- 1School of Public Health, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Ethiopia
- 2Department of Statistics, College of Natural Sciences, Mizan-Tepi University, Tepi, Ethiopia
- 3School of Public Health, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
- 4Department of Data Repository and Governance, National Data Management Center for Health, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- 5Department of Public Health, Mizan-Aman Health Science College, Mizan-Aman, Ethiopia
Background: Virological failure remains a public health concern among patients with human immunodeficiency virus (HIV) after treatment initiation. Ethiopia is one of the countries that aims to achieve the global target of 90-90-90 that aims to achieve 90% virological suppression, but there is a paucity of evidence on the determinants of virological failure. Therefore, the study is intended to assess determinants of virological treatment failure among patients on first-line highly active antiretroviral therapy (HAART) at Mizan Tepi University Teaching Hospital (MTUTH), Southwest Ethiopia.
Method: A hospital-based unmatched case-control study was conducted from 11 November to 23 December 2020, among 146 cases and 146 controls. All cases and controls were selected randomly using computer-generated random numbers based on their medical record numbers. During the document review, data were collected using checklists, entered into Epi-data version 4.0.2, and analyzed by SPSS version 25. A multivariable logistic regression analysis was done to identify the independent determinants of virological treatment failure.
Results: In this study, being male (adjusted odds ratio (AOR) = 1.89, 95% CI: 1.04, 3.47), substance use (AOR = 2.67, 95% CI: 1.40, 4.95), baseline hemoglobin (Hgb) < 12 mg/dl (AOR = 3.22, 95% CI: 1.82, 5.99), poor drug adherence (AOR = 3.84, 95% CI: 1.77, 5.95), restart ART medication (AOR = 2.45, 95% CI: 1.69, 7.35), and opportunistic infection (OI) while on HAART (AOR = 4.73, 95% CI: 1.76, 12.11) were determinants of virological treatment failure.
Conclusion: The study revealed that the sex of the patient, history of substance use, baseline Hgb < 12 mg/dl, poor drug adherence, restart after an interruption, and having OI through the follow-up period were determinants of virological failure. Therefore, program implementation should consider gender disparity while men are more prone to virological failure. It is also imperative to implement targeted interventions to improve drug adherence and interruption problems in follow-up care. Moreover, patients with opportunistic infections and restart HAART need special care and attention.
Background
The human immunodeficiency virus (HIV) continues to be a global public health challenge, affecting over 38 million people and infecting 1.5 million new people at the end of 2020 (1). Despite the decreased incidence of HIV infection in Ethiopia, more than 616,105 people are estimated to live with the virus in 2020, which puts the country among the worst affected in sub-Saharan Africa (2).
Mortality due to HIV has decreased since the era of highly active antiretroviral therapy (HAART). Evidence has shown that individuals on HAART with an undetectable viral load, the absence of an advanced clinical finding, and a high CD4 count are less likely to transmit HIV to other people and reduce HIV-associated morbidity and mortality. However, due to many factors, patients experience deterioration and treatment failure (3).
Virological failure is defined as a persistently detectable viral load exceeding 1,000 copies/ml (i.e., two consecutive viral load measurements within a 3-month interval, with adherence support between measurements) after at least 6 months of starting a new ART regimen (4). Patients who experienced virological failure were found to have an increased risk of clinical progression to AIDS and mortality when compared with patients with a complete virological response (3). Virological failure is a more informative parameter to determine treatment failure and is a common problem among patients on first-line ART (3, 5).
Optimization of viral load testing capacities is an important measure for the global control of the HIV epidemic, particularly in resource-limited countries, such as Ethiopia (6). Studies showed that virological failure is common in different countries, particularly in Sub-Saharan countries: 32% in Malawi (7), 16% in Swaziland (8), 24.6% in Kenya (9), 24% in Mozambique (10), 41.3% in Gabon (11), 11.9% in Rwanda (12), 11% in Uganda (13), and 14.8% in Ethiopia (14).
Globally, the United Nations Program on HIV/AIDS (UNAIDS) 90-90-90 is committed to having 90% of people on HAART virally suppressed by 2030, and as a result, HIV treatment failure would be prevented. Despite this ambitious goal, according to a systematic analysis of national HIV treatment cascades in 69 countries by 2016, viral suppression was between 7% in China and 68% in Switzerland (15). In Sub-Saharan countries, 83% were virally suppressed, contributing to a 29% reduction in new HIV infections between 2010 and 2016 (16). Mathematical modeling predicts that if the levels of NNRTI drug resistance exceed 10% in sub-Saharan Africa, drug resistance is predicted to be responsible for an additional 105,000 new HIV infections, 135,000 AIDS deaths, and US$ 650 million in ART drug costs between 2016 and 2020 (16).
Ethiopia has limited resources available for diagnosing treatment failure and monitoring patient response with viral load, which is the gold standard method for monitoring treatment effectiveness. Nevertheless, this is not feasible since patients initiate treatment with very advanced diseases. Therefore, identifying the determinants of virological failure and reducing its incidence would help to realize the 90-90-90 treatment target and achieve sustainable development goal 3. It is also important for the patients to prevent the progression of AIDS and have better efficacy of the second-line regimen. However, there is an evidence gap in identifying the determinants in the study area.
Therefore, this study aimed to assess determinants of virological treatment failure among patients on first-line HAART at Mizan Tepi University Teaching Hospital (MTUTH) and provide a wider understanding of the determinants of virological treatment failure, and enable early identification and control of this factor, which results in decreasing patients' risk of treatment failure.
Method and material
Study design and setting
A hospital-based case-control study was conducted from November to December 2020 at the Mizan Tepi University Teaching Hospital ART clinic. The hospital is located in Mizan-Aman town, which is located 561 km away from Addis Ababa toward the southwest of the country. The hospital has been serving more than 3 million people from two regions in southwestern Ethiopia. There were 3,805 registered patients on HAART from 2012 to 2020 in the ART clinic. The clinic provides voluntary counseling and testing (VCT), provider imitative counseling and testing (PICT), and prevention of mother to child transmission (PMTCT) services.
Study population
All adult patients infected with HIV (>15 years) who had taken ART for at least 6 months with two consecutive documented viral load test results at MTUTH between December 2012 and November 2020 were our source populations. Patients who had viral load results >1,000 or more in two consecutive viral load tests were considered as cases, and controls were those who had viral load results <1,000 in two consecutive viral load tests.
Sample size and sampling procedure
The sample size was determined using Epi Info 7.2 Stat-Cal by taking age as a predictor variable for virological failure based on the study done in Gonder University Referral Hospital (3) assuming a 1:1 case to control ratio, 5% margin of error, and 80% of study power. The final calculated sample size was 292 (146 cases and 146 controls). After filtering the illegible patients' cards, sampling frames were generated using ART medical record numbers for both cases and controls separately. There were a total of 207 patients with viral load results >1,000 or more and 3,521 patients who had viral load results <1,000 in the study period. Finally, cases and controls that fulfilled the inclusion criteria were randomly selected using computer-generated random numbers based on the medical record number of the patient. Cases and controls with 20% missing study variables were excluded from the study.
Study variables
The dependent variable (outcome of interest) was a virological failure. Age, marital status, sex, religion, educational status, place of residence, substance use, adherence, non-disclosure of HIV status, baseline WHO staging, baseline CD4 count, chronic illness, baseline hemoglobin (Hgb), history of opportunistic infection (OI), baseline body mass index (BMI), duration on ART, ART regime, previous exposure to ART, and restart after interruption were independent variables.
Data collection method
Data were extracted via document review using a structured checklist prepared in English adapted from the Ethiopian Federal Ministry of Health ART clinic intake and follow-up form, and were collected by the three health officers after consent was received from the MTUTH medical director office, the ART department, and card room to extract cards and to get the necessary information. The information was extracted from the patient's cards.
Data quality control
To assure the quality of data, we used the Federal Ministry of Health (FMOH) ART clinic intake checklist, and the collected data were reviewed and checked for completeness before data entry.
Operational definition
Virological failure: Viral load above 1,000 copies/ml based on two consecutive viral load measurements in 3 months, with adherence support following the first viral load test as diagnosed by ART physicians (3, 17).
ART drug adherence: We used the information from the card that was filled out by the physicians as good, fair, and poor adherence. This was assessed by patients' self-report about missed doses within a month, missing more than three doses from BID doses, and missing more than one dose from daily doses was considered poor adherence (18).
Opportunistic infection: People living with HIV and who developed either herpes zoster infection, candidiasis, cryptococcal meningitis, chronic diarrhea, encephalopathy, herpes simplex, Pneumocystis jiroveci pneumonia, pneumonia, pulmonary tuberculosis, extra-pulmonary tuberculosis, intestinal parasitosis, toxoplasmosis, or upper respiratory tract infections were considered as having opportunistic infections (19, 20).
Data processing and analysis
First, data were checked for coding errors, completeness, and missing values. Then, data were entered into Epidata Manager version 4.0.2 and were analyzed using SPSS version 25. Descriptive statistics, such as frequencies, means, standard deviations (SD), and proportions were done for different variables. Bivariable logistic regression analyses were done to select a candidate variable for multi-variable logistic regression analysis. Variables with a p-value < 0.25 during the bivariable analysis were included in the multivariable logistic regression analysis. Model adequacy was done by the Hosmer and Lemeshow test (p = 0.62) and multicollinearity between independent variables was checked by variance inflation factor (VIF) (4). Variables with a p-value <0.05 were considered statistically significant determinants of virological failure in the final multivariable logistic regression model.
Results
Socio-demographic characteristics
In total, two hundred ninety-two patients (146 cases and 146 controls) were involved in this study. The mean age of cases was 37.14 (SD = 10.47) and 37.45 (SD = 8.372) years for control groups while ART was initiated. Furthermore, fifty-nine (40.4%) cases and 50 (34.2%) controls were in the age range of 35 years or less in their ART initiation time. Regarding marital status, eighty-eight (60.3%) cases and 95(65%) controls were married at ART initiation. Moreover, seventy-six (52%) cases and 54 (37%) controls were men. In total, fifty-five (37.7%) cases and 58 (39.7%) controls had not attended school (Table 1).

Table 1. Socio-demographic characteristics of patients positive with HIV on highly active antiretroviral therapy (HAART) at Mizan Tepi University Teaching Hospital (MTUTH), 2020.
Behavioral, medical, and nutritional related factors
Among the study participants, only 65 (44.5%) cases and 58 (39.7%) controls disclosed their HIV status. In this study, seventy-seven (52.7%) cases and 40 (27.4%) controls had a history of substance use, and 29 (19.9%) cases and eight (5.5%) controls had a history of poor adherence to ART drugs. Regarding the medical-related factors, 27 (18.5%) cases and 17 (11.6%) controls had CD4 count ≤200 cells/mm3 at the ART initiation, and 40 (27.4%) cases and 12 (8.2%) controls had an underlying chronic illness. Moreover, 95 (65.1%) cases and 52 (35.6%) controls had the baseline hemoglobin of <12 mg/dl. Furthermore, forty-three (29.4%) cases and 30 (20.5%) had a baseline BMI ≤18.4 kg/m2, while 96 (65.8%) cases and 101 (69.2%) controls had a baseline BMI of 18.5–24.99 kg/m2 (Table 2).
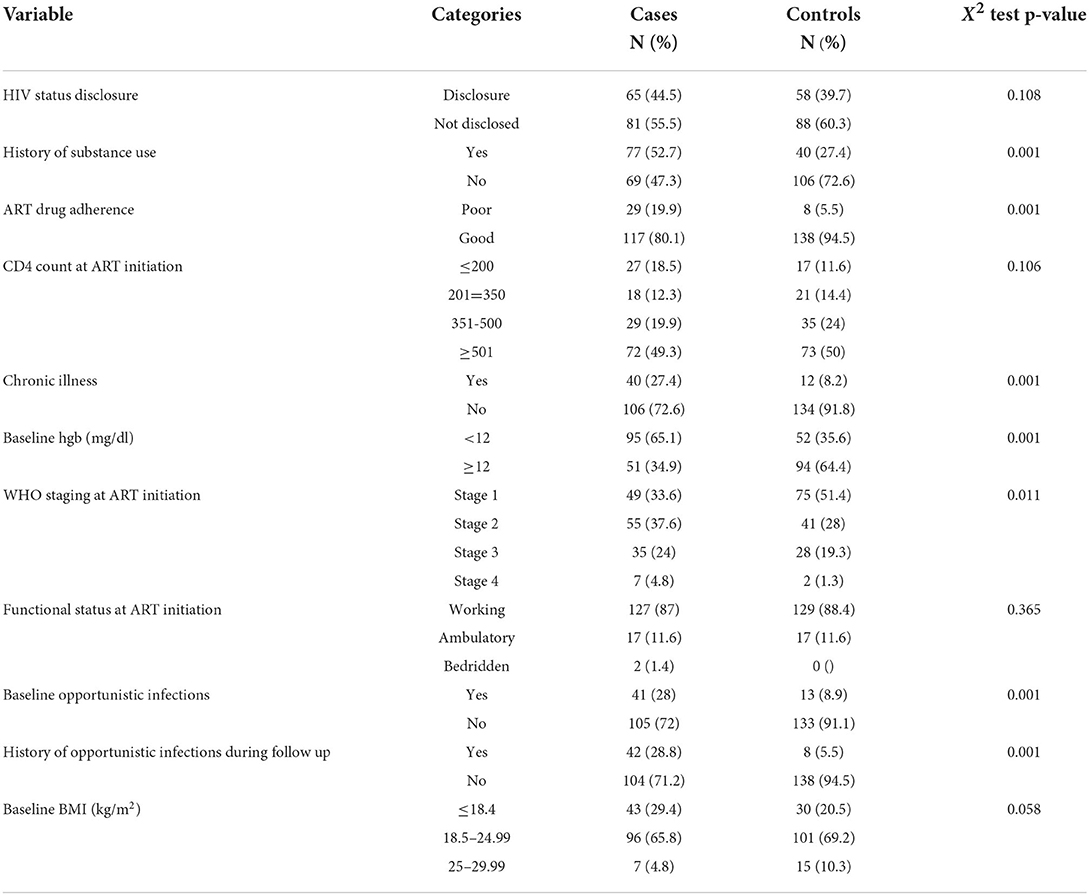
Table 2. The behavioral, clinical, and nutritional characteristics of patients with HIV treated at MTUTH in 2020.
Drug-related factors
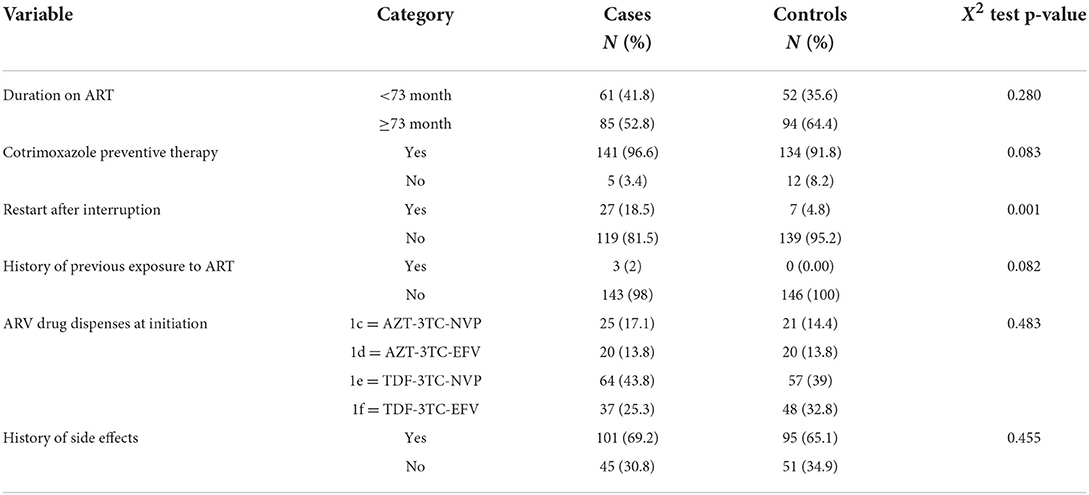
Among the study participants, 61 (41.8%) cases and 52 (35.6%) controls were <73 months on ART, and the majority of cases and controls had taken Current Procedural Terminology (CPT). Similarly, 27 (18.5%) cases and seven (4.8%) controls had a history of the restart after interruption. In total, sixty-four (43.8%) cases and 57 (39%) controls had taken the TDF-3TC-NVP regimen at ART initiation. In addition, 101 (69.2%) cases and 95 (65.1%) controls had experienced side effects (Table 3).
Determinants of virological failure
After adjusting for possible confounders, a multivariable logistic regression analysis showed that sex of the patient, history of substance use, baseline Hgb, restart after the interruption, poor drug adherence, and OI through follow-up had a statistically significant association with virological failure (Table 4).
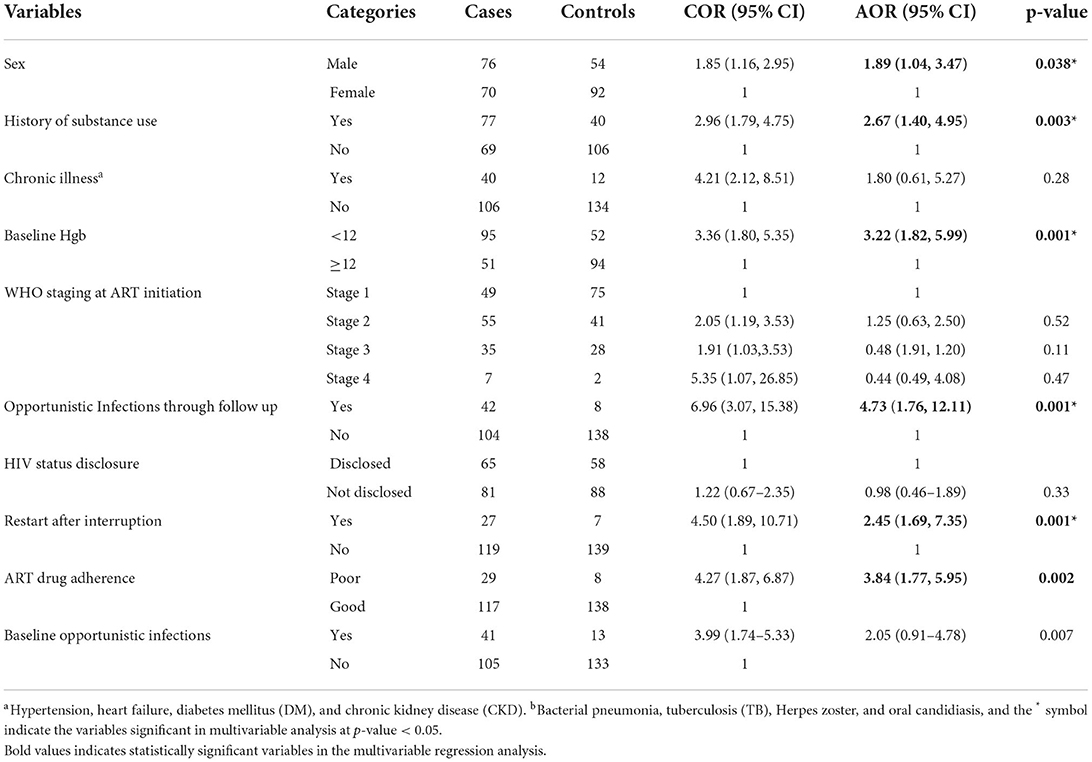
Table 4. The bivariable and multivariable logistic regression analyses for factors associated with virological failure among first-line HAART at MTUTH in 2020.
In this study, virological failure was 1.89 times higher among men (adjusting odds ratio (AOR) = 1.89, 95% CI: 1.04, 3.47) compared with female patients. The odds of virological failure among those who had a history of substance use were 2.6 times higher compared with those who had no history of substance use (AOR = 2.67, 95% CI: 1.40, 4.95). Patients with Hgb <12 mg/dl were 3 times at higher odd (AOR = 3.22, 95% CI: 1.82, 5.99) compared with those who had baseline Hgb levels ≥12 mg/dl. In addition, patients with poor drug adherence (AOR = 3.84, 95% CI: 1.77, 5.95), and restart ART medication (AOR = 2.45, 95% CI: 1.69, 7.35) after interruption were at higher odds for virological failure. Moreover, the odds of virological failure were 4-fold higher (AOR = 4.73, 95% CI: 1.76, 12.11) among those who had a history of OI through the follow-up period compared with those who had no history of OI.
Discussion
This study aimed to assess determinants of virological failure among patients on the first-line HAART at MTUTH. It showed that the sex of the patient, histories of substance use, baseline Hgb, restart after interruption of HAART, poor drug adherence, and opportunistic infections through follow-up were identified as determinants of virological failure.
The sex of the patient was identified as a determinant of virological failure. In this study, men were at a higher odd of developing virological failure. This finding is consistent with a study conducted in Spain (21) and the United States (22). However, this finding is inconsistent with a large cohort study conducted in Canada (23). The discrepancy could be explained by the differences in sample size and baseline clinical parameters. It could also be because men are more likely to use substances when compared with women (24); this, in turn, makes men more vulnerable to virological failure.
In this study, the odds of virological failure among those who had a history of substance use were higher compared with those who had no history of substance use. This finding is supported by the study conducted at Jimma (17), Addis Ababa (25), and Vietnam (26). This might be since using HAAT drugs and substances simultaneously would be prone to mental health problems resulting in poor adherence characterized by failure to take medication properly; moreover, interrupting the medication which leads finally to first-line ART treatment failure (27). Specifically, in our study area, this might be due to the more use of substances (such as, Bordea, Tella, Teji, Beer, and mostly younger groups used Khat as a recreational activity) since it is available easily in the area most as a cultural drink, and this might increase the negligence and carelessness of patients on using HAART properly.
Optimal treatment adherence is necessary for viral suppression and a good treatment outcome. This study identified poor drug adherence tripled the chance of virological failure among patients with first-line HAART. This finding is consistent with multiple previous shreds of evidence (3, 6, 28, 29). Those patients with poor drug adherence had no adequate drug concentration for sustained viral suppression. Similarly, our study showed patients with restarted after an interruption of HAART drugs had higher odds of developing virological failure. People often discontinue HAART drugs due to stigma, side effects, and lack of psychosocial support (30). Evidence showed interruption of HAART for a short period was associated with increased plasma viral load (pVL), which makes it difficult to achieve optimal viral suppression. This finding is in agreement with studies conducted in Ethiopia and the United Kingdom (30, 31).
Furthermore, the odds of virological failure among those who had baseline Hgb < 12 mg/dl were higher compared with those who had baseline Hgb ≥12mg/dl. This finding is supported by the study conducted at Jimma, Ethiopia (32), Cameron (33), and South Africa (34). This might be because patients with anemia are more likely to have advanced immune suppression and a higher rate of co-morbidities that could impose a negative effect on response to ARV treatments. Evidence from other studies also showed anemia at ART initiation is associated with increased mortality, disease progression (poor virological response), and reduced quality of life (32).
In addition, the odds of virological failure were higher among those who had a history of OI throughout the follow-up period compared with those who had no history of OI throughout the follow-up period. This finding is in line with the studies conducted in Jimma (17) and Arba Minch towns of Ethiopia (35) which showed patients with opportunistic infections, such as TB, appear to be at a higher risk of virological failure. This finding also consisted of a study conducted in Nigeria (36). Opportunistic infections could lead to depletion of CD4 cells and result in ART treatment failure (37). It could also be due to secondary infections impairing the immune system and providing a suitable environment for viral replication resulting in increased viral load or prone the patients to virological failure. Small sample size and missing of some important variables were some of the limitations of this study. Therefore, the study should be cautiously interpreted given the above limitations.
Conclusion
The study revealed that the sex of the patient, history of substance use, baseline Hgb < 12 mg/dl, poor drug adherence, restart after the interruption, and having OI through the follow-up period were determinants of virological failure. Therefore, program implementation should consider gender disparity while men are more prone to virological failure. It is also imperative to implement targeted interventions to improve drug adherence and interruption problems in follow-up care. Moreover, patients with opportunistic infection and restart after HAART interruption need special care and attention.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Mizan Tepi University College of Medicine and Health Sciences Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
EY and AA conceived the study and supervised data collection. BB carried out the statistical analysis and drafted the manuscript. TY, AD, MA, ZA, AW, and GM participated in statistical analysis and reviewing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank data collectors and supervisors for their cooperation. We also extend our gratitude to Hospital administrators and staff for their kind support during data collection time.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIDS, Acquired Deficiency Syndrome; ART, Antiretroviral Therapy; BMI, Body Mass Index; BSZ, Bench Sheko Zone; FMOH, Federal Ministry of Health; GDP, Global Domestic Product; HAART, Highly Active Antiretroviral Therapy; HIV-1, Human Immunodeficiency Virus 1; HIV, Human Immunodeficiency Virus; MTUTH, Mizan Tepi University Teaching Hospital; NNRTI, Non Nucleoside Reverse Transcriptase Inhibitor; OI, Opportunistic Infection; PICT, Provider imitative counseling and testing; PLWHA, People Living with HIV/AIDS; PMTCT, Prevention of Mother to Child Transmission; PVL, Plasma Viral Load; SNNPRS, Southern Nation Nationalities and People Regional State; TB, Tuberculosis; VCT, Voluntary counseling and Testing; WHO, World Health Organization.
References
1. Global HIV. & AIDS Statistics −2021 Fact Sheet. Joint United Nations Programme on HIV/AIDS. (2021).
2. EthiopiaCountry Operational Plan. Strategic Direction Summary. (2021). Available online at: https://www.state.gov/wp-content/uploads/2021/09/Ethiopia_SDS_Final-Public_Aug-11-2021.pdf (accessed April 06, 2021).
3. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case-control study. HIV AIDS. (2017) 9:153–9. doi: 10.2147/HIV.S139516
4. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed. (2016).
5. Seid A, Cherie N, Ahmed K. Determinants of virologic failure among adults on second line antiretroviral therapy in wollo, Amhara Regional State, Northeast Ethiopia. HIV AIDS. (2020) 12:697–706. doi: 10.2147/HIV.S278603
6. Meshesha HM, Nigussie ZM, Asrat A, Mulatu K. Determinants of virological failure among adults on first-line highly active antiretroviral therapy at public health facilities in Kombolcha town, Northeast, Ethiopia: a case-control study. BMJ Open. (2020) 10:e036223. doi: 10.1136/bmjopen-2019-036223
7. Gupta-wright A, Fielding K, Oosterhout JJ Van, Alufandika M, Grint DJ, Chimbayo E, et al. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi : an observational cohort study. Lancet HIV. (2020) 7:e620–8. doi: 10.1016/S2352-3018(20)30172-7
8. Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Adult on factores associated with virological failure and supression after enhanced adherence counsling,in childeren,adoulecent ART for HIV in Swaziland. PLoS ONE. (2015) 450:1–12. doi: 10.1371/journal.pone.0116144
9. Hassan AS, Nabwera HM, Mawaringa SM. Obonyo CA, Sanders EJ, De WTFT, et al. HIV−1 virological failure and acqaired drug resistance among first line antiretroviral expriancing adults at rular HIV clinic in costal kenya. AIDS Res Ther. (2014) 11:9. doi: 10.1186/1742-6405-11-9
10. Poul C, Maculuve S, Ceden S, Luis L, Roden J, Clotet B, et al. Determinantes of virological failure and antiretroviral drug resistant in Mozanbique. J Antimicrob Chemotherapy. (2017) 70:2639–47. doi: 10.1093/jac/dkv143
11. Vella C, Eymard-duvernay S, Sica J, Makosso L, Mongo AD, Boue V, et al. Short report virological failure rates and HIV-1 drud resistance patterns on patients in first line ART treatment in semirular and rular Gabon. J Int AIDS Soc. (2012) iv:17985.
12. Ndahimana A, Riedel DJ, Mwumvaneza M, et al. Drug resistance mutations after the first 12 months on ART and determinantes of virilogical failure in rwanda. Top Med Int Heal. (2016) 21:928–35. doi: 10.1111/tmi.12717
13. Bulage L, Ssewanyana I, Nankabinwa V, Nsubuga F, et al. Factors associated with virological non supression among HIV positive patents on ART in Uganda, Aug 2014–Jul 2015. BMC. (2017) 17:1–11. doi: 10.1186/s12879-017-2428-3
14. Gelaw B, Mulatu G, Tesfa G, Marew C, Chekole B, Alebel A. Magnitude and associated factors of virological failure among children on ART in Bahir Dar Town public health facilities, Northwest Ethiopia: a facility based cross-sectional study. Ital J Pediatr. (2021) 47:84. doi: 10.1186/s13052-021-01030-7
15. Endalamaw A, Mekonen M, Geremew D, Yehualashet FA, Tesera H, Habtewold TD. HIV/AIDS treatment failure and associated factors in Ethiopia: a meta-analysis. BMC Public Health, (2020) 20:82. doi: 10.1186/s12889-020-8160-8
16. Ahmed M, Merga H, Jarso H. Predictors of virological treatment failure among adult HIV patients on first-line antiretroviral therapy in Woldia and Dessie hospitals, Northeast Ethiopia: a case-control study. BMC Infect Dis. (2019) 19:305. doi: 10.1186/s12879-019-3924-4
17. Asfaw AB, Nigussie A, Shewanow T, Gudina EK, Getnet M, Amdisa D. Determinants of first-line antiretroviral treatment failure among patients on antiretroviral therapy in public hospitals Jimma, Southwest Ethiopia a determinants of first-line antiretroviral treatment failure among patients on antiretroviral therapy in P. Rehabil Sci. (2019) 4:13–24. doi: 10.11648/j.rs.20190402.11
18. Ethiopia national consolidated hiv prevention comprehensive guidlines for care and treatment Augest. (2018).
19. Dereje N, Moges K, Nigatu Y, Holland R. Prevalence and predictors of opportunistic infections among HIV positive adults on antiretroviral therapy (On-ART) vs. pre-ART in Addis Ababa, Ethiopia: a comparative cross-sectional study. HIV AIDS. (2019) 11:229–37. doi: 10.2147/HIV.S218213
20. Kim YJ, Woo JH, Kim MJ, Park DW, Song J-Y, Kim SW, et al. Opportunistic diseases among HIV-infected patients: a multicenter-nationwide Korean HIV/AIDS cohort study, 2006 to 2013. Korean J Intern Med. (2016) 31:953–60. doi: 10.3904/kjim.2014.322
21. Collazos J, Asensi V, Cartón JA. GEEMA for the GE para el EM de la A. Sex differences in the clinical, immunological virological parameters of HIV-infected patients treated with HAART. AIDS. (2007). Available online at: https://journals.lww.com/aidsonline/Fulltext/2007/04230/Sex_differences_in_the_clinical_immunological_and.6.aspx doi: 10.1097/QAD.0b013e3280b0774a (accessed April 06, 2021).
22. Finkel DG, John G, Holland B, Slim J, Smith SM. Women have a greater immunological response to effective virological HIV-1 therapy. AIDS. (2003). Available online at: https://journals.lww.com/aidsonline/Fulltext/2003/09050/Women_have_a_greater_immunological_response_to.32.aspx. doi: 10.1097/00002030-200309050-00032 (accessed April 06, 2021).
23. Cescon A, Patterson S, Chan K, Palmer AK, Margolese S, Burchell AN, et al. Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study. PLoS ONE. (2013) 8:e83649–e83649. doi: 10.1371/journal.pone.0083649
24. Ortego C, Huedo-Medina TB, Santos P, Rodríguez E, Sevilla L, Warren M, et al. Sex differences in adherence to highly active antiretroviral therapy: a meta-analysis. AIDS Care. (2012) 24:1519–34. doi: 10.1080/09540121.2012.672722
25. Niemeyer K, King A, Mengistu S, Hennig N. Pridictors of ART failure in an urban HIV/AIDS clinic in Addis Abeba, Ethiopian. Lancet Gobal Heal. (2016) 4:S6. doi: 10.1016/S2214-109X(16)30011-0
26. Pollack TM, Duong HT, Pham TT, Do CD, Colby D. Ciggatte smoking is associated with high HIV viral load among adults presenting for ART in vietnam. PLoS ONE. (2017) 12:1–11. doi: 10.1371/journal.pone.0173534
27. Azmach NN. Adherence to ART and associated factors among adults ARV users in Arba minch hospital. Cent African J public Heal. (2017) 3:19–26. doi: 10.11648/j.cajph.20170302.12
28. Derseh BT, Shewayerga B, Dagnew Mekuria A, Admasu Basha E. Virological treatment failure among adult HIV/AIDS patients from selected hospitals of North Shoa Zone, Amhara Region, Ethiopia. Infect Drug Resist. (2020) 13:4417–25. doi: 10.2147/IDR.S280966
29. Jean Louis F, Buteau J, François K, Hulland E, Domerçant JW, Yang C, et al. Virologic outcome among patients receiving antiretroviral therapy at five hospitals in Haiti. PLoS ONE. (2018) 13:e0192077. doi: 10.1371/journal.pone.0192077
30. Teklu AM, Yirdaw KD. Patients who restart antiretroviral medication after interruption remain at high risk of unfavorable outcomes in Ethiopia. BMC Health Serv. Res. (2017) 17:1–8. doi: 10.1186/s12913-017-2172-9
31. Burton CT, Nelson MR, Hay P, Gazzard BG, Gotch FM, Imami N. Immunological and virological consequences of patient-directed antiretroviral therapy interruption during chronic HIV-1 infection. Clin Exp Immunol. (2005) 142:354–61. doi: 10.1111/j.1365-2249.2005.02918.x
32. Asefa A, Asaye Z, Girma A, Hiko D. Predictors of clinical and immunological failure among patients on first-line antiretroviral therapy (ART) in Southwest Ethiopia. HIV/AIDS - Res Palliat Care. (2019) 11:377–86. doi: 10.2147/HIV.S234113
33. Meriki HD, Tufon KA, Afegenwi MH, Nyindem BA, Atanga PN, Anong DN, et al. Immuno-haematologic and virologic responses and predictors of virologic failure in HIV-1 infected adults on first-line antiretroviral therapy in Cameroon. Infect Dis poverity. (2014) 3:1–11. doi: 10.1186/2049-9957-3-5
34. Muzah BP, Takuva S, Maskew MD-MS. Risk factors for discordant immune response among HIV-infected patients initiat- ing antiretroviral therapy. A retrospective cohort study. Afr J HIV Med. (2012) 13:168–72. doi: 10.4102/sajhivmed.v13i4.110
35. Enderis BO, Hebo SH, Debir MK, Sidamo NB, Shimber MS. Predictores of time to first line ART failure among adult patients living with HIV in public health facility of Arba Minch town, southern Ethiopia. Ethiop J Heal Sci. (2019) 29:175–86.
36. Musa BM, Musa B, Muhammed HIN. Incidence of tuberculosis and immunological profile of TB / HIV co- infected patients in Nigeria. Med Ann Thorac. (2015) 10:185–92. doi: 10.4103/1817-1737.160838
Keywords: HIV/AIDS, virological failure, HAART, case-control, Ethiopia
Citation: Bogale B, Asefa A, Destaw A, Midaksa G, Asaye Z, Alemu Gebremichael M, Wolde AA, Yimer E and Yosef T (2022) Determinants of virological failure among patients on first line highly active antiretroviral therapy (HAART) in Southwest Ethiopia: A case-control study. Front. Public Health 10:916454. doi: 10.3389/fpubh.2022.916454
Received: 09 April 2022; Accepted: 26 September 2022;
Published: 25 October 2022.
Edited by:
Gbenga Kayode, University College, Bristol, United KingdomReviewed by:
Venkataramana Kandi, Kaloji Narayana Rao University of Health Sciences, IndiaJagdish Chandra, ESIC Model Hospital and PGIMSR, India
Copyright © 2022 Bogale, Asefa, Destaw, Midaksa, Asaye, Alemu Gebremichael, Wolde, Yimer and Yosef. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biruk Bogale, YnVyYWJvZ2FsZUBnbWFpbC5jb20=; YmlydWtAbXR1LmVkdS5ldA==
 Biruk Bogale
Biruk Bogale Adane Asefa
Adane Asefa Alemnew Destaw
Alemnew Destaw Gachana Midaksa
Gachana Midaksa Zufan Asaye2
Zufan Asaye2 Mathewos Alemu Gebremichael
Mathewos Alemu Gebremichael