- 1Department of Epidemiology and Health Statistics, Fujian Provincial Key Laboratory of Environment Factors and Cancer, School of Public Health, Fujian Medical University, Fuzhou, China
- 2Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou, China
- 3Laboratory Center, The Major Subject of Environment and Health of Fujian Key Universities, School of Public Health, Fujian Medical University, Fuzhou, China
- 4Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Background: Dietary fiber and vitamin C has been reported to play a possible role in tumorigenesis. However, few studies have estimated their association with oral cancer risk. In this project, we investigated the relationship between dietary fiber and vitamin C and oral cancer risk in adults in Southern China.
Methods: 382 patients newly diagnosed with oral cancer were matched to 382 hospital derived controls by frequency matching in age and sex. Pre-diagnostic consumption of dietary fiber and vitamin C intake were measured through food frequency questionnaire. Association between nutrients intake and oral cancer risk were evaluated by logistic regression. OR value and 95% confidence interval was calculated.
Results: Intake of dietary fiber and vitamin C was significantly lower in oral cancer patients (8.15 g/day) than in control participants (8.88 g/day). Increased dietary fiber or vitamin C intake was linked to a decreased incidence of OC after adjustment of age, marital status, residence, BMI, occupation, education, tobacco smoking, alcohol consumption and family history of cancer Ptrend< 0.001). Compared with the lowest tertile, the adjusted OR of the top tertile of dietary fiber was 0.47 (95 % CI 0.32, 0.68). While the adjusted OR of the highest tertile was 0.60 (95 % CI 0.42, 0.87) compared with the lowest tertile of vitamin C.
Conclusions: Dietary intake of fiber and vitamin C were lower in oral cancer patients than in control participants. Dietary fiber and vitamin C were inversely related to risk of oral cancer risk.
Background
Oral cancer accounted for 40% of cancers of the head and neck, which includes squamous cell carcinomas of the tongue, floor of the mouth, buccal mucosa, lips, hard and soft palate, and gingival (1). At the same time, oral cancer has the characteristics of strong invasiveness and poor prognosis (2). There were 354,864 new cancer cases and 177,384 deaths per year worldwide, with a higher rate in developing countries (3–5). Smoking, alcohol consumption, poor dental hygiene, the human papillomavirus, and betel nut consumption are most commonly reported risk factors of oral cancer (6–12). However, in addition to the conventional risk factors described above, other environmental factors including dietary and nutrients have also been reported in recent research (13–15). The significance of vegetables and fruits abundant in dietary fiber and vitamins in carcinogenesis is garnering more attention among dietary factors (16, 17).
The findings of a significant systematic study released by the World Cancer Research Fund (WCRF) in 2019 indicated that adopting a diet rich in wholegrains, vegetables, fruit, and legumes may protect against cancer (18). As they include micronutrients with chemo preventive qualities, such as antioxidant activity, these foods are intended to help prevent certain malignancies. Vitamin C (L-ascorbic acid) is a water-soluble antioxidant that assumes a significant job in the body (19). A great volume of investigations have demonstrated that vitamin C possesses anti-oxidant, anti-inflammatory, and immunity-boosting qualities (19–21). In this proposal, dietary fiber, which is frequently acquired from grains, fruits, and vegetables, is also an essential element of a balanced meal. Dietary fiber was thought to be made up of polysaccharides with a high degree of polymerization that were resistant to digestion and absorption in the upper intestine and may subsequently be fermented in the gut (22), and have been linked to a lower risk of a range of tumors, including bladder cancer (23, 24), pancreatic cancer (25, 26), colorectal cancer (27–29), lung cancer (30, 31). However, evidence of a link between dietary fiber and head and neck cancers, notably oral cancer, is scarce.
In 2015 (32), the International Head and Neck Cancer Epidemiology (INHANCE) consortium reported that taking vitamin C from food can prevent oral cancer (32), and then in 2017 (33), they reported that taking more fiber might reduce the risk of HNC. These two studies, however, are mostly directed at whites and a limited number of Japanese, and there are very few publications on the connection between dietary fiber and the incidence of oral cancer in developing areas.
Thus the study was conducted to explore the potential link between dietary fiber and vitamin C consumption and the incidence of oral cancer in southern China. The aforementioned nutrients, we expected, would be inversely connected to the risk of oral cancer.
Materials and Methods
Study Population and Data Collection
A hospital-based case-control study was conducted in Fujian Province, China. From September 2016 to July 2020, oral cancer patients were recruited at the Department of Mouth & Maxillofacial Surgery, First Affiliated Hospital of Fujian Medical University. All patients were newly diagnosed primary oral cancer verified by histology. Patients with recurring or metastasized cancer, as well as those who had already had chemotherapy or radiation, were excluded. During the same time period, control subjects were selected from the same hospital's health examination center and were frequency matched to cases by age (10-year group) and sex. All subjects included were Chinese Han men and women between the ages of 18 and 80 who had resided in Fujian for at least 10 years.
In accordance with the Declaration of Helsinki, all subjects provided written informed permission. The investigation protocol was granted by the Institutional Review Board of Fujian Medical University (approval number: 2011053; approval date: March 10, 2011). Face-to-face interviews with study subject were performed by professional interviewers (unless the subject was too ill to respond). Finally, 382 oral patients and 382 control participants were included in the study.
Dietary Assessment
Food frequency questionnaire was used to evaluate individuals' usual dietary habits during the previous year (FFQ). Majority food category include staple foods, beans and bean products, vegetables, fruits, animal foods, bacteria, algae, and nuts, beverages, and soups. The researchers gathered data on portion size and food consumption frequency. Subjects were informed food images to assist them measure their dietary consumption. By multiplying the intake frequency by the intake quantity and dividing it by 365 days, the average amount of each food item ingested (g/d) was determined. The Chinese Food Composition Tables (34) was used to determine dietary fiber (g/day) and vitamin C (mg/day) consumed.
Statistical Analysis
For categorical variables, differences of demographic features and dietary data between cases and controls were assessed using the χ2-test or Fisher's exact test. The t-test was used to compare differences of continuous variables between the two groups when the data was normally distributed, and the Wilcoxon rank sum test was used when the data was skewed. Adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were determined with the lowest tertile of dietary fiber and vitamin C consumption as reference. The regression residual approach was used to adjust nutrient intake by total energy consumption. All of the data was analyzed using Stata software version 15.0. All p-values in this study are two-sided, and statistical significance was evaluated at the 0.05 level.
Result
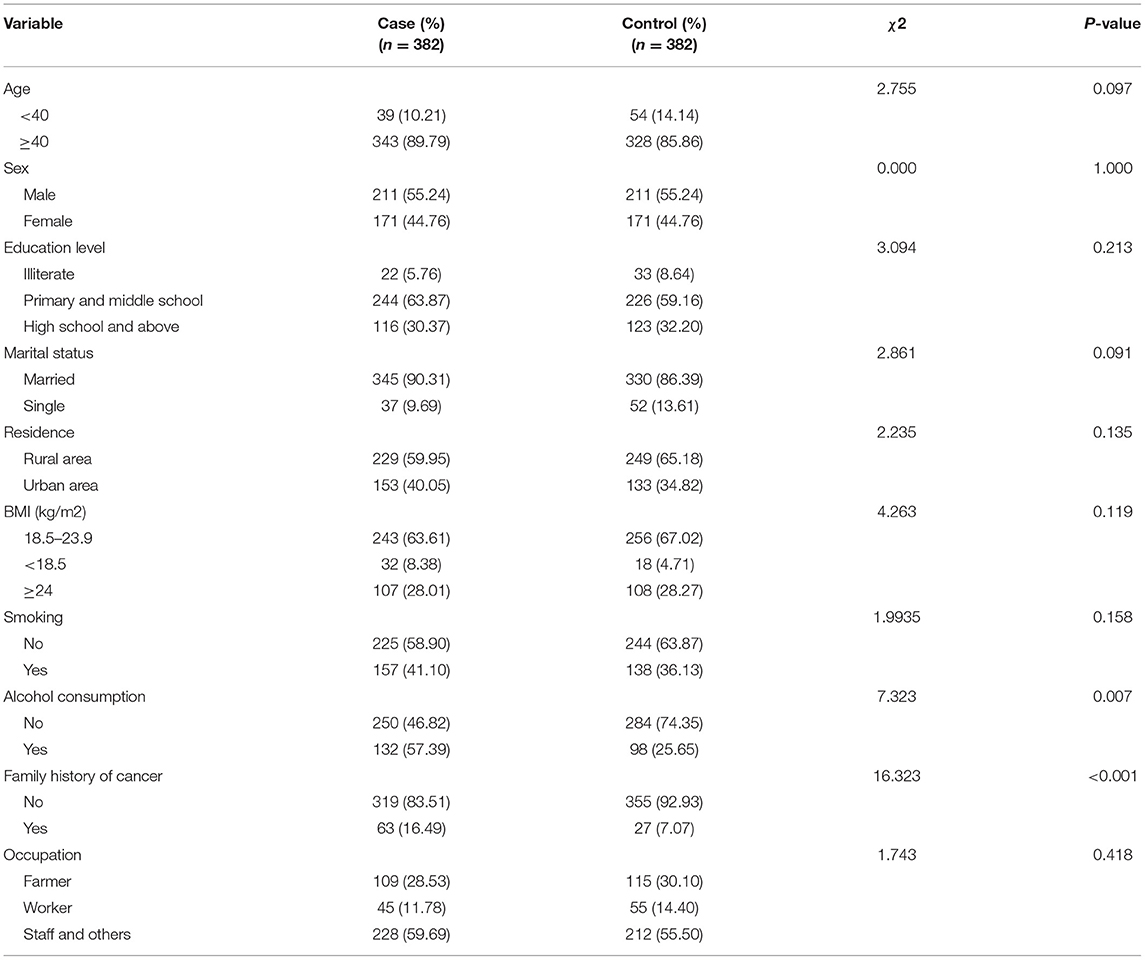
There were 764 individuals in total, of which 422 were men and 342 were women. Table 1 lists the demographic information of all participants in detail (Table 1). The median ages of the patient and control groups were 49 and 56 years, respectively. When compared to control people, the oral cancer group had a higher proportion of participants with alcohol consumption and a family history of cancer. The case and control groups had comparable distributions in ethnic group, education level, marital status, origin, BMI, smoking status, tea consumption, and employment.
Compared with the controls, the intake of dietary fiber and vitamin C was significantly lower among cases (Figures 1A,B). The average daily intake of dietary fiber in the control group was 8.88 (g/day), while that in the case group was 8.15 (g/day). As for vitamin C, the average daily intake of the control group was 118.27 (mg/day), while that of the case group was 108.86 (mg/day). In the correlation analysis between daily average intake of dietary fiber, vitamin C and food groups, vegetables (r = 0.6560, P < 0.001) and fruits (r = 0.5463, P < 0.001) were the major source of dietary fiber, followed by soy foods, algae, nuts and grain, which are all related to dietary fiber intake, as data shown in Table 2. Vegetables (r = 0.8419, P < 0.001), fruits (r = 0.4022, P < 0.001), algae and nuts (r = 0.2052, P < 0.001) showed strong correlation with Vitamin C intake. Grains, soy food, meat and eggs, dairy, pickles and processed meat are not significantly related to vitamin C intake.
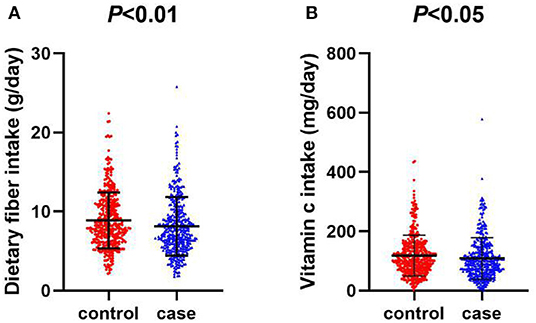
Figure 1. (A) Scatter diagram of daily intake of dietary fiber between oral cancer and control group. (B) Scatter diagram of daily intake of vitamin C between oral cancer and control group.
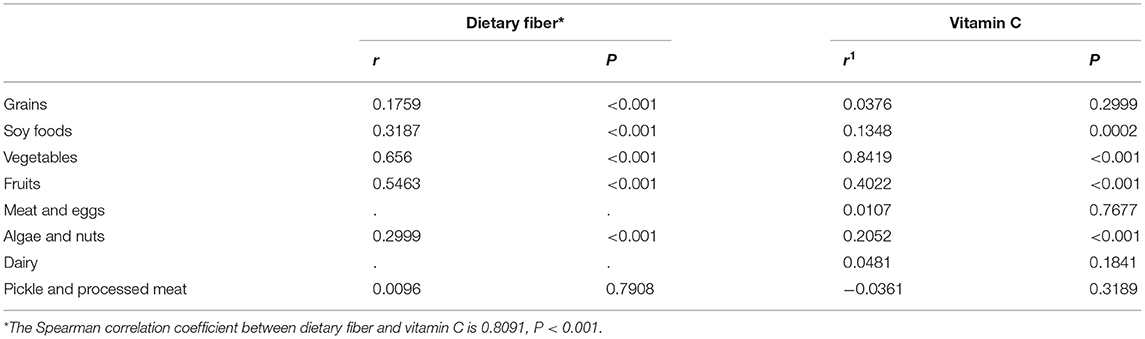
Table 2. Spearman correlation coefficient between daily average intake of dietary fiber and vitamin C and food groups in all study subjects.
Table 3 presents the odds ratio (OR) and 95% confidence interval (CI) for oral cancer across the tertile categories for the intake of dietary fiber and vitamin C. An inverse association between the intake of dietary fiber, vitamin C and risk of oral cancer was observed. In the crude mode, the ORs for the highest triplet compared with the lowest triplet intake were 0.52 (95% CI: 0.37–0.74; Ptrend = 0.0006) for dietary fiber, and the ORs was 0.65 (95% CI: 0.46–0.92; Ptrend = 0.0502) for vitamin C. After adjusting for age, marital status, residence, BMI, occupation and education, the individuals in the highest tertile of the intake of dietary fiber tended to have lower odds for oral cancer (OR = 0.51; 95% CI: 0.35–0.73; Ptrend = 0.005) compared with those in the lowest tertile and a consistent result was observed in vitamin C (OR = 0.63; 95% CI: 0.44–0.90; Ptrend = 0.0928). The result remained significant after further adjustment for tobacco smoking, alcohol consumption and family history of cancer, with ORs= 0.47 (95% CI: 0.32–0.68; Ptrend< 0.001) for dietary fiber, and 0.60 (95% CI: 0.42–0.87; Ptrend< 0.001) for vitamin C. Moreover, similarly association was observed when we treated the intake of dietary fiber and vitamin C as a continuous variable in the rude model, and adjusted models. The OR value of dietary fiber was 0.9337 (95% CI: 0.8950–0.9741, Ptrend< 0.001), while the OR value of vitamin C was 0.9974 (95% CI: 0.9952–0.9996, Ptrend< 0.001) in model 2.
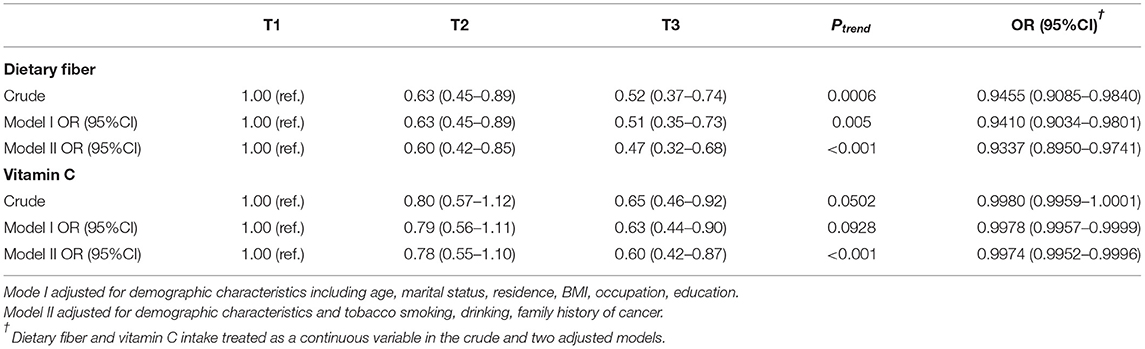
Table 3. Association between dietary fiber and vitamin C intake and oral cancer by logistic regression.
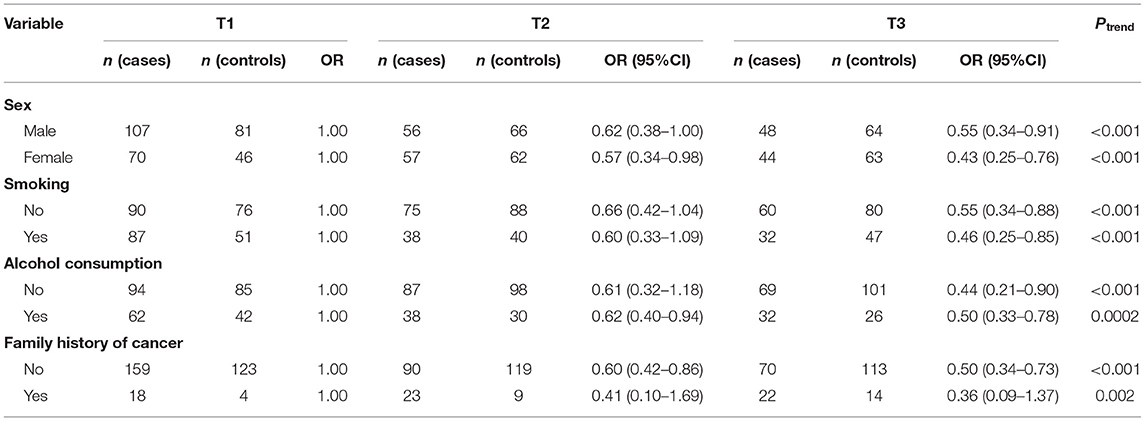
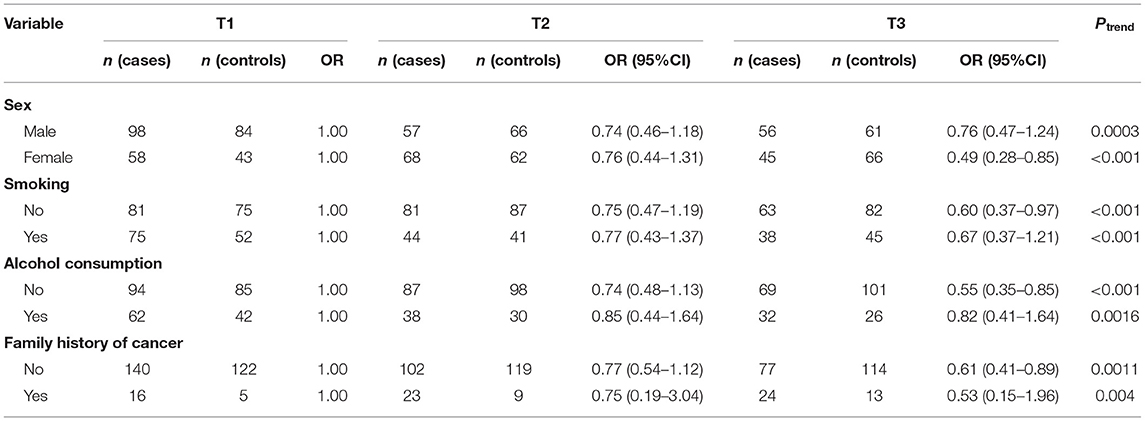
We also investigated whether the relationship between dietary fiber and vitamin C intake and oral cancer varies with different status of sex, smoking, alcohol consumption and family history of cancer (Tables 4, 5). The association between dietary fiber intake and oral cancer risk was more significant in female participants (OR = 0.43; 95% CI: 0.25–0.76; Ptrend< 0.001) and those without alcohol consumption (OR = 0.44, 95% CI: 0.21–0.90; Ptrend< 0.001). While association between intake of vitamin C and the risk of oral cancer remained significant in female (OR = 0.49, 95% CI: 0.28–0.85, Ptrend< 0.001) and those without alcohol consumption (OR = 0.55, 95% CI: 0.35–0.85; Ptrend< 0.001) compared with male and drinkers.
Discussion
In this case-control designed study, we observed that higher dietary fiber and vitamin C consumption were related to a lower risk of oral cancer after adjustment of potential confounding factors. These findings are in accordance with the conclusions of two previous meta-analysis studies (32, 33).
Kawakita et al., etc., summarized and analyzed 10 case-control studies participating in the International Head and Neck Cancer Epidemiology consortium in a meta-analysis in 2017 (33), in which the majority of the participants were white and an inverse relationship between fiber consumption and oral cancer was identified. In a recent prospective research comprising 101,700 individuals, more than 70% of whom were white, they discovered that fiber consumption may protect against the formation of oral cancer (35). Meanwhile, in a prospective longitudinal cohort analysis of newly diagnosed patients with head and neck cancer, dietary fiber consumption was found to be adversely linked with all-cause mortality (36). And a study (35) reported the relationship between fiber intake and HNC risk by using data from screening tests for prostate cancer, lung cancer, colorectal cancer and ovarian cancer (PLCO). These findings back with previous research that suggests dietary fiber might reduce the incidence of HNC. There are relatively few reports on the relationship between dietary fiber consumption and cancer in developing area. A case-control study of 851 cases of nasopharyngeal carcinoma and 1,502 controls conducted in China (37) reported that dietary fiber intake was negatively correlated with the risk of nasopharyngeal carcinoma. Another study from China showed (38) that a high dietary fiber intake, particularly from vegetables and fruit, was negatively correlated to the incidence of colorectal cancer in Chinese people. Only one case-control study was carried out in Beijing in 1993 to report the relationship between dietary fiber and oral cancer (39), according which, fiber from fruits and vegetables has a substantial inverse connection with the likelihood of oral cavity cancer, whereas fiber from other sources does not. Our study from southeast China confirmed these findings, demonstrating that dietary fiber and vitamin C consumption were both negatively proportionate to the incidence of oral cancer.
Although further researches are needed to validate this conclusion, numerous molecular pathways have been postulated to explain why fiber consumption protects against oral cancer. Dietary fiber appears to have an anti-inflammatory effect (40, 41), through the creation of anti-proliferative and pro-apoptosis short-chain fatty acids by gut bacteria (42). By decreasing nuclear factor kappa-B activation and increasing anti-microbial peptide release, these short-chain fatty acids can lower pro-inflammatory cytokine expression (43, 44), whereas inflammation plays a key role in the etiology of precancerous lesions in the mouth (45, 46). Another possibility is that fiber-rich foods usually contain higher antioxidants, because higher fiber intake mostly origin from diet patterns rich in fruits, vegetables and beans (47). Likewise, dietary fiber may just be a sign of a balanced lifestyle in whole. The intake of dietary fiber has a substantial connection with the consumption of vitamin C (Table 2), according to our research. Vegetables and grains are the most abundant sources of dietary fiber, followed by fruits, algae, nuts and soybeans, which are all linked to dietary fiber consumption.
As for the relationship between vitamin C and cancer, many researchers have reported the beneficial effects of vitamin C on cancer (48–51) in both digestive tract cancer (52–55) and head and neck cancer (32, 56). In the Netherlands Cohort Study in 2015 (56), the incidence of HNC and HNC-subtypes was shown to be inversely related to vitamin C consumption. However, the results of a randomized double-blind RCT experiment in Japan in 2015 reported that vitamin C supplementation had no significance in preventing oral cancer (57). In this study, 46 Japanese participants with oral leukoplakia were randomly assigned to an experimental group and given low-dose β -carotene combined with vitamin C supplement to treat and prevent oral leukoplakia from malignant transformation. Two patients in the experimental arm and three in the control arm had oral cancer throughout the median 60-month follow-up period. Therefore, they claim that vitamin C cannot prevent oral cancer. However the number of subjects involved in this study and follow-up time are not justified enough to draw a definite conclusion. The inconsistent conclusions observed may attributed to variation in cancer types, research design, study population, sample size, methods of assessing dietary nutrition and confounding factors adjusted.
However, potential limitations of our study should also be notified. Firstly, we could not rule out the possibility of selection bias due to the hospital-based case-control study design, although the hospital covered most of the oral cancer cases from the selected area and we recruited control participant from the same hospital. Secondly, it was meaningful to study the relevance as a case-control study, but this information was not available. While the case and control participation rates were high, it is still possible that participating controls were more health conscious than the general population, thus biasing risk estimates away from the null. Thirdly, recall bias and measurement error in dietary assessment using an FFQ is difficult to avoid in a case-control study. Thus, the associations observed between dietary fiber, vitamin C and oral cancer should be interpreted cautiously.
In conclusion, the current investigation found that greater dietary fiber and vitamin C intakes were linked to a lower incidence of oral cancer in a southeastern Chinese population. Given the limitations of hospital-based case-control studies, further well-designed, particularly large-scale cohort studies, are needed to corroborate our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JW (1st author), FL, and YF conceptualized the original idea for the study and have been involved in data collection, data analysis, and manuscript drafting. JQ assisted in statistical analysis. All authors have made substantial contributions to conception and design of the study, have read and approved the manuscript.
Funding
This study was supported by Fujian Natural Science Foundation Program under Grant 2020J01639, Technology Development Fund from the Department of Education of Fujian Province Grant Number: 2019L3006 and Fujian Provincial Health Technology Project under Grant No. 2018-1-57.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciate the oral cancer patients and control participants that contributed to this study.
References
1. Singh P, Rai A, Verma AK, Alsahli MA, Rahmani AH, Almatroodi SA, et al. Survival-based biomarker module identification associated with oral squamous cell carcinoma (OSCC). Biology. (2021) 10:760. doi: 10.3390/biology10080760
2. Ren ZH, Xu JL, Li B, Fan TF, Ji T, Zhang CP. Elective versus therapeutic neck dissection in node-negative oral cancer: Evidence from five randomized controlled trials. Oral Oncol. (2015) 51:976–81. doi: 10.1016/j.oraloncology.2015.08.009
3. Bray FJ, Ferlay I, Soerjomataram Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Shrestha AD, Vedsted P, Kallestrup P, Neupane D. Prevalence and incidence of oral cancer in low- and middle-income countries: a scoping review. Eur J Cancer Care. (2020) 29:e13207. doi: 10.1111/ecc.13207
5. Yang Y, Zhou M, Zeng X, Wang C. The burden of oral cancer in China, 1990-2017: an analysis for the Global Burden of Disease, Injuries, and Risk Factors Study. BMC Oral Health. (2021) 21:44. doi: 10.1186/s12903-020-01386-y
6. Su SY, Chen WT, Chiang CJ, Yang YW, Lee WC. Oral cancer incidence rates from 1997 to 2016 among men in Taiwan: Association between birth cohort trends and betel nut consumption. Oral Oncol. (2020) 107:104798. doi: 10.1016/j.oraloncology.2020.104798
7. Mahapatra S, Chaly PE, Mohapatra SC, Madhumitha M. Influence of tobacco chewing on oral health: a hospital-based cross-sectional study in Odisha. Indian J Public Health. (2018) 62:282–6. doi: 10.4103/ijph.IJPH_327_17
8. Sahoo PK, Sarkar S, Ghosh D, Mahata S, Pal R, Mistry T, et al. Premalignant and malignant lesions of oral cavity in eastern India: a hospital-based study. Eur J Cancer Prevent. (2020) 30:393–9. doi: 10.1097/CEJ.0000000000000640
9. Fu JY, Wu CX, Zhang CP, Gao J, Luo JF, Shen SK, et al. Oral cancer incidence in Shanghai —- a temporal trend analysis from 2003 to 2012. BMC Cancer. (2018) 18:686. doi: 10.1186/s12885-018-4582-4
10. Chuang SL, Wang CP, Chen MK, Su WW, Su CW, Chen SL, et al. Malignant transformation to oral cancer by subtype of oral potentially malignant disorder: a prospective cohort study of Taiwanese nationwide oral cancer screening program. Oral Oncol. (2018) 87:58–63. doi: 10.1016/j.oraloncology.2018.10.021
11. Jansen L, Buttmann-Schweiger N, Listl S, Ressing M, Holleczek B, Katalinic A, et al. Differences in incidence and survival of oral cavity and pharyngeal cancers between Germany and the United States depend on the HPV-association of the cancer site. Oral Oncol. (2018) 76:8–15. doi: 10.1016/j.oraloncology.2017.11.015
12. Hong HS, Akhavan J, Lee SH, Kim RH, Kang MK, Park NH, et al. Proinflammatory cytokine TNFα promotes HPV-associated oral carcinogenesis by increasing cancer stemness. Int J Oral Sci. (2020) 12:3. doi: 10.1038/s41368-019-0069-7
13. Rodríguez-Molinero J, Migueláñez-Medrán BDC, Puente-Gutiérrez C, Delgado-Somolinos E, Martín Carreras-Presas C, Fernández-Farhall J, et al. Association between oral cancer and diet: an update. Nutrients. (2021) 13:1299. doi: 10.3390/nu13041299
14. Green JM, Ciancio MJ, Goral J, Pytynia M, Pitstick L, Meyer A, et al. Dietary fat and male sex increase histopathological changes in a mouse model of oral cancer. Oral Dis. (2021) 27:215–25. doi: 10.1111/odi.13542
15. Bell EB, Reis IM, Cohen ER, Almuhaimid T, Smith DH, Alotaibi F, et al. Green salad intake is associated with improved oral cancer survival and lower soluble CD44 levels. Nutrients. (2021) 13:372. doi: 10.3390/nu13020372
16. Makiuchi T, Sobue T, Kitamura T, Ishihara J, Sawada N, Iwasaki M, et al. The relationship between vegetable/fruit consumption and gallbladder/bile duct cancer: a population-based cohort study in Japan. Int J Cancer. (2017) 140:1009–19. doi: 10.1002/ijc.30492
17. Zhao Z, Yin Z, Zhang C. Lifestyle interventions can reduce the risk of Barrett's esophagus: a systematic review and meta-analysis of 62 studies involving 250,157 participants. Cancer Med. (2021) 10:5297–320. doi: 10.1002/cam4.4061
18. Shams-White MM, Brockton NT, Mitrou P, Romaguera D, Brown S, Bender A, et al. Operationalizing the 2018 world cancer research fund/american institute for cancer research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrients. (2019) 11:1572. doi: 10.3390/nu11071572
19. Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. (2018) 28:698–708. doi: 10.1016/j.tcb.2018.04.001
20. Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, et al. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. (2013) 36:4104–8. doi: 10.2337/dc13-0750
21. Wong SK, Chin KY, Ima-Nirwana S. Vitamin C: a review on its role in the management of metabolic syndrome. Int J Med Sci. (2020) 17:1625–38. doi: 10.7150/ijms.47103
22. Korczak R, Slavin JL. Definitions, regulations, and new frontiers for dietary fiber and whole grains. Nutr Rev. (2020) 78:6–12. doi: 10.1093/nutrit/nuz061
23. Edefonti V, La Vecchia C, Di Maso M, Crispo A, Polesel J, Libra M, et al. Association between nutrient-based dietary patterns and bladder cancer in Italy. Nutrients. (2020) 12:1584. doi: 10.3390/nu12061584
24. Yu EYW, Wesselius A, Mehrkanoon S, Brinkman M, van den Brandt P, White E, et al. Grain and dietary fiber intake and bladder cancer risk: a pooled analysis of prospective cohort studies. Am J Clin Nutr. (2020) 112:1252–66. doi: 10.1093/ajcn/nqaa215
25. Zheng J, Guinter MA, Merchant AT, Wirth MD, Zhang J, Stolzenberg-Solomon RZ, et al. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev. (2017) 75:883–908. doi: 10.1093/nutrit/nux038
26. Bidoli E, Pelucchi C, Zucchetto A, Negri E, Dal Maso L, Polesel J, et al. Fiber intake and pancreatic cancer risk: a case-control study. Ann Oncol. (2012) 23:264–8. doi: 10.1093/annonc/mdr060
27. Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP diet and health study cohort. Am J Clin Nutr. (2020) 112:603–12. doi: 10.1093/ajcn/nqaa161
28. Oh H, Kim H, Lee DH, Lee A, Giovannucci EL, Kang SS, et al. Different dietary fibre sources and risks of colorectal cancer and adenoma: a dose-response meta-analysis of prospective studies. Br J Nutr. (2019) 122:605–15. doi: 10.1017/S0007114519001454
29. Hidaka A, Harrison TA, Cao Y, Sakoda LC, Barfield R, Giannakis M, et al. Intake of dietary fruit, vegetables, and fiber and risk of colorectal cancer according to molecular subtypes: a pooled analysis of 9 studies. Cancer Res. (2020) 80:4578–90. doi: 10.1158/0008-5472.CAN-20-0168
30. Yang JJ, Yu D, Xiang YB, Blot W, White E, Robien K, et al. Association of dietary fiber and yogurt consumption with lung cancer risk: a pooled analysis. JAMA Oncol. (2020) 6:e194107. doi: 10.1001/jamaoncol.2019.4107
31. Tao J, Jatoi A, Crawford J, Lam WWT, Ho JC, Wang X, et al. Role of dietary carbohydrates on risk of lung cancer. Lung Cancer. (2021) 155:87–93. doi: 10.1016/j.lungcan.2021.03.009
32. Edefonti V, Hashibe M, Parpinel M, Turati F, Serraino D, Matsuo K, et al. Natural vitamin C intake and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer. (2015) 137:448–62. doi: 10.1002/ijc.29388
33. Kawakita D, Lee YA, Turati F, Parpinel M, Decarli A, Serraino D, et al. Dietary fiber intake and head and neck cancer risk: A pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Int J Cancer. (2017) 141:1811–21. doi: 10.1002/ijc.30886
34. Jiang H, Zhang J, Du W, Su C, Zhang B, Wang H. Energy intake and energy contributions of macronutrients and major food sources among Chinese adults: CHNS 2015 and CNTCS 2015. Eur J Clin Nutr. (2021) 75:314–24. doi: 10.1038/s41430-020-0698-0
35. Kawakita D, Lee YA, Gren LH, Buys SS, La Vecchia C, Hashibe M. Fiber intake and the risk of head and neck cancer in the prostate, lung, colorectal and ovarian (PLCO) cohort. Int J Cancer. (2019) 145:2342–8. doi: 10.1002/ijc.32162
36. Maino Vieytes CA, Mondul AM, Li Z, Zarins KR, Wolf GT, Rozek LS, et al. Dietary fiber, whole grains, and head and neck cancer prognosis: findings from a prospective cohort study. Nutrients. (2019) 11:2304. doi: 10.3390/nu11102304
37. Mai ZM, Ngan RK, Kwong DL, Ng WT, Yuen KT, Ip DK, et al. Dietary fiber intake from fresh and preserved food and risk of nasopharyngeal carcinoma: observational evidence from a Chinese population. Nutr J. (2021) 20:14. doi: 10.1186/s12937-021-00667-8
38. Zhong X, Fang YJ, Pan ZZ, Lu MS, Zheng MC, Chen YM, et al. Dietary fiber and fiber fraction intakes and colorectal cancer risk in Chinese adults. Nutr Cancer. (2014) 66:351–61. doi: 10.1080/01635581.2013.877496
39. Zheng T, Boyle P, Willett WC, Hu H, Dan J, Evstifeeva TV, et al. A case-control study of oral cancer in Beijing, People's Republic of China. Assoc Nutr Intakes Foods Food Groups. (1993) 29:45–55. doi: 10.1016/0964-1955(93)90010-C
40. Saeed MA, Gribben KC, Alam M, Lyden ER, Hanson CK, LeVan TD. Association of dietary fiber on asthma, respiratory symptoms, and inflammation in the adult national health and nutrition examination survey population. Ann Am Thorac Soc. (2020) 17:1062–8. doi: 10.1513/AnnalsATS.201910-776OC
41. Sang S, Idehen E, Zhao Y, Chu Y. Emerging science on whole grain intake and inflammation. Nutr Rev. (2020) 78:21–8. doi: 10.1093/nutrit/nuz079
42. McNabney SM, Henagan TM. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. (2017) 9:1348. doi: 10.3390/nu9121348
43. Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. (2013) 114:525–31. doi: 10.1002/jcb.24402
44. Zhang S, Meng G, Zhang Q, Liu L, Yao Z, Wu H, et al. Dietary fiber intake and risk of prediabetes in China: results from the TCLSIH Cohort Study. Br J Nutr. (2021) 21:1–20. doi: 10.1017/S0007114521003779
45. Katsanos KH, Roda G, McBride RB, Cohen B, Colombel JF. Increased Risk of Oral Cancer in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol Hepatol. (2016) 14:413–20. doi: 10.1016/j.cgh.2015.09.041
46. Peng HY, Hsiao JR, Chou ST, Hsu YM, Wu GH, Shieh YS, et al. MiR-944/CISH mediated inflammation via STAT3 is involved in oral cancer malignance by cigarette smoking. Neoplasia. (2020) 22:554–65. doi: 10.1016/j.neo.2020.08.005
47. Dreher ML. Whole fruits and fruit fiber emerging health effects. Nutrients. (2018) 10:1833. doi: 10.3390/nu10121833
48. Sunil Kumar BV, Singh S, Verma R. Anticancer potential of dietary vitamin D and ascorbic acid: A review. Crit Rev Food Sci Nutr. (2017) 57:2623–35. doi: 10.1080/10408398.2015.1064086
49. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. (2018) 108:1069–91. doi: 10.1093/ajcn/nqy097
50. Harris HR, Bergkvist L, Wolk A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br J Cancer. (2013) 109:257–64. doi: 10.1038/bjc.2013.269
51. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. (2014) 50:1223–31. doi: 10.1016/j.ejca.2014.02.013
52. Bo Y, Lu Y, Zhao Y, Zhao E, Yuan L, Lu W, et al. Association between dietary vitamin C intake and risk of esophageal cancer: a dose-response meta-analysis. International journal of cancer. (2016) 138:1843–50. doi: 10.1002/ijc.29838
53. Kang JH, Luben R, Alexandre L, Hart AR. Dietary antioxidant intake and the risk of developing Barrett's oesophagus and oesophageal adenocarcinoma. Br J Cancer. (2018) 118:1658–61. doi: 10.1038/s41416-018-0113-y
54. Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int J Cancer. (2012) 130:147–58. doi: 10.1002/ijc.25989
55. Li P, Zhang H, Chen J, Shi Y, Cai J, Yang J, et al. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int J Cancer. (2014) 135:1444–53. doi: 10.1002/ijc.28777
56. de Munter L, Maasland DH, van den Brandt PA, Kremer B, Schouten LJ. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. (2015) 102:420–32. doi: 10.3945/ajcn.114.106096
Keywords: oral cancer, dietary fiber, vitamin C, food frequency questionnaires, case-control
Citation: Wang J, Fan Y, Qian J, Wang S, Li Y, Xu M, Chen F, Wang J, Qiu Y, Lin L, He B and Liu F (2022) Relationship Between Dietary Fiber and Vitamin C Intake and Oral Cancer. Front. Public Health 10:880506. doi: 10.3389/fpubh.2022.880506
Received: 21 February 2022; Accepted: 25 April 2022;
Published: 12 May 2022.
Edited by:
Kewal Krishan, Panjab University, IndiaReviewed by:
Deepika Rani, Panjab University, IndiaArun K. Garg, Panjab University, India
Jingya Jane Pu, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2022 Wang, Fan, Qian, Wang, Li, Xu, Chen, Wang, Qiu, Lin, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengqiong Liu, bGZxQGZqbXUuZWR1LmNu; Baochang He, aGJjNTE3QDE2My5jb20=
†These authors have contributed equally to this work
 Jing Wang1,2†
Jing Wang1,2† Jiawen Qian
Jiawen Qian Sijie Wang
Sijie Wang Yanni Li
Yanni Li Fa Chen
Fa Chen Jing Wang
Jing Wang Yu Qiu
Yu Qiu Lisong Lin
Lisong Lin Fengqiong Liu
Fengqiong Liu