- 1Borena Zone Health Department Office, Malaria and NTD, Yabelo, Ethiopia
- 2School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 3Department of Environmental Health Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Visceral leishmaniasis (VL) is a vector-borne protozoan neglected tropical disease. In some parts of Ethiopia, it is a public health problem and its main causative agent is the Leishmania donovani complex. The objective of the study was to determine the seroprevalence of VL and factors associated among the asymptomatic pastoral community of Dire District, Borena Zone, Oromia Region, Ethiopia. A community-based study was conducted among 432 pastoralist communities from June to July 2021. A systematic random sampling method was used to select households. Pretested structured questionnaires and face-to-face interviews were used to collect data. A single finger-prick blood sample was collected and tested for Leishmania donovani complex using an immune-chromatographic test (rk39-ICT). A logistic regression model was used to assess factors associated with VL infection and a p-value of < 0.05 was considered statistically significant. A total of 432 study participants were included (their mean age was 26.69) and 218 (50.5%) were females. The overall seroprevalence of VL was 33/432(7.6%) (95%CI: 5.32–15.60). Sero-prevalence was significantly associated with high family size (>5) (adjusted odds ratios (AOR) = 5.134; 95% CI: 2.032–9.748), sleeping or/and staying under acacia tree (AOR = 2.984; 95%CI = 1.074–8.288), presence of cracked house walls (AOR = 1.801; 95%CI: 1.026–4.926), presence of termite hills (AOR = 1.938; 95%CL: 1.002–7.050), availability of water points (AOR = 3.893; 95%CI: 1.034–7.426) and presence of domestic animals (AOR = 2.124; 95% CI: 2.341–5.108). It is recommended that community awareness on the transmission and prevention methods of Leishmania donovani complex and taking appropriate interventions on the identified factors play a greater role to prevent and control infection in the area. Further investigation is also needed to characterize the pathogens and risk factors and tackle the problem.
Introduction
Leishmaniases are a set of diseases caused by near to 22 species of the protozoan genus Leishmania and are transmitted between humans and other mammalian hosts (1). Clinically, there are two major forms of leishmaniasis, cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL). VL is the severe form of leishmaniasis, fatal if not treated, and is among tropical neglected protozoan diseases and caused by Leishmania donovani complex (L. donovani and L. infantum; are the causative agents of VL in Ethiopia) (2, 3) and Leishmania donovani is the principal causative agent (4).
Infection and transmission of VL are through the bite of the female phlebotomine sand-fly and inoculation of the infective stage of the parasite (5). Humans, hyraxes, and domestic dogs are the most commonly infected hosts, and they can also be potential reservoirs (6, 7). Domestic dogs might be the most important reservoir of both L. donovani and L. infantum in eastern Africa (6, 8). However, the transmission of L. donovani is generally thought to be anthroponotic in the East African region, and in Ethiopia, it is thought to be partially zoonotic and anthroponotic, the nature of which varies with geographical areas (8, 9). Thus, the decisive reservoir of VL in East Africa and Ethiopia remains to be determined and wellstudied. In addition, multiple factors like seasonal migration, biological, environmental, and areas of lower socioeconomic status are factors that contribute to disease transmission in the area (10). The presence of sandflies and gorges in the area, open waste disposal, the presence of a reservoir host, and asymptomatic cases are important risk factors for the spread of the disease (11).
Globally, the disease affects ~350 million people in 98 countries; where most of them are in developing regions. However, ~90% of the global VL burden is attributed to a few countries: Bangladesh, Brazil, India, Ethiopia, Sudan, and South Sudan (12). The report indicated that there are many foci of VL infection in eastern African countries (13, 14).
In Ethiopia, VL is predominantly found in the lowlands with varying degrees of endemicity and the vectors responsible for disease transmission are Phlebotomus martini, Phlebotomus celiae, and Phlebotomus orientalis (15). Approximately 3.2 million people in the population are at risk of VL and north-eastern, north-western, western, and south-eastern parts of Ethiopia are geographically suitable for disease transmission (16). Annually, 3,700–4,000 estimated VL cases occur in Ethiopia (17). The northern and northwest parts of Ethiopia have the highest burden, which accounts for nearly 30–40% of the total number of Ethiopian VL patients; of which around 30% of VL patients are malnourished and co-infected with HIV (18). While the southern foci (the south-western savannah and the south-eastern semi-arid lowlands) account for ~20% of the total VL burden in Ethiopia (13).
According to the Oromia Regional Health Bureau report, VL is endemic in Borena, Guji, and Bale zone (19). The Borena zone started to report VL cases in 2012 from Aero, Dire, and Miyo Districts. Currently, the problems could be extended to other districts but still more cases were from Dire districts and as a result, outbreaks of VL occurred in 2020 (20). In a nutshell, limited studies have determined the magnitude of VL due to the Leishmania donovani complex and its associated factors in Ethiopia. Moreover, no studies have been conducted to determine the status of seroprevalence and associated factors with VL in the study area. Thus, this study was conducted to determine the seroprevalence of the Leishmania donovani and factors associated with the asymptomatic pastoral community of the Dire District, Borena Zone, and Oromia region in Ethiopia.
Methods
Study area and period
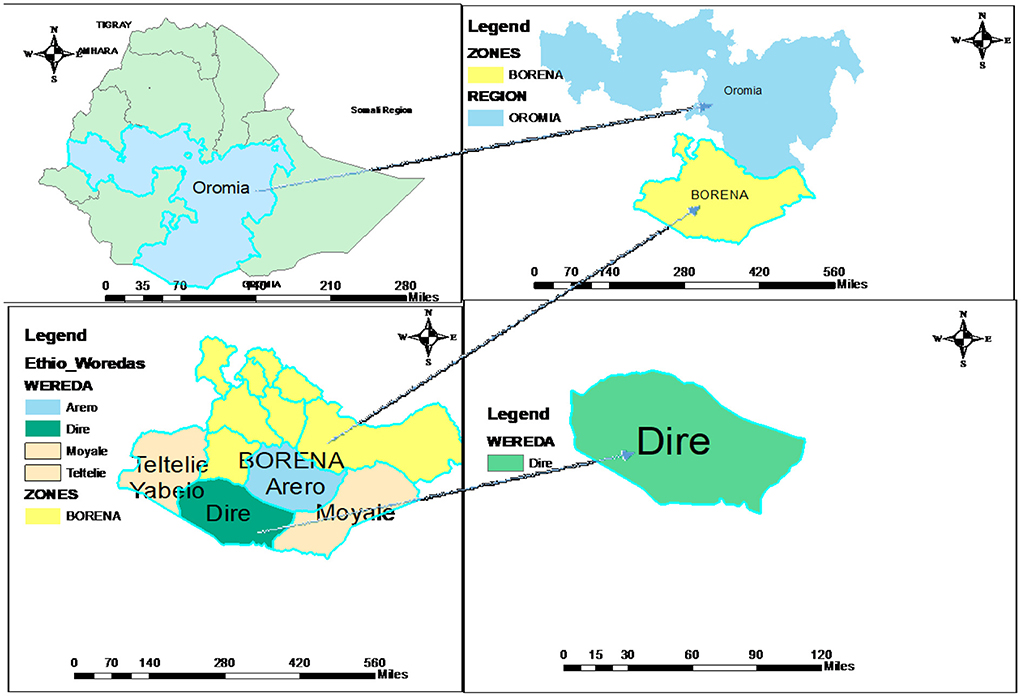
The study was carried out in Dire District in the Borena Zone. The Dire District is one of the 13 Districts in the administrative divisions of the Borena zone and is located 100 km from Yabalo town, the capital of the Borena zone, and 675 km from Addis Ababa. The woreda has 13 kebeles (smallest administrative unit) and one administrative town (Figure 1). According to the 2021 projection, the district has about 53,376 populations. Pastoralists remain the dominant livelihood system of the people, though some of the community members are gradually tending to Agro-pastoralist (livestock and crop production) livelihoods as a coping mechanism. The average rainfall varies from 300 mm in the low area to more than 740 mm in higher altitudes. The district has 1 hospital, 5 health centers, 17 health posts, and 6 private clinics, and the potential health service coverage is estimated to be 100% (21). The study was conducted from June to July 2021.
Study design and population
A community-based cross-sectional study design was conducted. The selected household (HH) members of the kebeles who fulfill the inclusion criteria were included in this study. Visceral leishmaniasis confirmed cases and treatment during data collection and individuals who had a previous history of VL disease were excluded.
Sample size determination
The sample size was determined using Epi-info version 7 software and the presence of animals in the yards with an exposed outcome of 41.1% (22), a confidence level of 95%, a margin of error of 5%, power of 80%, and the ratio of exposed to unexposed 1:1 were considered. In addition, the multistage sampling technique was used after adjusting for the design effect by a factor of 1.5 and 10% of the non-response rate; the final sample size became 432.
Sampling procedure
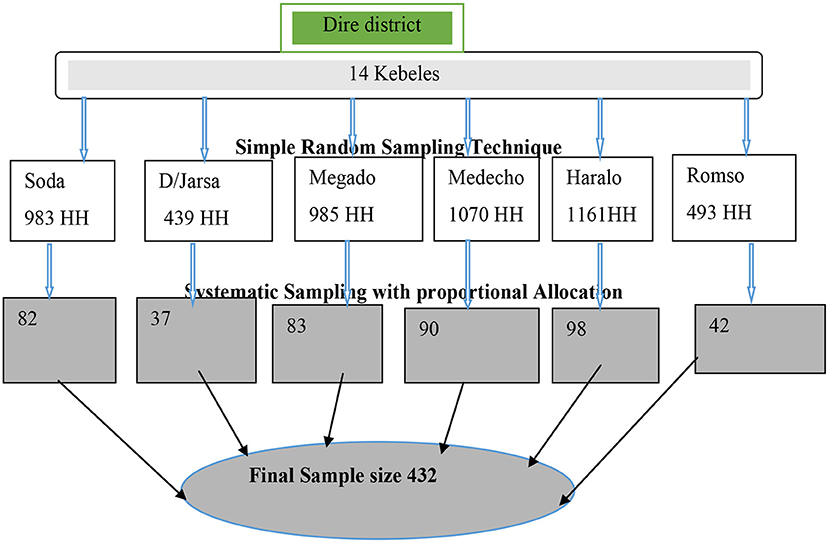
Kebeles and households (HHs) were used as primary and secondary sampling units, respectively to reach the study participants of the multistage sampling technique. From a total of 14 kebeles, 6 kebeles were selected using a simple random sampling method. The allocation of the sample to the kebeles was undertaken proportionally based on the number of HHs in the selected kebeles (Figure 2). The list of HHs in each of the study kebeles was obtained from Health posts (Health Extension workers). The sampling interval was established based on the total number of households in the kebeles and the 12th HH was selected from each kebele. Households were substituted with the nearest neighbor in cases of refusal to participate or if the HH was not inhabited. The selected HHs were given a unique identification number.
Data collection instruments and procedure
Four trained data collectors with college-level diploma graduates, two supervisors, and two Laboratory professionals from the Leishmaniasis treatment center at Yabalo Hospital and Mega Health center participated in the study. All data collectors were fluent speakers of the local language. Data were collected using a pre-structured questionnaire adopted from previous literature (22–24). Initially, the questionnaire was developed in English and translated into Afan Oromo and then back to English to check for its consistency.
Data were collected by face-to-face interviews using a structured Afan Oromo version questionnaire. The data and blood samples were collected from one person using the lottery method approach and were selected from any of the family members of the selected HHs. If a selected individual was < 18 years old the questionnaire-related data were collected from parents whose age was >18 years but a blood sample was collected from the selected person themself. If the selected person was adult individual both data and the blood sample were collected at the same time.
Sample collection and processing
Blood samples were collected from an individual selected from HH members using lottery methods. During sample collection, each participant's finger was cleaned using alcohol and cotton. A single finger prick blood specimen (8–12 μL) was collected and added to the absorbent pad well with 150 μl (2–3 drops) of the chase buffer provided with the kit (25, 26). Following the manufacturer's instructions, a serological diagnosis of L. donovani complex was then performed by using the rk39 ICT (IT LEISH, Bio-Rad, France) (27). Finally, the results were read after 10–20 min and interpreted as: reactive when both control and test lines appear; non-reactive when only the control line appears; invalid when no control line appears. Using whole blood samples, the sensitivity and specificity of the rK39 immunochromatographic test used for this study were 99 and 100% respectively based on the manufacturer's instruction.
Data analysis
Data entries were made using EpiData 3.1 version and then exported to Statistical Package for Social Science (SPSS) V-20 computer software for analysis. Descriptive statistic was used to summarize data. Independent variables with a p-value of 0.20 or less in the bivariable logistic regression were included in the multivariable logistic regression and each covariate effect was adjusted. Variables with a p-value ≤ 0.05 were considered for the significant association.
Data quality control
Designing proper data collection tools, and training for data collectors, laboratory professionals, and supervisors were measures taken to assure data quality. Before the actual data collection, the questionnaire was tested on 5% of the respondents in a neighboring district. During data collection, questionnaires were reviewed and checked for completeness, accuracy, and consistency on the daily basis. The VL serological test kit (the rk39) was checked for expiration date and batch before using the test and its standard operating procedures were strictly followed.
Ethical consideration
Ethical clearance was obtained from Haramaya University, College of Health and Medical Sciences Institutional Health Research Ethics Review Committee (IHRERC) (Ref. No. IHRERC/078/2021). A letter of support was obtained from Borena Zone Administration and Zonal health office, as well as the Dire district Administrations and Health Offices. Moreover, study participants were informed about the purpose of the study, and their consent was obtained before data collection. For children and adolescents aged < 18 years, informed voluntary written signed consent was obtained from their parent or legal guardian. In addition, oral assent was obtained from the child and/or adolescents aged < 18 years. Adolescents aged 18 years and adults were provided informed voluntary written and signed consent by themselves. All informed consent documents were provided in the local language and the confidentiality of the study participants was kept.
Results
Socio-demographic characteristics
A total of 432 study participants were included. Their mean age was 26.69 (standard deviation (SD) = ±7.8) years (age range: 1 to 85 years) and 218 (50.5%) were female. The highest proportion of the age group was 10–19 years (31.5%), Muslim religion followers (47%), illiterate (49.3%), and married (61.1%). One hundred eighty-five (42.8%) of the HHs had an estimated family income between 500 and 1,000 Ethiopian Birr and 40.0% of the household's family size ranged from 3 to 5 (Table 1).
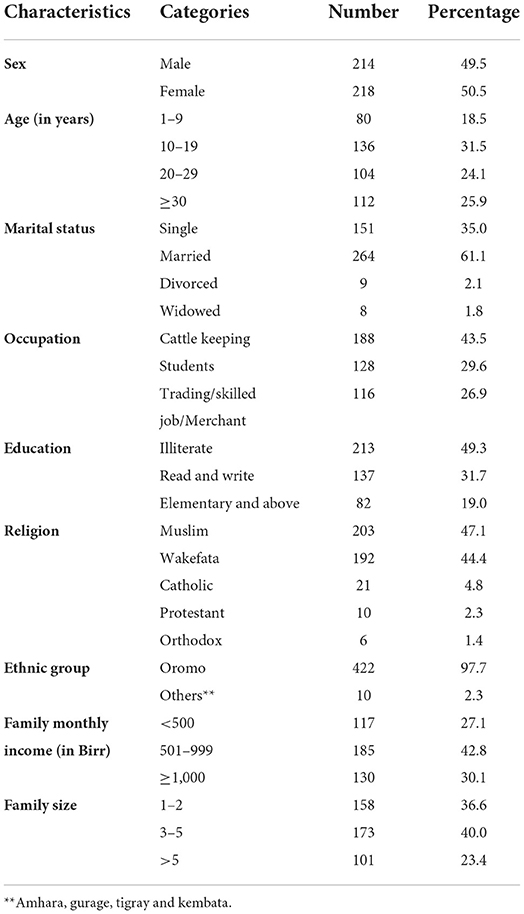
Table 1. Socio demographic characteristics of study participants of Dire District, Borena zone, Oromia Region, Ethiopia, 2021 (n = 432).
Behavioral characteristics
Among the study participants about 20.1, 66.2, 48.6, 55.1, 37, and 30.3% were from a family having a history of infection with VL, have travel history to other VL endemic areas, know about the prevention of VL, have sleep on the floor/ground, have slept/stayed day and night under an acacia tree, and know about the transmission and control of VL, respectively (Table 2).
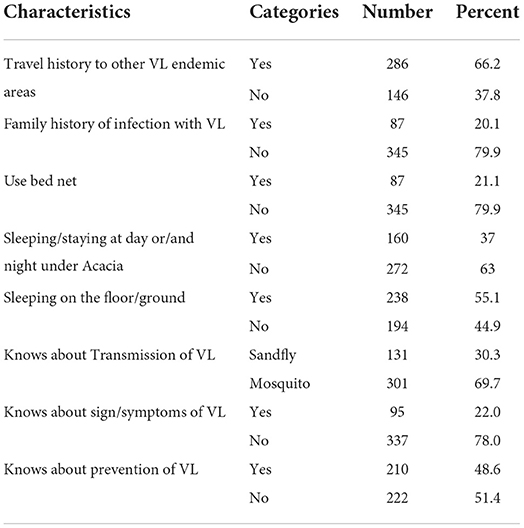
Table 2. Behavioral related factors among study participants of Dire District, Borena zone, Oromia Region, Ethiopia, 2021 (n = 432).
Environmental, housing conditions, and animal ownership related factors
Among the study participants; 281 (65%), 252 (58.4%), 215 (49.8%), 235 (54.4%), 224 (51.9%), and 235 (54.4%) had animal shelter inside the compound, their house roof had a thatch construction, had cracked house walls, the presence of crack soil near the sleeping place, the presence of termite hills near the house, and the presence of at least one or more domestic animals near the sleeping place, respectively (Table 3).
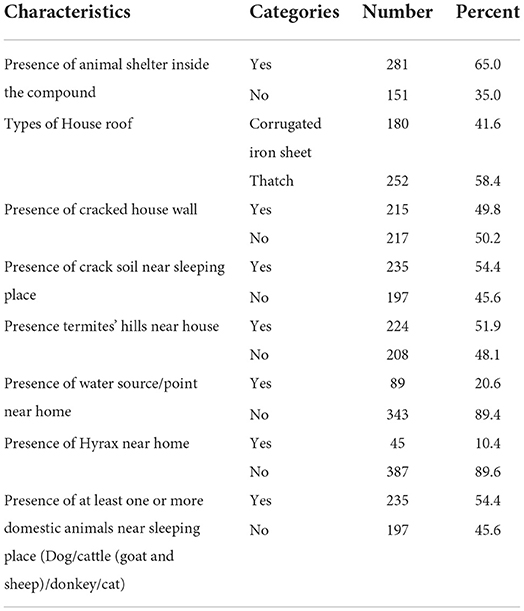
Table 3. Environmental, housing condition, and animal related factors among study participants of Dire District, Borena zone, Oromia Region, Ethiopia, 2021 (n = 432).
Prevalence of visceral leishmaniasis
The overall seroprevalence of L. donovani complex was 33/432(7.6%) (95%CI: 5.32–15.60). This infection was found to be more prevalent in men [24/33(11.2%)], participants aged 10-to 19 years old 8/33(12.6%), those with cattle keeping occupations [23/33(12.2%)], a family monthly income of < 500 Ethiopian birrs [15/33(12.8%)], and a family size >5 [15/33(18.3%)]. It was relatively more prevalent in study participants from having a family history of infection with VL [13/33(15.0%)], living in house roof types of thatch [24/33(9.5%)], presence of hyrax near home [7/33(15.6%)], presence of water source/point near home [16/33(18.4%)] and presence of at least one or more domestic animals near sleeping place [24/33(10.2%)].
Factors associated with seroprevalence of visceral leishmaniasis
In the bivariate logistic analysis variables, including sex, occupation, family monthly income, family size, travel history to other VL endemic areas, family history of infection with VL, sleeping or/and staying at night and/or day under the acacia tree, know about prevention of VL, presence of animal shelter inside the compound, presence of cracked house wall, presence of termite hills near the house, presence of water source/point near home and presence of at least one or more domestic animals near the sleeping place in the compound were significantly associated with seroprevalence of Leishmania donovani complex infection and considered as candidates for multivariable analysis (Table 4).
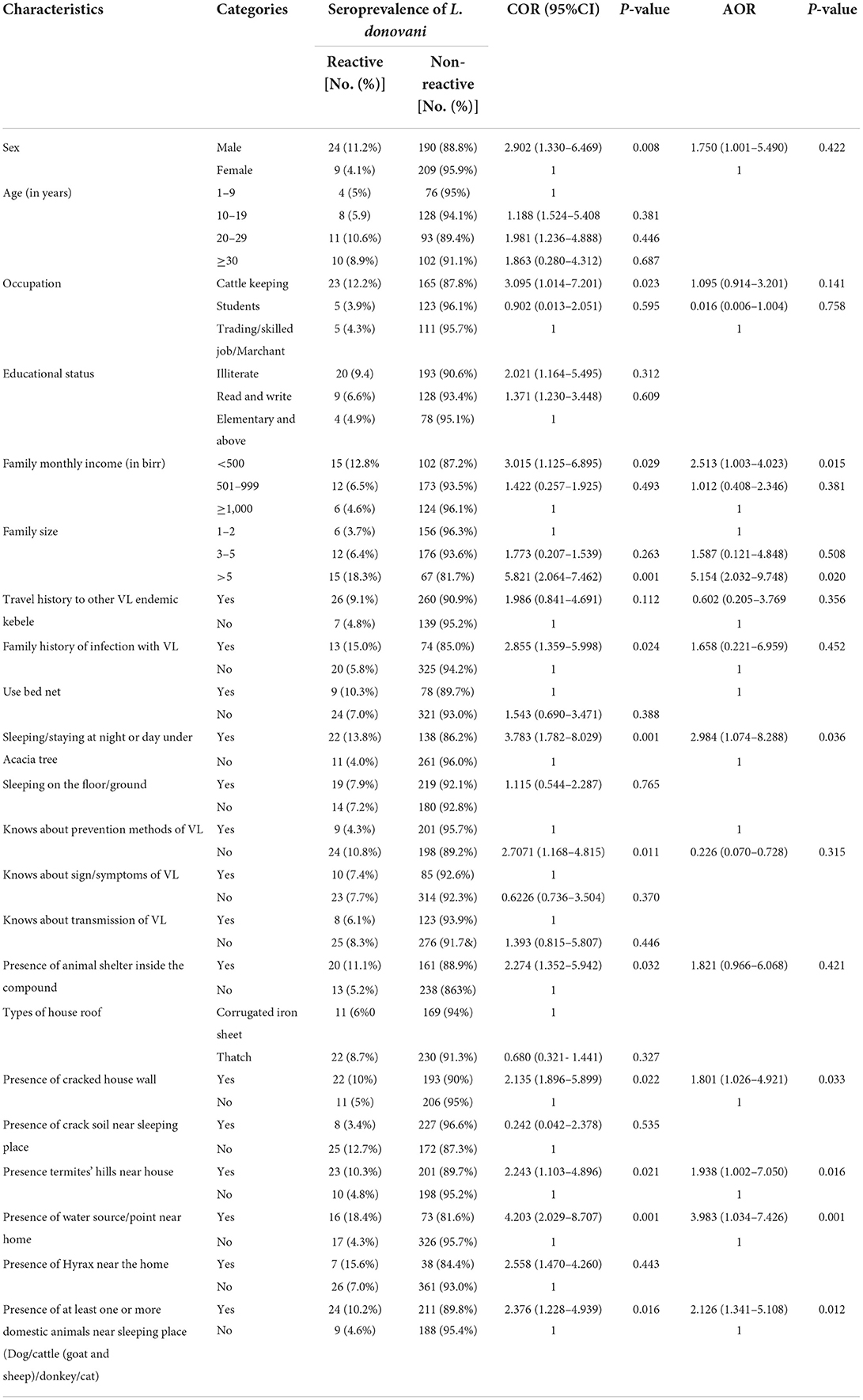
Table 4. Bivariable and multivariable logistic regression analysis factors associated with seroprevalence of visceral leishmaniasis in Dire District, Borena zone, Oromia Region, Ethiopia, 2021.
In multivariable logistic regression; < 500 Ethiopian Birr family monthly income, family size greater than five, sleeping/staying at night and/or day under the acacia tree, presence of cracked house wall, presence of termite hills near the house, presence of water/point source near home, and presence of at least one or more domestic animals near the sleeping place were statistically significant (Table 4).
Individuals with a family monthly income of < 500 Ethiopia Birr were almost 2.5 times (AOR: 2.513; 95%CI: 1.002–4.023) more likely to be infected with Leishmania donovani complex compared with individuals from a family in which the monthly income was ≥1,000 Ethiopia Birr. Individuals from a family of more than five people were 5.1 times more likely (AOR: 5.154; 95%CI: 2.032–9.748) to develop infection compared to households with a family size that was less or equal to two. Participants being sleeper/stayed at night or/and day under an acacia tree were almost 3 times (AOR: 2.984; 95%CL: 1.074–8.288) more likely to acquire VL infection compared with their counterparts (Table 4).
In terms of being more likely to develop VL infection compared, the odds of the presence of a cracked house wall meant people were 1.8 times more likely to contract it (AOR: 1.801; 95%CI:1.026–4.921). Having a termite hill near the house meant people were almost 2 times more likely (AOR: 1.938; 95%CI:1.002–7.050). Water sources/points near the home meant that participants were 4 times more likely (AOR: 3.983; 95%CI:1.034–7.426), and the presence of at least one or more domestic animals near the sleeping place meant people were 2.126 times more likely to contract infection (AOR: 2.126; 95%CI: 1.341–5.108) (Table 4).
Discussion
This study was undertaken to assess the seroprevalence of VL and its associated factors among the asymptomatic pastoral community of the Dire District in the Borena Zone of the Oromia Region, Ethiopia. The overall seroprevalence of the Leishmania donovani complex was 7.6% (95%CI: 5.32–15.60). Risk factors that aggravate the occurrence of VL infection include earning < 500 Ethiopian Birr for the monthly family income, having a family size greater than five, sleeping/staying at night and/or day under an acacia tree, the presence of cracked house walls, the presence of termite hills near the house, the presence of water points or sources near the home, and the presence of at least one or more domestic animals near the sleeping place.
In the current study, the overall seroprevalence of L. donovani complex was 7.6%. Similar results are reported from studies conducted in Benishangul Gumz (6.9%) (28), Tigray (8.8%) (29), the Gode district of Shebel zone of the Somali region (12.7%) (30), and the west Armachiho district (7.6%) (26). However, these results were higher than those reported in previous studies conducted in the Benishangul-Gumz (3.2%) (25) and Raya Azebo district of northeastern Ethiopia (0.87%) (31). However, they were less when compared to reports from Adadile district (31.1%) (30), Addis Zemen North West Ethiopia (39.1%) (22), and SNNPR (36.0.4%) (32). These differences might be due to the varied implementation of different intervention strategies, settlement of the community, and the different study population and design, as well as factors such as the socioeconomic status of the area and living conditions of the communities studied.
In the current study, a family size of more than 5 members in the household had a statistically significant association with the seroprevalence of VL infection. This study finding was consistent with those conducted in northwestern Ethiopia (22) and north-central Ethiopia (33). The higher amount of carbon dioxide and odors released in the homestead could attract sandflies and increase the chance of asymptomatic cases.
In this study, a low family monthly income was a statistically significant association, with seroprevalence of VL compared to their counterparts. These results were contrary to the studies conducted in the Pokot territory of Kenya and Uganda (34). In previous studies, living in a straw-roofed house was the house characteristic most associated with asymptomatic infection. This could be related to socioeconomic status or to the potential that straw roofs may provide resting places for sand flies and increase their survival in the homestead area (35). The housing condition and overall environment are favorable for breeding and resting sites of sandflies.
According to a study conducted in Kenya and Uganda, poor nutritional status has been associated with a higher risk of developing VL (36). In the present study, there was a strong association between the seroprevalence of L. donovani complex and the presence of poor housing conditions such as house wall cracks. This finding is supported by a study by Yared et al. that confirmed individuals who sleep regularly near cracked walls are at higher risk of VL infection (22). The possible reason for this might be that cracked walls are ideal breeding and resting sites for sand flies. Furthermore, mud-type houses have been identified as risk factors for asymptomatic VL infection as sand flies prefer mud crack walls for breeding and resting sites (37).
The findings of the current study show that sleeping or/and staying day or/and night under an acacia tree was significantly associated with seroprevalence of L. donovani complex. This is consistent with a previous study carried out in the Shebele zone of the Somali region (30) and the Gedaref state of Sudan (24). This might be due to the sand flies using the acacia vegetation tree as breeding/resting sites and having access to bite people while they sleep or stay under the tree. Another study from Ethiopia conducted in Libo Kemkem and Fogera, also reported that resting under acacia trees was a risk factor for VL (8) as acacia vegetation provides suitable breeding/resting sites for Phlebotomine sand flies (8, 38).
In this study, the presence of water points/sources near the home increased the likelihood of VL infection by 3 fold. This finding was supported by studies in Tigray Northern Ethiopia (39), and in north-central Ethiopia (40). In our study, more than 50% of the seroprevalence of the L. donovani complex was found in participants from Magado Kebele. The reason for the increment of cases in this area might be related to the settlement of the community near the water point of Ele_Bora. This may create favorable conditions for the breeding of sandflies. Moreover, all the cases from other kebele had a travel history that involved going to the water point in the study area.
In the current study, the presence of a domestic animal in the yard had a statistically significant association with the seroprevalence of L. donovani complex infection. The majority of the Leishmania species are involved in zoonotic transmission. Infected animal reservoir hosts transmitted pathogens to the human population and spillover events result in zoonotic diseases (41). This finding was supplemented by the study conducted in Armachiho district (26) and Shebel zone, Somali region, Ethiopia (30). The presence of a domestic animal in the yard increases the chance of getting an infection might be due to the zoonotic nature of the disease (42). Some of the domestic animals may attract vectors to homesteads and increase the probability of human exposure to sandflies. In the study area, the communities are pastoralists, and the presence of domestic animal shelters in their compounds may attract sand fly vectors to breed and rest there. In the current study; the presence of livestock and their manure in the compound could be a favorable breeding area for the sand fly's vectors.
The present study revealed that the presence of termite hills near houses had a statistically significant association with an increase of leishmaniasis infections. This is supported by the study conducted in the Pokot territory of Kenya and Uganda (34), the Shebele zone of the Somali region (30), and Armachiho district, Ethiopia (26). The termite's hill represents ideal conditions, providing breeding and resting sites for sandflies. The findings of this study might due to the majority of the landmarks being covered by the termite hills.
The presence of cracked soil was not significant. This is supported by a study conducted in Gedaref state, Sudan (24). This study is contrary to that study done in the Armachiho district (26) and Tigray regions in northern Ethiopia (22). The reason for these differences might be that the majority of the land is covered by sandy soil, which fills the cracked soil and may not be favorable for sandflies.
In the present study, utilization of bed net was not a statistically significant association with the seroprevalence of VL. On the contrary, a study conducted in Dessie town north-central Ethiopia (40) reported a statistically significant association. The reason for this discrepancy might be that access to and utilization of bed nets in the study area were very low, and only 18% of people had a bed net.
A travel history of visiting other endemic areas was not significantly associated with the seroprevalence of VL. This is contrary to a study conducted in the Somali region in southern Ethiopia (43) and Dessie town in north central Ethiopia (40). The possible reason for these differences might be that the community of the study area were pastoralists and they did not typically travel to other endemic areas, but there was an area that acted as a source of infection, the so-called Ele-Bora water source used for cattle.
The strength of this study was that it examined a hard-to-reach area. It determined the seroprevalence of VL infection at the asymptomatic pastoralist community level using both structured questionnaires and serological tests. The limitations of this study are that the laboratory methods used could not be rolled out past the current infection. Since this was a single snapshot measurement of exposure and outcome, it is difficult to derive causal relationships from the cross-sectional study.
In Ethiopia, despite a significant number of people being at risk of contracting VL, the zoonotic nature of the disease, and the spread of the disease to non-endemic areas due to environmental and climate changes, there is no active and routine screening of leishmaniasis cases. Thus, the health sectors and concerned bodies need to create tailored programs and intervention packages to make the community more aware and enable them to prevent the transmission and control of the disease, increasing the diagnosis, screening, and surveillance of VL using different laboratory-based approaches is essential for providing up-to-date evidence and design policy to control disease in the endemic area and at the national level.
Conclusion
The seroprevalence of VL among the asymptomatic pastoralist community was comparable with the majority of previous studies. The main factors associated with the seroprevalence of VL infection were sleeping or/and staying day or/and night under an acacia tree, an increased family size of more than five members, the presence of cracked house walls and termite hills, and the presence of water sources/points and domestic animals near the home. Awareness of VL transmission and prevention in pastoral communities and taking appropriate interventions, and focusing on factors that aggravate VL infection are recommended for preventing and controlling disease transmission in the area. Further comprehensive studies on the epidemiology and magnitude of the disease, and investigations of other contributing risk factors in the area using advanced methods and laboratory investigations are required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Haramaya University, College of Health and Medical Sciences Institutional Health Research Ethics Review Committee (IHRERC). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
HK undertook the data collection and the study idea. HK, FW, AG, and TG designed the manuscript, interpreted the results and drafted the manuscript, and including tables and figures. All authors critically reviewed the manuscript and approved the final submitted version.
Funding
Haramaya University postgraduate directorate covered financial support for research data collection.
Acknowledgments
The authors thank the Haramaya University College of Health and Medical Sciences, Institutional Health Research Ethical Review Committee for providing the ethical clearance to conduct the study. We also thank study participants for their cooperation and for providing the necessary information during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. (2018) 145:430–42. doi: 10.1017/S0031182016002092
2. Alvar JS. Leishmaniasis and poverty. Trends Parasitol. (2006) 22:552. doi: 10.1016/j.pt.2006.09.004
3. Leta S, Dao THT, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. (2014) 8:e3131. doi: 10.1371/journal.pntd.0003131
5. de Menezes JP, Saraiva EM, da Rocha-Azevedo B. The site of the bite: Leishmania interaction with macrophages, neutrophils and the extracellular matrix in the dermis. Parasit & Vectors. (2016) 9:264. doi: 10.1186/s13071-016-1540-3
6. Dawit G, Girma Z, Simenew K. A review on biology epidemiology and public health significance of leishmaniasis. J Bacteriol Parasitol. (2013) 4:2. doi: 10.4172/2155-9597.1000166
7. Lemma W. Zoonotic leishmaniasis and control in Ethiopia. Asian Pac J Trop Med. (2018) 11:313. doi: 10.4103/1995-7645.233178
8. Bashaye S, Nombela N, Argaw D, Mulugeta A, Herrero M, Nieto J, et al. Risk factors for visceral leishmaniasis in a new epidemic site in Amhara Region, Ethiopia. Am J Tropic Med Hygiene. (2009) 81:34. doi: 10.4269/ajtmh.2009.81.34
9. Jones CM, Welburn SC. Leishmaniasis beyond East Africa. Front Veterin Sci. (2021) 2021:65. doi: 10.3389/fvets.2021.618766
10. Ayehu A, Aschale Y, Lemma W, Alebel A, Worku L, Jejaw A, et al. Seroprevalence of Asymptomatic Leishmania donovani among laborers and associated risk factors in agricultural camps of west armachiho district, northwest ethiopia: a cross-sectional study. J Parasitol Res. (2018) 2018:5751743. doi: 10.1155/2018/5751743
11. WHO. Control of the Leishmaniasis. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniasis. World Health Organization: Geneva, Switzerland (2010).
12. World Health Organisation Expert C. Control of the leishmaniases. World Health Organ Tech Rep Ser. (2010) 949:1–186.
13. Gadisa E, Tsegaw T, Abera A, Elnaiem D-e, den Boer M, Aseffa A, et al. Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasites Vect. (2015) 8:381. doi: 10.1186/s13071-015-0987-y
14. Hailu T, Argaw D, Boelaert S. Leishmaniasis. In: Neglected Tropical Diseases- Sub-Saharan Africa (2016).
15. Gebre-Michael T, Balkew M, Berhe N, Hailu A, Mekonnen Y. Further studies on the phlebotomine sandflies of the kala-azar endemic lowlands of Humera-Metema (north-west Ethiopia) with observations on their natural blood meal sources. Parasit Vectors. (2010) 3:1–7. doi: 10.1186/1756-3305-3-6
16. Tsegaw GE, Seid A, Abera A, Teshome A, Mulugeta A, Herrero M, et al. Identification of Environmental parameters and risk maping of visceral leishmaniasis by using geographical information system and statistical approach. Geospat Health. (2013) 7:299–308. doi: 10.4081/gh.2013.88
17. Deribe KM. Gebre T, Hailu A, Ali A, AsefFa A, Davey G. The burden of neglected Tropical disease in Ethiopia and Elimination. Parasite Vect. (2012) 5:240. doi: 10.1186/1756-3305-5-240
18. Diro E, Lynen L, Assefa M, Takele Y, Mengesha B, Adem E, et al. Impact of the use of a rapid diagnostic test for visceral leishmaniasis on clinical practice in Ethiopia: a retrospective study. PLoS Negl Trop Dis. (2015) 9:e0003738. doi: 10.1371/journal.pntd.0003738
20. BZHO. BZHO(Borena Zonal Health Office)report of VL cases in 2020 in Borena zone,Oromia,Ethiopia (2020).
21. DDHO. DDHO(Dire district halt office). district profile of Dire, Borena zone, Oromia, Ethiopia (2021).
22. Yared K, Araya G, Wossenseged L, Essayas A, Oscar DK, Meshasha B, et al. Risk factors of Visceral Leishmaniasis a case control study north-weastrn Ethiopia. Parasit Vect. (2014) 7:470. doi: 10.1186/s13071-014-0470-1
23. Oryan T, Alidadi S, Akbari M. Risk factors associated with leishmaniasis. Trop Med Surg. (2014) 2:3. doi: 10.4172/2329-9088.1000e118
24. Nackers MYK, Salih N, Elhag MS, Elbadawi ME, Hammam O. Determinants of visceral leishmaniasis: a casecontrol study in Gedaref State, Sudan. PLoS Negl Trop Dis. (2015) 9:11. doi: 10.1371/journal.pntd.0004187
25. Bejano S, Kumar A, Damte W, Mulugeta M, Genetu GM. Prevalence of asymptomatic visceral leishmaniasis in human and dog, Benishangul Gumuz regional state, Western Ethiopia. Parasites Vect. (2021) 14:39. doi: 10.1186/s13071-020-04542-z
26. Ayehu Y, Wossenseged L, Animut A, Ligabaw W, Ayalew J, Abebe GB. Seroprevalence of asymptomatic leishmania donovani among laborers and associated risk factors in agricultural camps of West Armachiho District, Northwest Ethiopia. Hindawi. (2018) 2018:12.
27. FMOH(Federal Minstry of health). Guideline for Diagnosis,treatment and prevention of leishimanisis in Ethiopia Adi Abeba (2013).
28. Abera GT, Tsegaw A. Visceral leishmaniasis in Benishangul-Gumuz regional state, Western Ethiopia: reemerging or emerging. Trop Med Hygiene. (2016) 95:104. doi: 10.4269/ajtmh.15-0738
29. Bsrat BM, GE. Serological investigation of visceral Leishmania infection in human and its associated risk factors in Welkait District W. Ethiopia. Parasite Epidemiol Control. (2018) 3:13–20. doi: 10.1016/j.parepi.2017.10.004
30. Alebie A, Worku A, Yohannes S, Urga B, Hailu A, Tadesse D. Epidemiology of visceral leishmaniasis in Shebelle Zone of Somali Region, eastern Ethiopia. Parasit Vect. (2019) 12:209. doi: 10.1186/s13071-019-3452-5
31. Tadese A, Hailu A, Bekele F, Belay S. An epidemiological study of visceral leishmaniasis in North East Ethiopia using serological and leishmanin skin tests. PLoS ONE. (2019) 14:e0225083. doi: 10.1371/journal.pone.0225083
32. Ali A. Visceral leishmaniasis in Ethiopia.I. Cross-sectional leishmanin skin test in an endemic locality. Ann Trop Med Parasitol. (1993) 87:157–61. doi: 10.1080/00034983.1993.11812749
33. Tewart B. Community views on cutaneous leishmaniasis in Istalif, Afghanistan: implications for treatment and prevention. Int Q Community Health Educ. (2009) 29(2):123. doi: 10.2190/IQ.29.2.c
34. Reithinger A, Mohsen M, Leslie T. Risk factors for anthroponotic cutaneous Leishmaniasis at the household level in Kabul, Afghanistan. PLoSNegl Trop Dis. (2010) 4:e639. doi: 10.1371/journal.pntd.0000639
35. Custodio GAS, Cruz I, Moreno J. Factors associated with leishmania asymptomatic infection: results from a cross-sectional survey in highland Northern Ethiopia. PLoS neglected tropical diseases. (2012) 6:e1813. doi: 10.1371/journal.pntd.0001813
36. Kolaczinski R, Worku E, Ocheng A, Kasimiro J. Risk factors of visceral leishmaniasis in East Africa: a case-control study in Pokot territory of Kenya and Uganda. Int J Epidemiol. (2008) 37:344–52. doi: 10.1093/ije/dym275
37. Rashid JR, Wasunna MK. Kirigi G. Spatial clustering and epidemiological aspects of visceral leishmaniasis in two endemic villages, Baringo District, Kenya. Am J Tropic Med Hygiene. (2006) 74:308–17. doi: 10.4269/ajtmh.2006.74.308
38. Bantie A, Tessema F, Massa DYT. Factors associated with visceral leishmaniasis infection in North Gondar Zone, Amhara Region, North West Ethiopia. Case Control Study. (2014) 2:560–8. doi: 10.11648/j.sjph.20140206.20
39. Yohannes ZAEB. Prevalence and environmental determinants of cutaneous leishmaniasis in rural communities in Tigray, northern Ethiopia. PLoS Negl Trop Dis. (2019) 13:9. doi: 10.1371/journal.pntd.0007722
40. Eshetu A, Mamo D. Cutaneous leishmaniasis in north-central Ethiopia: trend, clinical forms, geographic distribution, and determinant. Tropic Med Health. (2020) 48:39. doi: 10.1186/s41182-020-00231-w
41. Hong A, Zampieri RA, Shaw JJ, Floeter-Winter LM, Laranjeira-Silva MF. One health approach to leishmaniases: understanding the disease dynamics through diagnostic tools. Pathogens. (2020) 9:809. doi: 10.3390/pathogens9100809
42. Lemma T, Bizuneh A, Tekie S. Preliminary study on investigation of zoonotic visceral leishmaniasis in endemic foci of Ethiopia by detecting Leishmania infections in rodents. Asian Pacific J Tropic Med. (2017) 10:418–22. doi: 10.1016/j.apjtm.2017.03.018
Keywords: seroprevalence, associated factors, Leishmania donovani, Dire District, Ethiopia, visceral leishmaniasis
Citation: Ketema H, Weldegebreal F, Gemechu A and Gobena T (2022) Seroprevalence of visceral leishmaniasis and its associated factors among asymptomatic pastoral community of Dire District, Borena zone, Oromia Region, Ethiopia. Front. Public Health 10:917536. doi: 10.3389/fpubh.2022.917536
Received: 11 April 2022; Accepted: 21 June 2022;
Published: 21 November 2022.
Edited by:
Javier Moreno, Instituto de Salud Carlos III (ISCIII), SpainReviewed by:
Iraj Sharifi, Kerman University of Medical Sciences, IranBahador Sarkari, Shiraz University of Medical Sciences, Iran
Copyright © 2022 Ketema, Weldegebreal, Gemechu and Gobena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdella Gemechu, YWJkZ2VtZWNodUBnbWFpbC5jb20=
 Haile Ketema1
Haile Ketema1 Fitsum Weldegebreal
Fitsum Weldegebreal Abdella Gemechu
Abdella Gemechu Tesfaye Gobena
Tesfaye Gobena