
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 15 August 2022
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.923161
Background: Gestational hypertension (GH) is a common disease that seriously threatens the safety and health of pregnant women and their newborns. Physical exercise (PE) is widely recognized as a health maintenance method and it has numerous benefits. Studies on the association between PE and the risk of GH in obese and overweight pregnant women have generated controversial findings. This updated meta-analysis was performed to reassess the effects of PE on GH.
Methods: The articles from inception to April 2022, presenting studies investigating exercise intervention and pregnancy outcomes were explored across several online databases. Heterogeneity among the included studies was estimated and tested by Q test and I2 statistic. Risk ratios (RRs) and 95% confidence intervals (CI) were calculated through either random-effect or fixed-effect models. Subgroup analyses, sensitivity analyses, and publication bias diagnoses were also conducted.
Results: Twelve with 1,649 subjects were included. PE was associated with a reduced risk of GH in obese and overweight pregnant women (Pooled RR = 0.58, 95% CI = 0.42–0.81, P = 0.001; I2 = 24.3%). Subgroup analysis found significant trends amongst Eastern countries (RR = 0.59, 95% CI = 0.36–0.96, P = 0.033). Sensitivity analysis suggested the results were stable. No publication bias was detected based on Begg's test and Egger's test.
Conclusion: PE was associated with reduced risk of GH in obese and overweight pregnant women, especially in Eastern countries. More well-designed studies are still needed to further elaborate on these associations.
Systematic review registration: CRD42022326183.
Over the past few decades, the number of overweight and obese people has been increasing, especially among women of reproductive age (1–3). Approximately 25% of women are overweight after childbirth and 20% of women are obese before pregnancy worldwide (4). However, it is reported that maternal obesity is associated with an increased risk of adverse pregnancy complications for both mother and baby (5–7). Studies have shown that children born to obese pregnant women might have a comparatively greater risk of suffering from cardiovascular disease later in life (8). Besides, neonatal mortality, preeclampsia, gestational hypertension (GH), gestational diabetes (GDM), and cesarean delivery also commonly occurred in overweight and obese pregnant women (5, 9–11).
GH is a common disease that seriously threatens the safety and health of pregnant women and their newborns (12). The incidence of this disease is about 10% worldwide (13). The higher incidence not only comes from the physical condition of pregnant women but also from the pressure of life, work, and family (14). Patients commonly experience symptoms such as hypertension, proteinuria, and edema (15). As the condition further deteriorates, patients may suffer from convulsions, coma, cerebral hemorrhage, heart failure, diffuse intravascular coagulation, and even death in severe cases (15, 16). In recent years, as the topic of hypertension in pregnancy has been discussed, the attention to this issue has gradually increased. The pathogenesis of GH is not fully established, so there is no complete cure for this disease, but preventative techniques are available (17, 18).
Exercise is widely recognized as a health maintenance method and it has numerous benefits (19, 20). It is acknowledged that exercise may accelerate metabolism, build cardiorespiratory capacity, improve immunity, and relieve mental stress (21–23). More notably, exercise can reduce complications in pregnant women by preventing weight gain or promoting weight loss (24, 25). However, the controversy of whether physical exercise (PE) may reduce the risk of GH in overweight and obese pregnant women always exists. In a previously published meta-analysis by Xing et al. (26), it was noted that exercise intervention reduced the risk of GH in overweight/obese pregnant women, while in the meta-analysis by Muhammad et al. (27) and meta-analysis by Du et al. (28), contrary conclusions were proposed. They found that PE was associated with a statistically non-significant decreased risk of GH. In the previous meta-analyses, the number of the included original studies was relatively small and subgroup analyses were not performed. Considering the serious consequences of GH, including the harm to maternal and infants, as well as the controversial conclusions from previous meta-analyses, it is of great value to conduct an updated meta-analysis to reassess this association.
The Preferred Reporting Items declared by the Systematic Review and Meta-Analysis (PRISMA) was utilized to conduct this updated meta-analysis (29).
The articles from inception to April 2022, presenting studies investigating exercise intervention and pregnancy outcomes were explored across several online databases, including Web of Science, Cochrane Library, Embase, PubMed, China Biomedical Database (CBM), VIP (Chinese) database, China National Knowledge Infrastructure, and Wanfang Data. The searching terms used were as follows: (exercise OR training OR activity OR exercise intervention) AND (pregnancy OR pregnant woman OR delivery) AND (essential hypertension OR hypertension OR hypertension in pregnancy OR gestational hypertension) AND (RCT OR randomized clinical trials OR randomized controlled clinical trial OR randomized controlled trial OR randomized controlled trials OR randomized experiment OR rct). The keywords used in the Chinese databases were replaced by the Chinese words with the same meaning as the English searching terms. For a comprehensive search to collect more qualified articles, studies were also collected through references from original published studies and relevant reviews. The records were processed with literature management software (EndNote, version 20) to exclude duplicates and to further screen the literature.
Studies could be included in this the subsequent analysis if the following inclusion criteria were fulfilled: (1) the subjects of the study were pregnant women; (2) the pregnant women were overweight or obese (no specific definition as long as the original studies reported the participants were overweight or obese); (3) the intervention was exercise (no limitation on the type of exercise); (4) The outcome of GH should be reported (no specific definition as long as the original studies reported the participants have GH) (5) the study design was restricted to randomized controlled trial study. The exclusion criteria were also set: (1) Preclinical study; (2) observational studies; (3) reviews, case reports, meta-analysis, guidelines; (4) Duplicate articles; (5) Unable to extract data.
Two authors (H. Tao and M. Liu) independently carried out the data extraction process using a pre-specified Excel form. Any dissonance was resolved with a senior supervisor (E. Xie) through discussion and consensus. Information extracted contents were listed as follows: (1) Basic information for the included articles (the first author's name, year of publication, geographic locations, the quality of the studies). (2) Baseline characteristics of the subjects in the eligible literature (sample size, ethnicity, pre-gestational BMI, gestational week, mothers' age). (3) Specific details of interventions (intervention measure and frequency). (4) The outcome indicators and outcome measures of interest (the event number in the experimental group, the total number in the experimental group, the event number in the control group, and the total number in the control group).
The quality of the included RCTs was assessed using the Jadad scoring scale for four indicators: literature random sequence generation, allocation concealment, blinding, and whether details of study participant withdrawal or dropout were described. Studies scoring 4–7 were considered of high quality and 1–3 were considered low-quality studies (30, 31).
The Stata version 15.1 statistical software (Stata Corporation, College Station, TX) was utilized in this meta-analysis. Since GH is a dichotomous variable, the results were combined using the Mantel-Haenszel method and expressed as the pooled RR with the corresponding 95% confidence interval (CI). Heterogeneity among the included studies was estimated and tested by Q test and I2 statistic. Random-effects model would be applied if the test demonstrated a substantial level of heterogeneity (I2 > 50%), otherwise, a fixed-effects model would be applied (I2 < 50%) (32, 33). Subgroup analyses was performed according to geographic locations, sample size, exercise intervention measures and dietary interventions. Sensitive analysis was conducted to explore whether the result would depend on a particular study (34). Funnel plots, Egger's test, and Begg's test were used to estimate the potential for publication bias (35, 36). Statistical significance was set at P< 0.05.
Of the 1,724 potentially relevant documents initially searched (644 citations from PubMed, 473 citations from Embase, 32 citations from Cochrane Library, 204 citations from Web of Science, and 371 citations from all the Chinese databases), 928 remained after excluding duplicates. After subsequent scanning of the titles and abstracts, 871 articles were eliminated, mainly for the irrelevance to the aim of this meta-analysis and non-conformity of the study type. Full texts of the 57 records that remained were scrutinized. Finally, 12 articles (35–46) that met the criteria were selected. The flowchart of the searching and selecting process was presented in Figure 1.
Twelve documents involving maternal outcomes in pregnant women were included in this meta-analysis. These studies were published between 2014 and 2021. Of all, five were conducted in China, three were from Italy, one was from Norway, one was from Spanish, one was from Denmark and the rest one was from New Zealand. More detailed characteristics and information were summarized in Table 1.
Twelve articles (37–48) regarding the relationship between exercise and GH were included in this meta-analysis. The result presented the RR through the fixed-effects model, which indicated that exercise was associated with a lower risk of GH in overweight or obese pregnant women (Pooled RR = 0.58, 95% CI = 0.42–0.81, P = 0.001; I2 = 24.3%; Figure 2).
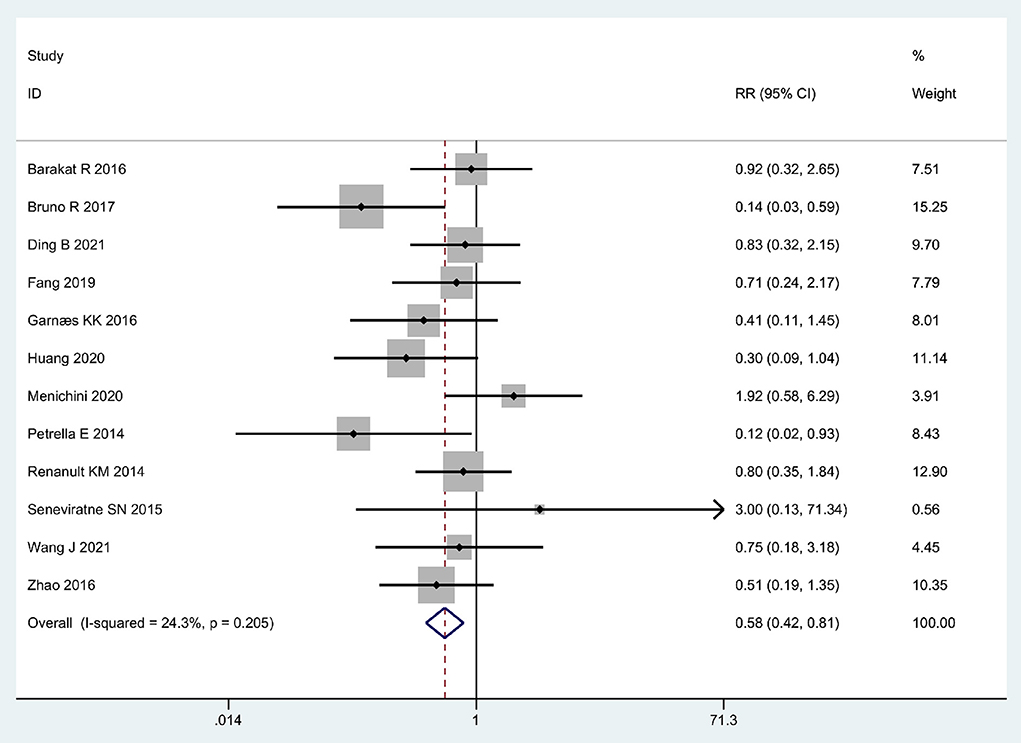
Figure 2. Forrest plot: Association between physical exercise and the risk of GH in obese and overweight pregnant women.
In the subgroup analyses performed by geographic locations, a statistically significant association was observed in eastern countries (RR = 0.59, 95% CI = 0.36–0.96, P = 0.033), while no statistically significant association was detected in Western countries (RR = 0.60, 95% CI = 0.29–1.26, P = 0.175). In terms of sample size, the outcomes were statistically significant in both the groups with sample size > 120 and ≤120 group (RR = 0.60, 95% CI = 0.40–0.90, P = 0.014; RR = 0.55, 95% CI = 0.32–0.95, P = 0.032, respectively). For Exercise intervention measures, seven studies with intervention of mixed physical exercise indicated a significant impact of exercise on reducing the risk of developing GH in in obese and overweight pregnant women RR = 0.38, 95% CI = 0.24–0.62, P = 0.001); however, in the five studies with intervention of walking only, the association between walking and incidence of GH was not statistically significant (RR = 0.90, 95% CI = 0.57–1.43, P = 0.650). In case of dietary interventions, the pooled RR for combining dietary interventions with PE was 0.55, (95% CI = 0.39–0.79, P = 0.001), and the pooled RR for no dietary interventions was 0.74 (95% CI = 0.35–1.57, P = 0.403). All these results of subgroup analyses were represented in Table 2.
After leaving an individual study out at a time, the fluctuation of the pooled RRs was found to be between 0.53 and 0.66 with an upper limit of 95% CI constantly remaining <1, and P-value constantly remained <0.05, which indicated high stability of this meta-analysis (Figure 3). By changing the fixed-effect model to the random-effect model, the overall result was not altered significantly (Pooled RR = 0.62, 95% CI = 0.41–0.92), which further confirmed the stability. No apparent evidence of asymmetry was observed by visual inspection of the funnel plot (Figure 4). Begg's test (Z = 1.03; P = 0.304), Egger's test (t = – 0.79; P = 0.450) for GH showed that publication bias might not exist.
Meta-analysis is considered an essential statistical tool to synthetically evaluate the effects of intervention for disease more precisely. Thus, we performed this updated meta-analysis, which identified a 0.58-fold decrease in the risk of developing GH in obese and overweight pregnant women. This finding was inconsistent with the previous meta-analysis by Muhammad et al. (27) and by Du et al. (28), they both found that exercise intervention would not reduce the risk of GH in overweight/obese pregnant women. However, the meta-analysis by Xing et al. (26) yielded a similar conclusion to our study. In the previous meta-analysis, the searching process may not have been comprehensive and therefore the results obtained do not seem to be very accurate. Comparing this study with the previous meta-analyses (26–28), in this study, we conducted a more detailed and comprehensive search, included more articles, had a larger sample size, and therefore had greater statistical validity and more feasible results.
Besides, subgroup analysis confirmed the relevance between exercise and the decreased risk of GH, because the outcomes were statistically significant in both the groups with sample size > 120 and ≤120 groups. In terms of the geographic locations, a positive association was observed in eastern countries while studies in Western countries showed no such association. This might be explained by discrepancies in different ethnicities between Asian and Western countries. The different lifestyles and dietary habits of different regions cause differences in the characteristics of the general population, and therefore the characteristics of pregnant women, differ significantly from one another. A meta-analysis (49) using the 2009 IOM guidelines in the global population indicated that the highest mean gestational weight gain (GWG) and pre-pregnancy BMI were in Western countries and the lowest were in Eastern countries. It is assumed that a higher BMI before pregnancy and a higher GWG during pregnancy may also be a risk factors for GH. However, due to the limitation of the number of articles, a stratified analysis based on pre-pregnancy BMI was not possible. In the case of the exercise pattern, a statistically significant reduction of 62% was observed in mixed exercise style, whereas no significant association was detected in walking only. Such results suggest that pregnant women should be exposed to different types of moderate-intensity exercise during pregnancy than walking only. It is recommended that pregnant women can combine walking, jogging, yoga, and tai chi to reduce the incidence of GH. More specific sports intensities and durations need to be explored in more depth. In case of dietary interventions, we found that combining dietary interventions with PE had better effect for pregnant women, which suggested that dietary interventions are as important as exercise interventions in the daily care of overweight or obese pregnant women. Dietary interventions can change the structure of pregnant women's diet by reducing the fat content and glucose load of the diet and increasing the protein and fiber content. These low glycemic index diets reduce weight gain during pregnancy and also reduce the risk of GH (50).
The exercise of pregnant women is greatly reduced and the intake of food is increased, so there are a lot of calories in the body that cannot be consumed, which is easy to lead to fat accumulation and obesity symptoms. These pregnant women are prone to GH due to abnormal lipid metabolism (51). It is known that adipose tissue is involved in metabolic syndromes such as obesity and hyperlipidemia, which can result in inflammatory changes that then cause increased oxidative stress. This may lead to endothelial dysfunction, and ultimately to clinical disorders such as GH (52). In clinical, many pregnant women are not aware of the harmful effects of obesity, which requires clinicians to strengthen guidance to control the BMI of obese pregnant women, of which exercise intervention is the simplest and most important measure. In an observational study by Hutcheon et al., the reasonable range of weight control was reported. In normal-weight women, lowest risk of adverse perinatal outcome was observed at a weight-gain z score of 20.2 SDs. With a non-inferiority margin of 20%, risks of adverse outcome were not meaningfully increased from the 20.2-SD reference value between z scores of 20.97 and +0.33 SDs (which corresponded to 11.3–18.4 kg). In overweight women, the recommended range was much broader: 22.11 to +0.29 SDs (4.4–18.1 kg) (53). Individualized exercise instruction can be performed for pregnant women, and appropriate exercise programs can be developed based on their exercise status and changes in body mass at different times during pregnancy.
Several inherent limitations need to be cited when interpreting the results of this meta-analysis. First, the exercise duration and intensity were different across the included studies, and due to the limited number of the included studies, results cannot be stratified by these factors. Third, the majority of studies were conducted in Europe and Asia, with limited research in other regions. Last, it is not possible to conduct a stratified analysis based on pre-pregnancy BMI. Despite the limitations, the strength of this article should be highlighted. To begin with, compared with the previous meta-analysis, more studies were enrolled. The large sample size provided stronger statistical power. Second, more comprehensive subgroup analyses were performed in this study. Third, no obvious publication bias was detected.
In conclusion, exercise is associated with a reduced risk of GH in overweight and/or obese pregnant women. Exercise is a convenient intervention in general life. Given the prevalence of GH is increasing, the effect of general exercise on GH in our study is promising. However, more well-designed studies are warranted to further elaborate on these associations.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
EX, CL, and QZ conceived and designed the research, provided critical opinions, and revised the manuscript. HT and ML designed the research, performed the statistical analysis, interpret data, and wrote the manuscript. All authors approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.923161/full#supplementary-material
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
2. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
3. Paulo HA, Mosha D, Mwanyika-Sando M, Mboya IB, Madzorera I, Killewo J, et al. Role of dietary quality and diversity on overweight and obesity among women of reproductive age in Tanzania. PLoS ONE. (2022) 17:e0266344. doi: 10.1371/journal.pone.0266344
4. Robbins CL, Zapata LB, Farr SL, Kroelinger CD, Morrow B, Ahluwalia I, et al. Core state preconception health indicators - pregnancy risk assessment monitoring system and behavioral risk factor surveillance system, 2009. MMWR Surveill Summ. (2014) 63:1–62.
5. Haque R, Keramat SA, Rahman SM, Mustafa MUR, Alam K. Association of maternal obesity with fetal and neonatal death: Evidence from South and South-East Asian countries. PLoS ONE. (2021) 16:e0256725. doi: 10.1371/journal.pone.0256725
6. Åmark H, Westgren M, Sirotkina M, Hulthén Varli I, Persson M, Papadogiannakis N. Maternal obesity and stillbirth at term; placental pathology-A case control study. PLoS ONE. (2021) 16:e0250983. doi: 10.1371/journal.pone.0250983
7. Lewandowska M. Maternal obesity and risk of low birth weight, fetal growth restriction, and macrosomia: multiple analyses. Nutrients. (2021) 13:1213. doi: 10.3390/nu13041213
8. Razaz N, Villamor E, Muraca GM, Bonamy AE, Cnattingius S. Maternal obesity and risk of cardiovascular diseases in offspring: a population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol. (2020) 8:572–81. doi: 10.1016/S2213-8587(20)30151-0
9. Kivelä J, Sormunen-Harju H, Girchenko PV, Huvinen E, Stach-Lempinen B, Kajantie E, et al. Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. (2021) 106:e4372–88. doi: 10.1210/clinem/dgab475
10. Bicocca MJ, Mendez-Figueroa H, Chauhan SP, Sibai BM. Maternal obesity and the risk of early-onset and late-onset hypertensive disorders of pregnancy. Obstet Gynecol. (2020) 136:118–27. doi: 10.1097/AOG.0000000000003901
11. Castaneda C, Marsden K, Maxwell T, Ten Eyck P, Kuwaye D, Kenne KA, et al. Prevalence of maternal obesity at delivery and association with maternal and neonatal outcomes. J Matern Fetal Neonatal Med. (2021) 1–8. doi: 10.1080/14767058.2021.1988563
12. Ouyang Y, Liu X, He Z, Huang D. Effect of high-quality nursing on postpartum hemorrhage and quality of life in puerperants with gestational hypertension. Am J Transl Res. (2022) 14:304–10.
13. Lewandowska M. The Association of Familial Hypertension and Risk of Gestational Hypertension and Preeclampsia. Int J Environ Res Public Health. (2021) 18:7045. doi: 10.3390/ijerph18137045
14. Yang L, Zhang W, Zhang L, Zhang S, Yang Y, Wang Q, et al. Gestational hypertension risk evaluation based on epidemiological, biochemical, and hemodynamic factors. Clin Exp Obstet Gynecol. (2013) 40:61–5.
15. Gestational hypertension and preeclampsia: ACOG practice bulletin summary number 222. Obstet Gynecol. (2020) 135:1492–5. doi: 10.1097/AOG.0000000000003892
16. National Guideline Alliance (UK). Evidence Review for Monitoring Gestational Hypertension: Hypertension in Pregnancy: Diagnosis Management: Evidence Review B. London: National Institute for Health and Care Excellence (NICE) (2019).
17. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. (2018) 218:287–93.e1. doi: 10.1016/j.ajog.2017.11.561
18. Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. (2022) 226:S1108–19. doi: 10.1016/j.ajog.2020.08.045
19. Shirzad M, Tari B, Dalton C, Van Riesen J, Marsala MJ, Heath M. Passive exercise increases cerebral blood flow velocity and supports a postexercise executive function benefit. Psychophysiology. (2022) e14132. doi: 10.1111/psyp.14132
20. Thijssen DHJ, Uthman L, Somani Y, van Royen N. Short-term exercise-induced protection of cardiovascular function and health: why and how fast does the heart benefit from exercise? J Physiol. (2022) 600:1339–55. doi: 10.1113/JP282000
21. Cheung C, Wyman JF, Peden-McAlpine C. Long-term yoga and aerobic/strength exercise adherence in older women with knee osteoarthritis: a mixed methods approach. Int J Yoga Therap. (2022) 32:4. doi: 10.17761/2022-D-20-00033
22. Brown M, Rébillard A, Hart NH, O'Connor D, Prue G, O'Sullivan JM, et al. Modulating tumour hypoxia in prostate cancer through exercise: the impact of redox signalling on radiosensitivity. Sports Med Open. (2022) 8:48. doi: 10.1186/s40798-022-00436-9
23. Granero-Jiménez J, López-Rodríguez MM, Dobarrio-Sanz I, Cortés-Rodríguez AE. Influence of physical exercise on psychological well-being of young adults: a quantitative study. Int J Environ Res Public Health. (2022) 19:4282. doi: 10.3390/ijerph19074282
24. Xu MY, Guo YJ, Zhang LJ, Lu QB. Effect of individualized weight management intervention on excessive gestational weight gain and perinatal outcomes: a randomized controlled trial. PeerJ. (2022) 10:e13067. doi: 10.7717/peerj.13067
25. Ashtree DN, Osborne DA, Lee A, Umstad MP, Saffery R, Craig JM, et al. Gestational weight gain is associated with childhood height, weight and BMI in the Peri/Postnatal Epigenetic Twins Study. J Dev Orig Health Dis. (2022) 1–9. doi: 10.1017/S2040174422000113
26. Xing Y, Wang X, Zhang W, Jiang H. The effect of exercise on maternal complications and birth outcomes in overweight or obese pregnant women: a meta-analysis. Ann Palliat Med. (2020) 9:4103–12. doi: 10.21037/apm-20-2097
27. Muhammad HFL, Pramono A, Rahman MN. The safety and efficacy of supervised exercise on pregnant women with overweight/obesity: a systematic review and meta-analysis of randomized controlled trials. Clin Obes. (2021) 11:e12428. doi: 10.1111/cob.12428
28. Du MC, Ouyang YQ, Nie XF, Huang Y, Redding SR. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: a meta-analysis. Birth. (2019) 46:211–21. doi: 10.1111/birt.12396
29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
30. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
31. Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. (1999) 20:448–52. doi: 10.1016/S0197-2456(99)00026-4
32. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
33. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
35. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
37. Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. (2016) 214:649.e1–8. doi: 10.1016/j.ajog.2015.11.039
38. Bruno R, Petrella E, Bertarini V, Pedrielli G, Neri I, Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: a randomized controlled trial. Matern Child Nutr. (2017) 13:e12333. doi: 10.1111/mcn.12333
39. Ding B, Gou B, Guan H, Wang J, Bi Y, Hong Z. WeChat-assisted dietary and exercise intervention for prevention of gestational diabetes mellitus in overweight/obese pregnant women: a two-arm randomized clinical trial. Arch Gynecol Obstet. (2021) 304:609–18. doi: 10.1007/s00404-021-05984-1
40. Fang L, Li W. Effect of diet and exercise intervention on pregnancy outcome of obese pregnant women. Chin J Front Med. (2019) 11:82–5.
41. Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP trial). PLoS Med. (2016) 13:e1002079. doi: 10.1371/journal.pmed.1002079
42. Huang H. Effect of diet and exercise intervention on pregnancy outcome of obese pregnant women (Chinese). Clin Med. (2020) 40:21–3.
43. Menichini D, Petrella E, Dipace V, Di Monte A, Neri I, Facchinetti F. The impact of an early lifestyle intervention on pregnancy outcomes in a cohort of insulin-resistant overweight and obese women. Nutrients. (2020) 12:1496. doi: 10.3390/nu12051496
44. Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med. (2014) 27:1348–52. doi: 10.3109/14767058.2013.858318
45. Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. (2014) 210:134.e1–9. doi: 10.1016/j.ajog.2013.09.029
46. Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. (2016) 123:588–97. doi: 10.1111/1471-0528.13738
47. Wang J, Wen H, Shang L, Qiu S, Yu T, Yuan S. Effect of diet and exercise instruction in early pregnancy on pregnancy outcome in overweight or obese pregnant women (Chinese). China Health Nutr. (2021) 31:70–1.
48. Zhao J, Li P, Li Y, Zhou A, Wang H, Wang Z, et al. Effects of exercise and dietary interventions on maternal and infant outcomes in obese pregnant women (Chinese). Modern J Integrat Trad Chin Western Med. (2016) 25:3926–28. doi: 10.3969/j.issn.1008-8849.2016.35.017
49. Martínez-Hortelano JA, Cavero-Redondo I, Álvarez-Bueno C, Garrido-Miguel M, Soriano-Cano A, Martínez-Vizcaíno V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2020) 20:649. doi: 10.1186/s12884-020-03335-7
50. Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. (2015) 3:767–77. doi: 10.1016/S2213-8587(15)00227-2
51. Zhou A, Xiong C, Hu R, Zhang Y, Bassig BA, Triche E, et al. Pre-pregnancy BMI, gestational weight gain, and the risk of hypertensive disorders of pregnancy: a cohort study in Wuhan, China. PLoS ONE. (2015) 10:e0136291. doi: 10.1371/journal.pone.0136291
52. Swank ML, Caughey AB, Farinelli CK, Main EK, Melsop KA, Gilbert WM, et al. The impact of change in pregnancy body mass index on the development of gestational hypertensive disorders. J Perinatol. (2014) 34:181–5. doi: 10.1038/jp.2013.168
Keywords: physical exercise, gestational hypertension, meta-analysis, pregnancy, obese
Citation: Xie E, Tao H, Liu M, Li C and Zhao Q (2022) The effect of exercise on the prevention of gestational hypertension in obese and overweight pregnant women: An updated meta-analysis. Front. Public Health 10:923161. doi: 10.3389/fpubh.2022.923161
Received: 19 April 2022; Accepted: 25 July 2022;
Published: 15 August 2022.
Edited by:
Maria Fiatarone Singh, The University of Sydney, AustraliaReviewed by:
Kamran Hessami, Harvard Medical School, United StatesCopyright © 2022 Xie, Tao, Liu, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enli Xie, ZW5saXhpZTE5NzlAMTYzLmNvbQ==; Changchun Li, aG5jaGFuZ2NodW5AMTI2LmNvbQ==; Qi Zhao, MTM2OTkyMzk5NkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.