
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 22 June 2022
Sec. Aging and Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.901068
With the rapidly aging population, frailty, characterized by an increased risk of adverse outcomes, has become a major public health problem globally. Several frailty guidelines or consensuses recommend screening for frailty, especially in primary care settings. However, most of the frailty assessment tools are based on questionnaires or physical examinations, adding to the clinical workload, which is the major obstacle to converting frailty research into clinical practice. Medical data naturally generated by routine clinical work containing frailty indicators are stored in electronic health records (EHRs) (also called electronic health record (EHR) data), which provide resources and possibilities for frailty assessment. We reviewed several frailty assessment tools based on primary care EHRs and summarized the features and novel usage of these tools, as well as challenges and trends. Further research is needed to develop and validate frailty assessment tools based on EHRs in primary care in other parts of the world.
In the context of an aging population, frailty is a major public health problem globally (1). Frailty is defined as a state of increased vulnerability to stressors, at the core of which is a decline in the physiological reserves, or even decompensation of multiple organ systems, leading to an increased risk of adverse outcomes (2, 3). This might include falls, hospitalisations, long-term care, disabilities, and mortality (2, 3). A systematic review involving over 60,000 community-dwelling older adults from the US, Canada, Australia, China, and some European countries showed that the average prevalence of frailty was 10.7%, and this percentage nearly tripled among those older than 85 years (4).
It is possible to reverse frailty through intervention (1, 5), and the early identification of patients with frailty is a prerequisite, especially using validated frailty assessment tools (6). Two typical models for frailty assessment that were initially proposed in 2001 are Fried's frailty phenotype and the frailty index (7, 8). The frailty phenotype evaluates whether patients have physical frailty through five clinical presentations, including low grip strength, slow gait speed, unintended weight loss, low physical activity, and self-reported exhaustion (7). The frailty index is based on the cumulative age-related health deficits model proposed by Rockwood and Mitnitski (8). Health deficits are defined in this model as a broad range of health problems that increase with age, such as diseases, symptoms, signs, disability, and abnormal laboratory results, and over 30 deficits are enough for assessment (8, 9). The frailty index score can be calculated by dividing the number of health deficits a patient has by the total number of health deficits (8, 9). Based on the theory of the cumulative health deficit model selecting items from the existing comprehensive geriatric assessment (CGA) questionnaire, could develop a clinically useful frailty index named the FI-CGA (10, 11). Other common frailty instruments have been proposed, such as the FRAIL scale, the Clinical Frailty Scale (CFS), and the Groningen Frailty Indicator (GFI) (3).
Several frailty guidelines or consensuses recommend frailty screening for older adults (3, 12–14), especially in primary care settings, using some commonly validated frailty assessment tools (Table 1). However, screening or evaluating frailty is not implemented in routine clinical practice (16). An important reason for this is that most frailty screening and assessment tools are based on specific questionnaires or physical examinations, which add to the clinical workload (17, 18). Furthermore, some frailty assessment tools consist of numerous multi-dimensional items and may require the guidance of geriatric specialists and training for assessors, which are the main obstacles to the conversion of frailty research to clinical practice (e.g., CGA, the “golden standard” to diagnose frailty, is complex and labor intensive and can't be readily available in day-to-day practice) (17).
Medical data naturally generated by routine work, such as symptoms, signs, diagnoses, abnormal laboratory tests, and lifestyle information, are stored in the EHRs (some of this information is formally coded) (19, 20). These data could be indicators of frailty assessment tools, especially the frailty index based on the cumulative deficit model, providing new resources and possibilities for frailty assessment (18, 20). Using frailty assessment tools based on EHRs (EHR-based frailty assessment tools) does not require additional resources and may bridge the gap between frailty research and clinical frailty assessment (17, 20). An integrative review in 2021 compared the EHR-based frailty assessment tools from different settings, especially the items and the mechanisms involved. It mentioned the advantages of primary care EHRs in constructing frailty assessment tools but failed to describe the application of relevant tools in detail (21). Similarly, some studies had pointed out that primary care EHRs are more comprehensive and representative compared with EHRs in hospitals (22, 23). However, there is currently a lack of a detailed summary of new studies focusing on frailty identification by primary care EHRs. This narrative review aims to describe the current status of identifying frail patients in primary care through EHRs, as well as challenges and future directions.
In 2013, Clegg et al. described the possibility and necessity of constructing frailty instruments based on the existing clinical data set in primary care (2). Applying the cumulative deficit frailty model as the theoretical framework (8), they developed and validated the eFI based on EHRs in ResearchOne and The Health Improvement Network (THIN) primary care databases in the UK. Thirty-six health deficits (Table 2) were used to calculate individuals' eFI scores, which defined the categories of fit, mild frailty, moderate frailty, and severe frailty (18) (Table 3). In the internal validation cohort (ResearchOne databases), including over 200,000 people aged 65–95, those with severe frailty (eFI scores > 0.36) had a 5-fold greater 1-year risk of death (the hazard ratio (HR) was 4.52), unplanned hospitalization (HR 4.73), and nursing home admission (HR 4.76) than the fit group (eFI scores 0–0.12) (17, 18). The external validation cohort (THIN databases) showed broadly similar predictive validity for mortality (for severe frailty, 1-year mortality HR 4.50) (18).
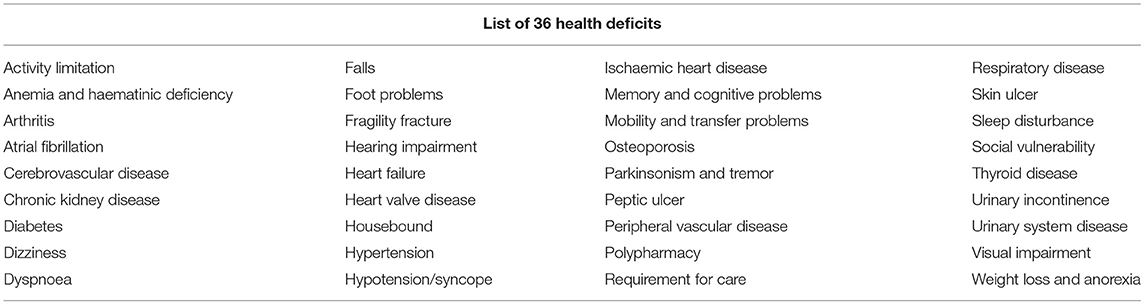
Table 2. List of 36 health deficits in the eFI by Clegg et al. (18).
Several studies have investigated the psychometric properties of the eFI. Hollinghurst et al. used the Secure Anonymised Information Linkage (SAIL) Databank, including 469,000 people aged 65–95, to further validate the eFI (24). HR trends for mortality consist of the initial findings from the previous two databases, which showed robust predictive validity of the eFI (24). Three British studies compared the eFI with several frailty tools to investigate its construct validity for frailty identification (25–27). One pilot study showed that those who were referred to the CGA clinic had higher mean eFI scores (0.33 vs. 0.23) (25). Another study including 353 older adults (≥75) showed that the eFI had a strong Spearman correlation coefficient with the research standard FI and Edmonton Frail Scale, as well as a moderate correlation with the CFS and phenotype model (26). However, in a similar study including 265 older people (mean 85.6), the eFI may have overestimated frailty status in comparison with CFS [odds ratio (OR) is 5.43] (27). A Canadian study showed that the manually calculated eFI from primary care EHRs in Canada had a strong correlation with the FI-CGA (Pearson correlation coefficient is 0.72) (28). In terms of feasibility and acceptability, the manually calculated eFI only needed patients' EHRs and 10–20 min to conduct, which is less intrusive than the PRISMA-7 or 4-m walk test and accepted by clinicians such as those in the previous British pilot study (25). Similarly, it is feasible to extract an eFI with a high discriminative ability (the area under the curve (AUC) is 0.9; vs. frailty phenotype) from routinely collected Australian primary care data, although 15% of records were difficult to extract (29). In general, the eFI is a valid, practical, sensitive, and time-efficient [in 5 min (25)] screening tool with which to identify frailty.
The eFI was soon recommended in the GP contract in England (in the new 2017/18 quality and outcomes framework, which is a fundamental part of the general medical services contract) (30). GPs can calculate eFI scores easily by using the existing software to identify at-risk patients in daily clinical practice under the guidance of webinar recordings (Watch the video for the operation of the eFI calculator on http://help.visionhealth.co.uk/DLM550/Visionplus/index.htm#77063) and other online resources from the website of NHS and then make a diagnosis by reviewing an individual's complete clinical history (30–32).
In 2013, Drubbel et al. constructed a 36-deficit frailty index using the International Primary Care Classification (ICPC) codes and a polypharmacy deficit from the primary care EHR database in Utrecht, Netherlands (33), marking the formation of the first EHR-based frailty assessment tool (also called electronic Frailty Index-Utrecht, and eFI-U). The study, which included 1,679 older patients demonstrated that the Drubbel-FI had moderate predictive power for adverse outcomes (c-statistic was 0.702) (33). Further study found that the Drubbel-FI had moderate overlap with the Groningen Frailty Indicator (GFI) (Pearson correlation coefficient is 0.544) and may cover different aspects or stages of frailty (34). To date, the Drubbel-FI has only been used as a frailty screening tool in some studies in the Netherlands, with 36 or 50 deficits and different cut-off values in different studies (35–37). It may take time for this tool to be put into clinical practice in primary care.
The Care Assessment Need score (CAN) was initially developed by the Department of Veterans Affairs (VA) as a risk prediction tool for hospitalization and mortality in veteran populations in the US (38). The Veterans Health Administration, which is part of the VA, is a national integrated healthcare system that captures and stores claims and healthcare data in a centralized database (39). The CAN score can be automatically generated from EHRs in the VA primary care database (38). Due to the similarity in data elements and calculating methods between the CAN model and deficit accumulation model, Ruiz et al. validated the CAN score as a frailty identification tool in 2018 (38, 40). The CAN score was significantly increased from the robust to prefrail and frail groups and showed a moderate association with the FRAIL scale (Spearman correlation coefficient is 0.437) (38). Compared with the 40-item CGA-FI, a study including 184 patients over age 65 demonstrated that the CAN score had acceptable diagnostic accuracy (AUC 0.736) and had high sensitivity and negative predictive value at the 55th percentile of cut-off scores (40). In short, the CAN score may be a useful tool for frailty identification in primary care but is only available in the US VA system.
Another computer-generated VA instrument, the Veterans Affairs Frailty Index (VA-FI) was constructed with 31 deficits based on claims data (including diagnostic and procedure codes) from national VA administrative, Medicare, and Medicaid data in 2019 (41). A study including nearly 3 million veterans who regularly visited VA clinics revealed that the 2-year HRs of mortality for prefrail, mildly frail, moderately frail, and severely frail patients were 1.51, 2.36, 3.68, and 6.62, respectively (41). The original deficits of VA-FI were mainly identified from the international classification of diseases, the ninth revision (ICD-9 codes) in claims data, and the updated VA-FI with ICD-10 codes (VA-FI-10) maintained content validity, stability, and predictive validity for mortality (42). To be precise, the VI-FI is a claims-based frailty assessment tool rather than an EHR-based frailty assessment tool such as the CAN score. Therefore, it may be applied to different medical systems with claim data (including ICD diagnoses, etc.) and be further implemented in larger populations.
The adapted 54-item eFI was constructed by Pajewski et al. using data from the Medicare Accountable Care Organization (ACO) in the USA (ACO is an organization of clinically integrated health care providers who give coordinated high-quality care to their Medicare patients). It involved health deficits that were previously validated in the eFI (18) and FI-LAB (based on routine physical and laboratory tests) (43), such as diagnostic codes, laboratory measurements, and medications (except the functional deficits from healthcare center visit data) (44). In this study, which included 12,998 patients, the adapted eFI independently predicted mortality, hospitalisations, emergency department (ED) visits, and falls in primary care (44). It seems feasible to apply an EHR-based FI in the managed care population in the US. However, further extensive studies are needed to investigate the psychometric properties of the adapted eFI.
In theory, EHR-based frailty assessment tools can automatically generate frailty scores for any person with EHRs, enabling frailty screening at the population level. Both the eFI and VF-FI were implemented in large sample (200,000–3,000,000) population studies (18, 24, 41). Clegg et al. found estimates of prevalence for mild, moderate, and severe frailty of 35–37, 12–16, and 3–4%, respectively, in British older adults from the ResearchOne and THIN databases (18). Similar results were found in the Welsh population in the SAIL Database (24). Another British study, which included 2,177,656 participants, demonstrated that 10% of adults aged 50–64 and 43.7% of older adults were affected by frailty (eFI > 0.12) (45). For older veterans in the US, 28.3% were prefrail, 19.7% were mildly frail, 12.7% were moderately frail, and 14.3% were severely frail in 2012 (41). The abundance of cases in large population studies may offer immense research opportunities, such as assessing differences in the prevalence of frailty among different ethnic groups in multiethnic countries. A study including 13,510 older adults in London demonstrated that the overall prevalence of frailty (eFI sores > 0.24) was 18.1%, with the highest prevalence in the Bangladeshis population (32.9%) and the lowest in the black population (4.0%) (46). In contrast, those with a higher prevalence of frailty in the United States are African Americans and Hispanics in a sample of over 16,000 older US veterans (47).
Patients with regular follow-up in primary care have continuous EHRs that can repeatedly generate frailty scores at different time points and provide information on changes in frailty with aging (5). This may aid research that previously seem difficult, such as investigating changes in frailty prevalence and frailty incidence, frailty transition, and frailty trajectories. The prevalence of frailty in older US veterans has increased over time (41). The overall rate rose from 32 to 47% in 2002–2012, and the proportion of veterans diagnosed with severe frailty increased from 4.3 to 14.1% (41). Consistent with the high prevalence, a high incidence of frailty was also found in older veterans (47). Frailty transition periods reveal a shorter time between frailty categories (47). The Welsh study described a median transition time from fit to mild, mild to moderate, and moderate to severe frailty of 2,165, 1,155, and 898 days, respectively, implying that frailty may accelerate in later stages (24).
As frailty is a dynamic and reversible state, understanding frailty trajectories within individuals and across the population is necessary. A British case-control study with a sample of 25,000 patients (≥75) investigated frailty trajectories by eFI scores and their impact on mortality (within 12 months) (48). Three different frailty trajectories were identified, followed by stable, moderately increasing, and rapidly rising frailty trajectories (48). A rapid rising frailty trajectory (starting at 0.21 at baseline, with monthly eFI score increases of 0.022 until the curve flattens) was associated with a 180% increase in mortality (OR 2.84) (48). A similar study, which included 214,250 US veterans with annual VA-FI scores in the 5 years before death (79.4 mean age at death), identified nine frailty trajectories (including 2 stable, 3 gradually increasing, 3 rapidly increasing, and 1 recovering trajectory) (49). Monitoring frailty trends, transitions, and trajectories may help physicians identify suitable candidates for prevention, treatment, or palliative care at the appropriate time and further study the etiology of frailty, such as the association between some common frailty risk factors (such as area-level deprivation) (50) and frailty trajectories.
Although frailty and chronic diseases are two different concepts, they are closely related (51–54). According to the deficit accumulation model, chronic disease(s), multimorbidity, or comorbidity are important components of frailty (51, 55). Moreover, the eFI was recommended in the 2016 UK National Institute for Health and Care Excellence multimorbidity guidelines as one of the validated tools to identify adults with multimorbidity who are at risk of adverse events (56).
Consistent with previous studies on frailty and chronic disease(s), studies using EHR-based frailty assessment tools in primary care also observed a positive association between frailty and chronic disease. Among 3 million older US veterans, frailty evaluated by VI-FI was associated with an increased risk of cardiovascular disease (CVD) mortality at all levels of frailty and an increased risk of myocardial infarction and stroke (57). Similarly, frailty was found to be prevalent in patients with atrial fibrillation (AF) (58), hypertension (59, 60), and multiple myeloma (61, 62), and higher frailty levels were associated with increased mortality in those patients (58–62). In addition, frailty, rather than comorbidities, was one of the main predictors of all-cause admissions in patients with heart failure in England (63).
Previous studies have suggested that frailty needs to be considered in the treatment of chronic disease(s) based on individuals' frailty status (64). It is observed that frailty appears to influence whether older patients with chronic disease(s) choose to have surgeries. A recent British study, which included 28,025 individuals with hip osteoarthritis in primary care, showed that increased pre-existing multimorbidity (especially defined by the eFI) was associated with a decreased likelihood of undergoing total hip arthroplasty (65). However, several real-world studies using EHR-based frailty assessment tools showed that the impact of frailty was not considered in drug treatment for patients with chronic diseases, leading to inappropriate pharmacological treatment in the most vulnerable patients (58, 59, 66). In a sample of 244,328 community-dwelling Dutch people aged 70 and older, lipid-lowering drug prescriptions decreased with age but increased with higher frailty levels, which may imply potential overtreatment (66). Two British studies using the eFI may provide some insights into antihypertensive treatment among older adults with hypertension (59, 60). A study including over 140,000 hypertension patients aged 80 and older in England showed that the mortality rates were greatest at SBP <110 mm Hg (59). Another study with 415,980 patients aged above 75 found that the risk of all-cause mortality was lower when blood pressure was 150–159 mmHg than 130–139 mmHg in those severely frail individuals aged 75–84 years (60). These results suggest that hypertensive patients with automatically generated high frailty scores should be monitored closely as their antihypertensive therapy needs to be used with caution to avoid overtreatment. A more complicated situation is reflected in the management of stroke prevention in frail patients with AF who are taking oral anticoagulants (OACs). One study, which included a half-million older adults in England showed that among patients with AF and high stroke risk, OACs prescription was more common in those with frailty (58). Considering frail patients' higher risk of death, gastrointestinal bleeding, and falls, stratified stroke prevention based on frailty scores may benefit the most vulnerable patients with AF (58). On the other hand, frailty assessment may also be beneficial for patients who are at high risk for developing frailty and taking strong anticholinergic medications (67).
In summary, it is feasible to evaluate frailty in populations with chronic diseases by using EHR-based frailty assessment tools, as demonstrated in these primary care studies (See the brief list of the studies involved in Table 4). The research results further suggest the necessity of assessing frailty, and the automatically generated frailty scores can provide a reference for clinical decision-making [e.g., as a modifier of the risk-benefit ratio in pharmacological treatment (64)] and as a signal to refer a patient for a comprehensive geriatric assessment that will help develop an individualized treatment plan that will consider his or her chronic disease(s).
The novel frailty assessment tools may also help to identify patients with high medical needs and the generated frailty score could be an indicator of healthcare usage. A British cohort study, which enrolled 22,859 older adults with eFI scores, showed that an increased level of frailty is associated with increased acute hospital admission, more community referrals, and more requirements for care plans. Similarly, an analysis of linked routine primary care records from approximately 100,000 participants aged 65–95 showed that frailty was associated with increased hospital admissions, increased GP consultations, and longer inpatient stays (68). The total additional costs for older people with frailty were approximately £6 billion per year across the UK (68). The results imply that eFI scores could be an indicator of community service usage and might aid in the allocation of healthcare resources. In addition, EHR-based frailty assessment tools could also identify patients with other specific medical needs. A study involving 265,195 people over the age of 80 found that among women, the incidence of each fracture type was high and increased with the frailty category (69). Strategies for fracture prevention should target older women with frailty (69). Similarly, frailty (by the adapted eFI) was associated with greater post-acute care needs, higher 30-day readmission rates, and higher all-cause mortality within 6 months for non-urgent surgery (70). Furthermore, adapted eFI scores >0.32 may identify patients most likely to benefit from in-home pharmacist medication reviews, as shown in a small sample study (71). At last, the eFI has also been attempted to predict in-patient mortality after hospitalization or ICU admission for critically ill community-dwelling patients, but maybe less predictive value than the hospital frailty risk score (HRFS, constructed from hospital data, mainly ICD codes) (72, 73). Similarly, building a new frailty tool using hospital discharge diagnostic data (ICD-10 codes) may address quick frailty assessment in patients returning from hospital to the community (74). Further application research on such tools may promote the development of evidence-based healthcare services targeting frailty to prevent adverse events and reduce health costs.
Although automatically generated frailty scores are a time-efficient, low-cost process that takes frailty assessment closer to clinical practice, the existing deficiencies may present challenges to the application of EHR-based frailty assessment tools in different health systems around the world. First, variables or deficits of frailty are normally recorded as unstructured data that are difficult to extract, and some deficits are not fully available (29) in primary care EHRs. Bery et al. analyzed 135 frailty assessment tools (containing 593 frailty variables) published between 2011 and 2018 and pointed out that only 22 frailty tools may rely solely on EHRs and administrative claims data (75). Similarly, Sultana et al. found that clinical assessments of cognition (Mini-Mental State Examination score), mobility, and cachexia were not routinely recorded (<3% among 314,191 elderly persons) in the Dutch Integrated Primary Care Information (IPCI) database (36). Deficits such as functional limitations or mobility (33, 62, 76), social determinants of health, and health attitudes (33, 75) are not commonly recorded in primary care records, and symptoms and signs are not well-recorded (75), which may lead to a narrower FI score range. that underestimates frailty (33). In contrast, temporary diseases or conditions that have been cured may be recorded as deficits and may also lead to a higher estimate of frailty (27). Second, EHR-based frailty assessment tools also face the challenges of variation in EHR software and EHR data quality in different organizations (77) due to the lack of standards in how each EHR database records, processes, and stores data. Further training of eFI on health providers, validation of data quality, and software improvements are also needed to support better use of these tools and reduce the variability of data across facilities. Furthermore, tools like eFI are highly dependent on a well-established and homogeneous primary health service system. In other words, some countries may not be in the position to apply those tools, and only the eFI is currently in clinical use. However, the current diagnosis of frailty has been highly survey-based rather than using the eFI even in the UK. A study found most patients did not have eFI scores when coded as frail (only diagnosed by clinicians using tools such as the PRISMA-7 or the GFI) (77). Therefore, more research is needed to improve EHR-based frailty tools and EHR systems and provide corresponding support measures to promote the application of these tools.
Technological advances such as AI algorithms can make full use of a wide breadth of data derived from EHRs for the detection of diseases or conditions and may have potential value in screening frailty. Recent studies of patients in residential care facilities in Australia have shown that artificial intelligence technology may be a feasible approach for evaluating frailty (sensitivity 0.978, specificity 0.891, compared with the eFI) (78). AI is also credited with improving the predictive performance of a modified frailty index based on Hong Kong hospital data (79). However, in the primary care setting, Williamson et al. used data from the Canadian Primary Care Sentinel Surveillance Network (n = 875 adults aged over 65 years) to develop the EHR-based frailty definition by machine learning methods and showed that it had the poor predictive ability (sensitivity 28%, specificity 94%) compared with the CFS (80). However, another study based on the same database with a larger sample improved the predictive ability of this tool by using the XGBoost model (stands for eXtreme Gradient Boosting, is a gradient-boosted decision tree machine learning algorithm) and changing the decision threshold (sensitivity 78.14%, specificity 74.41%) (81). In addition, machine learning, such as the natural language processing algorithm, may help extract frailty variables from unstructured data in EHRs (82) and make full use of EHRs in primary care. Future research is required to better understand the use of AI techniques to support frailty identification within primary care.
As mentioned, several important deficits may not be available in EHRs in primary care, while some new variables may be complements and even indicators that could independently identify frailty. A British study including 154 patients over 65 showed that home visits, although not commonly coded in EHRs, could be a good frailty screening tool (sensitivity 87.23%, specificity 61.68%) compared with clinical diagnosis. Similarly, a sample of 159,325 patients from the British EHRs database showed that inflammatory markers [such as C reactive protein (CRP)] are strong predictors of all-cause mortality in primary care, with a comparable C-statistic (maximum value was 0.89 when containing age, sex, and CRP) to several previously developed frailty indices. In addition, EHR-based frailty case-finding may also rely on the innovation of software and the redesigned EHR system. A recent study, using reprogrammed primary care IT in the UK, created the Pathfields Tool that incorporated suspected frail patients (e.g., patients over age 90 with diagnoses of dementia and/or severe frailty by eFI or home visit) into the Pathfields High-Risk Cohort (83). Compared with an eFI score of more than 0.24, the Pathfields Tool identified more patients with previously undiagnosed frailty (confirmed by the CFS scale) (83). Another study used the openEHR framework to represent frailty in an aging population, which may help further the development of aging population-oriented systems (84).
As mentioned, in countries where EHR-based frailty assessment tools already exist in primary care, their widespread use in clinical practice can capture and monitor population-level frailty levels, distribution, and trajectories and better allocate limited public health resources to the most vulnerable older adults. This may help bridge the gap between population health and public health services for older adults (75). Population-based health management may also require a more proactive integrated medical service pattern, and a study in the Netherlands provides a good example. This Dutch cluster-randomized controlled trial included 7,638 participants from 39 general practices with a follow-up of 12 months. Both interventions, using an electronic frailty screening tool plus standard GP care or the tool plus a nurse-led care programme, showed high probabilities of being cost-effective compared with usual care (37). It is also worth noting that Kaiser Permanente (KP), like the previously mentioned ACO, is another example of integrated care. It has numerous primary care settings and hospitals and generates enormous EHRs stored in KP HealthConnect (85, 86). These integrated EHRs are of enormous value to the electronic frailty assessment research, such as a recent study comparing the electronic frailty tools for the prediction of adverse outcomes of abdominal surgery using EHRs from KP (including a comparison of HFRS and eFI) (87). In addition, the KP pyramid, a population-based chronic care model, may inspire the application of tools like eFI in chronic disease risk stratification management (86, 88).
In the meantime, the assessment of frailty is necessary for specific groups, such as patients with chronic disease, which is underrepresented in current guidelines (63, 75). Designing different frailty assessment tools for specific groups based on records appears equally feasible without additional resources and may more accurately assess their risk (75). Similarly, the impact of chronic diseases or conditions also needs to be considered in interventions for frailty. For example, a dietitian acting as a first contact practitioner for frail patients rather than a GP may be a cost-effective way to alter the nutritional status of frail patients (eFI 0.26–0.36) (89). Additionally, depression interacts with frailty (by the eFI) to further reduce the daily functioning of frail patients, suggesting that the clinical management of frailty should integrate physical and mental healthcare (90).
The development of EHR-based frailty assessment tools will also have additional implications for healthcare systems, especially in countries where primary care is underdeveloped. Although the advantages of these tools are obvious, in these countries, EHR-based frailty assessment studies may first be generated from secondary or tertiary hospital databases given the relatively poor quality of primary care EHRs. For example, the only two current studies of the eFI in China were all based on hospital data systems (91, 92). Additionally, they may be generated from EHRs for specific groups, such as VA-FI and CAN scores for veterans, from health check-up data, such as the Korean Frailty Index (THE frailty index) (93), or pure claim data, such as the Claims-based frailty index (94). However, due to the lack of continuity of care or care limited to a particular group, these EHR data may not enable the accurate and continuous assessment of frailty and/or reflect the frailty of the entire population. The urgency of the frailty screening of the population may prompt the development of primary care and the integration of primary care and hospital data or claim data (The KP HealthConnect might be a good example), which will ultimately bring healthcare reform.
The EHR-based frailty assessment tools do not require additional work or resources. In addition to accurately predicting adverse events, they can also achieve novel scientific and clinical uses, especially providing a reference for population health management and health resource allocation. However, only the eFI has been put into use in clinical practice in the UK thus far, and other tools need more validation. More importantly, challenges such as the poor quality of some EHRs and differences between EHR systems should be addressed urgently. Some new technologies, such as AI, and the development of new frailty indicators and frailty-related health systems may bring solutions and changes, while further research is needed.
Research on EHR-based frailty assessment tools in primary care is still in its infancy globally. Our assumptions and conjectures may not be fully objective because selection and evaluation biases are not known in this narrative review. However, given the advantages of primary care in the screening and management of frail patients, we consider more countries need to develop and validate frailty assessment tools based on their primary care EHRs to better address the public health challenges posed by an increasing aged population.
JL and XL conceived the review. JL drafted/wrote the manuscript. CZ, JS, QZ, and XL revised and edited all the version of the manuscript. YY and XF revised the sections. All authors contributed to manuscript revision and approved the submitted version.
This research was supported by National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z20191009), 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH005), Science and Technology Department of Sichuan Province (2020YFSY0293), Science and Technology Plan Project of Sichuan Province (No. 2021JDR0296), the Scientific Research Project of the Chengdu Health, Health Commission of Sichuan Province (ZH2020-104), and Family Planning Commission (No. 2021062).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank JS for his unique suggestions for revisions to this article, as well as the help of improving language of the manuscript.
1. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. (2019) 394:1376–86. doi: 10.1016/S0140-6736(19)31785-4
2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
3. Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. (2019) 23:771–87. doi: 10.1007/s12603-019-1273-z
4. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x
5. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
6. Ambagtsheer RC, Beilby JJ, Visvanathan R, Dent E, Yu S, Braunack-Mayer AJ. Should we screen for frailty in primary care settings? A fresh perspective on the frailty evidence base: a narrative review. Prev Med. (2019) 119:63–9. doi: 10.1016/j.ypmed.2018.12.020
7. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Series A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
8. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. (2001) 1:323–36. doi: 10.1100/tsw.2001.58
9. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Series A Biol Sci Med Sci. (2007) 62:722–7. doi: 10.1093/gerona/62.7.722
10. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. (2004) 52:1929–33. doi: 10.1111/j.1532-5415.2004.52521.x
11. Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. (2005) 17:465–71. doi: 10.1007/BF03327413
12. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) 14:392–7. doi: 10.1016/j.jamda.2013.03.022
13. Dent E, Lien C, Lim WS, Wong WC, Wong CH, Ng TP, et al. The Asia-pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. (2017) 18:564–75. doi: 10.1016/j.jamda.2017.04.018
14. Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. (2014) 43:744–7. doi: 10.1093/ageing/afu138
15. Abbasi M, Rolfson D, Khera AS, Dabravolskaj J, Dent E, Xia L. Identification and management of frailty in the primary care setting. Can Med Assoc J. (2018) 190:E1134–E40. doi: 10.1503/cmaj.171509
16. Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE, et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. (2019) 67:1559–64. doi: 10.1111/jgs.15928
17. Rockwood K. Screening for grades of frailty using electronic health records: where do we go from here? Age Ageing. (2016) 45:328–9. doi: 10.1093/ageing/afw057
18. Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. (2016) 45:353–60. doi: 10.1093/ageing/afw039
19. Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. (2016) Suppl 1(Suppl. 1):S48–61. doi: 10.15265/IYS-2016-s006
20. Zaslavsky O, Thompson H, Demiris G. The role of emerging information technologies in frailty assessment. Res Gerontol Nurs. (2012) 5:216–28. doi: 10.3928/19404921-20120410-02
21. Lim A, Choi J, Ji H, Lee H. Frailty assessment using routine clinical data: an integrative review. Arch Gerontol Geriatr. (2021) 99:104612. doi: 10.1016/j.archger.2021.104612
22. Davis KAS, Mueller C, Ashworth M, Broadbent M, Jewel A, Molokhia M, et al. What gets recorded, counts: dementia recording in primary care compared with a specialist database. Age Ageing. (2021) 50:2206–13. doi: 10.1093/ageing/afab164
23. Levit LA, Kaltenbaugh MW, Magnuson A, Hershman DL, Goncalves PH, Garrett-Mayer E, et al. Challenges and opportunities to developing a frailty index using electronic health record data. J Geriatr Oncol. (2021) 12:851–4. doi: 10.1016/j.jgo.2021.02.008
24. Hollinghurst J, Fry R, Akbari A, Clegg A, Lyons RA, Watkins A, et al. External validation of the electronic Frailty Index using the population of Wales within the Secure Anonymised Information Linkage Databank. Age Ageing. (2019) 48:922–6. doi: 10.1093/ageing/afz110
25. Lansbury LN, Roberts HC, Clift E, Herklots A, Robinson N, Sayer AA. Use of the electronic Frailty Index to identify vulnerable patients: a pilot study in primary care. Br J Gen Pract. (2017) 67:e751–6. doi: 10.3399/bjgp17X693089
26. Brundle C, Heaven A, Brown L, Teale E, Young J, West R, et al. Convergent validity of the electronic frailty index. Age Ageing. (2019) 48:152–6. doi: 10.1093/ageing/afy162
27. Broad A, Carter B, McKelvie S, Hewitt J. The convergent validity of the electronic Frailty Index (eFI) with the Clinical Frailty Scale (CFS). Geriatrics. (2020) 5:88. doi: 10.3390/geriatrics5040088
28. Abbasi M, Khera S, Dabravolskaj J, Vandermeer B, Theou O, Rolfson D, et al. A cross-sectional study examining convergent validity of a frailty index based on electronic medical records in a Canadian primary care program. BMC Geriatr. (2019) 19:109. doi: 10.1186/s12877-019-1119-x
29. Ambagtsheer RC, Beilby J, Dabravolskaj J, Abbasi M, Archibald MM, Dent E. Application of an electronic Frailty Index in Australian primary care: data quality and feasibility assessment. Aging Clin Exp Res. (2019) 31:653–60. doi: 10.1007/s40520-018-1023-9
30. England NHS. National Health Service England. Supporting-Routine-Frailty-Identification-and-Frailty-Through-the-Gp-Contract-20172018. (2022). Available online at: https://www.england.nhs.uk/publication/supporting-routine-frailty-identification-and-frailty-through-the-gp-contract-20172018 (accessed June 9, 2022).
31. England NHS. National Health Service England. Efi (2022). Available online at: https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/efi/ (accessed June 9, 2022).
32. England NHS. National Health Service England. frailty-risk-identification (2022). Available online at: https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/frailty-risk-identification (accessed June 9, 2022).
33. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. (2013) 68:301–8. doi: 10.1093/gerona/gls161
34. Drubbel I, Bleijenberg N, Kranenburg G, Eijkemans RJC, Schuurmans MJ, de Wit NJ, et al. Identifying frailty: do the Frailty Index and Groningen Frailty Indicator cover different clinical perspectives? A cross-sectional study. BMC Fam Pract. (2013) 14:64. doi: 10.1186/1471-2296-14-64
35. Ravensbergen WM, Blom JW, Evers AW, Numans ME, de Waal MW, Gussekloo J. Measuring daily functioning in older persons using a frailty index: a cohort study based on routine primary care data. Br J Gen Pract. (2020) 70:e866–73. doi: 10.3399/bjgp20X713453
36. Sultana J, Leal I, de Wilde M, de Ridder M, van der Lei J, Sturkenboom M, et al. Identifying data elements to measure frailty in a dutch nationwide electronic medical record database for use in postmarketing safety evaluation: an exploratory study. Drug Safety. (2019) 42:713–9. doi: 10.1007/s40264-018-00785-z
37. Bleijenberg N, Drubbel I, Neslo RE, Schuurmans MJ, ten Dam VH, Numans ME, et al. Cost-effectiveness of a proactive primary care program for frail older people: a cluster-randomized controlled trial. J Am Med Dir Assoc. (2017) 18:1029–36.e3. doi: 10.1016/j.jamda.2017.06.023
38. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. (2018) 30:1241–5. doi: 10.1007/s40520-018-0910-4
39. Price LE, Shea K, Gephart S. The veterans affairs's corporate data warehouse: uses and implications for nursing research and practice. Nurs Adm Q. (2015) 39:311–8. doi: 10.1097/NAQ.0000000000000118
40. Ruiz JG, Priyadarshni S, Rahaman Z, Cabrera K, Dang S, Valencia WM, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. (2018) 18:106. doi: 10.1186/s12877-018-0802-7
41. Orkaby AR, Nussbaum L, Ho YL, Gagnon D, Quach L, Ward R, et al. The burden of frailty among U.S. Veterans and Its Association With Mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. (2019) 74:1257–64. doi: 10.1093/gerona/gly232
42. Cheng D, DuMontier C, Yildirim C, Charest B, Hawley CE, Zhuo M, et al. Updating and validating the US Veterans Affairs Frailty Index: Transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. (2021) 76:1318–25. doi: 10.1093/gerona/glab071
43. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. (2014) 12:171. doi: 10.1186/s12916-014-0171-9
44. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a medicare accountable care organization. J Gerontol A Biol Sci Med Sci. (2019) 74:1771–7. doi: 10.1093/gerona/glz017
45. Fogg C, Fraser SDS, Roderick P, de Lusignan S, Clegg A, Brailsford S, et al. The dynamics of frailty development and progression in older adults in primary care in England (2006-2017): a retrospective cohort profile. BMC Geriatr. (2022) 22:30. doi: 10.1186/s12877-021-02684-y
46. Pradhananga S, Regmi K, Razzaq N, Ettefaghian A, Dey AB, Hewson D. Ethnic differences in the prevalence of frailty in the United Kingdom assessed using the electronic Frailty Index. Aging Med. (2019) 2:168–73. doi: 10.1002/agm2.12083
47. Ganta N, Sikandar S, Ruiz SJ, Nasr LA, Mohammed YN, Aparicio-Ugarriza R, et al. Incidence of frailty in community-dwelling United States older veterans. J Am Med Dir Assoc. (2021) 22:564–9. doi: 10.1016/j.jamda.2020.10.019
48. Stow D, Matthews FE, Hanratty B. Frailty trajectories to identify end of life: a longitudinal population-based study. BMC Med. (2018) 16:171. doi: 10.1186/s12916-018-1148-x
49. Ward RE, Orkaby AR, Dumontier C, Charest B, Hawley CE, Yaksic E, et al. Trajectories of frailty in the 5 years prior to death among U.S. Veterans Born 1927-1934. J Gerontol A Biol Sci Med Sci. (2021) 76:e347–53. doi: 10.1093/gerona/glab196
50. Stow D, Hanratty B, Matthews FE. The relationship between deprivation and frailty trajectories over 1 year and at the end of life: a case-control study. J Public Health (Oxf). (2021) fdab320. doi: 10.1093/pubmed/fdab320 [Epub ahead of print].
51. Zazzara MB, Vetrano DL, Carfi A, Onder G. Frailty and chronic disease. Panminerva Med. (2019) 61:486–92. doi: 10.23736/S0031-0808.19.03731-5
52. Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. (2011) 27:39–52. doi: 10.1016/j.cger.2010.08.003
53. Villacampa-Fernández P, Navarro-Pardo E, Tarín JJ, Cano A. Frailty and multimorbidity: two related yet different concepts. Maturitas. (2017) 95:31–5. doi: 10.1016/j.maturitas.2016.10.008
54. Mei F, Gao Q, Chen F, Zhao L, Shang Y, Hu K, et al. Frailty as a predictor of negative health outcomes in chronic kidney disease: a systematic review and meta-analysis. J Am Med Dir Assoc. (2021) 22:535–43.e7. doi: 10.1016/j.jamda.2020.09.033
55. Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev. (2019) 180:107–16. doi: 10.1016/j.mad.2019.04.005
56. NICE. National Institute for Health and Care Excellence. Recommendations (2022). Available online at: https://www.nice.org.uk/guidance/NG56/chapter/Recommendations (accessed June 9, 2022).
57. Shrauner W, Lord EM, Nguyen XT, Song RJ, Galloway A, Gagnon DR, et al. Frailty and cardiovascular mortality in more than 3 million US Veterans. Eur Heart J. (2021) 43:818–26. doi: 10.1093/eurheartj/ehab850
58. Wilkinson C, Clegg A, Todd O, Rockwood K, Yadegarfar ME, Gale CP, et al. Atrial fibrillation and oral anticoagulation in older people with frailty: a nationwide primary care electronic health records cohort study. Age Ageing. (2021) 50:772–9. doi: 10.1093/ageing/afaa265
59. Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, et al. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: cohort study using electronic health records. Circulation. (2017) 135:2357–68. doi: 10.1161/CIRCULATIONAHA.116.026687
60. Masoli JAH, Delgado J, Pilling L, Strain D, Melzer D. Blood pressure in frail older adults: associations with cardiovascular outcomes and all-cause mortality. Age Ageing. (2020) 49:807–13. doi: 10.1093/ageing/afaa028
61. DuMontier C, Fillmore NR, Yildirim C, Cheng D, La J, Orkaby AR, et al. Contemporary analysis of electronic frailty measurement in older adults with multiple myeloma treated in the national us veterans affairs healthcare system. Cancers. (2021) 13:3053. doi: 10.3390/cancers13123053
62. Patel BG, Luo S, Wildes TM, Sanfilippo KM. Frailty in older adults with multiple myeloma: A study of US veterans. JCO Clin Cancer Inform. (2020) 4:117–27. doi: 10.1200/CCI.19.00094
63. Bottle A, Kim D, Hayhoe B, Majeed A, Aylin P, Clegg A, et al. Frailty and co-morbidity predict first hospitalisation after heart failure diagnosis in primary care: population-based observational study in England. Age Ageing. (2019) 48:347–54. doi: 10.1093/ageing/afy194
64. Onder G, Vetrano DL, Marengoni A, Bell JS, Johnell K, Palmer K. Accounting for frailty when treating chronic diseases. Eur J Intern Med. (2018) 56:49–52. doi: 10.1016/j.ejim.2018.02.021
65. Ferguson R, Prieto-Alhambra D, Peat G, Delmestri A, Jordan KP, Strauss VY, et al. Influence of pre-existing multimorbidity on receiving a hip arthroplasty: cohort study of 28 025 elderly subjects from UK primary care. BMJ Open. (2021) 11:e046713. doi: 10.1136/bmjopen-2020-046713
66. Kleipool EEF, Nielen MMJ, Korevaar JC, Harskamp RE, Smulders YM, Serne E, et al. Prescription patterns of lipid lowering agents among older patients in general practice: an analysis from a national database in the Netherlands. Age Ageing. (2019) 48:577–582. doi: 10.1093/ageing/afz034
67. Ruiz SJ, Cevallos V, Baskaran D, Mintzer MJ, Ruiz JG. The cross-sectional association of frailty with past and current exposure to strong anticholinergic drugs. Aging Clin Exp Res. (2021) 33:2283–9. doi: 10.1007/s40520-020-01742-6
68. Han L, Clegg A, Doran T, Fraser L. The impact of frailty on healthcare resource use: a longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing. (2019) 48:665–71. doi: 10.1093/ageing/afz088
69. Ravindrarajah R, Hazra NC, Charlton J, Jackson SHD, Dregan A, Gulliford MC. Incidence and mortality of fractures by frailty level over 80 years of age: cohort study using UK electronic health records. BMJ Open. (2018) 8:e018836. doi: 10.1136/bmjopen-2017-018836
70. Callahan KE, Clark CJ, Edwards AF, Harwood TN, Williamson JD, Moses AW, et al. Automated frailty screening at-scale for pre-operative risk stratification using the electronic frailty index. J Am Geriatr Soc. (2021) 69:1357–62. doi: 10.1111/jgs.17027
71. Stewart AE, Lovato JF, Zimmer R, Stewart AP, Hinely MT, Yang M. Development of a screening tool to identify patients likely to benefit from clinical pharmacist review in a home-based primary care population. J Am Pharm Assoc. (2020) 60:750–6. doi: 10.1016/j.japh.2020.03.008
72. Szakmany T, Hollinghurst J, Pugh R, Akbari A, Griffiths R, Bailey R, et al. Frailty assessed by administrative tools and mortality in patients with pneumonia admitted to the hospital and ICU in Wales. Sci Rep. (2021) 11:13407. doi: 10.1038/s41598-021-92874-w
73. Boreskie KF, Hay JL, Boreskie PE, Arora RC, Duhamel TA. Frailty-aware care: giving value to frailty assessment across different healthcare settings. BMC Geriatr. (2022) 22:13. doi: 10.1186/s12877-021-02722-9
74. Pogam ML, Seematter-Bagnoud L, Niemi T, Assouline D, Gross N, Trächsel B, et al. Development and validation of a knowledge-based score to predict Fried's frailty phenotype across multiple settings using one-year hospital discharge data: The electronic frailty score. EClinicalMedicine. (2022) 44:101260. doi: 10.1016/j.eclinm.2021.101260
75. Bery AK, Anzaldi LJ, Boyd CM, Leff B, Kharrazi H. Potential value of electronic health records in capturing data on geriatric frailty for population health. Arch Gerontol Geriatr. (2020) 91:104224. doi: 10.1016/j.archger.2020.104224
76. Wong ST, Katz A, Williamson T, Singer A, Peterson S, Taylor C, et al. Can linked electronic medical record and administrative data help us identify those living with frailty? Int J Popul Data Sci. (2020) 5:1343. doi: 10.23889/ijpds.v5i1.1343
77. Millares-Martin P. Large retrospective analysis on frailty assessment in primary care: electronic Frailty Index versus frailty coding. BMJ Health Care Inform. (2019) 26:4. doi: 10.1136/bmjhci-2019-000024
78. Ambagtsheer RC, Shafiabady N, Dent E, Seiboth C, Beilby J. The application of artificial intelligence (AI) techniques to identify frailty within a residential aged care administrative data set. Int J Med Inform. (2020) 136:104094. doi: 10.1016/j.ijmedinf.2020.104094
79. Ju C, Zhou J, Lee S, Tan MS, Liu T, Bazoukis G, et al. Derivation of an electronic frailty index for predicting short-term mortality in heart failure: a machine learning approach. ESC Heart Fail. (2021) 8:2837–45. doi: 10.1002/ehf2.13358
80. Tyler Williamson P, Aponte-Hao S, Mele B, Lethebe BC, Leduc C, Thandi M, et al. Developing and validating a primary care emr-based frailty definition using machine learning. Int J Popul Data Sci. (2020) 5:1344. doi: 10.23889/ijpds.v5i1.1344
81. Aponte-Hao S, Wong ST, Thandi M, Ronksley P, McBrien K, Lee J, et al. Machine learning for identification of frailty in Canadian primary care practices. Int J Popul Data Sci. (2021) 6:1650. doi: 10.23889/ijpds.v6i1.1650
82. Kharrazi H, Anzaldi LJ, Hernandez L, Davison A, Boyd CM, Leff B, et al. The value of unstructured electronic health record data in geriatric syndrome case identification. J Am Geriatr Soc. (2018) 66:1499–507. doi: 10.1111/jgs.15411
83. Attwood D, Boorer J, Ellis W, Earley M, Denovan J, Calkoen A, et al. The Pathfields tool: a frailty case-finding tool using primary care IT-implications for population health management. Age Ageing. (2020) 49:1087–92. doi: 10.1093/ageing/afaa119
84. Kalogiannis S, Deltouzos K, Zacharaki EI, Vasilakis A, Moustakas K, Ellul J, et al. Integrating an openEHR-based personalized virtual model for the ageing population within HBase. BMC Med Inform Decis Mak. (2019) 19:25. doi: 10.1186/s12911-019-0745-8
85. Selevan J, Kindermann D, Pines JM, Fields WW. What accountable care organizations can learn from kaiser permanente California's acute care strategy. Popul Health Manag. (2015) 18:233–6. doi: 10.1089/pop.2014.0157
86. Franchini M, Pieroni S, Cutilli A, Caiolfa M, Naldoni S, Molinaro S. The individual profile of pathology as a new model for filling knowledge gaps in health policies for chronicity. Front Med. (2019) 6:130. doi: 10.3389/fmed.2019.00130
87. Le ST, Liu VX, Kipnis P, Zhang J, Peng PD, Cespedes Feliciano EM. Comparison of electronic frailty metrics for prediction of adverse outcomes of abdominal surgery. JAMA Surg. (2022) 157:e220172. doi: 10.1001/jamasurg.2022.0172
88. Barcelo A, Luciani S, Agurto l, Ordunez P, Tasca R, Sued O. Improving Chronic Illness Care through Integrated Health Service Delivery Networks. (2012). Available online at: https://iris.paho.org/handle/10665.2/3187 (accessed June 9, 2022).
89. Hickson M, Child J, Collinson A. Impact of a dietitian in general practice: care of the frail and malnourished. J Human Nutr Dietetics. (2021) 35:145–53. doi: 10.1111/jhn.12942
90. Coventry PA, McMillan D, Clegg A, Brown L, van der Feltz-Cornelis C, Gilbody S, et al. Frailty and depression predict instrumental activities of daily living in older adults: a population-based longitudinal study using the CARE75+cohort. PLoS One. (2020) 15:e0243972. doi: 10.1371/journal.pone.0243972
91. Liang Y-D, Xie Y-B, Du M-H, Shi J, Yang J-F, Wang H. Development and validation of an electronic frailty index using routine electronic health records: an observational study from a general hospital in China. Front Med. (2021) 8:731445. doi: 10.3389/fmed.2021.731445
92. Shen Y, Wang Y, Shi Q, Hou L, Chen X, Dong B, et al. The electronic frailty index is associated with increased infection and all-cause mortality among older patients with primary lung cancer: a cohort study. Clin Interv Aging. (2021) 16:1825–33. doi: 10.2147/CIA.S335172
93. Kim YP, Choe YR, Park JH, Kim S, Won CW, Hwang HS. Frailty index associated with all-cause mortality, long-term institutionalization, and hip fracture. Eur Geriatr Med. (2019) 10:403–11. doi: 10.1007/s41999-019-00196-y
Keywords: frailty, assessment, EHRs, primary care, electronic frailty index
Citation: Luo J, Liao X, Zou C, Zhao Q, Yao Y, Fang X and Spicer J (2022) Identifying Frail Patients by Using Electronic Health Records in Primary Care: Current Status and Future Directions. Front. Public Health 10:901068. doi: 10.3389/fpubh.2022.901068
Received: 21 March 2022; Accepted: 31 May 2022;
Published: 22 June 2022.
Edited by:
Konstantin G. Arbeev, Duke University, United StatesCopyright © 2022 Luo, Liao, Zou, Zhao, Yao, Fang and Spicer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyang Liao, bGlhb3hpYW95YW5nQHdjaHNjdS5jbg==; orcid.org/0000000344099674; Qian Zhao, emhhb3FpYW5Ad2Noc2N1LmNu; orcid.org/0000000295405726
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.