- 1School of Public Health, Research Center for Medicine and Social Development, Chongqing Medical University, Chongqing, China
- 2First Clinical Medical College, Chongqing Medical University, Chongqing, China
- 3Chongqing Center for Disease Control and Prevention, Infectious Disease Control and Prevention Institute, Chongqing, China
- 4Department of Epidemiology and Health Statistics, West China School of Public Health, Sichuan University, Chengdu, China
Hyperuricemia is associated with substantial health and economic burden all over the world. Dietary habits are an important influencing factor of hyperuricemia. This study aimed to investigate the relationship between spicy food intake and hyperuricemia based on a large population. A total of 22,125 individuals aged 30–79 were enrolled in China Multi-Ethnic Cohort (CMEC), Chongqing region. Spicy food intake information was collected by a standardized questionnaire. The association between spicy food intake and hyperuricemia was estimated by multivariable logistic regression models and multiple linear regression models. Additionally, we explored these relations in subgroups stratified by sex and age. Furthermore, sensitivity analyses were conducted to verify the stability of current findings. After controlling for potential confounders, compared with participants who never consumed spicy food and consumed less hot, participants who ate 3–5 days per week and very hot had the highest risk of hyperuricemia; the ORs (95% CIs) were 1.28 (1.09, 1.5) and 1.22 (0.92, 1.63), respectively. Additionally, the corresponding ORs (95% CIs) for each level increment in the frequency and degree of pungency in spicy food intake were 1.04 (1.01, 1.07) (P trend = 0.009) and 1.15 (1.04, 1.26) (P trend = 0.004). Further in sex-stratified and age-stratified analysis, similar positive associations were observed among men and those aged 30–59, but no significant association was found among women and those aged 60–79. In the linear regression models, 3–5 days per week and moderate pungency in spicy food intake were associated with 5.21 μmol/L (95% CI: 1.72, 8.70) and 4.69 μmol/L (95% CI: 1.93, 7.45) higher serum urate level. Results in further subgroup analysis were generally consistent with the logistic regression models. This study suggests that spicy food intake may be a risk factor for hyperuricemia, especially in men and younger people, and more studies are warranted to verify the causal associations.
Introduction
Hyperuricemia is associated with substantial health and economic burden worldwide, which has attracted increasing recognition in recent years. In addition to mainly causing gout, hyperuricemia is often associated with several other diseases including chronic kidney disease, cardiovascular diseases, and metabolic syndrome (1–3). Its comorbidities also increase the risk of type 2 diabetes mellitus, hypertension, overweight and obesity, hypertriglyceridemia, and hypercholesterolemia (4). The prevalence of hyperuricemia was 14.6% in the United States and 16.6% in South Australia (5, 6). The latest data show that the overall prevalence of hyperuricemia in China is 13.3%, which has become a common metabolic disease after diabetes (7, 8). Compared with developing countries, the prevalence of hyperuricemia is generally higher in developed countries, but it has been increasing in many developing countries (9–11). With the development of the economy and society in China in the past 30 years, the eating habits of Chinese people have significantly changed. It is worth noting that dietary patterns were related to hyperuricemia. Certain foods rich in purine, alcohol, meat, seafood, a fat-rich diet, and soft drinks consumption can increase the risk of hyperuricemia (12, 13).
Spices have been an essential part of culinary cultures worldwide, with a long history in flavoring, coloring, and preserving food, as well as medicinal use (14, 15). Spiciness or pungency was regarded as one of the basic tastes in ancient Asia, especially in India and China (16, 17). It is reported that more than 30% of adults eat spicy food every day in China (18). As a mid-west city famous for its hot pot and spicy Sichuan cooking style, pepper's annual consumption per capita is up to 96.5 kg in Chongqing (19). There is increasing evidence that hyperuricemia or elevated serum uric acid (SUA) levels are associated with metabolic syndrome (MetS) and its components (20–22). Several previous studies have explored the relationship between spicy foods and metabolic diseases, indicating that the consumption of chili peppers was positively related to obesity, abnormal lipid metabolism, and insulin resistance (23–25). However, few previous studies have investigated the association between spicy food intake and hyperuricemia. Therefore, based on the China Multi-Ethnic Cohort (CMEC) data in Chongqing, the current study aimed to investigate the relation between spicy food intake and hyperuricemia.
Methods
Study Population
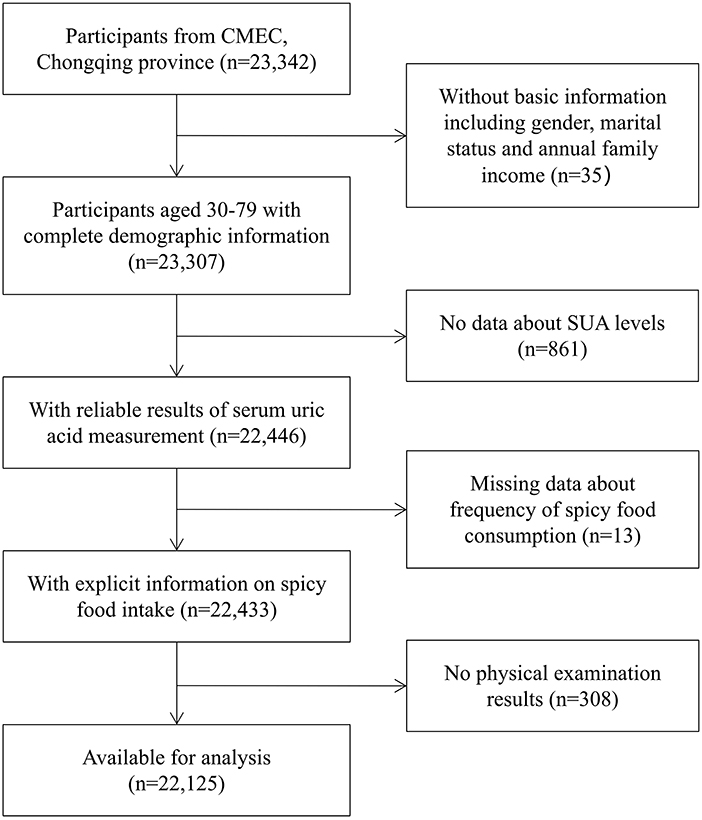
We gathered the data from the CMEC (Chongqing region). Details of the cohort study design have been presented previously (26). All participants in our study were randomly selected from 13 districts/counties. The inclusion criteria we applied were as follows: aged 30–79 years on the day of the investigation; lived in the local area for half a year or more and capable of completing the baseline survey; voluntarily participating in the study and signing informed consent; having the normal ability of expression and understanding. Overall, 23,342 individuals aged 30–79 were recruited at baseline. To explore the relationship between spicy food intake and hyperuricemia, 1,217 subjects were excluded because of the following reasons: missing basic demographic information; missing data on the determination of the status of hyperuricemia; incomplete information on spicy food intake; and missing data on physical examination results. Ultimately, the study enrolled 22,125 individuals for the current analysis. Details of participants' selection were presented in Figure 1.
Ascertainment of Hyperuricemia
Blood samples were collected from all participants via venepuncture after overnight fasting, then centrifuged and separated for later clinical laboratory testing, including fasting blood glucose (FBG), lipid profiles, and serum uric acid (SUA). The SUA was analyzed by the standard enzyme method, which was based on the generation of hydrogen peroxide from uric acid catalyzed by immobilized uricase, and then determined by the color reaction catalyzed by immobilized peroxidase. The intra-assay coefficient of variation for SUA was <6.5%. In this study, we defined hyperuricemia as increased SUA above 417 μmol/L (7 mg/dL) in men and above 357 μmol/L (6 mg/dL) in women, as described in previous studies (27, 28).
Assessment of Spicy Food Intake
The standardized questionnaire used to collect dietary intake information was delivered by well-trained staff through face-to-face interviews. Participants were asked, “How frequently did you have spicy foods during the past month?” with the following five response categories: “never,” “ <1 d/week”, “1–2 d/week”, “3–5 d/week,” or “6–7 d/week.” Among those weekly consumers (participants who consumed spicy food at least 1 day a week), participants were further asked, “Which kind of spicy food do you prefer?” with three response categories: “less hot,” “moderately hot,” or “very hot.”
Assessment of Covariates
A structured questionnaire was used to collect detailed information regarding sociodemographic characteristics and lifestyle factors. Besides the essential demographic characteristics including age, sex, educational level, marital status, and annual family income, the following variables that may affect the prevalence of hyperuricemia were explored in the current study: smoking status, alcohol consumption, physical activity, total energy intake, dietary pattern, body mass index (BMI), hypertension, type 2 diabetes mellitus, and dyslipidemia. All the introduced covariates were selected with reference to other studies on the association between spicy food and metabolic diseases (23–25, 29). Daily consumption of alcohol (grams of pure alcohol per day) was calculated based on the reported frequency and quantity of drinking. Participants' physical activity was converted into metabolic equivalent tasks (METs) spent on work, transportation, housework, and leisure time activities (30). The food frequency questionnaire (FFQ) contained 13 main food groups and was completed by all participants to collect their dietary intake data in the baseline survey. The average daily total energy intake (including protein, fat, and carbohydrates energy intake) for each participant was calculated based on dietary information according to the Chinese Food Composition Table 2004. The dietary pattern was estimated by the Dietary Approaches to Stop Hypertension (DASH) diet score, which focused on seven kinds of food, including fresh fruits, vegetables, beans, dairy, whole grain intake, red and processed meats, and sodium salt. Each kind of food was assigned one to five scores based on the quintile of the average food intake, and then all the food scores were added up to get an overall DASH score (31). BMI was calculated as weight divided by height squared (kg/m2). In the present study, hypertension was defined as having an average measured SBP/DBP ≥ 140/90 mmHg or self-reported history of hypertension diagnosed by doctors (32). Diabetes mellitus was defined as an FBG level of ≥ 7 mmol/L or a history of physician-diagnosed diabetes. Participants with any one of the following components were considered as having dyslipidemia: (1) serum total cholesterol (TC) ≥ 6.22 mmol/l; (2) triacylglycerol (TG) ≥ 2.26 mmol/l; (3) low-density lipoprotein cholesterol (LDLC) ≥ 4.14 mmol/l; (4) and high-density lipoprotein cholesterol (HDLC) < 1.04 mmol/l (33).
Statistical Analysis
The general characteristics of study participants were described according to the spicy food intake frequency (never, <1, 1–2, 3–5, 6–7 d/week) and the degree of pungency in spicy food (low, moderate, high), respectively. Means and standard deviations were presented for continuous variables, while categorical variables were expressed as percentages. ANOVA and chi-square tests were used to compare the differences in covariates among various groups of spicy food intake. The linear trend between increasing spicy food frequency and covariates was evaluated using Spearman correlation and Cochran-Mantel-Haenszel tests for continuous and categorical variables, respectively. Multivariable-adjusted logistic regression models were conducted to investigate the association between spicy food intake frequency, degree of pungency in spicy food, and hyperuricemia. And the results of the odds ratios (ORs) and 95% CIs were presented. A series of models were used to minimize the influence of confounding factors on this association. Model 1 was a crude model without any adjustments; model 2 adjusted for age, sex, marital status, educational level, and annual family income; model 3 additionally adjusted for smoking status, alcohol consumption, physical activity, total energy intake, DASH score, BMI, hypertension, type 2 diabetes mellitus, and dyslipidemia status. Considering the prevalence of hyperuricemia in men was higher than that in women and increased with age (8, 34, 35). Additionally, we explored these relations in subgroups stratified by sex and age. The interaction tests of spicy food intake on age, sex, educational level, marital status, annual family income, BMI, smoking, alcohol drinking, physical activity, more meat, and more fish were also examined in logistic regression models. Sensitivity analyses were conducted by excluding participants with self-reported peptic ulcer disease, coronary heart disease, stroke, and cancer (n = 1,992) to verify the stability of our findings. All statistical analyses were carried out using SPSS version 26.0. All statistical tests were two-sided, and a P < 0.05 was considered statistical significance.
Results
General Characteristics
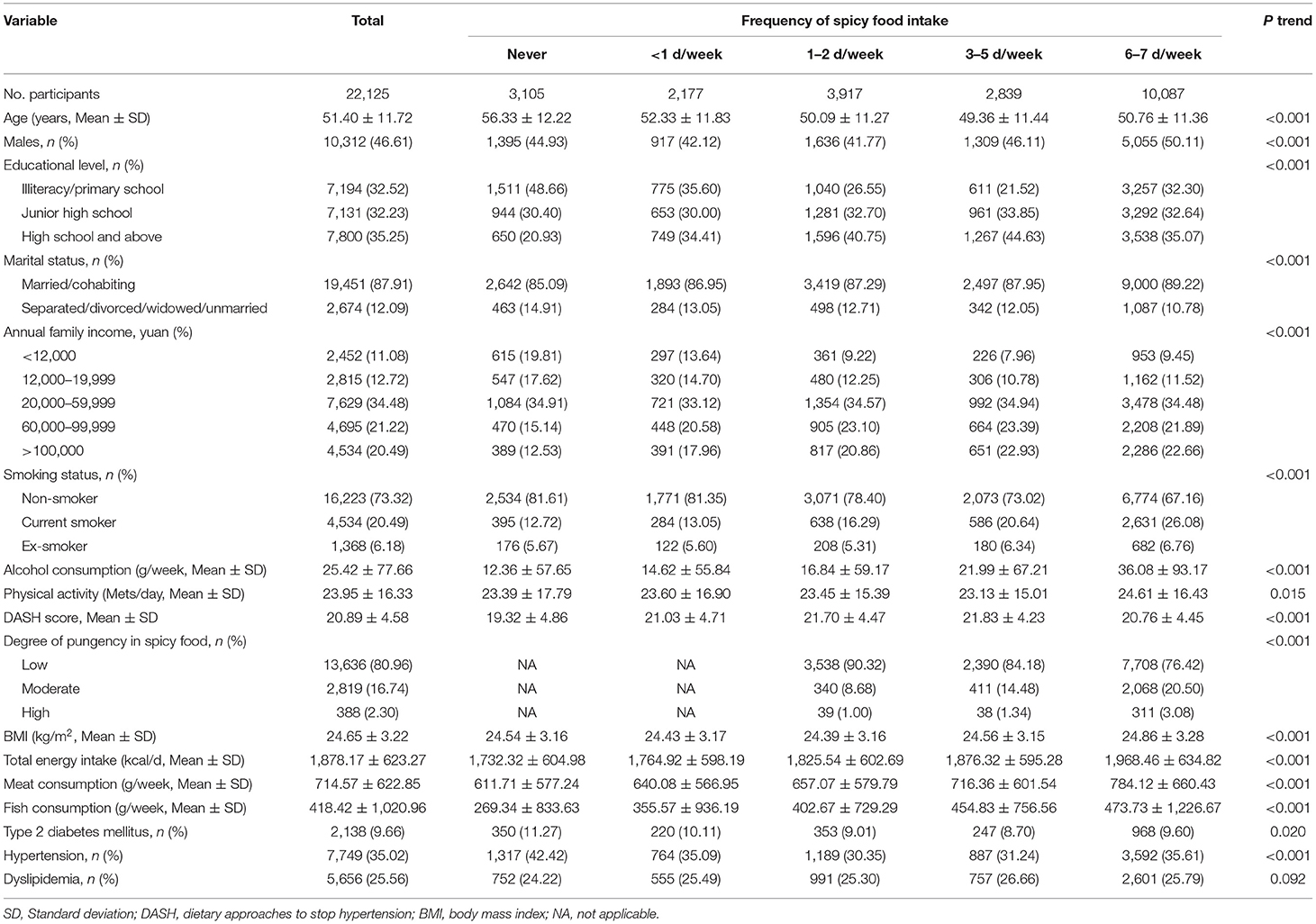
Table 1 presented the participants' general characteristics by five spicy food intake frequency groups. Of the 22,125 participants, the mean age was 51.4 ± 11.72 years. Compared with participants who never consumed spicy food, those who ate spicy food frequently were more likely to be younger, a man, married or cohabiting, with higher educational level, higher annual family income, have more physical activity, smokers, consume more alcohol, have higher total energy intake, higher BMI, and serum urate level, but less likely to have diabetes or hypertension. In addition, among weekly spicy food consumers, those who consumed spicy food more frequently tended to prefer the higher pungency of spicy food.
Association of Spicy Food Intake With Hyperuricemia
Table 2 revealed a positive relationship between spicy food intake frequency and hyperuricemia. After progressively controlling for potential confounders, compared with those who never consumed spicy food, the OR (95% CIs) of <1, 1–2, 3–5, and 6–7 d/week were 0.99 (0.83, 1.18), 1.03 (0.89, 1.2), 1.28 (1.09, 1.5), and 1.13 (0.99, 1.29), respectively (P trend = 0.009). The multilevel-adjusted analyses stratified by sex were further performed, and results showed that men who consumed spicy food <1, 1–2, 3–5, and 6–7 d/week were 1.02, 1.06, 1.44, and 1.23 times more likely to have hyperuricemia (P trend = 0.003). In contrast, this relationship was significantly attenuated among women (all P trend > 0.05). Moreover, a significant interaction between sex and frequency of spicy food consumption was found (Pinteraction = 0.004) (Supplementary Table S1). In addition, when the association was explored in a stratified analysis according to age, we found that among participants aged 30–59, the adjusted OR (95% CIs) of <1, 1–2, 3–5, and 6–7 d/week were 1.01 (0.8, 1.27), 1.08 (0.88, 1.31), 1.4 (1.14, 1.72), and 1.2 (1.01, 1.43) (P trend = 0.005). But no significant relationship was found among those aged 60–79 (all P trend > 0.05). There was no significant interaction between age and spicy food intake frequency (Pinteraction = 0.133) (Supplementary Table S1).
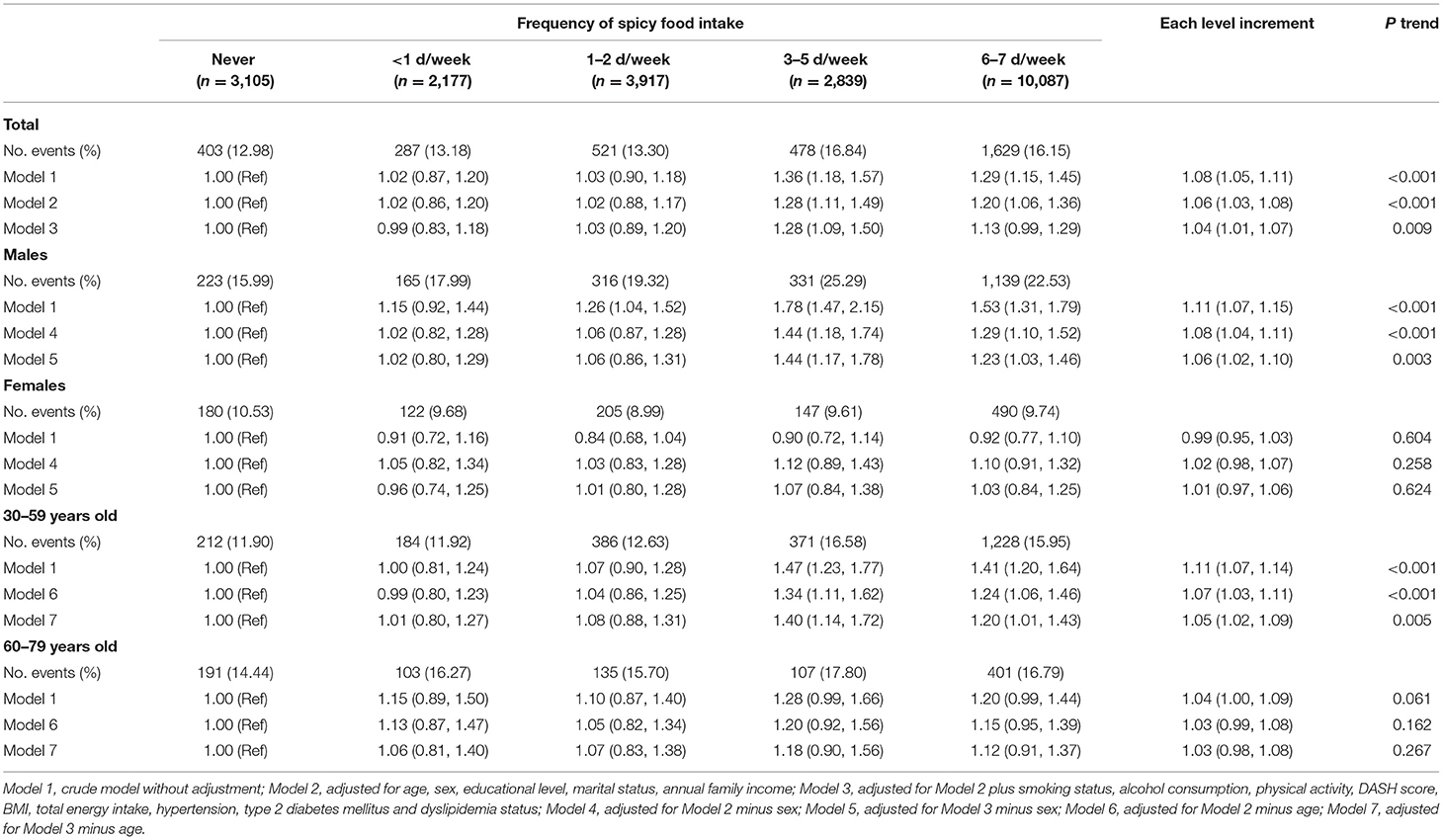
Table 2. Multivariable-adjusted associations (ORs and 95% CIs) between frequency of spicy food consumption and risk of hyperuricemia (N = 22,125).
Similar significant positive associations between the degree of pungency in spicy food and hyperuricemia were presented in Table 3. After adjusting for multiple variables, compared with participants who consumed less hot spicy food, the ORs (95% CIs) for those who ate moderately hot and very hot were 1.17 (1.05, 1.32) and 1.22 (0.92, 1.63), respectively. The corresponding OR (95% CI) for each level increment in the degree of pungency in spicy food was 1.15 (1.04, 1.26) (P trend = 0.004). Similarly, when exploring the relationship in the stratified analysis according to age and sex, a positive association was only found among men and participants aged 30–59, respectively (all P trend < 0.001). In addition, we found a significant interaction of age, sex, and spicy pungency on the risk of hyperuricemia (both Pinteraction < 0.05). More stratified analyses were performed according to other demographic characteristics, and the linear associations between frequency and degree of pungency in spicy food and hyperuricemia are shown in Supplementary Table S1.
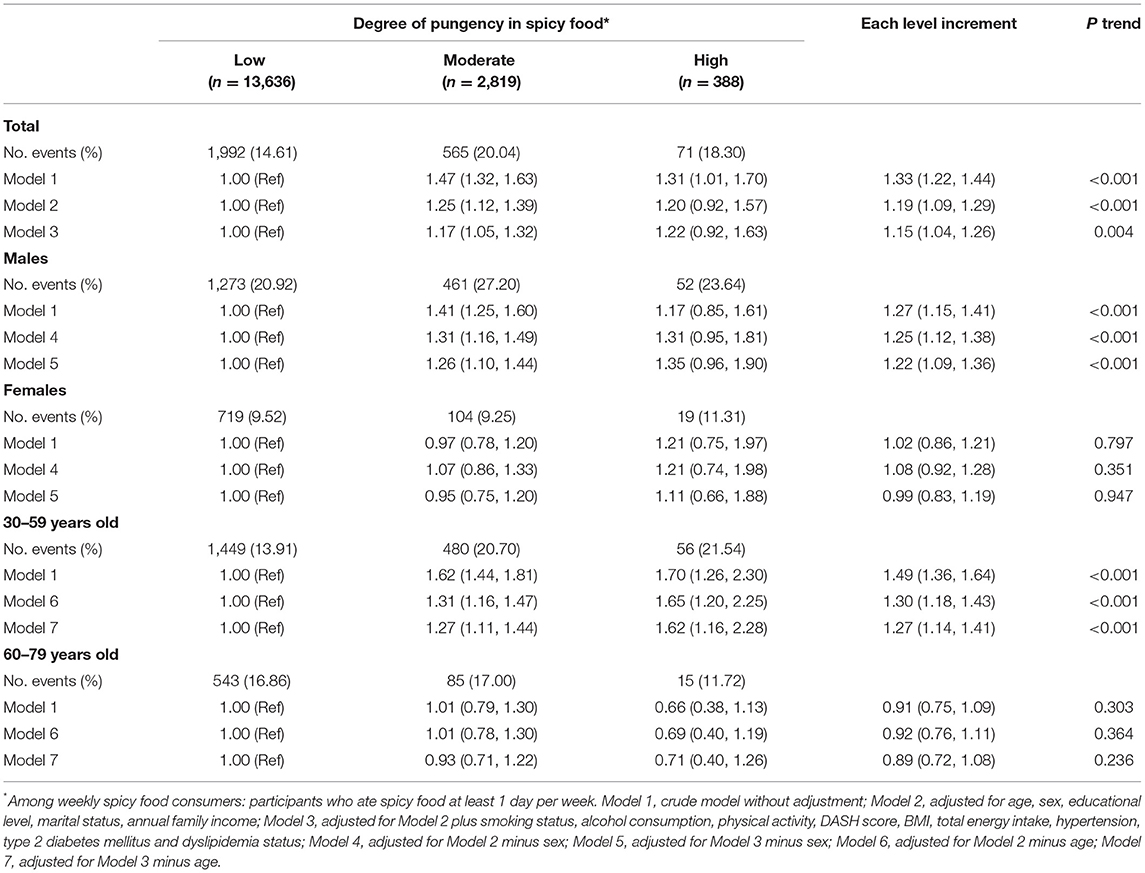
Table 3. Multivariable-adjusted associations (ORs and 95% CIs) between degree of pungency in spicy food consumption and risk of hyperuricemia (N = 16,843).
Association of Spicy Food Intake With Serum Urate Level
Table 4 showed the associations of spicy food intake with serum urate level. Those participants who consumed spicy food more frequently were more likely to have higher serum urate levels. Compared with those who never ate spicy food, 3–5 d/week was associated with the highest serum urate level; the corresponding adjusted β (95% CI) was 5.21 (1.72, 8.7). A similar positive association was observed among men; the 3–5 d/week intake frequency was related to 10.37μmol/L (95% CI: 4.6, 16.14) higher serum urate level. However, there were no significant associations among women (P trend = 0.92). In the subgroup analysis stratified by age, we found similar positive associations among participants aged 30–59 and 60–79.
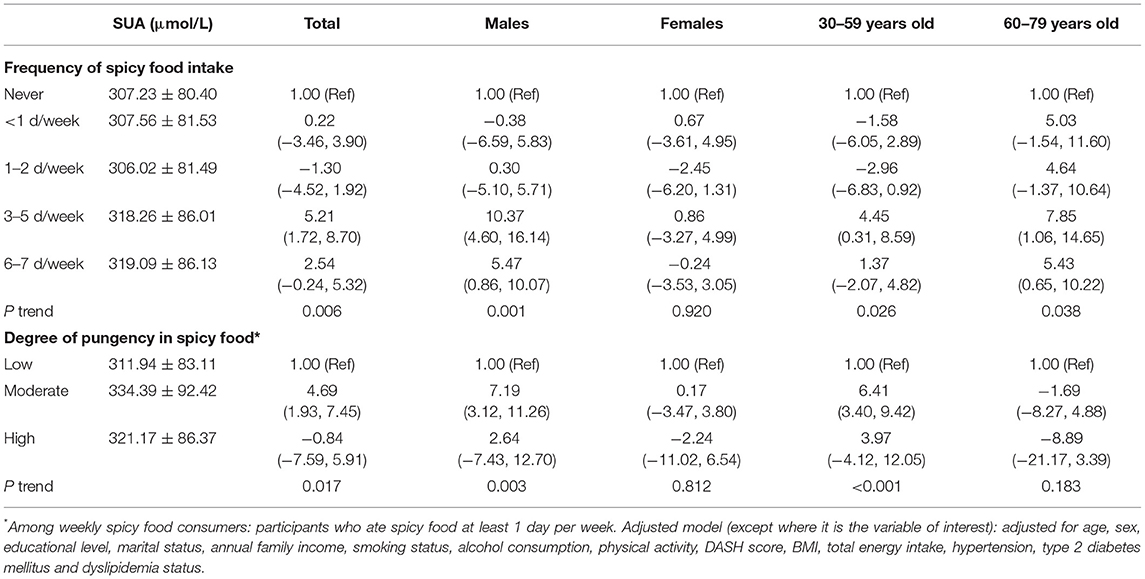
Table 4. Multivariable-adjusted associations (β coefficients and 95% CI) of frequency and degree of pungency in spicy food with serum urate level.
In addition, compared with participants who ate less hot spicy food, those who consumed a stronger degree of pungency had higher serum urate levels. The corresponding adjusted β (95% CI) for moderately hot consumers was 4.69 (1.93, 7.45). Similarly, the moderate degree of pungency in spicy food intake was associated with 7.19μmol/L (95% CI: 3.12, 11.26) higher serum urate level among men, but no significant association was observed among women (P trend = 0.812). When further performed the multilevel-adjusted analysis stratified by age, a similar positive relationship was found among participants aged 30–59 (P trend <0.001) but not among those aged 60–79 (P trend = 0.183).
Sensitivity Analyses
After excluding participants with self-reported peptic ulcer disease, coronary heart disease, stroke, and cancer, the results showed no marked change in the association of frequency of spicy food intake or degree of pungency in spicy food with the risk of hyperuricemia. Details were shown in Supplementary Tables S2, S3.
Discussion
The current study explored the relationship between spicy food intake and hyperuricemia in Chinese adults. The frequency and robust pungency in spicy food consumption was positively associated with hyperuricemia. Those positive correlations were independent of socioeconomic and lifestyle factors frequently associated with spicy food consumption and hyperuricemia, such as age, sex, marital status, annual family income, educational level, smoking status, alcohol consumption, BMI, physical activity, DASH score, and total energy intake.
In line with previous studies, participants who consumed spicy food more frequently were more likely to be young, a man, current smokers, alcohol drinkers, and have higher BMI and total energy intake (36–39). We found that both frequency and degree of pungency in spicy food were positively associated with the prevalence of hyperuricemia as well as the serum uric acid level, which was consistent with the results of the Henan rural cohort study (29). Many previous studies can support our results. It seems that spicy food intake can lead to high BMI and obesity, which in turn to high serum uric acid and hyperuricemia (23, 40, 41). The findings of CKB reported that the spicy food strength and frequency might associate with increased BMI and other adiposity measures among half a million Chinese people (40). Consistent with the CKB study, a study conducted in Henan Province of China revealed that spicy flavor and spicy food frequency were associated with a higher risk of general obesity in Chinese rural populations (23). Additionally, a cross-sectional study suggested that spicy food intake might increase abdominal obesity risk by increasing energy intake (42). It is worth noting that many studies have demonstrated that obesity is an independent contributor to hyperuricemia in many countries, such as in United States, China, Japan, and Korea (41, 43–45). Furthermore, previous studies also found that spicy food consumption was associated with abnormal lipid metabolism among adults, and older people in China, respectively (24, 46). Abnormal lipid metabolism can lead to dyslipidemia, which was observed as an essential risk factor for hyperuricemia in many cross-sectional studies and longitudinal studies (41, 43, 45).
There is no exact mechanism research to prove that spicy foods increase the risk of hyperuricemia so far. Based on some previous studies, we speculated on the underlying causes of this relationship as follows. Firstly, the critical reason for this connection may be illustrated by the meat-dominated diet along with chili intake in Chinese cuisines. In Chinese cuisine, spicy foods are more meat than vegetables (47). Excessive intake of fatty meat along with spicy foods may increase the risk of obesity, which is known to be an independent risk factor for hyperuricemia (40). More importantly, meat contains more purines than other foods. In China, mutton, fish, and especially hotpot, which contains a lot of purine, are often flavored to taste spicy (40, 48). Excessive purine intake accompanied by spicy food can directly lead to high serum uric acid, and it may further develop into hyperuricemia as a result (48). Secondly, spicy food would increase carbohydrate intake and might result in high BMI and weight increase. And we know that fat accumulation may cause excessive uric acid production (44). Unlike the typical Western diet, which is meat-based, the Chinese take rice or noodles as their staple food. Because of improving the flavor, taste, color, and smell of food, spicy food was believed to enhance the palatability of food and also can be used to treat loss of appetite (16). Moreover, increasing carbohydrate intake was often used to relieve the burning sensation caused by spicy food (23). Thirdly, chili sauce and chili oil are widely used for flavoring in China, such as in hot pots and pickles, which may lead to excessive fat intake and further raise blood lipid levels (49). The increase in blood lipid levels, especially triglyceride, will induce more free fatty acids, accelerate the decomposition of adenosine triphosphate, and increase the production of uric acid (50). Furthermore, many studies have shown that consumers of spicy food with higher frequency or more robust pungency degrees were more likely to be drinkers (24, 37, 46, 51). Previous studies have found that alcohol drinking can promote uric acid synthesis and then block the excretion of uric acid, eventually leading to hyperuricemia (44, 52). In conclusion, excessive fat, carbohydrates, oil, and alcohol intake with spicy food may increase the risk of hyperuricemia. But after the total energy intake (protein, carbohydrate, and fat), DASH dietary pattern, and alcohol consumption have been adjusted in the current study, significant associations still were observed. In addition, positive relationships between spicy food and hyperuricemia were generally similar across subgroups stratified by alcohol consumption, more meat, and more fish, which to some extent showed the relationship was independent of these factors. Thus, the exact mechanism between spicy food and hyperuricemia remains unknown. This cross-sectional study only observed the association between spicy food intake and hyperuricemia but failed to establish a causal relationship. Further research is needed to validate this relationship.
Compared with those who never ate spicy food and consumed less hot, participants who consumed 3–5 days per week and were very hot had the highest risk of hyperuricemia. The results of non-linearity in the linkage between spicy food intake and the risk of hyperuricemia may be due to the extremely unbalance distribution of spicy food intake in our study. We further explored these relations in sex-stratified subgroups. Our results showed that men but not women participants who consumed higher frequency and a stronger degree of pungency in spicy food had significantly higher serum uric acid levels and a higher risk of hyperuricemia. This difference may be because participants who consumed spicy food frequently in our study were usually men. Moreover, the significant interaction between sex and spicy food intake further suggests that men may be more sensitive to the effect of spicy food intake on the risk of hyperuricemia. In addition, we should not ignore that anti-androgen therapy and female sex hormones could protect against the development of hyperuricemia (53, 54). When exploring the association in subgroup analysis stratified by age, significant positive associations were mainly found among 30–59 years old. This difference may be because youngers were more likely to eat spicy food in this study. More research is warranted to confirm the gender-dependent and age-dependent association and elucidate its mechanism.
Our study has several advantages as an observational study exploring the relationship between spicy food intake and hyperuricemia in Chongqing, China. Firstly, this research was carried out in Chongqing, China, a city known for its hot pot with a very high consumption of chili peppers. Secondly, the current study included a large sample size and adjusted for a broad range of confounding factors. It may be more representative to explore this association here, and the results of this study provide some insights into the association between spicy food consumption and hyperuricemia. In addition, not only according to the previous diagnosis results self-reported by participants, the determination of hyperuricemia but also based on the laboratory measurement results of their blood uric acid level. This strategy minimizes the omissions in patients with hyperuricemia.
Some limitations of this study should be noticed. Firstly, as a cross-sectional survey, we could not demonstrate a causal association and did not distinguish the effect of the spicy flavor by itself from other accompanying dietary factors, as we did not ask about the specific kinds of spicy food, such as spicy meat or vegetables. But confounding factors were adjusted, and subgroup analyses were performed to improve the study. Further prospective studies in large populations and clinical research are needed to verify the relationships. Secondly, a food frequency questionnaire was used to obtain the information on participants' spicy food intake, which might have reporting and recall bias, and no accurate data on the chili intake was obtained. Further research is needed to elucidate the specific mechanism of the effect of capsaicin on serum uric acid. Thirdly, all the individuals in the current study were voluntarily participating and were more likely to have high educational levels and healthy lifestyles, which may lead to underestimating the prevalence of various diseases. It is necessary to further verify the current results in other random sampling populations in future studies. Finally, this study only focused on Chongqing in the southwest region of China, which may not be representative of the whole Chinese population. More research in other areas is warranted to explore the association between spicy food and hyperuricemia.
Conclusions
The present study demonstrated that frequency and degree of pungency in spicy food were positively associated with serum uric acid levels and the risk of hyperuricemia. In addition, this positive association was found significant in men and younger people but weaker in women and older people. Further prospective and intervention studies are warranted to verify the causal associations and elucidate specific mechanisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Ethics Committee of Sichuan University (No. K2016038), and all participants recruited in this study have signed informed consent.
Author Contributions
QL analyzed the data and drafted the initial manuscript. RD, LC, XB, MX, YW, and JX were involved in the investigation, literature search, data acquisition, data analysis, and critically revising of the manuscript for important intellectual content. XL, WT, and JQ were involved in the conception, design, and data acquisition. XD and XT were involved in the manuscript's conception and design, data acquisition, and editing. All authors agree to be accountable for the content of the work.
Funding
This work was supported by the National Key Research and Development Program of China (Grant Number: 2017YFC0907303), the Medical Scientific Research Project of Chongqing Health Commission (Grant Number: 2022WSJK021), and the Key Research and Development Project of the Science and Technology of Sichuan Province (Grant Number: 2020YFS0216).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to all the participants in this study and to all team members for their support of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.919347/full#supplementary-material
Abbreviations
CMEC, China Multi-Ethnic Cohort; CKB, China Kadoorie Biobank; BMI, Body mass index; METs, metabolic equivalent tasks; FFQ, Food Frequency Questionnaire; DASH, Dietary Approaches to Stop Hypertension; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; FBG, Fasting Blood Glucose; TC, Total Cholesterol; TG, Triglycerides; LDLC, Low-Density Lipoprotein Cholesterol; HDLC, High-Density Lipoprotein Cholesterol; SD, Standard deviation; ANOVA, Analysis of Variance; OR, Odds ratio; CI, Confidence interval.
References
1. Zhang H, Li Y, Mao Z, Liu X, Zhang X, Yang K, et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population: the RuralDiab study. Clin Chim Acta. (2018) 480:119–25. doi: 10.1016/j.cca.2018.02.003
2. Yadav D, Lee ES, Kim HM, Choi E, Lee EY, Lim JS, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean rural cohort. Atherosclerosis. (2015) 241:271–7. doi: 10.1016/j.atherosclerosis.2015.04.797
3. Nagahama K, Inoue T, Kohagura K, Kinjo K, Ohya Y. Associations between serum uric acid levels and the incidence of hypertension and metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens Res. (2015) 38:213–8. doi: 10.1038/hr.2014.161
4. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11:649–62. doi: 10.1038/nrrheum.2015.91
5. Singh G, Lingala B, Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology. (2019) 58:2177–80. doi: 10.1093/rheumatology/kez196
6. Ting K, Gill TK, Keen H, Tucker GR, Hill CL. Prevalence and associations of gout and hyperuricaemia: results from an Australian population-based study. Internal Med J. (2016) 46:566–73. doi: 10.1111/imj.13006
7. Chinese Society of Endocrinology. Guideline for the diagnosis and management of hyperuricemia and gout in China 2019. Chin J Endocrinol Metab. (2020) 36:1–13. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZHNF202001001&DbName=ZHYX2020
8. Liu R, Han C, Wu D, Xia X, Gu J, Guan H, et al. Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. (2015) 2015:762820. doi: 10.1155/2015/762820
9. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheumatism. (2011) 63:3136–41. doi: 10.1002/art.30520
10. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. (2016) 388:2039–52. doi: 10.1016/s0140-6736(16)00346-9
11. Lohsoonthorn V, Dhanamun B, Williams MA. Prevalence of hyperuricemia and its relationship with metabolic syndrome in Thai adults receiving annual health exams. Arch Med Res. (2006) 37:883–9. doi: 10.1016/j.arcmed.2006.03.008
12. Choi HK, Liu SM, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid - the Third National Health and Nutrition Examination Survey. Arthritis Rheumatism. (2005) 52:283–9. doi: 10.1002/art.20761
13. Han Q-X, Zhang D, Zhao Y-L, Liu L, Li J, Zhang F, et al. Risk factors for hyperuricemia in Chinese centenarians and near-centenarians. Clin Interventions Aging. (2019) 14:2239–47. doi: 10.2147/cia.S223048
14. Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Austral. (2006) 185:S4–24. doi: 10.5694/j.1326-5377.2006.tb00548.x
15. Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. (2008) 19:347–61. doi: 10.1016/j.jnutbio.2007.11.003
16. Nilius B, Appendino G. Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol. (2013) 164:1–76. doi: 10.1007/112_2013_11
17. Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. (2004) 38:377–84. doi: 10.1016/j.npep.2004.07.005
18. Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. (2015) 351:h3942. doi: 10.1136/bmj.h3942
19. Zou Z, Zou X. Geographical and ecological differences in pepper cultivation and consumption in China. Front Nutr. (2021) 8:718517. doi: 10.3389/fnut.2021.718517
20. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. (2007) 120:442–7. doi: 10.1016/j.amjmed.2006.06.040
21. Zhang Q, Zhang C, Song X, Lin H, Zhang D, Meng W, et al. A longitudinal cohort based association study between uric acid level and metabolic syndrome in Chinese Han urban male population. BMC Public Health. (2012) 12:419. doi: 10.1186/1471-2458-12-419
22. Osgood K, Krakoff J, Thearle M. Serum uric acid predicts both current and future components of the metabolic syndrome. Metab Syndr Relat Disord. (2013) 11:157–62. doi: 10.1089/met.2012.0151
23. Yang K, Li Y, Mao Z, Liu X, Zhang H, Liu R, et al. Relationship between spicy flavor, spicy food intake frequency, and general obesity in a rural adult Chinese population: the Ruraldiab study. Nutr Metab Cardiovasc Dis. (2018) 28:252–61. doi: 10.1016/j.numecd.2017.10.021
24. Xue Y, He T, Yu K, Zhao A, Zheng W, Zhang Y, et al. Association between spicy food consumption and lipid profiles in adults: a nationwide population-based study. Br J Nutr. (2017) 118:144–53. doi: 10.1017/s000711451700157x
25. Li J, Wang R, Xiao C. Association between chilli food habits with iron status and insulin resistance in a Chinese population. J Med Food. (2014) 17:472–8. doi: 10.1089/jmf.2013.2748
26. Zhao X, Hong F, Yin J, Tang W, Zhang G, Liang X, et al. Cohort Profile: the China Multi-Ethnic Cohort (CMEC) study. Int J Epidemiol. (2021) 50:721–l. doi: 10.1093/ije/dyaa185
27. Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. (2014) 26:186–91. doi: 10.1097/BOR.0000000000000028
28. Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol. (2000) 27:1045–50.
29. Dong X, Li Y, Yang K, Zhang L, Xue Y, Yu S, et al. Mediation effect of body mass index on the association between spicy food intake and hyperuricemia in rural Chinese adults: the Henan rural cohort study. BMC Public Health. (2020) 20:1629. doi: 10.1186/s12889-020-09736-9
30. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and Met intensities. Med Sci Sports Exerc. (2000) 32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009
31. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Internal Med. (2008) 168:713–20. doi: 10.1001/archinte.168.7.713
32. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
33. Joint Committee for Guideline Revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. (2018) 15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011
34. Liu H, Zhang XM, Wang YL, Liu BC. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J Nephrol. (2014) 27:653–8. doi: 10.1007/s40620-014-0082-z
35. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the united states and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
36. He T, Wang M, Tian Z, Zhang J, Liu Y, Zhang Y, et al. Sex-dependent difference in the association between frequency of spicy food consumption and risk of hypertension in Chinese adults. Eur J Nutr. (2019) 58:2449–61. doi: 10.1007/s00394-018-1797-8
37. Wen Q, Wei Y, Du H, Lv J, Guo Y, Bian Z, et al. Characteristics of spicy food consumption and its relation to lifestyle behaviours: results from 0.5 million adults. Int J Food Sci Nutr. (2021) 72:569–76. doi: 10.1080/09637486.2020.1849038
38. Choi SE, Chan J. Relationship of 6-n-propylthiouracil taste intensity and chili pepper use with body mass index, energy intake, and fat intake within an ethnically diverse population. J Acad Nutr Dietetics. (2015) 115:389–96. doi: 10.1016/j.jand.2014.09.001
39. Shi Z, Riley M, Taylor AW, Page A. Chilli consumption and the incidence of overweight and obesity in a Chinese adult population. Int J Obesity. (2017) 41:1074–9. doi: 10.1038/ijo.2017.88
40. Sun D, Lv J, Chen W, Li S, Guo Y, Bian Z, et al. Spicy food consumption is associated with adiposity measures among half a million Chinese people: the China Kadoorie Biobank study. BMC Public Health. (2014) 14:1293. doi: 10.1186/1471-2458-14-1293
41. Chen Y, Zhang N, Sun G, Guo X, Yu S, Yang H, et al. Metabolically healthy obesity also has risk for hyperuricemia among Chinese general population: a cross-sectional study. Obesity Res Clin Prac. (2016) 10(Suppl 1):S84–95. doi: 10.1016/j.orcp.2016.03.008
42. Yang K, Li Y, Xue Y, Wang L, Liu X, Tu R, et al. Association of the frequency of spicy food intake and the risk of abdominal obesity in rural Chinese adults: a cross-sectional study. BMJ Open. (2019) 9:e028736. doi: 10.1136/bmjopen-2018-028736
43. Fu S, Luo L, Ye P, Xiao W. Epidemiological associations between hyperuricemia and cardiometabolic risk factors: a comprehensive study from Chinese community. BMC Cardiovasc Disord. (2015) 15:129. doi: 10.1186/s12872-015-0116-z
44. Shiraishi H, Une H. The effect of the interaction between obesity and drinking on hyperuricemia in Japanese male office workers. J Epidemiol. (2009) 19:12–6. doi: 10.2188/jea.je20080016
45. McAdams-DeMarco MA, Law A, Maynard JW, Coresh J, Baer AN. Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC Musculoskeletal Disord. (2013) 14:347. doi: 10.1186/1471-2474-14-347
46. Yu K, Xue Y, He T, Guan L, Zhao A, Zhang Y. Association of spicy food consumption frequency with serum lipid profiles in older people in China. J Nutr Health Aging. (2018) 22:311–20. doi: 10.1007/s12603-018-1002-z
47. Sherman PW, Hash GA. Why vegetable recipes are not very spicy. Evolution Human Behav. (2001) 22:147–63. doi: 10.1016/S1090-5138(00)00068-4
48. Nan H, Qiao Q, Dong Y, Gao W, Tang B, Qian R, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. J Rheumatol. (2006) 33:1346–50.
49. Hou J, Liu X, Tu R, Dong X, Zhai Z, Mao Z, et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: a cross-sectional study. Environ Int. (2020) 136:105459. doi: 10.1016/j.envint.2020.105459
50. Peng T-C, Wang C-C, Kao T-W, Chan JY-H, Yang Y-H, Chang Y-W, et al. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int. (2015) 2015:127596. doi: 10.1155/2015/127596
51. Wang H, Chen L, Shen D, Cao Y, Zhang X, Xie K, et al. Association between frequency of spicy food consumption and hypertension: a cross-sectional study in Zhejiang Province, China. Nutr Metab. (2021) 18:70. doi: 10.1186/s12986-021-00588-7
52. Makinouchi T, Sakata K, Oishi M, Tanaka K, Nogawa K, Watanabe M, et al. Benchmark dose of alcohol consumption for development of hyperuricemia in Japanese male workers: an 8-year cohort study. Alcohol. (2016) 56:9–14. doi: 10.1016/j.alcohol.2016.08.002
53. Mumford SL, Dasharathy SS, Pollack AZ, Perkins NJ, Mattison DR, Cole SR, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. (2013) 28:1853–62. doi: 10.1093/humrep/det085
Keywords: spicy food intake, hyperuricemia, gender difference, age difference, adults
Citation: Luo Q, Ding R, Chen L, Bu X, Xiao M, Liu X, Wu Y, Xu J, Tang W, Qiu J, Ding X and Tang X (2022) The Association Between Spicy Food Intake and Risk of Hyperuricemia Among Chinese Adults. Front. Public Health 10:919347. doi: 10.3389/fpubh.2022.919347
Received: 13 April 2022; Accepted: 03 June 2022;
Published: 06 July 2022.
Edited by:
Hong Xue, George Mason University, United StatesReviewed by:
Peng Nie, Xi'an Jiaotong University, ChinaXiaozhong Wen, University at Buffalo, United States
Copyright © 2022 Luo, Ding, Chen, Bu, Xiao, Liu, Wu, Xu, Tang, Qiu, Ding and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Tang, dGFuZ3hpYW9qdW5AY3FtdS5lZHUuY24=; Xianbin Ding, eGlhbmJpbmRpbmdAMTI2LmNvbQ==
 Qinwen Luo1
Qinwen Luo1 Liling Chen
Liling Chen Xiaojun Tang
Xiaojun Tang