
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 22 July 2022
Sec. Clinical Diabetes
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.915883
This article is part of the Research Topic Advances in the Diagnosis and Prevention of Diabetic Neuropathy View all 15 articles
Background: Diabetic peripheral neuropathy (DPN) is one of the most common chronic complications of diabetes and the strongest initiating risk factor for diabetic foot ulceration. Early diagnosis of DPN through screening measures is, therefore, of great importance for diabetic patients. Recently, shear wave elastography (SWE) has been used as a method that is complementary to neuroelectrophysiological examination in the diagnosis of DPN. We aimed to conduct a meta-analysis based on currently available data to evaluate the performance of tibial nerve stiffness on SWE for diagnosing DPN.
Methods: Both PubMed, EMBASE, the Cochrane Library, and Web of Science were searched for studies that investigated the diagnostic performance of SWE for DPN up to March 1th, 2022. Three measures of diagnostic test performance, including the summary area under receiver operating characteristics curve (AUROC), the summary sensitivity and specificity, and the summary diagnostic odds ratios were used to assess the diagnostic accuracy of SWE. All included studies were published between 2017 and 2021.
Results: Six eligible studies (with 170 DPN patients, 28 clinically defined DPN patients, 168 non-DPN patients, and 154 control participants) that evaluated tibial nerve stiffness were included for meta-analysis. The summary sensitivity and specificity of SWE for tibial nerve stiffness were 75% (95% confidence interval [CI]: 68–80%) and 86% (95% CI: 80–90%), respectively, and the summary AUROC was 0.84 (95% CI: 0.81–0.87), for diagnosing DPN. A subgroup analysis of five two-dimensional SWE studies revealed similar diagnostic performance, showing the summary sensitivity and specificity of 77% (95% CI: 69–83%) and 86% (95% CI: 79–91%), respectively, and a summary AUROC value of 0.86 (95% CI: 0.83–0.89).
Conclusions: SWE is found to have good diagnostic accuracy for detecting DPN and has considerable potential as an important and noninvasive adjunctive tool in the management of patients with DPN.
Diabetes is one of the most common chronic diseases worldwide and has become an important public health problem recently (1). According to the data of International Diabetes Federation (IDF) (2), ~463 million adults around the world were suffering from diabetes in 2019, and the diabetic population is expected to reach 700 million people by 2045 (about 10% of the global population).
Diabetic peripheral neuropathy (DPN), the main type of diabetic neuropathy, is one of the most common and serious complication of diabetes and the strongest initiating risk factor for diabetic foot ulceration, occurring in about 50% of patients with diabetes (3, 4). This percentage is even higher, up to 60–90% in some areas (5, 6). DPN is the leading cause of lower-limb amputation and disabling neuropathic pain (7), which has a devastating effect on the quality of life and long-term survival of patients with diabetes and brings a heavy economic burden. It is worth noting that major amputations in patients with diabetes are associated with a low life expectancy, with a 5-year mortality ranging from 52 to 80% (8). Therefore, early diagnosis of the DPN in people with diabetes is of great importance for taking effective targeted measures, thereby preventing the development of foot ulcers and amputations.
However, in clinical settings, the assessment of DPN can be challenging and is mainly based on characteristic symptoms and signs (3, 9). At present, nerve conduction studies (NCS) is widely considered to be one of the gold standard methods for evaluating DPN (10). NCS is a quantifiable, objective, and sensitive method. Nevertheless, there are some limitations of this technique, such as invasiveness, time-consuming, high cost, and the need for qualified professionals to perform (9), which has largely restricted their practical applications. Notably, NCS is limited to evaluating large nerve fibers, while small nerve fibers are the first to be affected in DPN patients (9). So this technique does not assess early neuropathic changes (3). Moreover, NCS has usage difficultly for screening in large sample sizes (11). Therefore, there is a pressing need for portable, reliable, and valid tools to detect DPN.
Over the past few years, shear wave elastography (SWE) has gathered considerable attention. SWE is a non-invasive imaging technique that maps the elastic properties of tissues by assessing the velocity of shear wave propagation in the particular tissue (12). The shear wave speed is directly related to tissue stiffness (12). This modality offers a new type of high-quality ultrasound examination and has been widely applied in many organs such as the liver (13, 14), thyroid (15, 16), and the breast (17, 18). As an exciting and rapidly evolving adjunctive diagnostic tool to conventional ultrasound, SWE provides more quantitative information of tissue properties that used in the routine clinical evaluation of various traumatic and pathological conditions of the musculoskeletal system, which may contribute to diagnosis (19). Interestingly, a recent study in which SWE technique was used as a method that is complementary to neuroelectrophysiological examination in the diagnosis of DPN has been found that the stiffness of the affected nerves of diabetic patients with DPN was significantly greater than that of diabetic patients without DPN and healthy control individuals (20).
Although several studies have evaluated the diagnostic performance of SWE in detecting DPN, most have included a relatively small sample size (20–23). Furthermore, a consensus for the value of SWE that used as a biomarker in the diagnosis of DPN has not been reached. Generating an evidence-based summary of the SWE performance characteristics would be of high clinical importance for improved management of DPN in diabetic patients. Given this, in the present study, we aimed to conduct a meta-analysis based on currently available data to evaluate the diagnostic accuracy of SWE for the detection of DPN.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines were followed for reporting this systemic review and meta-analysis (24). Both PubMed, EMBASE, the Cochrane Library, and Web of Science were systematically searched from inception to March 1th, 2022 using the following keywords: ((“diabetes”) OR (“diabetic peripheral neuropathy”) OR (DPN) OR (“diabetic foot”) OR (“diabetic foot ulcers”) OR (DFU) OR (“diabetic complications”)) AND ((elastography)). In addition, references of the identified articles were manually examined for other relevant publications. In our study, the references were managed using EndNote X9 software (Clarivate Analytics, Philadelphia, PA, United States).
The original research articles were included in the present study if they conformed the following criteria: (1) the study examined the diagnostic performance of SWE for detecting DPN; (2) the SWE was included as an index test; (3) all the research patients were patients with diabetes; (4) the study enrolled at least 10 patients with diabetes, and; (5) at least one 2 × 2 table (i.e., true-positive, false-positive, false-negative, and true-negative) of test performance can be constructed using the data extracted from the study. Studies fulfilling any of the following criteria were excluded: (1) studies were not relevant to SWE diagnosis (e.g., studies that used only strain elastography); (2) reviews, guidelines, conference abstracts, and author comments; (3) animal studies; (4) data incomplete; (5) duplicate publications, and; (6) studies published in non-English or non-Science Citation Index (SCI) journals.
Two investigators (B.T. Dong and X.C. Yang) read the articles, and checked the study eligibility and quality independently. In our meta-analysis, Microsoft Excel 2019 was used to pre-design the data extraction form. The patients' data including number of patients, age, sex, and body mass index (BMI) were collected from each included article. Moreover, the outcome indicators including cut-off values, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic curve (AUROC) values were also extracted from the included studies.
As for the technical characteristics of SWE, the various aspects of this technique were assessed as follows: (1) vendors; (2) type of elastography; (3) probes; (4) target nerve; (5) the number of repeated measurements performed per patient; (6) the representative value of elasticity (mean or median); (7) number of readers; (8) blinding to the reference standard, and; (9) time interval between SWE and reference.
The quality of the included studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (25). The four steps of searching literatures, selecting studies, extracting data, and checking the study quality were separately performed by B.T. Dong and X.C. Yang in this meta-analysis. All discrepancies were resolved by consensus of these three authors (B.T. Dong, X.C. Yang, and G.R. Lyu).
Stata version 15.0 (STATA Corp., TX, USA) was selected to perform all statistical analyses. Review Manager (version 5.4.1; Cochrane Collaboration, https://training.cochrane.org/online-learning/core-software/revman/revman-5-download) software was used to assess the methodological quality of the included studies (26). First, we extracted the raw data from all the included studies, and then 2 × 2 tables were reconstructed for further analysis. In our meta-analysis, three measures of diagnostic test performance, including the summary AUROC, the summary diagnostic odds ratio (DOR), and the summary sensitivity and specificity, were used with the aim of examining the accuracy of SWE for diagnosing DPN. Positive likelihood ratio (LR) and negative LR were also calculated. For each summary statistic, we computed the 95% confidence intervals (CIs). In addition, we also conducted a subgroup analysis in order to evaluate the diagnostic performance of the typical type of SWE technique (two-dimensional SWE). Further, the summary receiver operating characteristic (SROC) curve was constructed using the data from the studies included in our meta-analysis to calculate the summary AUROC of SWE for detecting DPN.
The Spearman correlation coefficient was calculated to evaluate the threshold effect of the included studies. The existence of threshold heterogeneity was considered when the P < 0.05. To evaluate the non-threshold heterogeneity of included studies, the Cochran's Q-test and inconsistency index (I2) statistic was used. I2 value was calculated in our analysis, and then used to describe the amount of non-threshold heterogeneity. Using the Cochran's Q-test and I2 statistic, P < 0.05 indicated statistically significant heterogeneity; an I2 value >50% may be considered as substantial heterogeneity. Furthermore, the Deeks' funnel plot was used to assess the potential publication bias of the SWE studies with regard to their performance in detecting DPN, with a P < 0.05 suggested significant bias.
The flow diagram of study identification is shown in Figure 1. Using the search strategies presented, a total of 1,146 records were retrieved. After removal of 596 duplicates, 550 studies were initially screened. However, 544 studies were excluded for some reasons, such as reviews, only abstract, not relevant to DPN diagnosis, or not relevant to SWE diagnosis, etc. Finally, six studies were included for evaluation and meta-analysis (12, 20–23, 27).
Table 1 displays the basic characteristics of the studies included in this review. Most of the studies were published between 2019 and 2021, except one study that was published in 2017. One study cohort was from the Turkey, while the others were from the China. In addition, all six studies were conducted in a single-center setting. Among the included studies, there were 4 prospective studies. In terms of a reference standard used for diagnosis of DPN, three studies used the NCS, two used the electrophysiology examination, and one used the NCS and positive symptoms or signs of neuropathy. The articles included in this meta-analysis were published in six different journals, the mean impact factor of these journals was 4.638 (range: 1.889–11.105).
In total, 520 subjects were included in this meta-analysis. Specifically, they were 170 DPN patients, 28 clinically defined DPN patients (i.e., patients with clinical signs or symptoms of DPN but normal NCS), 168 non-DPN patients, and 154 control participants. In the DPN group, the study populations were all patients with type 2 diabetes mellitus (T2DM). Moreover, all the included subjects were adults. Figure 2 shows the distribution of research population of the included studies.
The technical characteristics of the SWE technique used in the included studies are summarized in Table 2. Among the included studies, SWE was performed using two types of devices, including Aixplorer in five studies and Acuson S2000 in one study. Based on the technique used in this meta-analysis, SWE can be categorized as either point SWE, i.e., Virtual Touch Q (Siemens AG, Erlangen, Germany), or as two-dimensional SWE, i.e., Aixplorer (SuperSonic Imagine, Aix-en-Provence, France). The SWE technique used in examination of nerves is illustrated in Figure 3. Of note, all the 6 included studies measured tibial nerve stiffness, and two of these reports also included median nerve stiffness and common peroneal nerve stiffness measurements, respectively. Furthermore, as the measure of nerve stiffness, four two-dimensional SWE studies used elasticity, expressed in kilopascals (kPa), and one two-dimensional SWE study and one point SWE study used the shear wave speed, which expressed in meters per second.
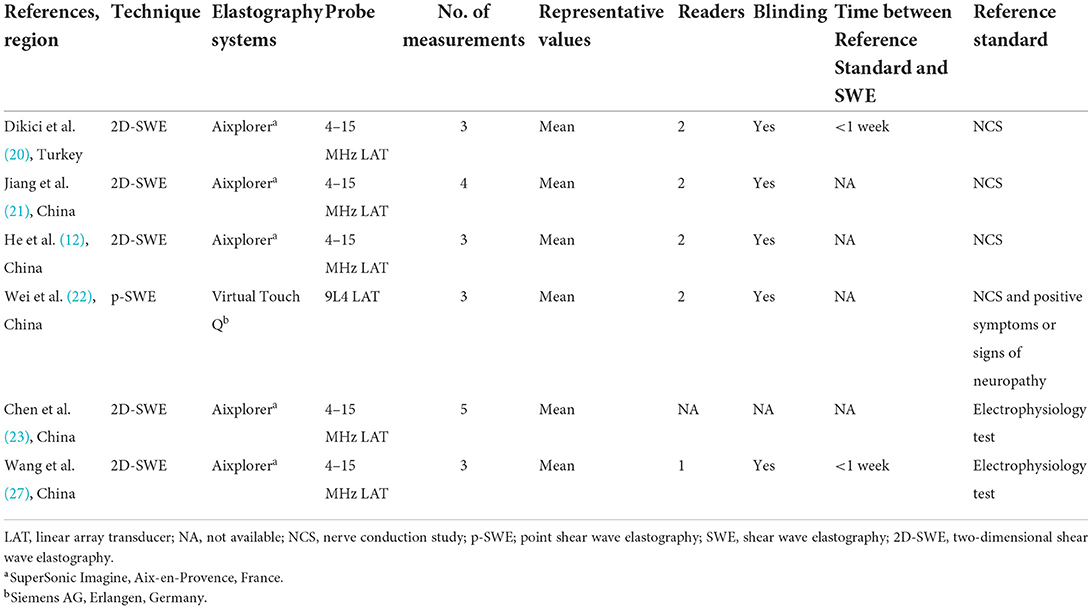
Table 2. Technical characteristics of the elastography/reference standard used in the included studies.
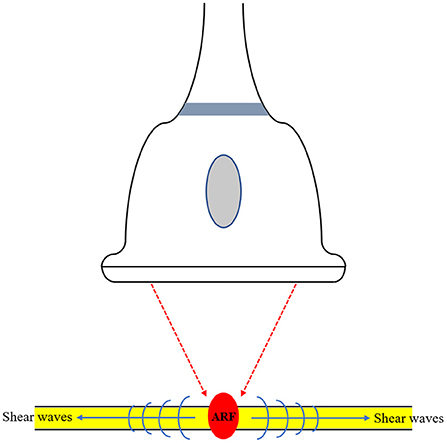
Figure 3. Illustration of the shear wave elastography (SWE) technique used in examination of nerves. SWE generates shear waves via acoustic radiation force (ARF). This technique provides more quantitative information in relation to the elasticity of the tissue.
Six studies investigated SWE diagnostic performance for the prediction of DPN. As is shown in Table 3, in the 6 studies evaluating the tibial nerve stiffness using SWE for diagnosing DPN, the mean AUROC value was 0.864 (range: 0.712–0.941). Figure 4 demonstrates that, for the tibial nerve stiffness using SWE, the summary sensitivity and specificity were 75% (95% CI: 68–80%) and 86% (95% CI: 80–90%), respectively. For diagnosing DPN, the summary AUROC of SWE for tibial nerve stiffness was 0.84 (95% CI: 0.81–0.87), as shown in Table 4. The summary DOR was 18 (95% CI: 10–33), when tibial nerve stiffness was used to diagnose DPN. It is worth mentioning that tibial nerve stiffness measured by SWE had a sensitivity and specificity value of 90.0 and 85.0%, respectively, and an AUROC value of 0.941, at a cut-off value of 51.1 kPa, for predicting DPN.
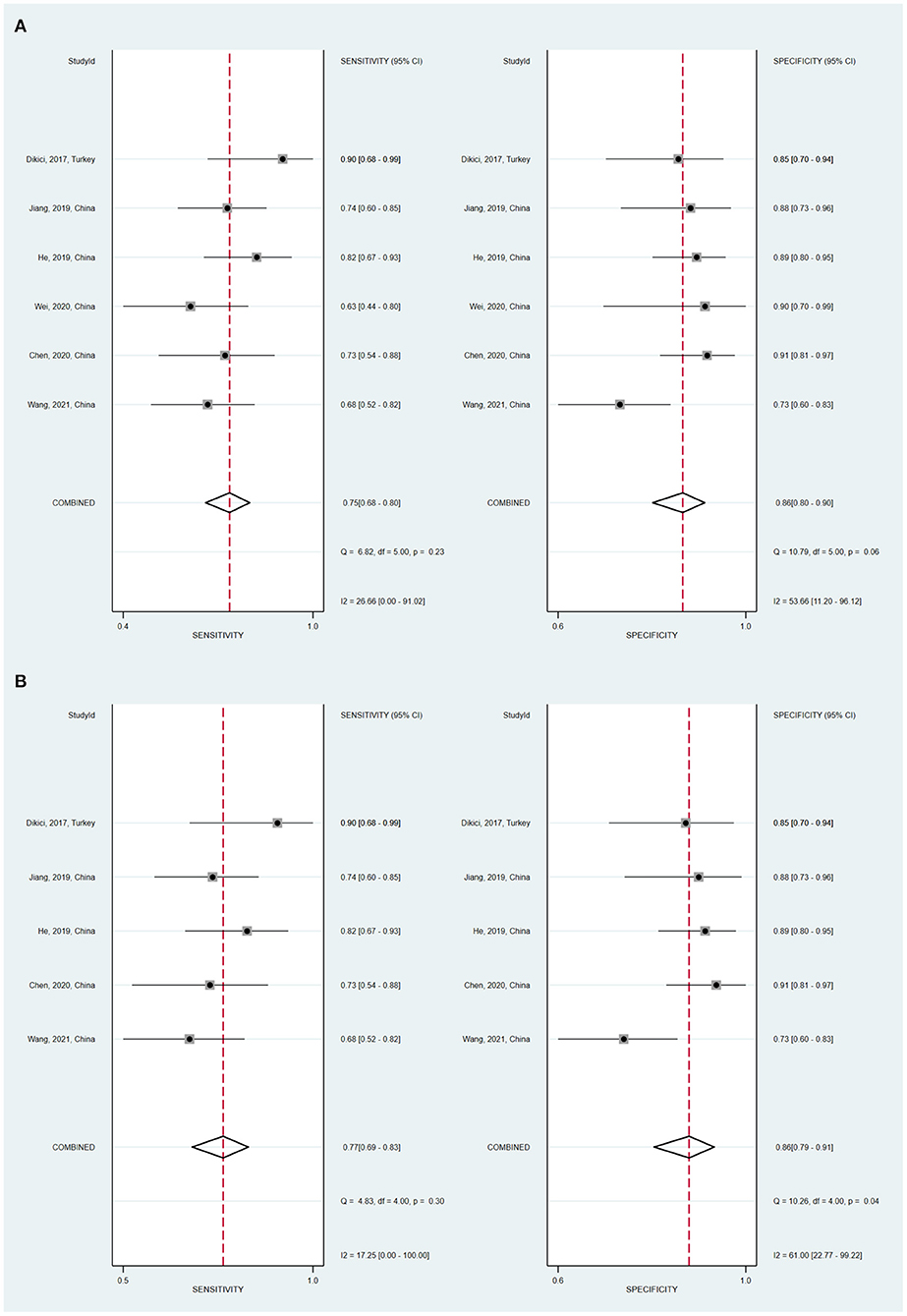
Figure 4. Coupled forest plots of the summary sensitivity and specificity of tibial nerve stiffness using shear wave elastography (SWE) (A) and two-dimensional SWE (B) for the diagnosis of diabetic peripheral neuropathy (DPN).

Table 4. Meta-analysis results of the stiffness of the tibial nerve using SWE for prediction of DPN.
Furthermore, a subgroup analysis of five two-dimensional SWE studies revealed similar diagnostic performance, showing the summary sensitivity and specificity of 77% (95% CI: 69–83%) and 86% (95% CI: 79–91%), respectively, and a summary AUROC value of 0.86 (95% CI: 0.83–0.89) (Figure 5). Table 5 shows that the summary DOR of two-dimensional SWE for tibial nerve stiffness was 20 (95% CI: 10–39), for predicting DPN.
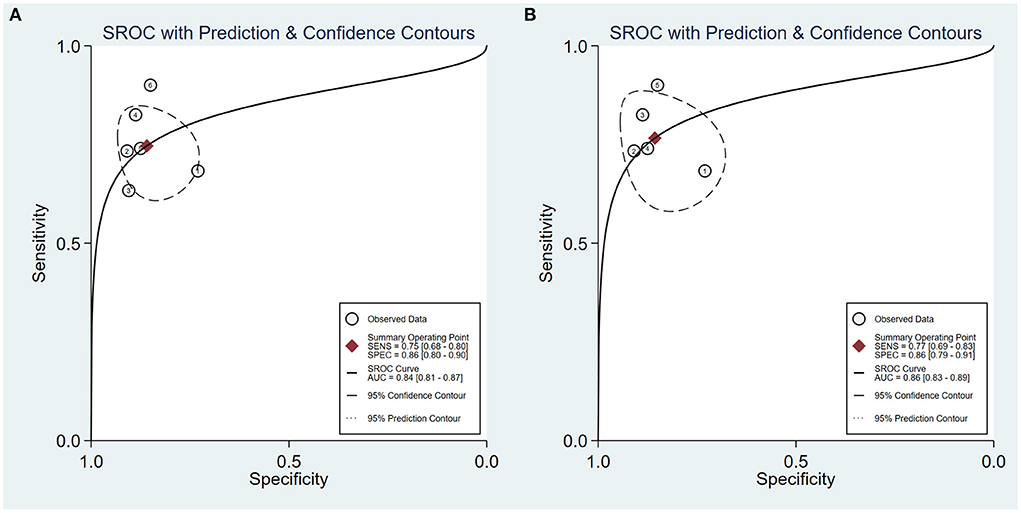
Figure 5. Summary receiver operating characteristic (SROC) curve of tibial nerve stiffness using shear wave elastography (SWE) (A) and two-dimensional SWE (B) for the diagnosis of diabetic peripheral neuropathy (DPN).
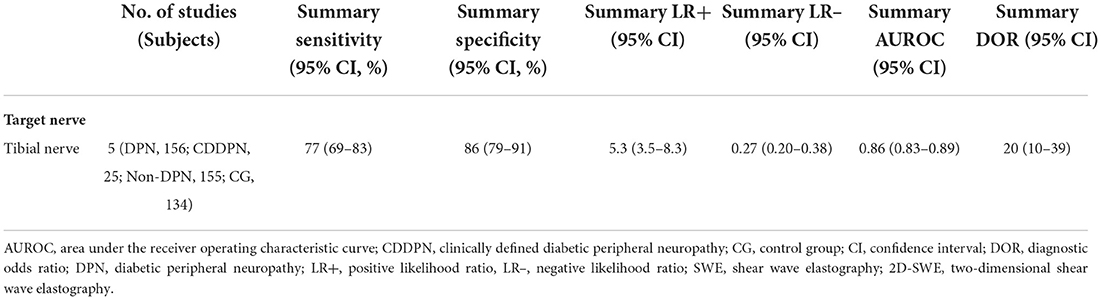
Table 5. Meta-analysis results of the stiffness of the tibial nerve using 2D-SWE for prediction of DPN.
Notably, there was one study in which SWE measurements were performed on both the median nerve and tibial nerve; this study simultaneously reported the diagnostic performance of median nerve stiffness (cut-off value, 4.1 m/s; sensitivity, 80.0%; specificity, 85.0%; AUROC, 0.899) and tibial nerve stiffness (cut-off value, 4.1 m/s; sensitivity, 81.3%; specificity, 88.7%; AUROC, 0.927). Additionally, another study also reported that common peroneal nerve measured by SWE had an AUROC of 0.653 for diagnosing DPN.
No threshold effect was found in the present meta-analysis, as depicted by the Spearman correlation coefficient value of 0.257 (P = 0.623). When SWE technique was used to diagnose DPN, the Cochran's Q-test (sensitivity, P = 0.23; specificity, P = 0.06) showed no statistically significant heterogeneity evidence both with regard to the summary sensitivity and specificity. This can be seen in Figure 4.
Deeks' funnel plot of SWE used to assess publication bias is illustrated in Figure 6. In this meta-analysis, there was no evidence of publication bias among the six included studies with a P value of 0.64.
DPN is the most important risk factor for the occurrence of diabetic foot ulcers, which seriously affect the quality of life and survival of patients with diabetes (3, 9). Early diagnosis of DPN is, therefore, of increasing importance. Unfortunately, in routine clinical practice, there are currently no simple biomarkers for early detection of DPN (3). In this regard, a novel diagnostic tool known as SWE technique may provide valuable alternatives, which has recently been introduced for the detection of DPN. In view of this, we wondered whether SWE can be used as a biomarker for the diagnosis of DPN. Our meta-analysis presents a comprehensive summary of the SWE performance characteristics for DPN, and, to our knowledge, this is the first meta-analysis of published studies that provides evidence of SWE being a novel and non-invasive tool for diagnosing DPN.
In this systematic review and meta-analysis, we identified six (170 DPN patients, 28 clinically defined DPN patients, 168 non-DPN patients, and 154 control participants) original articles with enough data to assess the performance of SWE for the prediction of DPN. Our study has revealed that tibial nerve stiffness measurement by SWE has good diagnostic performance for DPN, showing a summary sensitivity and specificity of 75 and 86%, respectively, and a summary AUROC of 0.84. A subgroup analysis of five two-dimensional SWE studies revealed similar diagnostic performance. The summary sensitivity and specificity were 77 and 86%, respectively, and the summary AUROC was 0.86. These results thus indicate that SWE can be used as a potential novel, useful, and quantitative tool for diagnosing DPN.
Tibial nerve is the most frequently involved site in diabetic polyneuropathy (20). Indeed, previous work has clearly shown that, when measured with SWE, the tibial nerve is stiffer in patients with diabetes. Several pathophysiological mechanisms may be used to explain changes in nerve stiffness as reflected by SWE measurements. The DPN developed because of the metabolic disorders associated with chronic hyperglycemia; edema within the nerve fascicle can increase intraneural pressure and then make the nerve stiffer (21, 27).
Of interest, even in diabetic patients without DPN, the stiffness of the tibial nerve was significantly higher than that of healthy control subjects (20). However, both neuroelectrophysiological examination and cross-sectional area (CSA) at ultrasound examination did not reflect such a change between diabetic patients without DPN and healthy control subjects (20). Notably, in the previous original study conducted by Jiang et al. (21), the results had shown that clinically defined DPN patients, i.e., patients with clinical signs or symptoms of DPN but normal NCS which often occurs in the early stages of DPN (28), had significantly greater tibial nerve stiffness than both diabetic patients without DPN and control subjects. These diabetic patients may have already suffered some nerve damage, although normal electrophysiological examination results exists (23). Which suggests that the SWE technique may be able to detect DPN before it becomes evident clinically or on NCS (29). This finding also shows that SWE, compared to electrophysiology test, exhibits a better correlation with clinical findings (21). Therefore, SWE technique has more potential value in early subelectrophysiological DPN detection. Early diagnosis of DPN is important, as early treatment at the earliest stages of DPN decreases both short-term and long-term morbidity (30, 31). Nevertheless, in view of the limited sample size, future studies using the SWE technique to assess the nerve stiffness in patients with clinically defined DPN are clearly needed.
In our meta-analysis, five of the six included studies used two-dimensional SWE technique for nerve stiffness assessment, and the other used point SWE technique. Both of these techniques rely on the acoustic radiation force impulse (ARFI) technique, which generates shear waves using focused, short-duration acoustic pulses (32). In contrast, two-dimensional SWE represents a relatively new ultrasound elastography technology for quantitative estimation of tissue stiffness (33). Two-dimensional SWE is a real-time and noninvasive imaging technique, which has distinct strengths for the evaluation of peripheral neuromuscular disorders. Surprisingly, one of the earliest studies using this technique showed that two-dimensional SWE displayed high sensitivity (90%) and specificity (85%) in the diagnosis of DPN, outperforming the CSA measurements (20). Thus, two-dimensional SWE has the considerable potential to be a promising non-invasive tool for diagnosing DPN. Considering the limited number of included studies, however, we were unable to compare these two SWE techniques in this meta-analysis. Additional studies are therefore needed to further investigate the performance differences between two-dimensional SWE and point SWE.
Notably, across the studies included in our meta-analysis, the cut-off values used to diagnose DPN varied. Several factors, such as imaging plane (longitudinal or axial) and the size of the region of interest while performing the elastography, limb position, different anatomic regions, and sometimes a variable distribution of the severity of diabetes, may have contributed to these differences. A previous study has examined the effect of limb position on the stiffness of the tibial nerve measurement by SWE technique (34). There may also be other factors that may simultaneously affect the cut-off value. In actual clinical practice, determining the optimal cut-off value for nerve SWE measurements is very important in order to ensure its general clinical applicability. Additionally, if used in combination with other methods such as the Toronto clinical scoring system (TCSS), SWE technique could potentially further improve the diagnostic value for DPN (27).
This meta-analysis has some limitations that should be noted. First, of the six included studies, five were from China. Therefore, the generalization of the present meta-analysis findings is relatively limited. Second, DPN is a multiple peripheral nerve disease; we only summarized the diagnostic value of SWE in the detection of DPN of the tibial nerve. It is worth stating that, in two of the six included studies (12, 23) that also reported the diagnostic performance of median nerve and common peroneal nerve, respectively, the results showed that the tibial nerve on SWE had better performance for diagnosing DPN. Third, our meta-analysis maybe have several intrinsic heterogeneities, such as techniques, reference standards, and SWE measurements. Furthermore, the optimal thresholds were not determined in this meta-analysis. Therefore, further studies with a larger sample size are needed. Finally, we only focused on the full-articles published with English, which may bias the results.
In conclusion, our meta-analysis demonstrated that SWE shows good performance in diagnosing DPN and has considerable potential as an important and noninvasive adjunctive tool in the management of patients with DPN. Further studies focusing on the identification of optimal cut-off value for nerve SWE measurements are required in order to ensure its general clinical applicability.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
BD, YC, and GL contributed to the study design and literature search. XY, HW, and YC completed the data analysis. BD, HW, and YC generated and improved the figures and tables. BD completed the manuscript. BD and GL proofread the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stohr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. (2021) 11:13686. doi: 10.1038/s41598-021-93062-6
2. International Diabetes Federation. IDF Diabetes Atlas, 9th Edn (2019). Available online at: https://www.diabetesatlas.org (accessed January 16, 2022).
3. Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol. (2021) 12:671257. doi: 10.3389/fendo.2021.671257
4. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. (2019) 19:86. doi: 10.1007/s11892-019-1212-8
5. Wang GF, Xu N, Yin D, Hui Y, Zhang JP, Han GJ. New advances in the diagnosis and treatment of diabetic peripheral neuropathy. Chin Gen Pract. (2012) 15:1661–3. doi: 10.3969/j.issn.1007-9572.2012.15.001
6. Qin L, Niu JY, Zhou JY, Zhang QJ, Zhou F, Zhang N, et al. Prevalence and risk factors of diabetic peripheral neuropathy in Chinese communities. Zhonghua Liu Xing Bing Xue Za Zhi. (2019) 40:1578–84. doi: 10.3760/cma.j.issn.0254-6450.2019.12.014
7. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/S2213-8587(19)30081-6
8. Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg. (2016) 55:591–9. doi: 10.1053/j.jfas.2016.01.012
9. Yu Y. Gold Standard for Diagnosis of DPN. Front Endocrinol. (2021) 12:719356. doi: 10.3389/fendo.2021.719356
10. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. (2009) 50:675–682.e1. doi: 10.1016/j.jvs.2009.05.017
11. Callaghan BC, Price RS, Chen KS, Feldman EL. The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: a review. JAMA Neurol. (2015) 72:1510–8. doi: 10.1001/jamaneurol.2015.2347
12. He Y, Xiang X, Zhu BH, Qiu L. Shear wave elastography evaluation of the median and tibial nerve in diabetic peripheral neuropathy. Quant Imaging Med Surg. (2019) 9:273–82. doi: 10.21037/qims.2019.02.05
13. Kakegawa T, Sugimoto K, Kuroda H, Suzuki Y, Imajo K, Toyoda H, et al. Diagnostic accuracy of two-dimensional shear wave elastography for liver fibrosis: a multicenter prospective study. Clin Gastroenterol Hepatol. (2021) 20:e1478–82. doi: 10.1016/j.cgh.2021.08.021
14. Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. (2018) 67:260–72. doi: 10.1002/hep.29179
15. Kim HJ, Kwak MK, Choi IH, Jin SY, Park HK, Byun DW, et al. Utility of shear wave elastography to detect papillary thyroid carcinoma in thyroid nodules: efficacy of the standard deviation elasticity. Korean J Intern Med. (2019) 34:850–7. doi: 10.3904/kjim.2016.326
16. Shang H, Wu B, Liu Z, Liu Y, Cheng W. The effectiveness of shear wave elastography in the diagnosis of PTMC. Technol Health Care. (2020) 28:221–6. doi: 10.3233/THC-191895
17. Altintas Y, Bayrak M, Alabaz Ö, Celiktas M. A qualitative and quantitative assessment of simultaneous strain, shear wave, and point shear wave elastography to distinguish malignant and benign breast lesions. Acta Radiol. (2021) 62:1155–62. doi: 10.1177/0284185120961422
18. Luo T, Zhang JW, Zhu Y, Jia XH, Dong YJ, Zhan WW, et al. Virtual touch imaging quantification shear-wave elastography for breast lesions: the diagnostic value of qualitative and quantitative features. Clin Radiol. (2021) 76:316.e1–316.e8. doi: 10.1016/j.crad.2020.10.016
19. Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. (2017) 37:855–70. doi: 10.1148/rg.2017160116
20. Dikici AS, Ustabasioglu FE, Delil S, Nalbantoglu M, Korkmaz B, Bakan S, et al. Evaluation of the tibial nerve with shear-wave elastography: a potential sonographic method for the diagnosis of diabetic peripheral neuropathy. Radiology. (2017) 282:494–501. doi: 10.1148/radiol.2016160135
21. Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic performance of two-dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy. Eur Radiol. (2019) 29:2167–74. doi: 10.1007/s00330-018-5858-4
22. Wei M, Ye X. Feasibility of point shear wave elastography for evaluating diabetic peripheral neuropathy. J Ultrasound Med. (2020) 39:1135–41. doi: 10.1002/jum.15198
23. Chen R, Wang XL, Xue WL, Sun JW, Dong XY, Jiang ZP, et al. Application value of conventional ultrasound and real-time shear wave elastography in patients with type 2 diabetic polyneuropathy. Eur J Radiol. (2020) 126:108965. doi: 10.1016/j.ejrad.2020.108965
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
27. Wang F, Zheng M, Hu J, Fang C, Chen T, Wang M, et al. Value of shear wave elastography combined with the Toronto clinical scoring system in diagnosis of diabetic peripheral neuropathy. Medicine. (2021) 100:e27104. doi: 10.1097/MD.0000000000027104
28. Bae JS, Kim BJ. Subclinical diabetic neuropathy with normal conventional electrophysiological study. J Neurol. (2007) 254:53–9. doi: 10.1007/s00415-006-0261-5
29. Wee TC, Simon NG. Ultrasound elastography for the evaluation of peripheral nerves: a systematic review. Muscle Nerve. (2019) 60:501–12. doi: 10.1002/mus.26624
30. Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract. (2001) 54:115–28. doi: 10.1016/S0168-8227(01)00278-9
31. Asadov R, Erdal A, Bugdayci O, Gündüz OH, Ekinci G. The effectiveness of ultrasonography and ultrasonographic elastography in the diagnosis of carpal tunnel syndrome and evaluation of treatment response after steroid injection. Eur J Radiol. (2018) 108:172–6. doi: 10.1016/j.ejrad.2018.09.027
32. Hu X, Huang X, Chen H, Zhang T, Hou J, Song A, et al. Diagnostic effect of shear wave elastography imaging for differentiation of malignant liver lesions: a meta-analysis. BMC Gastroenterol. (2019) 19:60. doi: 10.1186/s12876-019-0976-2
33. Tsuchida W, Yamakoshi Y, Matsuo S, Asakawa M, Sugahara K, Fukaya T, et al. Application of the novel estimation method by shear wave elastography using vibrator to human skeletal muscle. Sci Rep. (2020) 10:22248. doi: 10.1038/s41598-020-79215-z
Keywords: biomarker, diabetic peripheral neuropathy, diagnosis, stiffness measurement, shear wave elastography
Citation: Dong B, Lyu G, Yang X, Wang H and Chen Y (2022) Shear wave elastography as a quantitative biomarker of diabetic peripheral neuropathy: A systematic review and meta-analysis. Front. Public Health 10:915883. doi: 10.3389/fpubh.2022.915883
Received: 08 April 2022; Accepted: 01 July 2022;
Published: 22 July 2022.
Edited by:
Charumathi Sabanayagam, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Ren Xinping, Ruijin Hospital, Shanghai Jiao Tong University, ChinaCopyright © 2022 Dong, Lyu, Yang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guorong Lyu, bGdyX2ZldXNAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.