- 1Department of Pharmacy, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 2State Key Laboratory of Quality Research in Chinese Medicines, Macau University of Science and Technology, Macau, Macau SAR, China
- 3Department of Pharmacy, Nanjing Drum Tower Hospital Clinical College, Nanjing University of Chinese Medicine, Nanjing, China
- 4Department of Anesthesiology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
Background: Pain has become an important factor in evaluating patients' quality of life and clinical treatment. For gastric cancer (GC) patients, open radical gastrectomy (OG) causes significant trauma to the body, increases patients' pain after operation, and delays early recovery. The aim of this study was to investigate the predictive factors of acute pain after OG within postoperative 72 h.
Methods: From March 2020 to September 2021, 307 patients who underwent OG were included in the study in Nanjing Drum Tower Hospital. The predictors included demographic predictors, pathological data, surgical predictors, and intraoperative predictors. The pain scores at 12, 24, 48, and 72 h after operation were evaluated by numeric rating scale (NRS). The predictors of acute pain were determined by univariate and multivariate analysis.
Results: The average pain score (NRS) of patients showed a downward trend over time within 72 h after OG. Multivariate analysis indicated that total gastrectomy (OR 1.823, 95% CI 1.094–3.040, P < 0.05), AJCC TNM stage (II) (OR.232, 95% CI 0.062–0.872, P < 0.05), AJCC TNM stage(III) (OR.185, 95% CI 0.049–0.698, P < 0.05), BMI (kg/m2) (OR 1.75, 95% CI 1.029–2.976, P < 0.05), distant metastasis (OR 3.054, 95% CI 1.019–9.155, P < 0.05), intraoperative transfusion (OR 2.246, 95% CI 1.267–3.982, P < 0.01) were significant predictive factors for acute pain after OG.
Conclusion: Reasonable postoperative acute pain control was the prerequisite for accelerating the postoperative rehabilitation of patients. In order to reduce the occurrence of excessive or insufficient analgesia, it was necessary for patients who underwent OG to formulate appropriate analgesics according to risk factors.
Introduction
Gastric cancer (GC) is a common malignant tumor of the digestive system, posing a significant risk to human health. According to global cancer statistics, GC has the fifth-highest incidence rate, and was the third leading cause of cancer deaths (1). The only hope for curing cancer stomach was radical gastrectomy (2). Depending on the tumor's location, it could remove all or part of the stomach. According to the classification of surgical methods, radical gastrectomy could be mainly divided into laparoscopic radical gastrectomy (LRG) and OG. LRG has developed rapidly since Kitano reported it for early GC in 1994 and has many advantages, including reducing bleeding, alleviating pain, and accelerating recovery (3–6). The therapeutic effect of LRG in patients with GC was increasingly prominent, especially for patients with early GC. The incidence of postoperative complications was lower, and the prognosis was better than OG (7, 8). However, for patients with advanced GC, clinical application's therapeutic effect and safety were still controversial. Moreover, surgery cost is relatively high because of high requirements for the technical level of equipment and physicians. The effectiveness and safety of LRG have also become the focus of clinicians and patients. Studies have shown that OG is safer when enlarged lymph nodes (ESLNs) are >2.5 cm (9). OG could effectively remove the lesions of patients and remove the surrounding lymph nodes as much as possible to improve the prognosis of patients and the survival rate of patients. However, it causes great trauma to the body, which increases the patients' pain invisibly. Moderate to severe postoperative acute pain could cause a strong stress response in patients, leading to decreased immune function, and a greater risk of postoperative tumor recurrence and metastasis, which directly and indirectly affects the prognosis (10).
Therefore, the study of factors affecting postoperative acute pain has important clinical significance for optimizing postoperative acute pain management (11). Doctors, nurses, and pharmacists need to understand the influencing factors of postoperative analgesic effect of the operation, intervene with these factors, and formulate individualized analgesic schemes, so as to reduce the occurrence of excessive or insufficient analgesia. In this article, 307 patients with GC after OG were followed up, and the factors that may affect the postoperative analgesic effect were analyzed, so as to provide reference for the formulation of postoperative analgesic scheme.
Methods
Patient and Public Involvement
This study was a retrospective single-center real-world study without any intervention in the treatment. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, and the Ethics Committee agreed to waive the informed consent. GC patients who underwent OG at Nanjing Drum Tower Hospital from March 2020 to September 2021 were reviewed. Patients who met the following eligibility criteria were included: diagnosis of primary GC and accepted OG. All participants were Han Chinese. Patients with these conditions were excluded: remnant GC, history of other malignant tumors, quitting operation, and incomplete data.
Perioperative Anesthesia and Surgical Procedure
All the research predictors were from patients who were anesthetized by the same team of anesthesiologists and operated by the same team of physicians. All patients underwent general anesthesia and OG.
Anesthesia information: All patients underwent total intravenous anesthesia. No premedication. The intravenous infusion pathway was established after the patient reached the operating room. Anesthesia was induced with midazolam (0.1 mg/kg), etomidate (0.2 mg/kg), cisatracurium besylate (0.4 mg/kg), and sufentanil (0.4 mg/kg). Target-controlled infusion (TCI) pump was used to maintain anesthesia with a target blood concentration of 4~6 mg/mL propofol; some patients were given patient-controlled intravenous analgesia (PCA) after surgery.
All patients underwent OG. The patients were placed in the supine position as the surgical position and subjected to general anesthesia. The abdominal region of the patients was routinely disinfected. The 15–20 cm around the navel in the middle of the upper abdomen was taken as the surgical incision. The subcutaneous tissue of the patients was stripped layer by layer to expose the lesions. The anatomical position of the organs in the abdominal cavity was carefully explored. The ultrasonic knife was used to complete the operation of gastric dissociation. The operator should strictly abide by the principle of tumor-free operation. At the same time, the corresponding lymph tissue should be cleaned according to the specific position of the tumor tissue. After the operation, the bleeding was completely stopped, and the abdominal cavity was thoroughly rinsed with sterile distilled water. The incision was sutured after the operation and covered with sterile dressing. Finally, the drainage tube was placed on the abdominal wall.
Postoperative Analgesia
Postoperative patients received standard postoperative analgesia. PCA was given 10 min before the end of the operation. Fentanyl (adult: 15–20 mg/kg) was continuously infused, dexamethasone 10 mg, ondansetron 8 mg, diluted with normal saline, and the total volume was 100 ml. Dexamethasone and ondansetron prevent nausea or vomiting. The program was used for continuous infusion of background speed of 2 mL/h, a bolus dose of 0.5 mL, and lock for 15 min. Flurbiprofen axetil (50 mg b.i.d), parecoxib (40 mg b.i.d), or dezocine (10 mg b.i.d) as analgesics alleviate inflammation. If the patient complained of unbearable pain, intravenous pethidine was used as a rescue analgesic needed.
Pain Intensity Measurement
Pain monitoring during hospitalization. The measurements were assessed using the American Society of Pain Guidelines for Postoperative Pain Management and the Chinese Society of Anesthesia Guidelines for Postoperative Pain Management. Pain measurement was performed at multiple time points (12, 24, 48, 72 h after operation) after the operation. The pain intensity was measured by NRS. NRS pain intensity score ranged from 0 to 10, 0 was painless, 10 was the most painful. Due to the implementation of postoperative acute pain management in our hospital, only 29.3% of patients after OG with NRS score ≥3 under the joint action of medical care and pharmacists. NRS = 3 as the cut-off value was not suitable for this study. Therefore, the NRS <2 was classified as a good analgesic effect (no pain), NRS ≥ 2 was classified as a poor analgesic effect (pain). Evaluating and recording NRS scores at multiple time points. Postoperative vomiting was recorded during follow-up. All the administrations were completed by the same postoperative acute pain management team composed of trained pharmacists.
Predictors
The predictors included demographic predictors, pathological data, surgical predictors, and intraoperative predictors. We collected the participants' age, gender, BMI, diabetes, hypertension, previous abdominal surgery, pre-operative hemoglobin (g/L), pre-operative albumin (g/L), carcinoembryonic antigen, and pre-operative chemo- or radio-therapy before operation. We also recorded intraoperative information, such as American Society of Anesthesiologists physical status (ASA) score, total gastrectomy, or not intraoperative blood loss (ml), intraoperative fentanyl dosage (mg), intraoperative dexmedetomidine dosage (mg), and duration of operation (min). According to postoperative pathological data, we recorded tumor location, tumor size (cm), Lauren's histology, pathological grading, lymph node metastasis, depth of invasion, distant metastasis, lymphovascular invasion, and perineural invasion. Pathologic staging was evaluated according to the 8th American Joint Committee on Cancer (AJCC) staging system of GC.
Statistics Analysis
IBM SPSS Statistics software (version 25.0; Chicago, IL) was used for statistical analysis. All continuous predictors were expressed by mean ± SD or median and quartiles (25th, 75th). All classification predictors were represented by percentages.
According to the distribution characteristics of data, Student t test or Mann- Whitney U test was used for univariate analysis to evaluate the related factors of patients. Categorical predictors were analyzed using the chi-squared test. In order to determine the risk factors for predicting poor analgesic effect, binary logistic regression was performed for multivariate analysis. Values of P < 0.05 were considered statistically significant.
Results
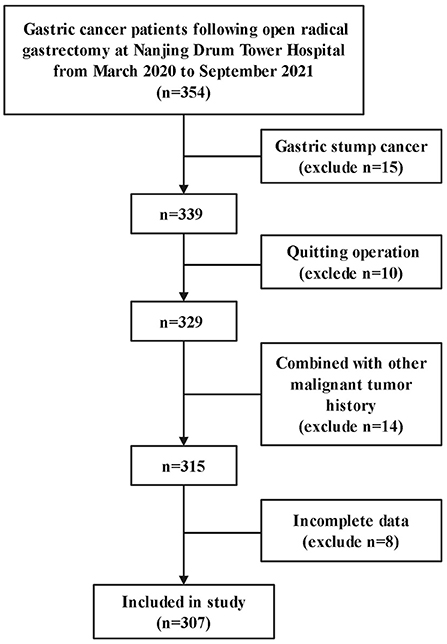
A total of 354 patients were close to participate in this study. 15 patients with gastric stump cancer, 10 patients who abandoned surgery, 14 patients with other malignant tumor histories, and 8 patients who had incomplete data were excluded from the study. Therefore, 307 patients were available for analysis (Figure 1).
Descriptive Statistics
Demographics information, underlying diseases, data on surgery, and ASA classification were collected by researchers. Descriptive statistics for the patient characteristics are presented in Table 1. The mean patient age was 76.97 ± 9.80 years old, and 70 of the patients (22.8%) were female; 183 (59.6%) GC patients received total gastrectomy; 117 (38.1%) patients had hypertension; 46 (15.0%) patients had diabetes. Within 72 h after operation, a total of 197 (64.3%) patients suffered pain (NRS ≥ 2). PCA was provided for 21 (6.8%) patients for postoperative analgesia. For all patients, the average pain score changes at 24, 48, and 72 h after the operation are shown in Figure 2.
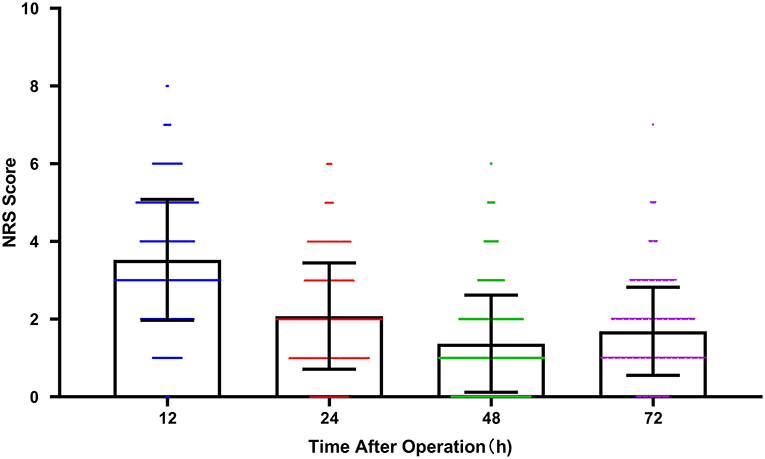
Figure 2. The average pain score (NRS) with time after operation at 12, 24, 48, and 72 h. Scatter plot with bar. The plot represented mean with SD. Color symbols represented individual values. (NRS, Numerical Rating Scale).
Univariate Analysis
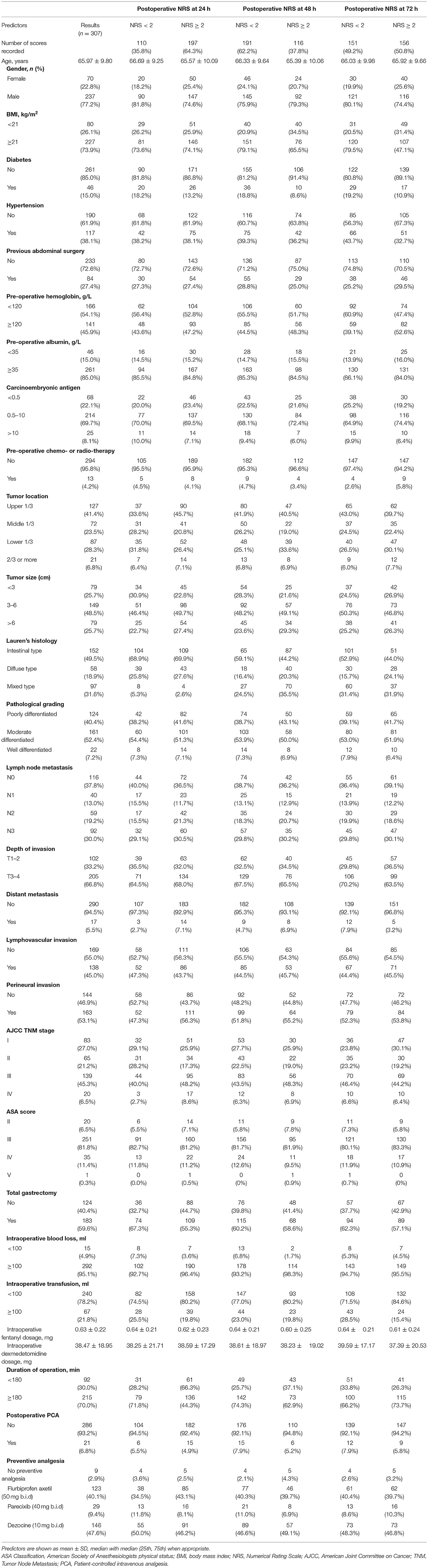
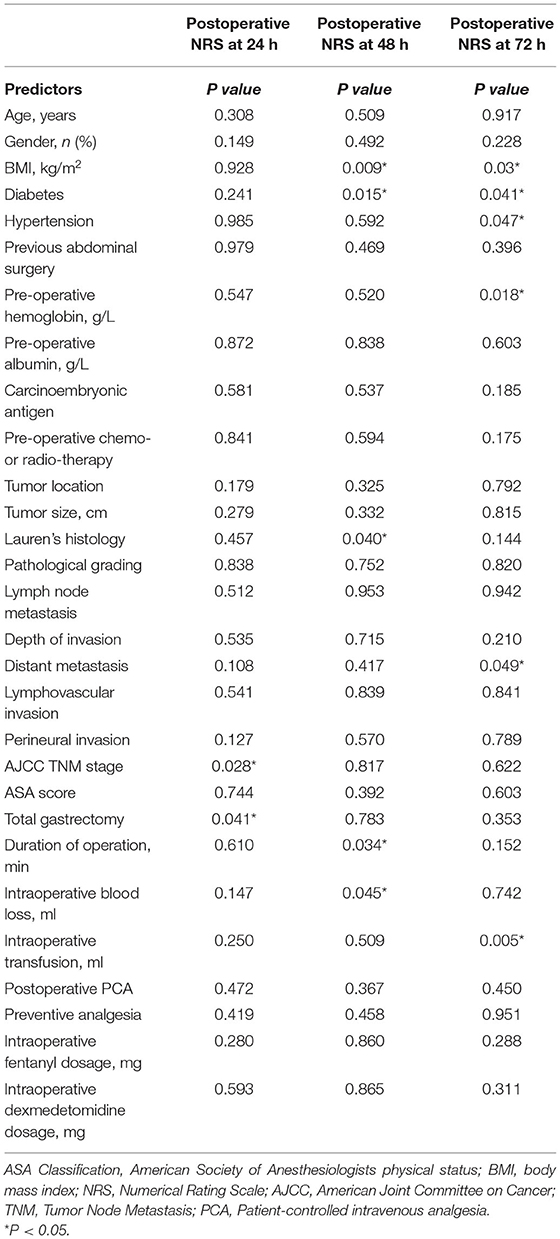
Our study assessed the pain scores at 24, 48, and 72 h after surgery. Table 2 showed the data analysis results. At postoperative 24 h, whether total gastrectomy was performed or not (P < 0.05), and AJCC TNM stage (P < 0.05) was related to postoperative acute pain after OG. At postoperative 48 h, BMI (P < 0.01), diabetes (P < 0.05), hypertension (P < 0.05), Lauren's histology (P < 0.05), intraoperative blood loss (P < 0.05), and duration of operation (P < 0.05) were related to postoperative acute pain. At postoperative 72 h, BMI (P < 0.05), diabetes (P < 0.05), pre-operative hemoglobin (P < 0.05), intraoperative blood transfusion (P < 0.01) were related to postoperative acute pain. BMI and diabetes were both associated with postoperative acute pain at 48 and 72 h. In addition, there was a difference in the patient sources between groups, but this difference did not reach statistical significance. We used these predictors in the multivariate analysis.
Multivariate Analysis
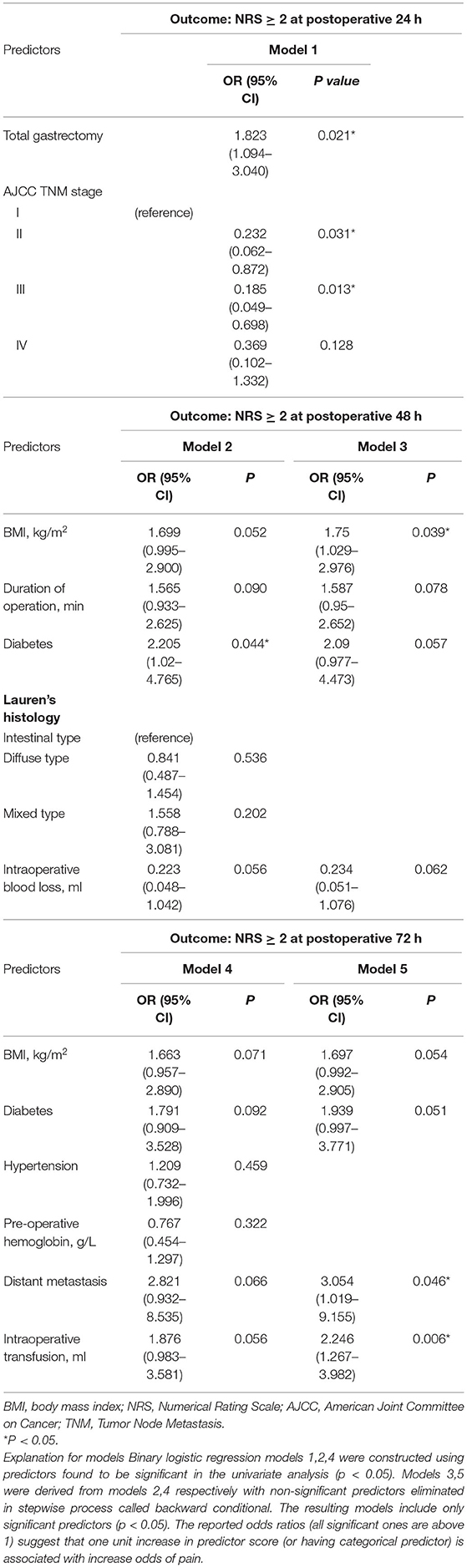
To determine the risk factors of pain after OG, binary logistic regression was used to investigate the predictors that showed a significant difference (P < 0.05) in the univariate analysis (Table 3 and Figure 3). After 24 h post-operation, the significant predictors included total gastrectomy (OR 1.823, 95% CI 1.094–3.040, P < 0.05), AJCC TNM stage (II) (OR 0.232, 95% CI 0.062–0.872, P < 0.05), and AJCC TNM stage (III) (OR 0.185, 95% CI 0.049–0.698, P < 0.05). After operation 48 h, the significant predictors included BMI (kg/m2) (OR 1.75, 95% CI 1.029–2.976, P < 0.05). After operation 72 h, the significant predictors included distant metastasis (OR 3.054, 95% CI 1.019–9.155, P < 0.05), intraoperative transfusion (OR 2.246, 95% CI 1.267–3.982, P < 0.01).
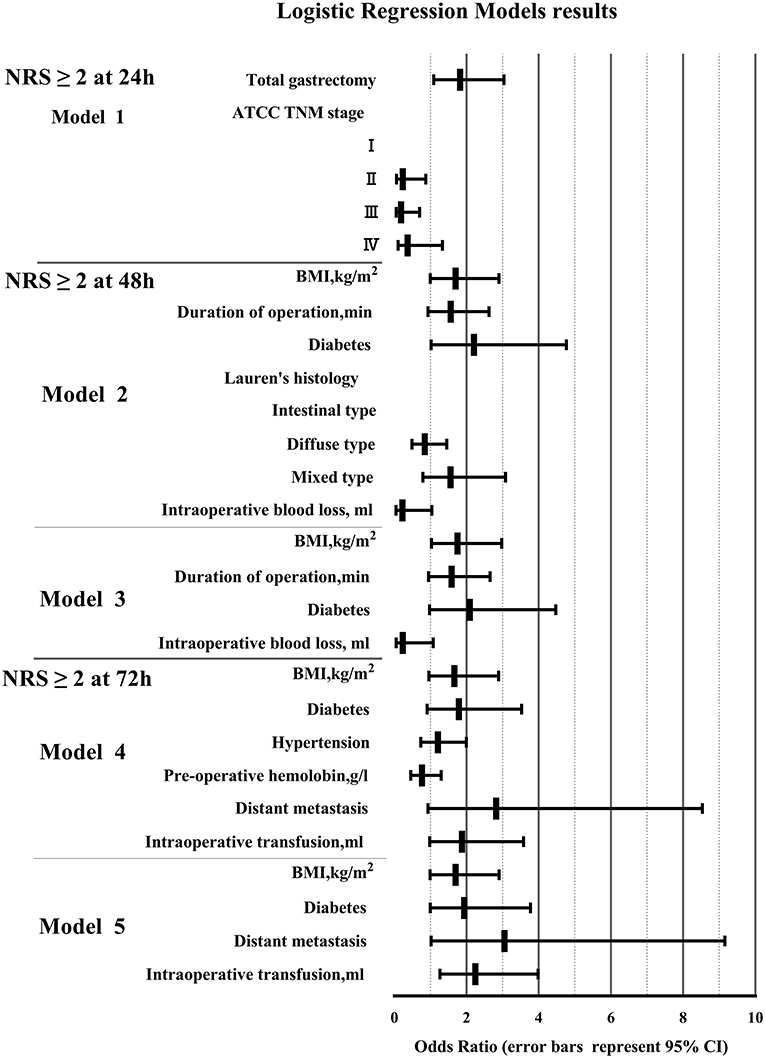
Figure 3. Binary logistic regression model results. (BMI, body mass index; NRS, Numerical Rating Scale; AJCC, American Joint Committee on Cancer; TNM, Tumor Node Metastasis).
Discussion
As one of the most common malignant tumors of the digestive system, GC posed a serious threat to people's lives and health (12). The results of this study showed that the pain scores of patients showed a downward trend over time within 72 h after surgery. However, the pain score at 72 h was slightly higher than that at 48 h, which may be related to wound dressing change and drainage tube removal. Some patients had a tolerance to analgesics, and the withdrawal of PCA (48–72 h after surgery).
To determine independent predictors of pain after OG within 72 h, we used binary logistic regression models after univariate analysis. There were so many variables included in this study, including demographics information, pathological data, and surgical data. Univariate analysis was carried out to screen out some variables which may be meaningful. And then binary logistic regression analysis was performed on variables with differences (P < 0.1). Binary logistic regression analysis used backward conditional, eliminated non-local variables step by step, and finally got 5 significant predictive factors (P < 0.05). It could not only explain the correlation between variables and postoperative acute pain after OG, but also reflect the strength of the correlation through OR value. In this study, total gastrectomy, AJCC TNM stage (I), BMI≥21 kg/m2, distant metastasis, intraoperative blood transfusion (≥100 ml) were risk factors for postoperative acute pain.
In our study, total gastrectomy or proximal or distal gastrectomy was an important factor affecting postoperative acute pain. Total gastrectomy had potential advantages in improving the long-term survival rate and reducing the incidence of residual GC (13). Compared with proximal or distal gastrectomy, total gastrectomy had a longer operation time and more intraoperative blood loss. Activated injury receptors or immune cells released a large number of endogenous inflammatory mediators (14). At the same time, injury receptors expressed one or more cell surface receptors, such as G protein-coupled receptors (GPCR) and N-methyl-D-aspartic acid (NMDA). These receptors specifically recognized the corresponding inflammatory mediators, enhancing the excitability nerve fibers, and improving the sensitivity of injury receptors to injurious stimuli (15). Laparoscopic distal gastrectomy for TNM stage I-III GC had less blood loss, less postoperative pain, and mild inflammatory response (16).
We found that BMI correlated with postoperative acute pain (P = 0.039) after OG. Most studies from Asian Centers used BMI value of 25 kg/m2 as the critical value for dividing patients into obesity, which was inconsistent with the current definition of obesity by the WHO (17). In a meta-analysis, the effect of obesity on the prognosis of GC after resection was studied, and BMI ≥ 30 was defined as obesity (18). Intraoperative blood loss was reported in 4 studies and was lower in the non-obese group, but the difference was not statistically significant (19–22). Similarly, non-obese patients could be observed in wound infection decreased trend, but this did not reach the level of statistical significance (22). Excessive visceral fat wrapped in the main blood vessels of the upper abdomen may affect the recognition of the best anatomical plane, and the operation time may be longer. Increased blood loss, increased risk of wound infection, and prolonged operation time were potential factors for postoperative acute pain.
Our study suggested that patients at different TNM stages of cancer may respond differently to postoperative acute pain. A retrospective study investigated the effect of postoperative systemic inflammation on prognosis in patients with TNM stage I GC, and suggested that early postoperative serum C-reactive protein (CRP) level (cut-off value was 13.9 mg/dL) could predict the long-term prognosis of radical gastrectomy (23). Saito et al. evaluated the effect of CRP peak level on prognosis in patients with advanced GC after radical gastrectomy and identified CRP peak level (cut-off value was 12 mg/dL) as an independent prognostic factor (24). CRP is synthesized by the liver, mainly regulated by interleukin-6, and may upregulate pro-inflammatory and anti-inflammatory cytokines (25). Recently, some studies have shown that postoperative systemic inflammation is significantly correlated with the postoperative prognosis of cancer patients through evaluating serum CRP level (25–27). The increase of postoperative CRP level in patients with GC may predict the increase of inflammatory level, and strong inflammatory response may cause serious postoperative acute pain.
According to the 8th AJCC TNM classification system, no matter the depth of tumor penetrating the gastric wall (T) and the number and state of lymph nodes (N), distant metastasis is divided into stage IV. Patients at the IV stage usually suffer from a long and painful illness. Postoperative patients in our hospital would use non-steroidal anti-inflammatory drugs combined with opioids analgesia. Opioids play an analgesic effect by simulating the physiological role of endogenous opioid peptides (28). Patients with advanced GC faced low cholesterol levels due to malnutrition. Low cholesterol levels may reduce the activity of opioids (29). Studies have shown that patients with lung cancer at low cholesterol levels need higher doses of opioids to achieve the same level of pain control (30). Our study also confirmed that patients with distant metastasis were more likely suffer acute pain than patients with early GC after surgery.
In our study, blood transfusion was an independent predictor of postoperative acute pain. Blood transfusion could save a life in many cases but had a negative influence on immune regulation, postoperative infection, and tumor metastasis, and recurrence (31). Immunomodulation of the innate and adaptive immune system occurred after exposure of the recipient to the many cell-bound and soluble antigens which were expressed on viable and decaying cells in the transfusion (32). Blood transfusion was associated with infectious complications following gastrointestinal surgery (33). The activation of inflammation during blood transfusion was closely related to the severity of postoperative pain. A meta-analysis also confirmed that the restrictive allogeneic blood transfusion strategy could reduce the perioperative infection rate without increasing the incidence of complications such as cardiac events or mortality (34). Retrospective analysis of a single central database also confirmed that perioperative blood transfusion was independently associated with poor prognosis in patients with GC (35).
Our study also had some limitations. We only evaluated and explored the possible factors affecting pain within 72 h after surgery. There was no study on the influencing factors of pain 3 days and long-term after surgery. At the same time, our research was limited to OG, and there was no study on the influencing factors of pain after LRG and robotic radical gastrectomy for GC. In addition, postoperative acute pain was affected by genetic polymorphism related to pharmacokinetics, pharmacodynamics of analgesics (36) and psychology, and we had not studied these influencing factors.
Pain has become an important factor in evaluating patients' quality of life and clinical treatment. Medical staff should predict the influencing factors of postoperative acute pain, formulate reasonable analgesic schemes, and reduce the occurrence of excessive analgesia and insufficient analgesia. Reasonable postoperative pain control was the prerequisite for accelerating the postoperative rehabilitation of patients.
Total gastrectomy, AJCC TNM stage (I), BMI (≥21, kg/m2), distant metastasis, and intraoperative transfusion (≥100 ml) were significantly associated with pain after OG within postoperative 72 h. To reduce the occurrence of excessive analgesia and insufficient analgesia, formulating appropriate analgesics according to these risk factors was necessary for patients who underwent OG.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Nanjing Drum Tower Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HX and JW: design. JW and HX: writing. ZM and JW: analysis. WG and MH: methodology. JW and WG: data curation. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 72104105) and Special Fund for Clinical Research of Nanjing Drum Tower Hospital (No. 2021-LCYJ-PY-07). HX was responsible for the overall content of these funding as guarantor. The guarantor accepted full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge all the patients who participated in this study.
Abbreviations
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists physical status; CRP, C-reactive protein; ESLNs, Enlarged lymph nodes; GC, Gastric cancer; GPCR, G protein-coupled receptors; LRG, Laparoscopic radical gastrectomy; NMDA, N-methyl-D-aspartic acid; NRS, Numerical rating scale; OG, Open radical gastrectomy; PCA, Patient-controlled intravenous analgesia; TCI, Target-controlled infusion; TNM, Tumor node metastasis; WHO, World health organization.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2020) 70:313. doi: 10.3322/caac.21609
2. Pandey K, Devi P, Das PK, Mohanty S, Goutam K, Samantara S, et al. Billroth I, a viable alternative in early distal gastric cancers: short-term results from an Indian Tertiary Care Center. Indian J Surg Oncol. 12:290–7. doi: 10.1007/s13193-021-01288-7
3. Xia X, Zhang Z, Xu J, Zhao G, Yu F. Comparison of postoperative lymphocytes and interleukins between laparoscopy-assisted and open radical gastrectomy for early gastric cancer. J Int Med Res. (2019) 47:303–10. doi: 10.1177/0300060518800909
4. Zheng HL, Lu J, Zheng CH, Li P, Xie JW, Wang JB, et al. Short- and long-term outcomes in malnourished patients after laparoscopic or open radical gastrectomy. World J Surg. (2018) 42:195–203. doi: 10.1007/s00268-017-4138-9
5. Zhang F, Lan Y, Tang B, Hao Y, Shi Y, Yu P. Comparative study of laparoscopy-assisted and open radical gastrectomy for stage T4a gastric cancer. Int J Surg. (2017) 41:23–27. doi: 10.1016/j.ijsu.2017.01.116
6. Jiao J, Liu S, Chen C, Maimaiti A, He Q, Hu S, et al. Comparative study of laparoscopic radical gastrectomy and open radical gastrectomy. J Minim Access Surg. (2020) 16:41–6. doi: 10.4103/jmas.JMAS_155_18
7. Yalav O, Topal U, Gumus S, Unal AG, Rencuzogullari A. Laparoscopic versus open total radical gastrectomy for advanced gastric cancer: surgical outcomes. Ann Ital Chir. (2020) 92:609–15.
8. Li B, Yu-Hong Wong I, Siu-Yin Chan F, Chan KK, Lai-Yin Wong C, Law TT, et al. Comparison of laparoscopic versus open gastrectomy for gastric cancer. Surg Oncol. (2020) 35:14–21. doi: 10.1016/j.suronc.2020.06.008
9. Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, et al. Which method is more suitable for advanced gastric cancer with enlarged lymph nodes, laparoscopic radical gastrectomy or open gastrectomy?. Gastric Cancer. (2018) 21:853–63. doi: 10.1007/s10120-018-0800-7
10. Kim SY, Kim NK, Baik SH, Min BS, Hur H, Lee J, et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study. Medicine. 95:e3602. doi: 10.1097/MD.0000000000003602
11. Fan Q, Xie H, Ma Z, Chen Z, Yan T, Ge W. Perioperative predictors of moderate and severe postoperative pain in idiopathic scoliosis patients following spinal correction and fusion operations. Medicine. (2018) 97:e13215. doi: 10.1097/MD.0000000000013215
12. Wang Z, Wang C. Laparoscopic radical gastrectomy and open radical gastrectomy for gastric cancer curative effect comparison. J Xinxiang Med Coll. (2020) 386–90. doi: 10.7683/xxyxyxb.2020.04.020
13. Zhao L, Ling R, Chen J, Shi A, Chai C, Ma F, et al. Clinical outcomes of proximal gastrectomy versus total gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Dig Surg. (2021) 38:1–13. doi: 10.1159/000506104
14. Ronchetti S, Migliorati G, Delfino DV. Association of inflammatory mediators with pain perception. Biomed Pharmacother. (2017) 96:1445–52. doi: 10.1016/j.biopha.2017.12.001
15. Gottesman-Katz L, Latorre R, Vanner S, Schmidt BL, Bunnett NW. Targeting G protein-coupled receptors for the treatment of chronic pain in the digestive system. Gut. (2021) 70:970–81. doi: 10.1136/gutjnl-2020-321193
16. Omori T, Yamamoto K, Hara H, Shinno N, Yamamoto M, Sugimura K, et al. A randomized controlled trial of single-port versus multi-port laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. (2021) 35:4485–93. doi: 10.1007/s00464-020-07955-0
17. Deurenberg P, Yap M. The assessment of obesity: methods for measuring body fat and global prevalence of obesity. Baillieres Best Pract Res Clin Endocrinol Metab. (1999) 13:1–11. doi: 10.1053/beem.1999.0003
18. Tsekrekos A, Lovece A, Chrysikos D, Ndegwa N, Schizas D, Kumagai K, et al. Impact of obesity on the outcomes after gastrectomy for gastric cancer: a meta-analysis. Asian J Surg. (2021) 45:15–26. doi: 10.1016/j.asjsur.2021.04.033
19. Jung JH, Ryu SY, Jung MR, Park YK, Jeong O. Laparoscopic distal gastrectomy for gastric cancer in morbidly obese patients in South Korea. J Gastric Cancer. (2014) 14:187–95. doi: 10.5230/jgc.2014.14.3.187
20. Wang Z, Zhang X, Liang J, Hu J, Zeng W, Zhou Z. Short-term outcomes for laparoscopy-assisted distal gastrectomy for body mass index ≥30 patients with gastric cancer. J Surg Res. (2015) 195:83–8. doi: 10.1016/j.jss.2014.12.044
21. Chen H, Sui W. Influence of obesity on short- and long-term outcomes after laparoscopic distal gastrectomy for gastric cancer. J BUON. (2017) 22:417−23.
22. Pata G, Solaini L, Roncali S, Pasini M, Ragni F. Impact of obesity on early surgical and oncologic outcomes after total gastrectomy with “over-D1” lymphadenectomy for gastric cancer. World J Surg. (2013) 37:1072–81. doi: 10.1007/s00268-013-1942-8
23. Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Nakade H, Miyao S, et al. Postoperative serum C-reactive protein level predicts long-term outcomes in Stage I Gastric Cancer. J Surg Res. (2019) 242:323–31. doi: 10.1016/j.jss.2019.04.075
24. Saito T, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, et al. Which is a more reliable indicator of survival after gastric cancer surgery: postoperative complication occurrence or C-reactive protein elevation?. J Surg Oncol. (2015) 112:894–9. doi: 10.1002/jso.24067
25. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
26. Yeung DE, Peterknecht E, Hajibandeh S, Hajibandeh S, Torrance AW. C-reactive protein can predict anastomotic leak in colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis. (2021) 36:1147–62. doi: 10.1007/s00384-021-03854-5
27. Kurokawa Y, Yamashita K, Kawabata R, Fujita J, Imamura H, Takeno A, et al. Prognostic value of postoperative C-reactive protein elevation versus complication occurrence: a multicenter validation study. (2020) Gastric Cancer. 23:937–43. doi: 10.1007/s10120-020-01073-5
28. Bodnar RJ. Endogenous opiates and behavior: 2018. Peptides. (2020) 132:170348. doi: 10.1016/j.peptides.2020.170348
29. Oh TK, Kim K, Jheon S, Lee HJ, Do SH. Association between perioperative cholesterol level and analgesia after video-assisted thoracoscopic surgery. Korean J Anesthesiol. (2019) 72:135–42. doi: 10.4097/kja.d.18.00122
30. Huang Z, Liang L, Li L, Xu M, Li X, Sun H, et al. Opioid doses required for pain management in lung cancer patients with different cholesterol levels: negative correlation between opioid doses and cholesterol levels. Lipids Health Dis. (2016) 15:47. doi: 10.1186/s12944-016-0212-9
31. Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol. 17:7. doi: 10.1186/s12957-018-1551-y
32. Whitlock EL, Kim H, Auerbach AD. Harms associated with single unit perioperative transfusion: retrospective population based analysis. BMJ. (2015) 350:h3037. doi: 10.1136/bmj.h3037
33. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. (2003) 54:908–14. doi: 10.1097/01.TA.0000022460.21283.53
34. Carson JL, Stanworth SJ, Dennis JA, Trivella M, Roubinian N, Fergusson DA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 12:CD002042. doi: 10.1002/14651858.CD002042.pub5
35. Liu X, Ma M, Huang H, Wang Y. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: a retrospective analysis of a single center database. BMC Cancer. (2018) 18:649. doi: 10.1186/s12885-018-4574-4
Keywords: gastric cancer, surgery, postoperative, acute pain, predictor
Citation: Xie H, Wei J, Ma Z and Ge W (2022) Predictive Factors for Acute Postoperative Pain After Open Radical Gastrectomy for Gastric Cancer. Front. Public Health 10:907222. doi: 10.3389/fpubh.2022.907222
Received: 29 March 2022; Accepted: 26 April 2022;
Published: 01 June 2022.
Edited by:
Xuefeng Xie, Anhui Medical University, ChinaReviewed by:
Chunsong Yang, Sichuan University, ChinaYi Fang, Peking University People's Hospital, China
Copyright © 2022 Xie, Wei, Ma and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Ge, NjIyMTIzMEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Han Xie
Han Xie Jingxuan Wei
Jingxuan Wei Zhengliang Ma
Zhengliang Ma Weihong Ge
Weihong Ge