/jcem.85.7.6689
59. Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. (1999) 84:899–905. doi: 10.1210/jc.84.3.899
60. Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM. Serum leptin concentration, body composition, and gonadal hormones during puberty. Int J Obes Relat Metab Disord. (1999) 23:678–85. doi: 10.1038/sj.ijo.0800902
61. Kimm SY, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, et al. Racial divergence in adiposity during adolescence: the NHLBI Growth and Health Study. Pediatrics. (2001) 107:e34. doi: 10.1542/peds.107.3.e34
62. Bell JA, Carslake D, Wade KH, Richmond RC, Langdon RJ, Vincent EE, et al. Influence of puberty timing on adiposity and cardiometabolic traits: a Mendelian randomisation study. PLoS Med. (2018) 15:e1002641. doi: 10.1371/journal.pmed.1002641
Keywords: fat mass, fat-free mass, pubertal timing, children and adolescents, propensity score
Citation: Zheng Y, Liang J, Zeng D, Tan W, Yang L, Lu S, Yao W, Yang Y and Liu L (2022) Association of body composition with pubertal timing in children and adolescents from Guangzhou, China. Front. Public Health 10:943886. doi: 10.3389/fpubh.2022.943886
Received: 31 May 2022; Accepted: 20 July 2022;
Published: 17 August 2022.
Edited by:
Jennifer Savage, The Pennsylvania State University (PSU), United StatesReviewed by:
Leigh C. Ward, The University of Queensland, AustraliaJieyun Yin, Soochow University, China
Copyright © 2022 Zheng, Liang, Zeng, Tan, Yang, Lu, Yao, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Liu, cHVwdWxpdTkxOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yijin Zheng1†
Yijin Zheng1† Jianping Liang
Jianping Liang Li Liu
Li Liu