- 1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Big Data Health Science, School of Public Health, Zhejiang University, Hangzhou, China
- 3Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, United States
Introduction: As an important pathogen causing diarrheal diseases, the burden and change in the death rate of norovirus-associated diseases (NADs) globally are still unknown.
Methods: Based on global disease burden data from 1990 to 2019, we analyzed the age-standardized death rate (ASDR) of NADs by age, region, country, and Socio-Demographic Index (SDI) level. The discrete Poisson model was applied in the analysis of NADs' spatiotemporal aggregation, the Joinpoint regression model to analyze the trend of death burden of NADs over 30 years, and a generalized linear model to identify the risk factors for the death rate from NADs.
Results: The ASDR of NADs significantly decreased by a factor of approximately 2.7 times, from 5.02 (95% CI: 1.1, 11.34) in 1990 to 1.86 (95% CI: 0.36, 4.16) in 2019 [average annual percent change (AAPC) = −3.43, 95% CI: −3.56, −3.29]. The death burden of NADs in 2019 was still highest in African regions despite a great decline in recent decades. However, the ASDR in high SDI countries presented an uptrend [0.12 (95% CI: 0.03, 0.26) in 1990 and 0.24 (95% CI: 0.03, 0.53) in 2019, AAPC = 2.52, 95% CI: 2.02–3.03], mainly observed in the elderly over 70 years old. Compared to children under 5 years old, the 2019 death rate of elderly individuals over 80 years old was much higher in high SDI countries. The generalized linear model showed that factors of the number of physicians (RR = 0.67), the proportions of children under 14 years old (RR = 1.21), elderly individuals over 65 years old (RR = 1.13), educational level (RR = 1.03) and urbanization proportion (RR = 1.01) influenced the ASDR of NADs.
Conclusions: The death burden of NADs has remained high in developing regions over the last three decades and has increased among the elderly in countries with high SDI levels, even though the global trend in NAD-associated deaths has decreased significantly in the past three decades. More effective public health policies against NADs need to be implemented in high SDI regions and for the elderly.
Introduction
Although the disability-adjusted life years (DALYs) and age-standardized death rate (ASDR) of diarrheal diseases decreased by 51% and 49%, respectively, from 1990 to 2010, diarrheal diseases remain the fourth cause of DALYs and seventh cause of death, resulting in approximately 1.45 million deaths worldwide every year (1, 2). Previous studies suggested that diarrheal diseases were the second leading cause of death among children under 5 (3–5). They represented 78% of all diarrhea deaths among children in the African, Southeast Asian, and Eastern Mediterranean regions (6). The mortality rates of diarrheal diseases in the developing regions referenced above were over 25% (4). According to an attribution study of diarrheal diseases, norovirus was a common pathogen of diarrheal diseases, with 13.8% of children under 5 years requiring hospitalization, second only to rotavirus (38.3%) and enteropathogenic Escherichia coli (15.3%) (7). Norovirus was considered related to almost one-fifth of all acute gastroenteritis cases across all age groups (8, 9). Patel MM et al. estimated that norovirus was detected in 12% of children under 5 years of age with severe diarrhea, which indicated that norovirus was the second most common cause of severe gastroenteritis after rotavirus (10). In developed countries that introduced a rotavirus vaccine program, norovirus surpassed rotavirus as the leading cause of acute gastroenteritis in children (11). The U.S. Centers for Disease Control and Prevention (CDC) also suggested that norovirus caused approximately 60% of acute gastroenteritis cases (with a known cause) in the United States every year (12).
However, insufficient attention has been given to the detection, diagnosis, and surveillance of norovirus. Payne et al. searched ICD-9-CM discharge diagnostic codes and found that there was no ICD-9-CM code for norovirus (008.63) among 278 laboratory-confirmed norovirus cases (13). Previous studies on the disease burden were limited in data by the effect of annual and seasonal epidemics and varied greatly in investigated age groups and locations (14). Consequently, high-quality studies on the disease burden, trends, and epidemic features of norovirus-associated diseases (NADs) are still missing, especially in developing regions. Based on global disease burden data, we investigated the global death burden and trend of NADs worldwide with the stratification of multiple factors from 1990 to 2019 and explored the correlations between ASDR and sociodemographic economic factors. This study aims to better understand the disease burden of NADs and identify the regions and populations with high death rates or those on the rise, providing insight to assist targeted policymaking and recommending actions to reduce the death rate of NADs.
Materials and Methods
Data Sources
Referring to the categories of diarrhea rates reported in clinical, case-control, and community studies, Global Burden of Disease (GBD) 2019 estimated the death numbers and rates of NADs globally by age, sex, cause, region, and Socio-Demographic Index (SDI) level (7). The Bayesian hierarchical meta-regression tool (Dismod-MR) and the Cause of Death Ensemble model (CODEm) were used to calculate the estimates of metrics. And the death rate of NADs was calculated from diarrhea data via a counterfactual approach called population attributable fraction (PAF) (15). We obtained the data from GBD 2019 using the Global Health Data Exchange, including the number of deaths, death rate, and ASDR of NADs with 95% uncertainty intervals (UIs) across 204 countries and territories and 21 GBD regions from 1990 to 2019. Jiang et al. obtained the genome of norovirus in 1993 (16). After that, molecular methods like the RT-PCR were developed. Therefore, the global screening data of NADs relying on molecular diagnostic techniques in the 1990s is likely to be biased. To eliminate the influence of data bias on the results, we made an additional analysis of data from 2005 to 2019. A total of 204 countries were divided into five SDI quintiles (high, high-middle, middle, low-middle, and low) according to economic growth, fertility rate, and educational attainment. The SDI ranges from 0 to 1, and the standard of classification was provided by GBD studies. All rates are reported per 100,000 person-years.
Spatial and Temporal Aggregation Analysis
The geographical distribution and spatiotemporal aggregation were performed to explore the space-time cluster of NADs worldwide from 1 January 1990 to 31 December 2019. Based on the discrete Poisson model, national units and time were scanned via the dynamic space-time two-dimensional cylinder scanning window. For each scanning window, the expected number of deaths was estimated from the actual number of deaths and the population. The log-likelihood ratio (LLR) and relative risk (RR) calculated from the actual and expected death numbers were used for estimating the presence and degree of aggregation. The cluster was classified according to the LLR value. The window with the largest significant LLR value statistically was the first cluster. P-values were calculated by Monte Carlo simulation, with P < 0.05 considered significant.
Joinpoint Regression Model Analysis
Joinpoint regression program (17) was developed by the National Cancer Institute (NCI). The Joinpoint regression model helped find the turning points that were statistically significant via the Monte Carlo permutation test. We used Joinpoint Regression Software (18) to calculate the annual percent change (APC) with 95% CIs and the average annual percent change (AAPC) to analyze the temporal trends of the age-standardized death rate of NADs by SDI quintiles. An APC or AAPC greater or smaller than 0 denoted an up or downtrend of the ASDR. The level of significance was set at P < 0.05.
Generalized Linear Model
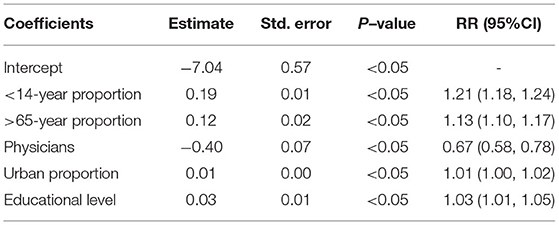
Based on previous studies, we selected 5 covariates that were statistically significant from 14 country-level sociodemographic covariates across 199 countries from 1990 to 2019. We used several types of generalized linear models to fit the age-standardized death rate and each country-level sociodemographic covariate. The best-fit model with a Gaussian-distributed corresponding variable was determined according to the Akaike information criterion. Fitted model equation:
where Yt denotes the age-standardized death rate; β0 is the intercept; β (<14-year proportion), β (>65-year proportion), β (urban proportion), β (physicians), and β (educational level) denote the population proportion under 14 and over 65 years old, the proportion of urbanization, the number of physicians per 1,000 persons and the average level of education, at least bachelor's or equivalent, respectively.
Software
We used Microsoft Excel 2019 for data extraction, sorting, and cleaning and R (version 3.2.3), SatScan (version 9.5), and Joinpoint (version 4.8.0.1) for further data analysis.
Results
The Number and ASDR of NADs in 1990 and 2019 With Change Rate and AAPC Over the 30 Years
In total, there were 135,798 NADs deaths worldwide in 2019, and the ASDR was 1.86 (95% UI: 0.36–4.16) per 100,000 person-years. Children younger than 5 years old (0.65, 95% UI: 0.18–1.49) and elderly individuals at least 80 years old (0.7, 95% UI: 0.08–1.76) had a higher ASDR than others (Table 1). The ASDR declined in most of the 21 GBD regions, whereas Australasia, high-income North America, and Western Europe increased from 1990 to 2019 with significant AAPCs (Table 1, Supplementary Figure S1).
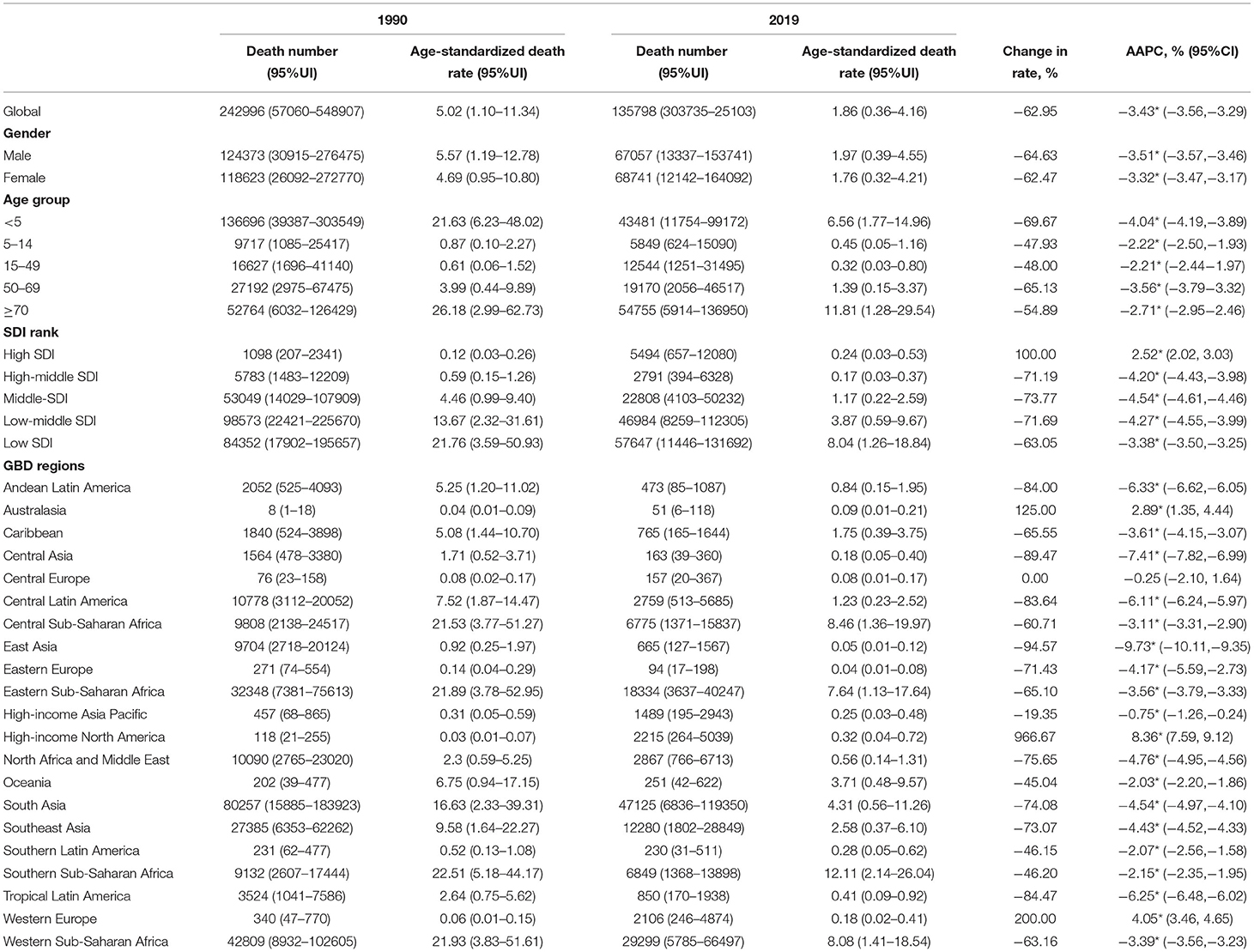
Table 1. The number and age-standardized death rate (ASDR) of norovirus-associated diseases (NADs) in 1990 and 2019 with change rate and average annual percent changes (AAPCs) over the 30 years.
Global Burden of NADs Among 204 Countries and Territories
Nationally, Lesotho showed the highest ASDR in 1990, followed by Angola. Austria and Greece had the lowest ASDR in 1990 (Figure 1A, Supplementary Figures S2–S6). By 2019, Lesotho still presented the greatest age-standardized death rate, and Greece still had the lowest ASDR (Figure 1B, Supplementary Figures S2–S6). Regionally, African regions had higher ASDRs in 1990 and 2019 (Figures 1A,B). Globally, the ASDR of most countries and territories decreased significantly over the past 30 years, except for North America, Western Europe, and Australia (Figure 1C). The ASDR in East Asia (AAPC = −9.45%, P < 0.05) presented the largest drop and the greatest annual percent change at the regional level. In contrast, the ASDR in high-income North America (AAPC = 8.19%, P < 0.05) increased with the greatest AAPC (Supplementary Figure S1).
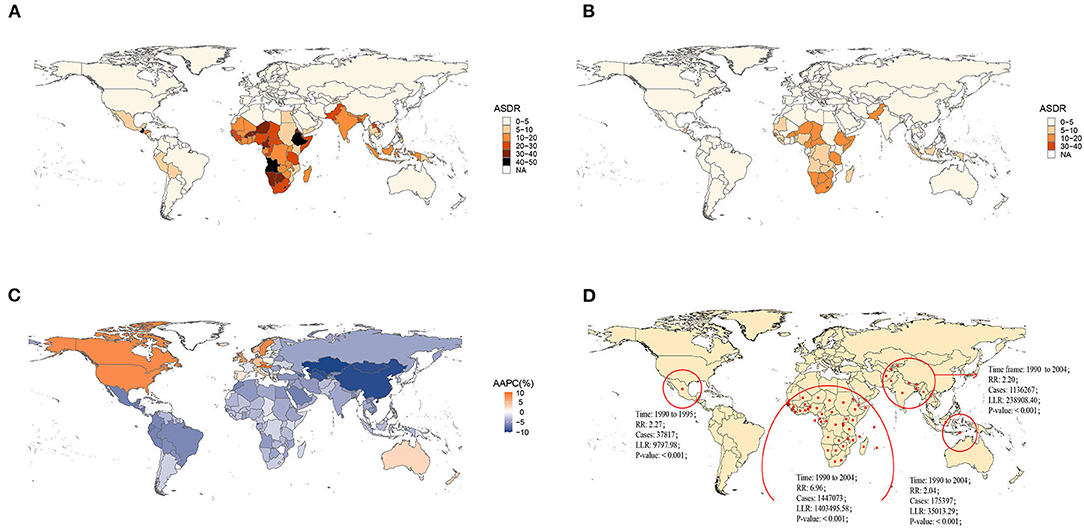
Figure 1. Global burden of norovirus-associated diseases (NADs) in 1990 and 2019 with the annual percent change rate and spatial and temporal aggregation over the 30 years. (A) Age-standardized death rate (ASDR) in 1990; (B) ASDR in 2019; (C) average annual percent changes (AAPCs) from 1990 to 2019; (D) Spatial and temporal aggregation from 1990 to 2019.
Four spatial and temporal aggregation areas were observed through spatial and temporal aggregation analyses. The first-level spatial and temporal aggregation areas were mainly located in African regions, and the gathering time was from 1 January 1990 to 31 December 2004. The actual number of cases reported in the regions was 1,447,073, while the number of expected cases was 268,296 (RR = 6.96, LLR = 1,403,495.58, P < 0.001). The second and third spatial and temporal aggregation areas were both in South Asia from 1 January 1990 to 31 December 2004, with relative risks of 2.2 (LLR = 238,908.40, P < 0.001) and 2.04 (LLR = 35,013.29, P < 0.001), respectively. The fourth spatial and temporal aggregation area covered Mexico and Guatemala from 1 January 1990 to 31 December 1995. The actual number of cases reported was 37,817, but the number of expected cases was 16,728 (RR = 2.27, LLR = 9,797.98, P < 0.001) (Figure 1D).
Age-Specific Temporal Trends of NADs Burden Among SDI Quintiles From 1990 to 2019
Socio-Demographic Index-stratified joinpoint analysis showed that the ASDR of NADs declined significantly since 1990 globally and in high-middle, middle, low-middle, and low SDI regions (Figure 2, Table 1). The proportions of NADs in diarrheal disease-associated deaths showed a decreasing or stabilizing trend in those regions (Supplementary Figures S7, S9–S12). However, the ASDR in high SDI regions presented different trends. As shown in Figure 2B, the ASDR showed an uptrend with different APCs. Between 1998 and 2005, the ASDR rose rapidly (APC = 10.67%, P < 0.05). Then, between 2005 and 2008, the ASDR continued to increase significantly (APC = 4.72%, P < 0.05). Since 2008, the rate presented a slight downtrend, however, it was still higher than that in the past, approximately doubling the burden compared to 1990 (Figure 2B). The proportion of NADs in diarrheal disease-associated deaths increased from 12.24% in 1990 to 15.69% in 2019, which has surpassed rotavirus to rank second among 13 pathogens in high SDI regions (Supplementary Figure S8).
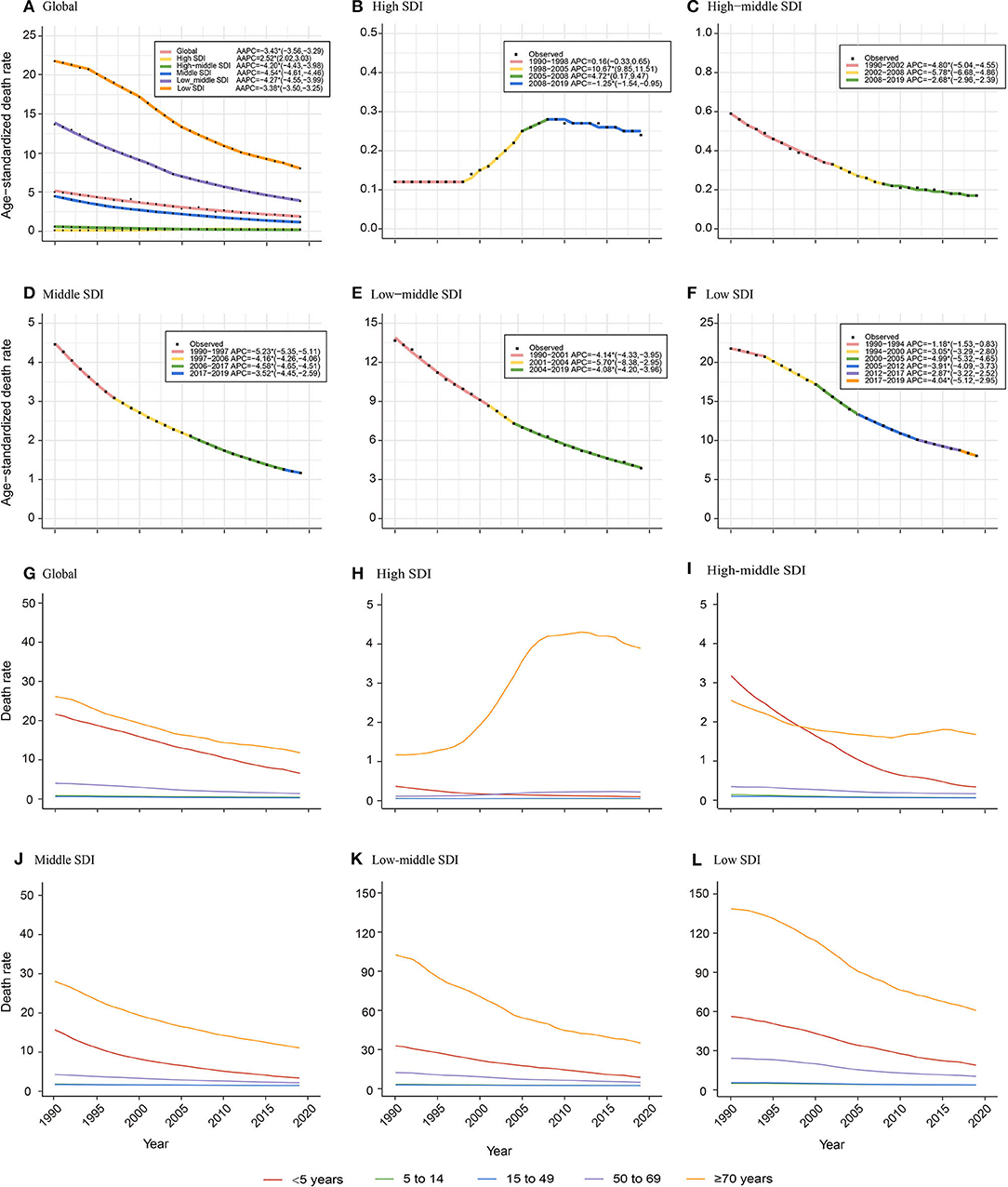
Figure 2. Age-specific temporal trends of ASDR globally and in different Socio-Demographic Index (SDI) regions over 30 years old. (A–F) Temporal trends of ASDR globally and in different SDI regions; (G–L) Age-specific temporal trends of death rate globally and in different SDI regions. The APCs with asterisks (*) are statistically significant (P < 0.05).
Globally, children under 5 years old and the elderly over 70 years old have born with the heaviest death burden from NADs over the 30 years old. The death rate of children younger than 5 years old presented a significant decrease since 1990. The death rate of the elderly over 70 years decreased globally and in high-middle, middle, low-middle, and low SDI regions. However, it showed an increasing trend among elderly over 70 years old showed in high SDI regions (Figure 2G).
Age Group Distribution of NADs Burden in 1990 and 2019 Among SDI Quintiles and Worldwide
The death rate was the highest in the elderly over 80 years old among all age groups globally and in five SDI regions both in 1990 and 2019. In adults and the elderly, the death rate presented a tendency to increase with age. Children under 5 years old were another peak population with a high death rate. The ratio of elderly over 80 years old to children under 5 years old increased from 1.6 to 7.6 in 1990 and 3.4 to 139.4 in 2019, which showed a growing trend in the proportion of death burden in elderly over 80 years old among all age groups (Figure 3). Compared to other SDI regions, the ratio was much higher in the high SDI region with a ratio of 139.4 (3.4 to 11.3 in other SDI regions) (Figure 3B).
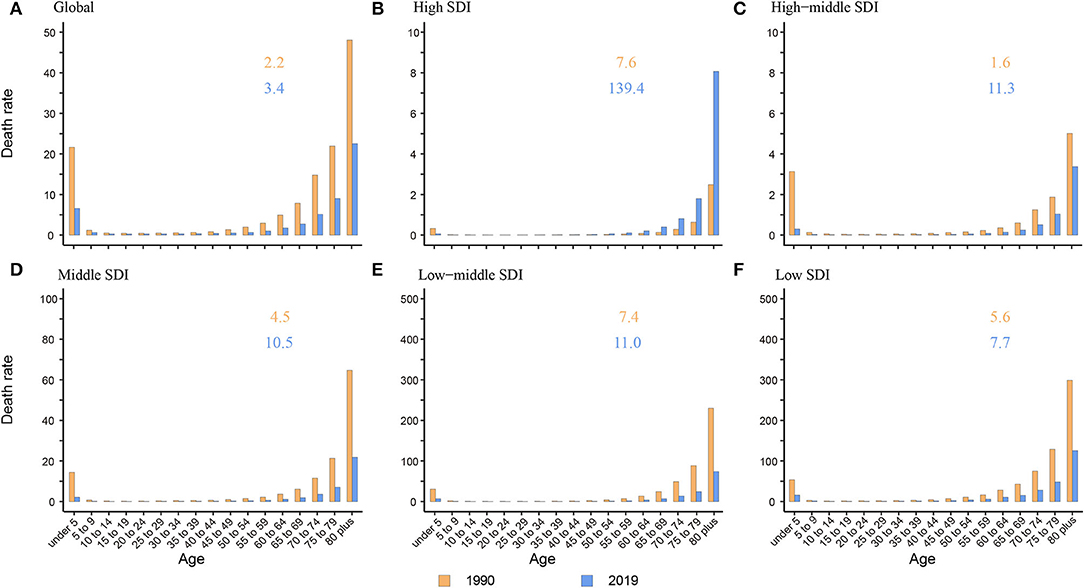
Figure 3. The death rate of NADs among children under 5 and elderly over 80 globally and in 5 SDI regions. (A) Global; (B) High SDI; (C) High-middle SDI; (D) Middle SDI; (E) Low-middle SDI; (F) Low SDI. The orange and blue numbers above the column denote the ratio of elderly over 80 years old to children under 5 years old in 1990 and 2019 separately.
Correlation Analysis of Sociodemographic Factors With the ASDR of NADs
The generalized linear model (GAM) found that the number of physicians per 1,000 persons had a significant negative effect on the ASDR of NADs, RR = 0.67 (95% CI: 0.58, 0.78). In contrast, the proportion of individuals under 14 (RR = 1.21) and over 65 years old (RR = 1.13), urbanization proportion (RR = 1.01) and average national educational level (RR = 1.03) were significantly positively correlated with the ASDR (Table 2). Furthermore, in low SDI regions, the ASDR declined sharply with the increasing SDI index. The downward trend flattened in the middle and high SDI regions (Supplementary Figure S13A). A correlation analysis showed that there was a negative correlation (R = −0.63, P < 0.05) between the ASDR of NADs and the SDI index at the national level in 2019 (Supplementary Figure S13B).
Discussion
Despite greatly declining over several decades, the ASDR in developing areas (e.g., Africa, and Southeast Asia) was still highest, adding up to more than 80% of the ASDR globally, which was consistent with that of previous studies (Supplementary Figure S1). Norovirus is mainly transmitted through the fecal-oral route with several other transmission routes, aerosolized viral particles (19, 20), food, water, and environmental pollution (21). The malnutrition and lack of vitamins, substandard quality of water, insufficient health facilities, and prolonged breastfeeding over 6 months in those areas could affect the incidence and death rate from diarrheal disease in different proportions (22–24). Compared to developed areas, diarrheal patients in developing areas may obtain fewer medical resources for pathogen detection and further treatment. Oral rehydration salts (ORS) and zinc supplementation are critical for diarrheal patients, whereas the coverage of drugs remains relatively low across the developing world, partly explaining the high death rate in these areas (25).
Due to the development of diagnostic techniques and medical interventions, the ASDR of diarrheal diseases in high-middle, middle, low-middle, and low SDI regions has fallen substantially in recent decades (Figures 2C-F). One unexpected finding was that the ASDR in high SDI regions, high-income North America, Western Europe, and Australasia presented a significant uptrend after 1998 (Figure 2B, Supplementary Figure S1). The ASDR of NADs surpassed rotavirus as the second-highest among 13 pathogens of infectious diarrhea in high SDI regions in 2019 (Supplementary Figure S8). Previous studies showed that the global prevalence of norovirus gastroenteritis has been associated with a single genotype named GII.4 since the 1990s (26–29). GII.4 strains caused several main outbreaks of norovirus between 1995 and 2008 with a 2- to the 3-year interval (30). The first wave was in 1995/1996 and was caused by the U5-95_US strain (26, 31–33). and then Australia in 1997–2000 (27). In 2002, the new GII.4 variants of “the Farmington Hills virus” caused epidemics in Europe (34), the United States (28), and Australia (26). In 2004, the “Hunter virus,” another GII.4 variant emerged and caused multiple outbreaks in Australia, New Zealand, Japan and Taiwan, and Europe (35, 36). According to Kroneman A, GII.4 genotype noroviruses were dominant in the United States, Europe, and Oceania from 1998 to 2007, accounting for 70%-80% of all outbreaks (37). Another study showed that hospitalizations and deaths were more likely to occur in outbreaks related to GII.4 strains, with an incidence ratio of 9.4 and a mortality rate of 3.1 (38). The outbreaks of the GII.4 variant in these developed countries might explain the increase of the death rate from 1990 to 2008 in high SDI regions (26, 39). In the rotavirus vaccine era, the burden rank of norovirus in 13 pathogens causing diarrheal diseases has gradually increased. Norovirus infection was shown to be more prevalent than rotavirus infection in children in the US recently (13). Similar increases have been observed in other countries (11, 40, 41). A longitudinal epidemiological study in the United States compared the positive rates of norovirus and rotavirus in the feces of diarrheal children before and after the introduction of the rotavirus vaccine in 8.5 years and concluded that the total prevalence of norovirus was 10.9% and increased after 2003, between 11% and 16.8% (42). More attention to norovirus is needed, including dedicating attention to the development of norovirus vaccines.
Like many other diseases, children and the elderly are the two age groups having relatively poor immunity and are more vulnerable to bacteria and viruses. We found that in high-middle, middle, low-middle, and low SDI regions, there were two death rate peaks in 2019: children under 5 and elderly individuals over 80 years old (Figure 3). Hall et al. also reported that norovirus-related hospitalization rates are u-shaped, and the incidence is highest among children under 5 years old (9.4 per 10,000) and over 65 years old (8.1 per 10,000) (11). Interestingly, in the high SDI region, there was only one death rate peak in 2019 of those over 80 years old without an insignificant young age peak. The death rate of children under 5 years old was almost equal to that of children over 5 years old and adults (Figure 3B). One plausible explanation is that the level of medical and health care in high SDI regions is relatively higher, lowering the death rate of children under 5 years. Additionally, with better medical and health security, more elderly individuals live in nursing homes and hospitals in high SDI areas. In an interesting coincidence, a previous study demonstrated that GII.4 was more likely to be transmitted via person-to-person transmission, especially in long-term care facilities (LTCFs) and hospital settings, and GI.7 and GII.12 were more likely to be transmitted via foodborne transmission. Moreover, in a total review of 2,895 outbreaks with known transmission routes in the United States from September 2009 to August 2013, person-to-person and foodborne transmission accounted for 83.7% and 16.1%, respectively (43). In the study by Scallan E and his colleagues, the proportion of foodborne noroviruses declined from 40% to 26%, consistent with estimates in other studies (44–46). The poor immunity, GII.4 strains, person-to-person transmission, and specific places all indicate that elderly individuals are currently the most vulnerable people. In addition, the growing death rate of the elderly in high SDI areas illustrates the need for more attention and interventions in older people in developed countries (Figure 2H).
As an important embodiment of medical resources, the assignment of doctors per 1,000 persons was closely associated with the death rate of diarrheal diseases. Previous studies have suggested that there is a negative correlation between the incidence of diarrheal diseases and educational level in developing countries (47–49). People with higher educational levels may have enough nutrition, health knowledge and good personal hygiene to prevent infections. There is less chance for these people to both transmit and be infected by norovirus. However, in the result of our generalized linear model, a higher ASDR was associated with a higher educational level (Table 2). This finding is supported by a population-based cohort study in the Netherlands (9). de Wit et al. explained that people who receive more education may also have higher incomes. They eat out more and have more opportunities to try specialty foods. Another study also showed that highly educated people were more likely to have risk behaviors of foodborne infection (50). In this study, the proportion of urbanization was positively correlated with the ASDR (Table 2). An explanation for this observation is that the population density in urban areas is higher. People residing in urban areas are more likely to have interpersonal transmission. A cross-sectional study of Haitian children also observed that children residing in urban areas reported diarrhea much more frequently than those from rural areas (51). High SDI regions and countries had lower ASDRs, also denoting that we need to make more effort on policy and medical interventions in low SDI areas (Supplementary Figure S13). The risk factors in the results can provide some suggestions for the specific implementation aspects.
Limitation
The death rate perhaps says more about the severe cases among all norovirus-infected patients. Although being proportional to the incidence, the death rate only partly reflects the burden and epidemic features of NADs. It is better to understand the disease burden of NADs from multiple indicators. The modeled data of norovirus in the GBD study was estimated based on the vital registration, verbal autopsy data, and surveillance system data. However, the detection of norovirus depended on the development of molecule diagnostics like RT-PCR, so the data from surveillance systems in the 1990s and early 2000s was probably biased, especially in the areas with scarce medical sources.
Conclusion
We analyzed the global death burden and epidemic features of NADs in different regions and countries among people of different ages. The overall burden of NADs is on the decline except for the high SDI region and the elderly. The death burden in developing areas is still much higher than that in developed areas. Overall, improving medical care in these places, strengthening the protection of vulnerable populations, and accelerating the development of targeted vaccines are important for easing the disease burden of NADs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SY designed the study. XZ, CC, YD, DY, DJ, XL, MY, CD, and LL collected data. XZ and CC analyzed data. XZ and YD checked the data and results. SY, XZ, CC, and DY interpreted the data and wrote the report. RH modified the language. SY and RH revised the report from the preliminary draft to submission. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant numbers: 82173577, 81672005, U1611264, and 81001271) and the Mega-Project of National Science and Technology for the 12th and 13th Five-Year Plan of China (grant numbers: 2018ZX10715-014-002 and 2014ZX10004008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.905172/full#supplementary-material
References
1. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-Adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
3. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. (2010) 375:1969–87. doi: 10.1016/S0140-6736(10)60549-1
4. Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PLoS ONE. (2012) 7:e29151. doi: 10.1371/journal.pone.0029151
5. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. (2012) 379:2151–61. doi: 10.1016/S0140-6736(12)60560-1
6. Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. (2008) 86:710–7. doi: 10.2471/BLT.07.050054
7. Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE. (2013) 8:e72788. doi: 10.1371/journal.pone.0072788
8. Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. (2014) 14:725–30. doi: 10.1016/S1473-3099(14)70767-4
9. de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ, Vinjé J, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol. (2001) 154:666–74. doi: 10.1093/aje/154.7.666
10. Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. (2008) 14:1224–31. doi: 10.3201/eid1408.071114
11. Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, et al. Norovirus disease in the United States. Emerg Infect Dis. (2013) 19:1198–205. doi: 10.3201/eid1908.130465
12. Elizabeth Robilotti Stan Deresinski and Benjamin A. Pinsky. Norovirus. Clin Microbiol Rev. (2015) 28:134–164. doi: 10.1128/CMR.00075-14
13. Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. (2013) 368:1121–30. doi: 10.1056/NEJMsa1206589
14. Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. (2012) 12:220. doi: 10.1186/1471-2458-12-220
15. Estimates Estimates of global regional and national morbidity mortality and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study. Lancet Infect Dis. (2015) 17:909–48. doi: 10.1016/S1473-3099(17)30276-1
16. Jiang X, Wang M, Wang K, Estes MK. Sequence and genomic organization of Norwalk virus. Virology. (1993) 195:51–61. doi: 10.1006/viro.1993.1345
17. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. (2000) 19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z
18. Liu X, Jiang J, Yu C, Wang Y, Sun Y, Tang J, et al. Secular trends in incidence and mortality of bladder cancer in China, 1990-2017: a joinpoint and age-period-cohort analysis. Cancer Epidemiol. (2019) 61:95–103. doi: 10.1016/j.canep.2019.05.011
19. Kirking HL, Cortes J, Burrer S, Hall AJ, Cohen NJ, Lipman H, et al. Likely transmission of norovirus on an airplane, October 2008. Clin Infect Dis. (2010) 50:1216–21. doi: 10.1086/651597
20. Wikswo ME, Cortes J, Hall AJ, Vaughan G, Howard C, Gregoricus N, et al. Disease transmission and passenger behaviors during a high morbidity Norovirus outbreak on a cruise ship, January 2009. Clin Infect Dis. (2011) 52:1116–22. doi: 10.1093/cid/cir144
21. Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, et al. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. (2012) 140:1161–72. doi: 10.1017/S0950268812000234
22. Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. (2011) 11 (Suppl. 3):S15. doi: 10.1186/1471-2458-11-S3-S15
23. Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. (2009) 30 (Suppl. 1):S12–40. doi: 10.1177/15648265090301S103
24. West KP Jr. Vitamin A deficiency disorders in children and women. Food Nutr Bull. (2003):24(Suppl. 4): S78–90. doi: 10.1177/15648265030244S204
25. Santosham M, Chandran A, Fitzwater S, Fischer-Walker C, Baqui AH, Black R, et al. Progress and barriers for the control of diarrhoeal disease. Lancet. (2010) 376:63–7. doi: 10.1016/S0140-6736(10)60356-X
26. Bull RA, Tu ET. C. J, McIver W D, Rawlinson P A White Emergence of a new norovirus genotype II4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. (2006) 44:327–33. doi: 10.1128/JCM.44.2.327-333.2006
27. White PA, Hansman GS, Li A, Dable J, Isaacs M, Ferson M, et al. Norwalk-like virus 95/96-US strain is a major cause of gastroenteritis outbreaks in Australia. J Med Virol. (2002) 68:113–8. doi: 10.1002/jmv.10177
28. Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, et al. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus–United States, 2002. J Infect Dis. (2004) 190:27–36. doi: 10.1086/420888
29. Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. (2004) 363:682–8. doi: 10.1016/S0140-6736(04)15641-9
30. Pang XL, Preiksaitis JK, Wong S, Li V, Lee BE. Influence of novel norovirus GII.4 variants on gastroenteritis outbreak dynamics in Alberta and the Northern Territories, Canada between 2000 and 2008. PLoS ONE. (2010) 5:e11599. doi: 10.1371/journal.pone.0011599
31. Tu ET, Bull RA, Greening GE, Hewitt J, Lyon MJ, Marshall JA, et al. Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b. Clin Infect Dis. (2008) 46:413–20. doi: 10.1086/525259
32. Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang XL, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001-2007. J Infect Dis. (2009) 200:802–12. doi: 10.1086/605127
33. Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis. (1999) 179:1334–44. doi: 10.1086/314783
34. Dingle KE. Mutation in a Lordsdale norovirus epidemic strain as a potential indicator of transmission routes. J Clin Microbiol. (2004) 42:3950–7. doi: 10.1128/JCM.42.9.3950-3957.2004
35. Kroneman A, Vennema H, Harris J, Reuter G, von Bonsdorff CH, Hedlund KO, et al. Increase in norovirus activity reported in Europe. Euro Surveill. (2006) 11:E061214.1. doi: 10.2807/esw.11.50.03093-en
36. Ramirez S, De Grazia S, Giammanco GM, Milici M, Colomba C, Ruggeri FM, et al. Detection of the norovirus variants GGII.4 hunter and GGIIb/hilversum in Italian children with gastroenteritis. J Med Virol. (2006) 78:1656–62. doi: 10.1002/jmv.20751
37. Kroneman A, Verhoef L, Harris J, Vennema H, Duizer E, van Duynhoven Y, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol. (2008) 46:2959–65. doi: 10.1128/JCM.00499-08
38. Desai R, Hembree CD, Handel A, Matthews JE, Dickey BWS, McDonald, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis. (2012) 55:189–93. doi: 10.1093/cid/cis372
39. Sakon N, Yamazaki K, Nakata K, Kanbayashi D, Yoda T, Mantani M, et al. Impact of genotype-specific herd immunity on the circulatory dynamism of norovirus: a 10-year longitudinal study of viral acute gastroenteritis. J Infect Dis. (2015) 211:879–88. doi: 10.1093/infdis/jiu496
40. Ferreira MS, Victoria M, Carvalho-Costa FA, Vieira CB, Xavier MP, Fioretti JM, et al. Surveillance of norovirus infections in the state of Rio De Janeiro, Brazil 2005-2008. J Med Virol. (2010) 82:1442–8. doi: 10.1002/jmv.21831
41. Bucardo F, Reyes Y, Svensson L, Nordgren J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE. (2014) 9:e98201. doi: 10.1371/journal.pone.0098201
42. Koo HL, Neill FH, Estes MK, Munoz FM, Cameron AH, L DuPont, et al. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J Pediatric Infect Dis Soc. (2013) 2:57–60. doi: 10.1093/jpids/pis070
43. Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinjé J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol. (2014) 52:147–55. doi: 10.1128/JCM.02680-13
44. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. (2011) 17:7–15. doi: 10.3201/eid1701.P11101
45. Adak GK, Meakins SM, Yip H, Lopman BA, O'Brien SJ. Disease risks from foods, England and Wales, 1996-2000. Emerg Infect Dis. (2005) 11:365–72. doi: 10.3201/eid1103.040191
46. Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, et al. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis. (2005) 11:1257–64. doi: 10.3201/eid1108.041367
47. Guerrant RL, Kirchhoff LV, Shields DS, Nations MK, Leslie J, de Sousa MA, et al. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. (1983) 148:986–97. doi: 10.1093/infdis/148.6.986
48. Stanton BF, Clemens JD. Socioeconomic variables and rates of diarrhoeal disease in urban Bangladesh. Trans R Soc Trop Med Hyg. (1987) 81:278–82. doi: 10.1016/0035-9203(87)90241-0
49. Huttly SR, Blum D, Kirkwood BR, Emeh RN, Feachem RG. The epidemiology of acute diarrhoea in a rural community in Imo State, Nigeria. Trans R Soc Trop Med Hyg. (1987) 81:865–70. doi: 10.1016/0035-9203(87)90055-1
50. Klontz KC, Timbo B, Fein S, Levy A. Prevalence of Selected Food Consumption and Preparation Behaviors Associated with Increased Risks of Food-borne Disease. J Food Prot. (1995) 58:927–30. doi: 10.4315/0362-028X-58.8.927
Keywords: norovirus-associated diseases, Global Burden of Disease, epidemic features, death rate, global trend analysis
Citation: Zhang X, Chen C, Du Y, Yan D, Jiang D, Liu X, Yang M, Ding C, Lan L, Hecht R and Yang S (2022) Global Burden and Trends of Norovirus-Associated Diseases From 1990 to 2019: An Observational Trend Study. Front. Public Health 10:905172. doi: 10.3389/fpubh.2022.905172
Received: 26 March 2022; Accepted: 10 May 2022;
Published: 17 June 2022.
Edited by:
Rodrigo DeAntonio, CEVAXIN Centro de Vacunación e Investigación, PanamaReviewed by:
Filemón Bucardo, National Autonomous University of Nicaragua, León, NicaraguaSaravanakumar Puthupalayam Kaliappan, Christian Medical College and Hospital, India
Copyright © 2022 Zhang, Chen, Du, Yan, Jiang, Liu, Yang, Ding, Lan, Hecht and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigui Yang, eWFuZ3NoaWd1aUB6anUuZWR1LmNu
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share first authorship
 Xiaobao Zhang1‡
Xiaobao Zhang1‡ Danying Yan
Danying Yan Daixi Jiang
Daixi Jiang Mengya Yang
Mengya Yang Cheng Ding
Cheng Ding Shigui Yang
Shigui Yang