
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 31 May 2022
Sec. Public Health Education and Promotion
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.898242
This article is part of the Research TopicDigital Information for Patient EducationView all 10 articles
Aims: To establish a predictive nomogram for clinical prognosis in cervical spondylotic myelopathy (CSM) with intramedullary T2-weighted increased signal intensity (ISI).
Methods: The clinical data of 680 patients with CSM with intramedullary T2-weighted ISI were retrospectively analyzed. The patients were divided into the modeling group (476) and the validation group (204) by using a random number table at a ratio of 7:3. The independent prognostic factors were screened using multivariate logistic regression analysis. The factors were subsequently incorporated into the establishment of the predictive nomogram. The area under the receiver operating characteristic (ROC) curve (AUC) was undertaken to estimate the discrimination of the predictive nomogram. The calibration curve and the Hosmer-Lemeshow test were used to assess the calibration of the predictive nomogram. The clinical usefulness of the predictive nomogram was evaluated by decision curve analysis (DCA).
Results: Based on the pre-operative Japanese Orthopedic Association (JOA) score, maximal canal compromise (MCC), and maximal spinal cord compression (MSCC), we established a predictive nomogram. The AUCs in the modeling group and validation group were 0.892 (95% CI: 0.861~0.924) and 0.885 (95% CI: 0.835~0.936), respectively, suggesting good discrimination of the nomogram. Calibration curves showed a favorable consistency between the predicted probability and the actual probability. In addition, the values of P of the Hosmer-Lemeshow were 0.253 and 0.184, respectively, suggesting good calibration of the nomogram. DCA demonstrated that the nomogram had good clinical usefulness.
Conclusion: We established and validated a predictive nomogram for the clinical prognosis in CSM with intramedullary T2-weighted ISI. This predictive nomogram could help clinicians and patients identify high-risk patients and educate them about prognosis, thereby improving the prognosis of high-risk patients.
Cervical spondylotic myelopathy (CSM) is a progressive degenerative disease defined by spondyloarthritis, congenital cervical canal stenosis, and ossification of the posterior longitudinal ligament (1–3). In the early stages of the disease, patients with CSM present mild symptoms that are easily ignored. That often causes a delayed diagnosis and irreversible neurologic damage. As the disease progresses, patients with CSM often exhibit a wide variety of symptoms and signs, such as numb hands, bilateral arm paresthesia, gait abnormality, and positive Hoffmann signs (4, 5). At present, the diagnosis of CSM is mainly based on clinical symptoms, signs, and clinical imaging. Magnetic resonance imaging (MRI) is an invaluable and irreplaceable modality for radiographic neurological assessment by visualizing the degree of spinal cord compression and the signal changes of the spinal cord (6, 7).
The presence of intramedullary increased signal intensity (ISI) was found in patients with cervical spondylotic myelopathy on T2-weighted images, suggesting spinal cord injury (8). ISI on T2-weighted MRI reflects chronic intramedullary compression lesions, such as neuronal cell death, demyelination, and reactive astrogliosis (9–11). MRI findings include intramedullary abnormal ISI on T2-weighted images indicating poor prognosis in CSM (12, 13).
At present, no consensus is reached on the independent prognostic factors of patients with CSM with intramedullary T2-weighted ISI, and it is still controversial. As a statistical predictive model, a nomogram estimates individualized risk based on independent prognostic factors. The nomogram could help clinicians and patients predict disease prognosis and timely identify the high-risk patients. Hence, we established and validated a predictive nomogram for the clinical prognosis in CSM with intramedullary T2-weighted ISI. We hope that this predictive nomogram could help clinicians and patients identify high-risk patients and educate them about prognosis, thereby improving the prognosis of high-risk patients.
We retrospectively collected and analyzed the data of patients with CSM with intramedullary T2-weighted ISI admitted to the Second Affiliated Hospital of Xi'an Jiaotong University between January 2012 and June 2021. All patients underwent a detailed examination on admission, while their imaging included cervical spine plain X-rays and MRI. Patients were selected according to the following inclusion criteria: (1) aged 18 years and above, (2) patients with CSM, (3) cervical MRI indicated spinal cord compression, and (4) cervical MRI showed intramedullary T2-weighted ISI. Patients were excluded based on the following criteria: (1) cervical spondylotic radiculopathy, (2) acute cervical spinal cord injury, (3) amyotrophic lateral sclerosis or syringomyelia, and (4) cardiopulmonary or coagulation dysfunction. Finally, 680 patients were included in the final analysis. The patients who met the inclusion criteria were divided into the modeling group (476) and the validation group (204) by using a random number table at a ratio of 7:3. A flow diagram is shown in Figure 1. The modeling group was further divided into two groups based on whether the 6-month post-operative improvement rate for the Japanese Orthopedic Association (JOA) score was ≥60%, namely, the poor prognosis group (115) and the good prognosis group (361). This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University, China (approval No. 2021226). Verbal informed consent was obtained from each participant. Since the data were anonymous and the study was retrospective, no written informed consent was obtained.
The pre-operative and post-operative neurological functions were assessed by using the JOA score. Prognosis status was evaluated by a 6-month post-operative improvement rate for the JOA score. The calculation formula of the JOA improvement rate is as follows: the JOA improvement rate = (post-operative JOA score—pre-operative JOA score)/(17—pre-operative JOA score) × 100% (14). A JOA score improvement rate of more than 75% is excellent, 50–74% is good, 25–49% is acceptable, and <25% is poor (15).
All patients underwent MRI on admission using either a 3.0-T MRI. Maximal canal compromise (MCC) was applied to assess the maximal canal stenosis, while maximal spinal cord compression (MSCC) was used to measure the maximal degree of spinal cord compression (16). The MCC was defined as the sagittal anteroposterior spinal canal diameter at the maximal spinal canal stenosis (Di) divided by the averaged sagittal anteroposterior spinal canal diameter between the non-pathological spinal canal above (Da) and non-pathological spinal canal below (Db) (Figure 2A). MCC was calculated as follows: MCC (%) = [1 – Di/(Da + Db)/2] × 100%. MSCC was defined as the sagittal anteroposterior spinal cord diameter at the maximal spinal cord compression (di) divided by the averaged sagittal anteroposterior spinal cord diameter between the non-pathological spinal cord above (da) and non-pathological spinal cord below (db) (Figure 2B). MSCC was calculated as follows: MSCC (%) = [1 – di/(da + db)/2] × 100%. During the radiographic assessment, radiographic measurements were obtained by two experienced researchers measuring MRI images of each patient, respectively. Student's t-test analysis was performed on the two groups of measurements. If there was no statistical significance, the mean values of the two groups of measurements were taken as the final radiographic measurements. Otherwise, the above assessment was repeated.
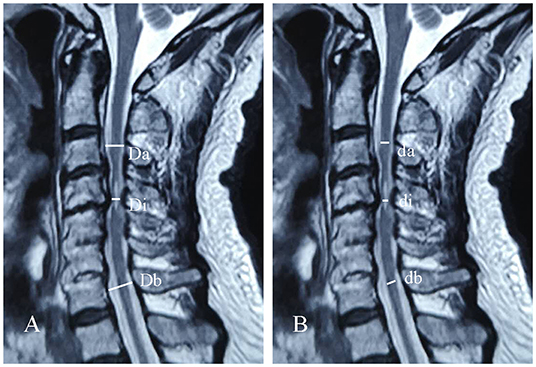
Figure 2. Schematic of maximal canal compromise (MCC) (A) and maximal spinal cord compression (MSCC) (B) in sagittal cervical MRI.
All patients underwent anterior or posterior cervical decompression surgery. Of these, 139 patients underwent anterior cervical corpectomy and fusion, and 541 patients underwent posterior cervical expansive open-door laminoplasty. All surgical procedures were performed by the corresponding author. The external head and neck stabilization was continued for 3 months after surgery. Patients underwent clinical review and evaluation of a neurological function at 6 months post-operatively.
The following patients' data were collected and analyzed: gender, age, body mass index (BMI), disease duration, hypertension, diabetes, pre-operative JOA score, MCC, MSCC, number of spinal cord compression segments, operative time, intraoperative blood loss, 6-month post-operative JOA score, and 6-month post-operative improvement rate for the JOA score.
Statistical analyses were performed using IBM SPSS Statistics software version 26.0 (SPSS Inc., Chicago, IL, United States) and R software version 4.1.1. The measurement data were expressed as mean ± standard deviation (SD), and the enumeration data were expressed in percentages. Continuous variables were analyzed using Student's t-test or the Mann-Whitney U-test, and categorical variables were analyzed using the χ2 test or Fisher's exact test. The independent prognostic factors of patients with CSM with intramedullary T2-weighted ISI were screened using univariate and multivariate logistic regression analysis in the modeling group. The independent prognostic factors were subsequently incorporated into the establishment of the predictive nomogram. The predictive nomogram was validated internally in the modeling group and externally in the validation group. The area under the receiver operating characteristic (ROC) curve (AUC) was undertaken to estimate the discrimination of the predictive nomogram. The calibration curve and the Hosmer-Lemeshow test were used to assess the calibration of the predictive nomogram. The clinical usefulness of the predictive nomogram was evaluated by decision curve analysis (DCA). The value of P < 0.05 denoted a statistically significant difference.
A total of 680 individuals, including 476 patients in the modeling group and 204 patients in the validation group, were included in the present study (Table 1). There were 361 patients whose 6-month post-operative improvement rate for the JOA score was ≥60% in the modeling group. The treatment success rate for patients with CSM with intramedullary T2-weighted ISI was 75.8%. No statistically significant differences in gender, age, BMI, and other clinical data were found between the modeling group and validation group (P > 0.05), which was comparable.
The modeling group was further divided into two groups based on whether the 6-month post-operative improvement rate for the JOA score was ≥60%, namely, the poor prognosis group (115) and the good prognosis group (361). Disease duration, pre-operative JOA score, MCC, and MSCC were statistically significant risk factors after univariate logistic regression analysis in the modeling group (Table 2). A multivariate logistic regression analysis was performed for statistically significant risk factors. Multivariate logistic regression analysis revealed that pre-operative JOA score [odds ratio (OR) = 1.601, P < 0.05], MCC (OR = 1.285, P < 0.05), and MSCC (OR = 1.611, P < 0.05) were independent prognostic factors (Table 3). The logistic regression model is Logit (P) = −33.322 + 0.470 Pre-operative JOA score + 0.251 MCC + 0.477 MSCC.
The independent prognostic factors were subsequently incorporated into the development of the predictive nomogram of patients with CSM with intramedullary T2-weighted ISI (Figure 3). In the predictive nomogram, the points corresponding to each independent prognostic factor were obtained, then the sum of the points was calculated as the total score, and the predicted risk corresponding to the total score was the probability of poor prognosis of patients with CSM with intramedullary T2-weighted ISI.
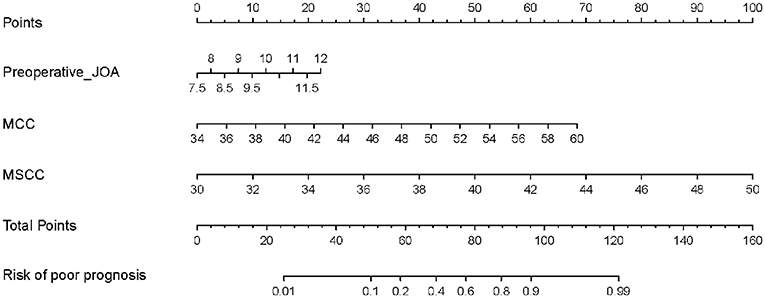
Figure 3. Predictive nomogram for clinical prognosis in cervical spondylotic myelopathy (CSM) with intramedullary T2-weighted increased signal intensity (ISI).
The AUC of the modeling group was 0.892 (95% CI: 0.861~0.924), P < 0.001 (Figure 4A). The cutoff value of the modeling group was 0.775, P < 0.001. The AUC of validation group was 0.885 (95% CI: 0.835~0.936), P < 0.001 (Figure 4B). The results of the AUC indicated that the discrimination of the predictive nomogram was good.
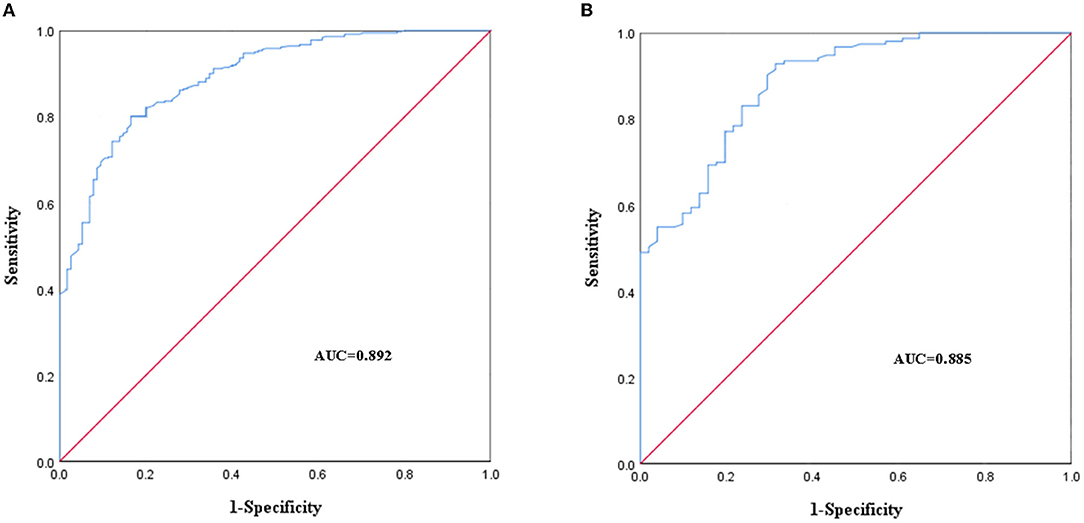
Figure 4. Receiver operating characteristic (ROC) curves of the predictive nomogram in the modeling group (A) and the validation group (B).
The calibration curves of the predictive nomogram showed a favorable consistency between the predicted probability and the actual probability in the modeling group (Figure 5A) and the validation group (Figure 5B). In addition, the results of the Hosmer-Lemeshow in the modeling group and the validation group were χ2 = 10.180 (P = 0.253) and χ2 = 11.319 (P = 0.184), respectively, suggesting the good calibration of the predictive nomogram.
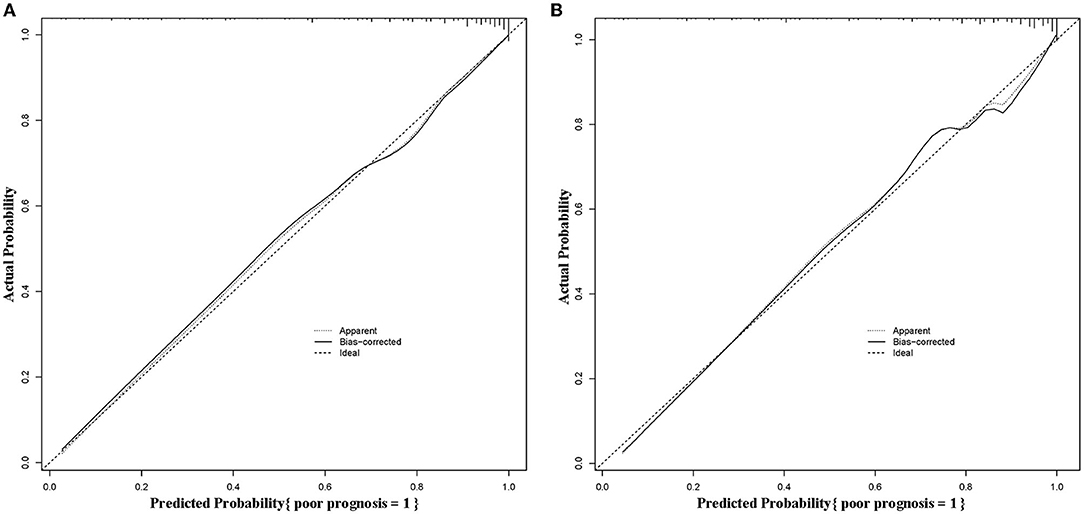
Figure 5. Calibration curves of the predictive nomogram in the modeling group (A) and the validation group (B).
A DCA of the predictive nomogram in the modeling group and the validation group is shown in Figure 6. DCA demonstrated that the predictive nomogram in the modeling group (Figure 6A) and the validation group (Figure 6B) presented similar net benefits at the range of threshold probability, with better net benefits than the two extreme lines when the threshold probability was 0.1~1.0. The black horizontal extreme line represented that in patients who received no intervention, the net benefit was 0. The black oblique extreme line represented that in patients who received the intervention, the net benefit was a backslash with a negative slope. The predictive nomogram had good clinical usefulness when it was in the above threshold probability. The cutoff value (0.775) obtained from the ROC curve of the modeling group was within the threshold probability range of the above two DCA curves, also indicating that the predictive nomogram has good clinical usefulness. Further analysis of the DCA curves of the predictive nomogram showed that the net clinical benefit of the modeling group and the validation group was 61 and 56%, respectively, when 0.775 was set as the threshold probability value for diagnosing poor prognosis and taking intervention. In other words, 61 and 56 of every 100 patients with CSM with intramedullary T2-weighted ISI who were diagnosed with poor prognosis using the predictive nomogram in the modeling group and the validation group would respectively have clinical benefits.

Figure 6. Decision curve analysis (DCA) of the predictive nomogram in the modeling group (A) and the validation group (B).
Take a patient with CSM with intramedullary T2-weighted ISI as an example. The prognostic factors for this patient were as follows: pre-operative JOA score = 10, MCC = 46, and MSCC = 39. The risk of poor prognosis for the patient was 0.823 (>0.775) according to the predictive nomogram (Figure 7). According to the DCA curve, effective interventions should be taken to reduce the risk of poor prognosis.
Cervical spondylotic myelopathy is a progressive, degenerative spine disease, and the most common cause of spinal cord dysfunction in adults worldwide (17). An MRI revealed cervical spinal stenosis and signal change of the cervical spinal cord. T2-weighted MRI showed corresponding intramedullary ISI suggesting cervical spinal cord injury (18, 19). From a histopathological view, the intramedullary ISI reflected the severity and extent of spinal cord lesions (20, 21). Orthopedic surgeons focused on the clinical prognostic factors of the patients with CSM with intramedullary T2-weighted ISI. The clinical prognosis of such patients was affected by various factors and was difficult to predict. In this study, we established and validated a predictive nomogram for the clinical prognosis in CSM with intramedullary T2-weighted ISI. Univariate and multivariate logistic regression analysis revealed that pre-operative JOA score, MCC, and MSCC were independent prognostic factors.
Patients with CSM who had intramedullary T2-weighted ISI presented with typical clinical symptoms, such as loss of hand dexterity, unstable gait, and sensory deficit in limbs (22, 23). A lot of quantitative measures that assess the severity of CSM have been developed to assess neurological function. Among them, the JOA score has been greatly popularized in spinal surgery (24). Zhang et al. (25) found that patients with CSM with intramedullary T2-weighted ISI usually had lower pre-operative JOA scores and less improved neurological function after surgery. The results of the present study revealed that the pre-operative JOA score was the independent prognostic factor of patients with CSM with intramedullary T2-weighted ISI. The pre-operative JOA score was incorporated into the development of the predictive nomogram of patients with CSM with intramedullary T2-weighted ISI. The predictive nomogram demonstrated a good degree of discrimination, calibration, and clinical usefulness. Patients with CSM with intramedullary T2-weighted ISI had lower pre-operative JOA scores and a higher probability of poor clinical prognosis after assessment by the predictive nomogram. The reasons causing the above phenomena might be that patients with lower pre-operative JOA scores usually had a poor pre-operative neurological function and delayed or even lost recovery of neurological function during the process of post-operative recovery. Therefore, it is necessary to predict the clinical prognosis of patients with CSM with intramedullary T2-weighted ISI using the pre-operative JOA score.
Maximal canal compromise is often used to evaluate the severity of cervical spinal stenosis in CSM. The measurement of MCC was that the anteroposterior canal diameter on midsagittal and axial T2-weighted images at the maximum compromise level was compared with the anteroposterior canal diameter at normal levels immediately above and below the level of injury (26, 27). Aarabi et al. (28) identified that there was a correlation between MCC and American Spinal Injury Association (ASIA) motor score recovery with a 1-year follow-up. In addition, they also found an inverse relationship between the degree of canal compromise and follow-up ASIA motor score. The results of the present study revealed that the MCC was the independent prognostic factor of patients with CSM with intramedullary T2-weighted ISI. The MCC was incorporated into the development of the predictive nomogram of patients with CSM with intramedullary T2-weighted ISI. After the validation of the predictive nomogram, it was found that patients with severe MCC had a greater risk of poor clinical prognosis. The reason for the above phenomena can be that the space between the spinal cord and spinal canal is severely limited when there issevere MCC. As a result, the spinal cord was more susceptible to ischemic injury resulting from compression. Furthermore, due to the persistent limitation of the spinal cord in severe MCC, the MCC was prone to progressive injury. Therefore, the post-operative recovery of neurological function in such patients was often unsatisfactory. Orthopedic surgeons should pay close attention to the severity of MCC in patients with CSM with intramedullary T2-weighted ISI in clinical diagnosis and treatment.
Maximal spinal cord compression is often used to evaluate the severity of cervical spinal cord compression in CSM. Tarawneh et al. (29) found that MSCC offered a certain prognostic value for the recovery of neurological function. Miyanji et al. (30) found that the extent of MSCC was significantly different between patients with complete and incomplete spinal cord injuries, with more substantial MSCC seen in the patients with complete spinal cord injury. The results of the present study revealed that the MSCC was the independent prognostic factor of patients with CSM with intramedullary T2-weighted ISI. The MSCC was incorporated into the development of the predictive nomogram of patients with CSM with intramedullary T2-weighted ISI. After the validation of the predictive nomogram, it was found that patients with more severe MSCC were more likely to have a poor clinical prognosis. The abovementioned phenomenon might be caused by the compression and the secondary ischemia of the spinal cord. That exacerbated the severity of spinal cord injury. Hence, the post-operative recovery of neurological function in such patients was often unsatisfactory. In clinical treatment, orthopedic surgeons should relieve or even remove spinal cord compression in a timely manner to improve the clinical prognosis of spinal cord injury.
There are some limitations to this study. First of all, this is a retrospective study. Further prospective studies are needed at a later stage. Second, this study was mainly carried out in one hospital at the present stage. A multi-center study has not been carried out. In future studies, the research team will conduct a prospective multi-center study. That will be the further improvement and verification of this study.
In conclusion, we established and validated a predictive nomogram for the clinical prognosis in CSM with intramedullary T2-weighted ISI. This predictive nomogram could help clinicians and patients identify high-risk patients and educate them about prognosis, thereby improving the prognosis of high-risk patients. Patients can predict their own prognosis based on their own clinical data related to the independent prognostic factors. Then, patients can consult with their doctors as early as possible and participate in the elaboration of therapeutic protocols.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University, China. The patients/participants provided their written informed consent to participate in this study.
JW and HPL contributed to study conception and design. JW and BHY contributed to data collection, data analysis, and manuscript drafting. All authors were involved in the revision of the manuscript and approved the final version of the article.
This study was supported by the Fundamental Research Funds for the Central Universities, No. ZRZD2017008 (to HPL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McCormick JR, Sama AJ, Schiller NC, Butler AJ, Donnally CJ 3rd. Cervical spondylotic myelopathy: a guide to diagnosis and management. J Am Board Fam Med. (2020) 33:303–13. doi: 10.3122/jabfm.2020.02.190195
2. Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. (2017) 7:572–86. doi: 10.1177/2192568217699208
3. Paracino R, Fasinella MR, Mancini F, Marini A, Dobran M. Review of laminoplasty vs. laminectomy in the surgical management of cervical spondylotic myelopathy. Surg Neurol Int. (2021) 12:44. doi: 10.25259/SNI_788_2020
4. Haddas R, Cox J, Belanger T, Ju KL, Derman PB. Characterizing gait abnormalities in patients with cervical spondylotic myelopathy: a neuromuscular analysis. Spine J. (2019) 19:1803–8. doi: 10.1016/j.spinee.2019.06.005
5. Inoue T, Soshi S, Kubota M, Marumo K. Efficacy of laminoplasty in improving sensory disturbances in patients with cervical spondylotic myelopathy: a prospective study. World Neurosurg. (2020) 134:e581–8. doi: 10.1016/j.wneu.2019.10.141
6. Witiw CD, Mathieu F, Nouri A, Fehlings MG. Clinico-radiographic discordance: an evidence-based commentary on the management of degenerative cervical spinal cord compression in the absence of symptoms or with only mild symptoms of myelopathy. Global Spine J. (2018) 8:527–34. doi: 10.1177/2192568217745519
7. Kim YH, Ha KY, Park HY, Cho CH, Kim HC, Heo Y, et al. Simple and reliable magnetic resonance imaging parameter to predict post-operative ambulatory function in patients with metastatic epidural spinal cord compression. Global Spine J. (2021) 10:21925682211000322. doi: 10.1177/21925682211000322
8. Nam TH, Lee JW, Yeom JS, Lee E, Kang Y, Ahn JM, et al. Increased signal intensity on post-operative T2-weighted axial images in cervical spondylotic myelopathy: patterns of changes and associated impact on outcomes. J Clin Neurosci. (2021) 90:244–50. doi: 10.1016/j.jocn.2021.06.007
9. Seki S, Kawaguchi Y, Nakano M, Yasuda T, Hori T, Noguchi K, et al. Clinical significance of high intramedullary signal on T2-weighted cervical flexion-extension magnetic resonance imaging in cervical myelopathy. J Orthop Sci. (2015) 20:973–7. doi: 10.1007/s00776-015-0757-x
10. Zhou LY, Yao M, Tian ZR, Liu SF, Song YJ, Ye J, et al. Muscone suppresses inflammatory responses and neuronal damage in a rat model of cervical spondylotic myelopathy by regulating drp1-dependent mitochondrial fission. J Neurochem. (2020) 155:154–76. doi: 10.1111/jnc.15011
11. Suleiman LI, Weber KA 2nd, Rosenthal BD, Bhatt SA, Savage JW, Hsu WK, et al. High-resolution magnetization transfer MRI in patients with cervical spondylotic myelopathy. J Clin Neurosci. (2018) 51:57–61. doi: 10.1016/j.jocn.2018.02.023
12. Zhang J, Wang L, Li J, Yang P, Shen Y. Predictors of surgical outcome in thoracic ossification of the ligamentum flavum: focusing on the quantitative signal intensity. Sci Rep. (2016) 6:23019. doi: 10.1038/srep23019
13. Zhang JT, Meng FT, Wang S, Wang LF, Shen Y. Predictors of surgical outcome in cervical spondylotic myelopathy: focusing on the quantitative signal intensity. Eur Spine J. (2015) 24:2941–5. doi: 10.1007/s00586-015-4109-5
14. Cheng XJ, Jin L, Wang X, Zhang W, Shen Y. Predictors of poor outcome in cervical spondylotic myelopathy patients underwent anterior hybrid approach: focusing on change of local kyphosis. J Orthop Surg Res. (2020) 15:369. doi: 10.1186/s13018-020-01905-1
15. Jiang Z, Wang A, Wang C, Kong W. Analysis of compliance and efficacy of integrated management of whole process in the choice of percutaneous full-endoscopic surgery for patients with cervical disc herniation. J Orthop Surg Res. (2020) 15:381. doi: 10.1186/s13018-020-01920-2
16. Karpova A, Craciunas S, Chua SY, Rabin D, Smith S, Fehlings MG. Accuracy and reliability of MRI quantitative measurements to assess spinal cord compression in cervical spondylotic myelopathy: a prospective study. Evid Based Spine Care J. (2010) 1:56–7. doi: 10.1055/s-0028-1100916
17. Machino M, Ando K, Kobayashi K, Nakashima H, Kanbara S, Ito S, et al. Prediction of outcome following laminoplasty of cervical spondylotic myelopathy: focus on the minimum clinically important difference. J Clin Neurosci. (2020) 81:321–7. doi: 10.1016/j.jocn.2020.09.065
18. Tachibana N, Oichi T, Kato S, Sato Y, Hasebe H, Hirai S, et al. Spinal cord swelling in patients with cervical compression myelopathy. BMC Musculoskelet Disord. (2019) 20:284. doi: 10.1186/s12891-019-2673-2
19. Matsushita A, Maeda T, Mori E, Yuge I, Kawano O, Ueta T, et al. Can the acute magnetic resonance imaging features reflect neurologic prognosis in patients with cervical spinal cord injury? Spine J. (2017) 17:1319–24. doi: 10.1016/j.spinee.2017.05.009
20. Alisauskaite N, Spitzbarth I, Baumgärtner W, Dziallas P, Kramer S, Dening R, et al. Chronic post-traumatic intramedullary lesions in dogs, a translational model. PLoS ONE. (2017) 12:e0187746. doi: 10.1371/journal.pone.0187746
21. Yoon H, Moon WJ, Nahm SS, Kim J, Eom K. Diffusion tensor imaging of scarring, necrosis, and cavitation based on histopathological findings in dogs with chronic spinal cord injury: evaluation of multiple diffusion parameters and their correlations with histopathological findings. J Neurotrauma. (2018) 35:1387–97. doi: 10.1089/neu.2017.5409
22. Abode-Iyamah KO, Stoner KE, Grossbach AJ, Viljoen SV, McHenry CL, Petrie MA, et al. Effects of brain derived neurotrophic factor Val66Met polymorphism in patients with cervical spondylotic myelopathy. J Clin Neurosci. (2016) 24:117–21. doi: 10.1016/j.jocn.2015.07.016
23. Gibson J, Nouri A, Krueger B, Lakomkin N, Nasser R, Gimbel D, et al. Degenerative cervical myelopathy: a clinical review. Yale J Biol Med. (2018) 91:43–8.
24. Hayama S, Nakaya Y, Nakano A, Fujishiro T, Yano T, Mizutani M, et al. Decompression status of the spinal cord after cervical laminoplasty in various body positions and neck postures observed using percutaneous ultrasonography: relationship with neurological recovery. J Orthop Sci. (2021) 26:S0949-2658(21)00188-3. doi: 10.1016/j.jos.2021.05.009
25. Zhang P, Shen Y, Zhang YZ, Ding WY, Wang LF. Significance of increased signal intensity on MRI in prognosis after surgical intervention for cervical spondylotic myelopathy. J Clin Neurosci. (2011) 18:1080–3. doi: 10.1016/j.jocn.2010.12.023
26. Lee S, Roh SW, Jeon SR, Park JH, Kim KT, Lee YS, et al. A prognostic factor for prolonged mechanical ventilator-dependent respiratory failure after cervical spinal cord injury : maximal canal compromise on magnetic resonance imaging. J Korean Neurosurg Soc. (2021) 64:791–8. doi: 10.3340/jkns.2020.0346
27. Chen YC, Kuo CH, Cheng CM, Wu JC. Recent advances in the management of cervical spondylotic myelopathy: bibliometric analysis and surgical perspectives. J Neurosurg Spine. (2019) 31:299–309. doi: 10.3171/2019.5.SPINE18769
28. Aarabi B, Alexander M, Mirvis SE, Shanmuganathan K, Chesler D, Maulucci C, et al. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J Neurosurg Spine. (2011) 14:122–30. doi: 10.3171/2010.9.SPINE09922
29. Tarawneh AM, D'Aquino D, Hilis A, Eisa A, Quraishi NA. Can MRI findings predict the outcome of cervical spinal cord injury? A systematic review. Eur Spine J. (2020) 29:2457–64. doi: 10.1007/s00586-020-06511-7
Keywords: cervical spondylotic myelopathy, intramedullary increased signal intensity, nomogram, digital tool, patient prognosis education
Citation: Wang J, Li H and Yang B (2022) Predictive Nomogram for Clinical Prognosis in Cervical Spondylotic Myelopathy With Intramedullary T2-Weighted Increased Signal Intensity: A Novel Digital Tool for Patient Prognosis Education. Front. Public Health 10:898242. doi: 10.3389/fpubh.2022.898242
Received: 17 March 2022; Accepted: 25 April 2022;
Published: 31 May 2022.
Edited by:
Xiaofei Zhang, Nankai University, ChinaReviewed by:
Demeng Xia, Shanghai University, ChinaCopyright © 2022 Wang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haopeng Li, bGhwXzM5OTNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.