- 1Medical School, Hangzhou Normal University, Hangzhou, China
- 2Affiliated Hospital, Hangzhou Normal University, Hangzhou, China
Background: Hypertension is rising as a major public health burden around the world. This study explored the association between single-nucleotide polymorphisms (SNPs) in the adenosine triphosphate (ATP)-Binding Cassette Subfamily A1 (ABCA1) gene and hypertension among Chinese Han adults.
Method: A total of 2,296 Han Chinese in southeast China were recruited for this study. We collected medical reports, lifestyle details, and blood samples from individuals. The polymerase chain reaction-ligase detection reaction (PCR-LDR) method was used to detect the genotypes of these SNPs in the ABCA1 gene.
Results: After adjusting some covariates, the additive and recessive models of the rs2472510 and rs2515614 were significantly associated with hypertension. The haplotypes TCTA (rs2297406-rs2472433-rs2472510-rs2515614) were associated with high SBP, and the haplotypes CCTA, TCTA, and TTTA were associated with high diastolic blood pressure (DBP).
Conclusion: The results of the relationship between the polymorphisms of rs2297406, rs2472433, rs2472510, and rs2515614 in ABCA1 and hypertension in southeastern China would provide a theoretical basis for genetic screening and disease prevention.
Introduction
Hypertension is becoming a major public health burden around the world. The latest data from the World Health Organization (WHO) estimated that 1.28 billion adults who aged 30–79 years worldwide have hypertension, over 1 billion people lived in low- and middle-income countries in 2019 (1). Essential hypertension (EH) is the most common type that affected 85% of those with hypertension (2). In addition, it is a major risk of cardiovascular and cerebrovascular diseases, such as angina, heart attack, stroke, heart failure, and kidney failure (3–7). Modifiable environmental elements of hypertension include unhealthy diets, physical inactivity, consumption of tobacco and alcohol, and obesity (8–10). Furthermore, genetic factors should play a key role in the etiology of hypertension and making an effect on cardiovascular diseases (11, 12).
Adenosine triphosphate-binding cassette transporter A1 (ABCA1) belongs to the ABC gene family, and the human ABCA1 gene has a total length of 149 kb that includes a 14,553-bp promoter, 146,581 bp of intron and exons, and a 1-kb 3′ flanking region (13). Intracellular cholesterol metabolic homeostasis is maintained by a complex network, regulating cholesterol biosynthesis, uptake, efflux, conversion, esterification, and cholesterol trafficking (14). The level of cholesterol in the inner leaflet of the plasma membrane was exactly controlled by the ABCA1, the main participant in cholesterol efflux (15). Therefore, loss of ABCA1 function could affect the cholesterol levels. Some research studies proposed that the abnormal ABCA1 cholesterol transporting function may cause hypertension by stimulating epithelial sodium channels (ENaCs), the vital components in the regulation of total body Na+ homeostasis, and might also lead to the occurrence of hypertension (16). In addition, Xu et al. surveyed that the expression level of the ABCA1 gene was significantly decreased in hypertensive patients who were newly diagnosed but untreated; Yamada et al. found that the −14C->T polymorphism of the ABCA1 gene had been appreciably affiliated with the prevalence of hypertension in Japanese individuals (17, 18).
Therefore, we performed a case-control study of the Chinese Han population to estimate the risk of hypertension with polymorphisms of ABCA1 in Zhejiang province and predict the genetic risk of this condition.
Method
Subjects
This study adopted a cluster random sampling method and randomly selected four townships in Ningbo City, Zhejiang Province. From April 2013 to July 2013, health was examined at these four townships (Hengjie, Gaoqiao, Gulin, and Jishigang) health service centers for persons ≥40 years of age. The inclusion criteria of this study were those who were 1) willing to participate in this study and signed an informed consent form, 2) aged 40 or above, and 3) Han Chinese. The exclusion criteria were as follows: 1) not signed the informed consent form, 2) under the age of 40, 3) other ethnic minorities in China, and 4) missing information during the data collection process. The final population included in the analysis was 2,296 individuals.
Diagnostic Criteria
The definition of hypertension refers to the “China Guidelines for the Prevention and Treatment of Hypertension (2018 Revised Edition)” that is compiled by the China Hypertension Prevention and Control Guidelines Revision Committee, and systolic blood pressure (SBP) greater than or equal to 140 mmHg or diastolic blood pressure (DBP) greater than or equal to 90 mmHg was determined as hypertension (19).
Epidemiological Investigation
The field of epidemiological investigation mainly adopts the method of a face-to-face interview. The survey content mainly includes general demographic information (such as name, gender, age, education level, etc.), lifestyle behaviors (such as smoking, drinking, dietary information, physical exercise, etc.), and physical examination (such as height, weight, waist circumference, etc.). Smoking history was defined as having smoked one or more cigarettes per day in the past 1 year or more. Drinking history was defined as the average daily consumption of white wine ≥50 g (1 tael), red wine ≥150 g (3 taels), or beer ≥ 500 g (1 catty) for more than 1 year.
The physical examination requirements were as follows: (1) Blood pressure (BP) measurement: BP of each respondent was measured after standing for 5 min. The measurement time interval was not less than 30 s, and the average value was recorded. (2) Height measurement: During the measurement, the subject stood upright with the head, shoulders, hips, and heels close to the measuring ruler. (3) Waist circumference measurement: During the measurement, the subject stood upright with the abdomen in a natural posture, and the tape measure was close to the skin to measure the narrowest part of the waist. (4) Weighing: Before weighing, each respondent takes off his coat and wears only necessary underwear. All measurements were performed three times and the average value was recorded. (5) Body mass index (BMI) was recorded as follows: weight (kg)/height squared (m2), and taking 18.5 < BMI < 24 as normal weight, 24 ≤ BMI < 28 as overweight, and BMI ≥ 28 as obesity.
Single-Nucleotide Polymorphism (SNP) Selection and Genotyping
A total of 2,429 blood samples were collected from the antecubital vein after the subjects had fasted for ≥8 h. Part of the collected samples was used to examine biochemical indicators, such as serum lipid levels, whereas the other part was transferred into a test tube containing anti-coagulant solution to extract DNA. Peripheral venous blood was collected by a professional nurse and all blood samples were stored at −80°C. The phenol-chloroform method was used to extract DNA from peripheral blood leukocytes. The DNA concentration was determined by spectrophotometry, and the DNA concentration was diluted to 10–30 ng/μl, which was used as a template for polymerase chain reaction (PCR) amplification. The PCR reaction volume contained 1 μl of DNA, 1.5 μl of 10× buffer, 1.5 μl of MgCl2, 0.3 μl of dNTPs, 0.15 μl of each primer, 0.2 μl of Taq enzyme, and water to make the total volume 15 μl. The amplification conditions were as follows: 94°C for 3 min; 35 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 15 s, and extension at 72°C for 30 s; and 72°C for 3 min. The ligation reaction volume comprised of 3 μl PCR product, 1 μl of 10× Taq DNA ligase buffer, 0.125 μl of Taq DNA ligase (40 U/μl), 0.01 μl of each discriminating probe, and water to make the total volume 10 μl. The reaction conditions were as follows: 30 cycles of 94°C for 30 s and 56°C for 3 min. To 1 μl of the extension product, 8 μl of loading buffer was added, followed by denaturation at 95°C for 3 min. Next, the reaction products were detected by electrophoresis, and the mixture was analyzed using a sequencer (ABI 3730XL). The polymerase chain reaction-ligase detection reaction (PCR-LDR) method was employed for genotyping detection. PCR was used to amplify the gene fragment containing the mutation site, and three probes were designed based on the SNP site to distinguish the genotype of the site. Two of the probes were completely complementary to the upstream sequence of the SNP site, except that they had different lengths, and the 3'end bases were different according to the genotype. The other probe (FAM fluorescently labeled) was complementary to the downstream sequence of the SNP. According to the characteristics of the ligase, the length of the ligated fragments obtained from different genotypes was different. GeneMapper software was used to analyze the data of the ligated fragments to obtain different genotypes. Primers, probe sequences, and product lengths for genotyping at each site are shown in Appendix Table 1.
Single nucleotide polymorphisms were mainly searched using the PubMed and GeneCards databases. The specific screening process was as follows: (1) literature related to gene polymorphisms and hypertension was searched in NCBI-PubMed, and SNPs were screened; (2) GeneView information was obtained for relevant SNPs from the GeneCards and NCBI databases, and then, missense mutations, 3′ untranslated region (3′ UTR), 5′UTR, or transcription factor-binding sites were selected. (3) The minor allele frequency (MAF) of SNPs in the Chinese population was detected from the HapMap database for the international human genome, and SNP sites with MAF values greater than 0.05 were screened.
Statistical Analysis
The genetic model analysis is one of the commonly used analysis methods to analyze the association between different alleles and diseases and is the primary association analysis method to determine how SNPs affect disease risk. Genetic models include additive models, dominant models, and recessive models. There were three possible genotypes for a major gene with two alleles: aa, aA, and AA, then if Y is the phenotype and G is the genotype, then the penetrance is expressed as Pr (Y | G) (penetrance function is a set of probability functions of phenotype to genotype), then the dominant genetic model is Pr(Y|aA) = Pr(Y|AA), and the recessive genetic model is Pr(Y)|aA) = Pr(Y |aa), the additive genetic model is Pr (Y | aA) at a position between Pr (Y | AA) and Pr (Y | aa). Statistical analysis was performed using SPSS 24.0 software (SPSS Inc., Chicago, IL, USA). The relationship between demographic information and hypertension was assessed using the χ2 test and t-test. The Person χ2 test was used to determine whether the distribution of points in the population conformed to the Hardy-Weinberg Equilibrium law. The genetic balance was considered to be achieved with p > 0.05, and inheritance was stable in the population. A binary logistic regression model was used to analyze the relationship between ABCA1 gene polymorphisms and hypertension. The forest map used the R language (package “forestplot”). Haploview 4.2 software was used for haplotype analysis. p < 0.05 indicated that the difference was statistically significant.
Results
Basic Characteristics of the Surveyed Population and Analysis of Factors Related to Hypertension
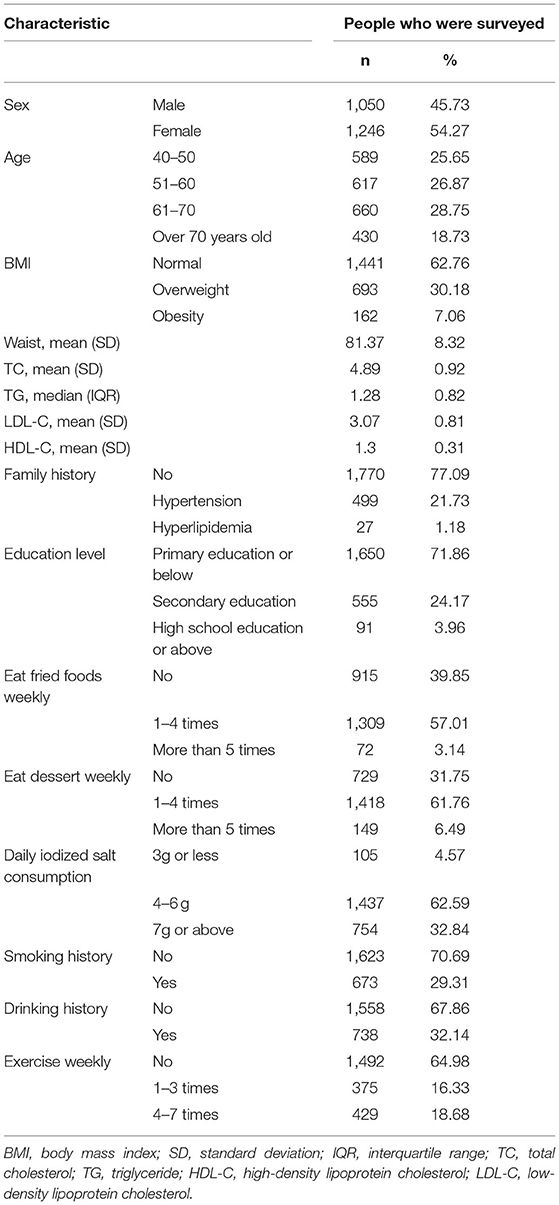
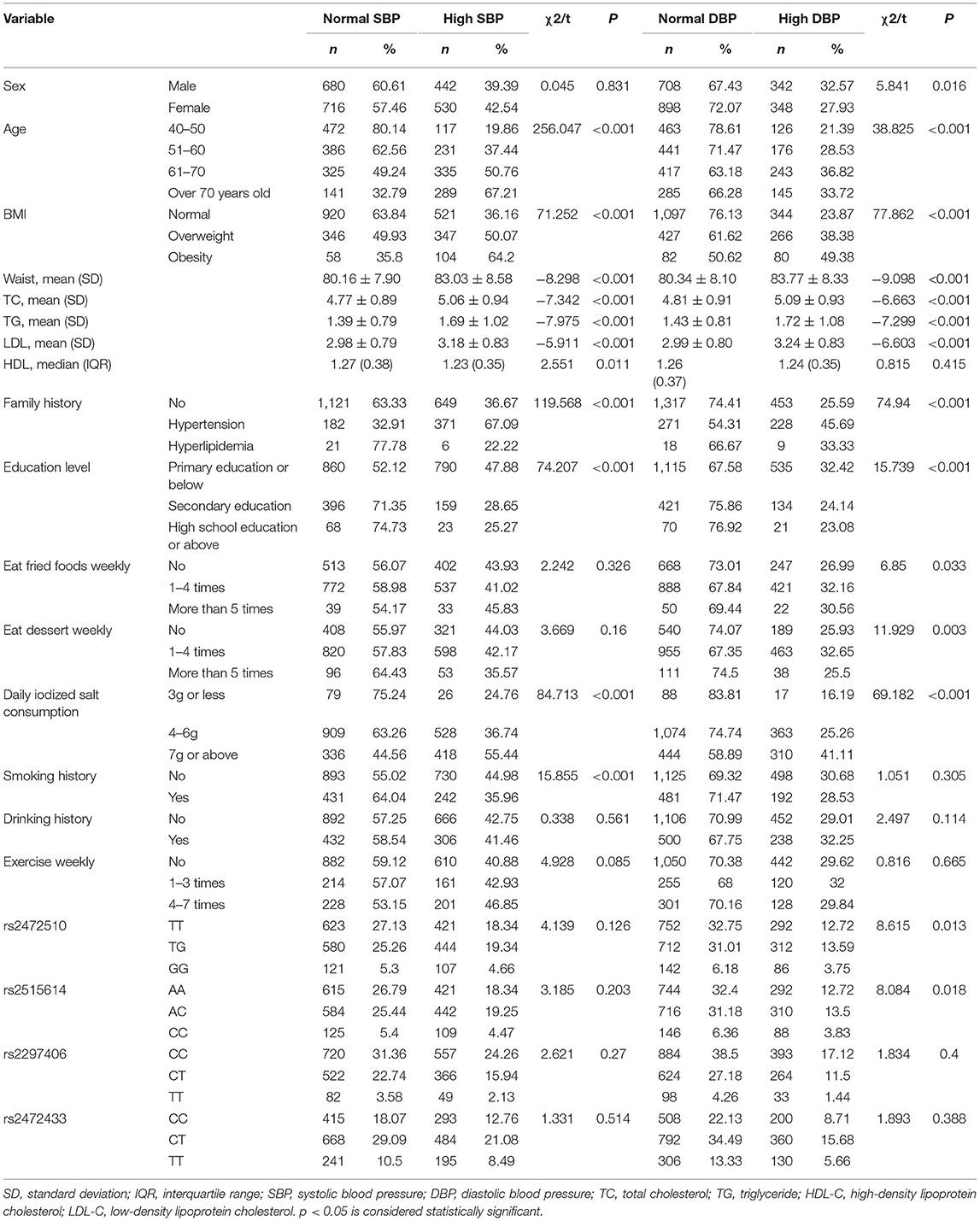
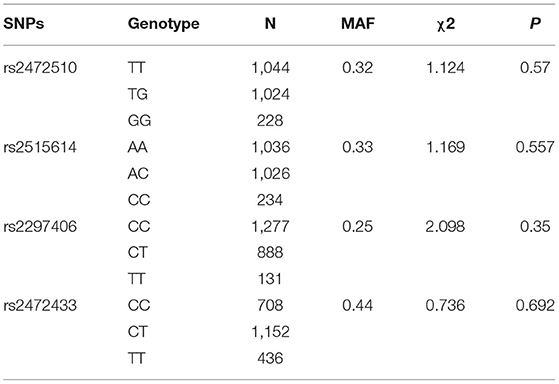
A total of 2,296 people were included in this study. Table 1 shows the basic characteristics of the respondents that include 1,050 men and 1,246 women. The age of the study population was 40 years or older and some blood indicators and lifestyle factors were also included in the table. Then, according to the classification criteria for hypertension, the population was divided into a case group and a control group, of which 972 (42.33%) had high systolic blood pressure (SBP) and 690 (30.05%) had high diastolic blood pressure (DBP). Table 2 shows the relationship between hypertension and the various factors analyzed. In the high SBP group, age, BMI, waist circumference, TC/TG/LDL/HDL, family history, education level, daily iodized salt consumption, and smoking history were associated with high SBP in this study population, while sex, frequency of eating fried foods weekly, frequency of eating dessert weekly, drinking history, and frequency of weekly exercises were not associated with high SBP. Sex, age, BMI, waist circumference, TC/TG/LDL, family history, education level, frequency of eating fried foods weekly, frequency of eating desserts weekly, and daily iodized salt consumption were associated with high DBP in this study population, whereas HDL level, smoking history, drinking history, and frequency of weekly exercise were not statistically different in the high DBP group. Simultaneously, the Hardy–Weinberg Equilibrium analysis was performed on the selected SNPs. The results show that rs2472510, rs2515614, rs2297406, and rs2472433 reach genetic equilibrium in the sample population of this study (Table 3).
Association Between ABCA1 Gene Polymorphisms and Hypertension
The relationship between different gene models under each ABCA1 locus and hypertension was analyzed by binary logistic regression. From the results shown in Figure 1, there is no relationship between the various genotypes under rs2472510, rs2515614, rs2297406, rs2472433, and high SBP. However, in rs2472510, the GG type (odds ratio [OR] = 1.56, 95% CI = 1.155–2.105, p = 0.004) in the additive model and the TG + GG type (OR = 1.2, 95% CI = 1.002–1.437, p = 0.047) in the dominant model were more likely to have high DBP as compared to the TT type, and in the recessive model, the TG + TT type (OR = 0.681, 95% CI = 0.513–0.905, p = 0.008) had a high risk of DBP, which was 0.681 times than that of the GG type. In rs2515614, the CC type (OR = 1.536, 95% CI = 1.141–2.067, p = 0.005) in the additive model was more likely to have high DBP than the AA type, and the AC + AA type (OR = 0.684, 95% CI = 0.516–0.906, p = 0.008) in the recessive model had a 0.684 times higher risk of high DBP than the CC type. Neither rs2297406 nor rs2472433 was associated with high DBP (Figure 2).
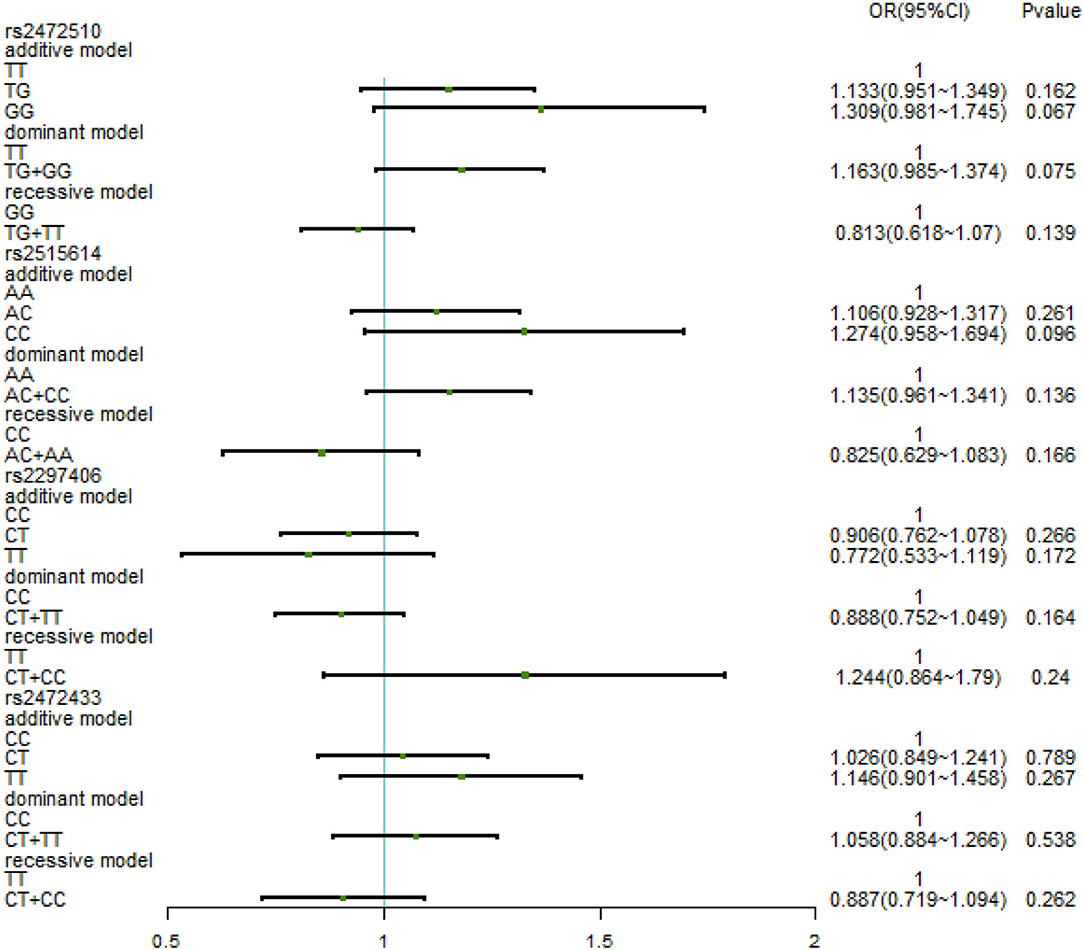
Figure 1. Binary logistic regression analysis for the association between genotypes of rs2472510, rs2515614, rs2297406, rs2472433, and high SBP.
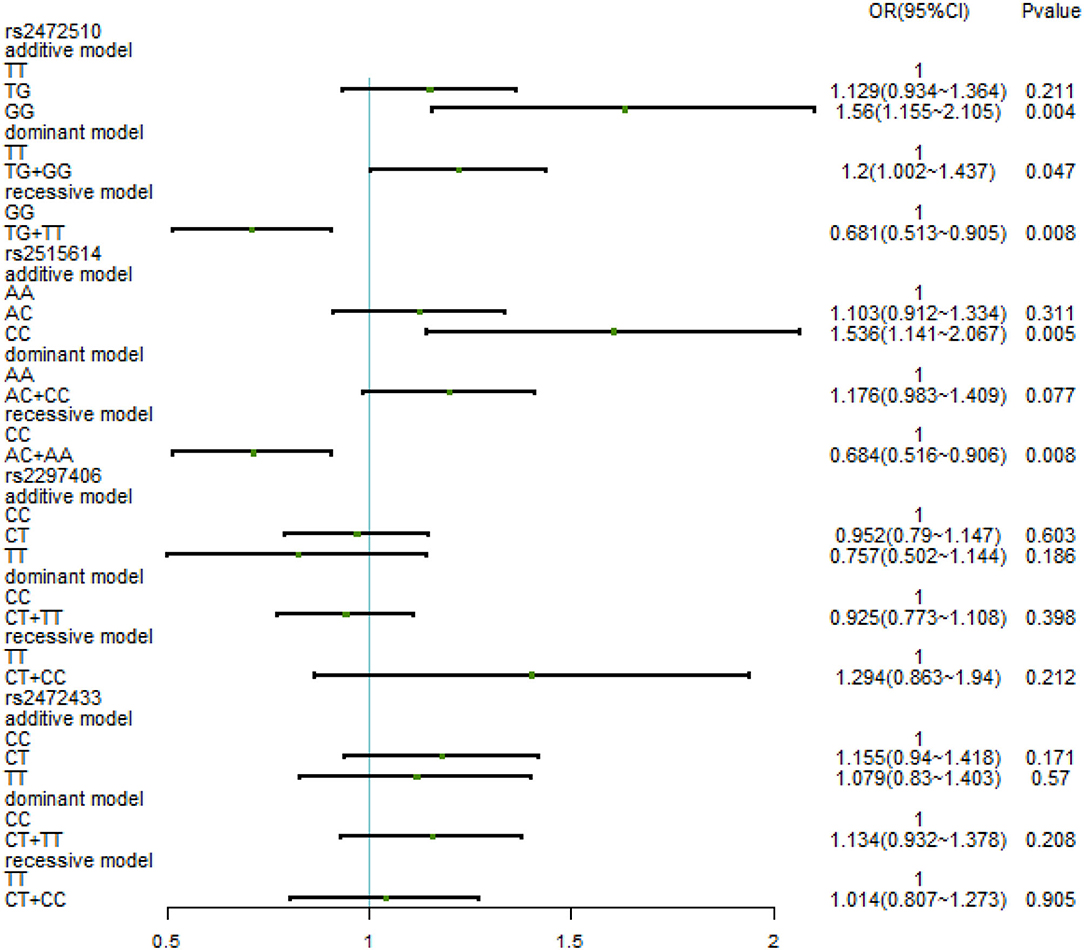
Figure 2. Binary logistic regression analysis for the association between genotypes of rs2472510, rs2515614, rs2297406, rs2472433, and high DBP.
After adjusting for factors associated with high SBP in the previous correlation analysis, such as age, BMI, waist circumference, TC/TG/LDL-C/HDL-C, family history, education level, daily iodized salt consumption, and smoking history, there was still no relationship between the various genotypes under the four SNPs and high SBP (Figure 3). In the high DBP group, adjustments were made for gender, age, BMI, waist circumference, TC/TG/LDL-C, family history of disease, education level, frequency of fried foods per week, times of desserts per week, and daily iodized salt consumption quantity. In rs2472510, the risk of high DBP in the GG type (adOR = 1.48, 95% CI = 1.072–2.042, p = 0.017) in the additive model was 1.48 times higher than that of the TT type, and in the recessive model, the risk of high DBP in the TG + TT type (adOR = 0.715, 95% CI = 0.527–0.97, p = 0.031) was 0.715 times than that of the GG type, but there was no statistical significance in the dominant model. In rs2615614, the risk of high DBP in the CC type (adOR = 1.472, 95% CI = 1.07–2.206, p = 0.017) was 1.472 times higher than that in the AA type in the additive model, while the risk of high DBP in the AC + AA type (adOR = 0.711, 95% CI = 0.526–0.962, p = 0.027) was 0.711 times higher than that in the CC type in the recessive model. The dominant genotype was not statistically significant. After adjusting for possible confounders, neither rs2297406 nor rs2472433 remained associated with high DBP (Figure 4).
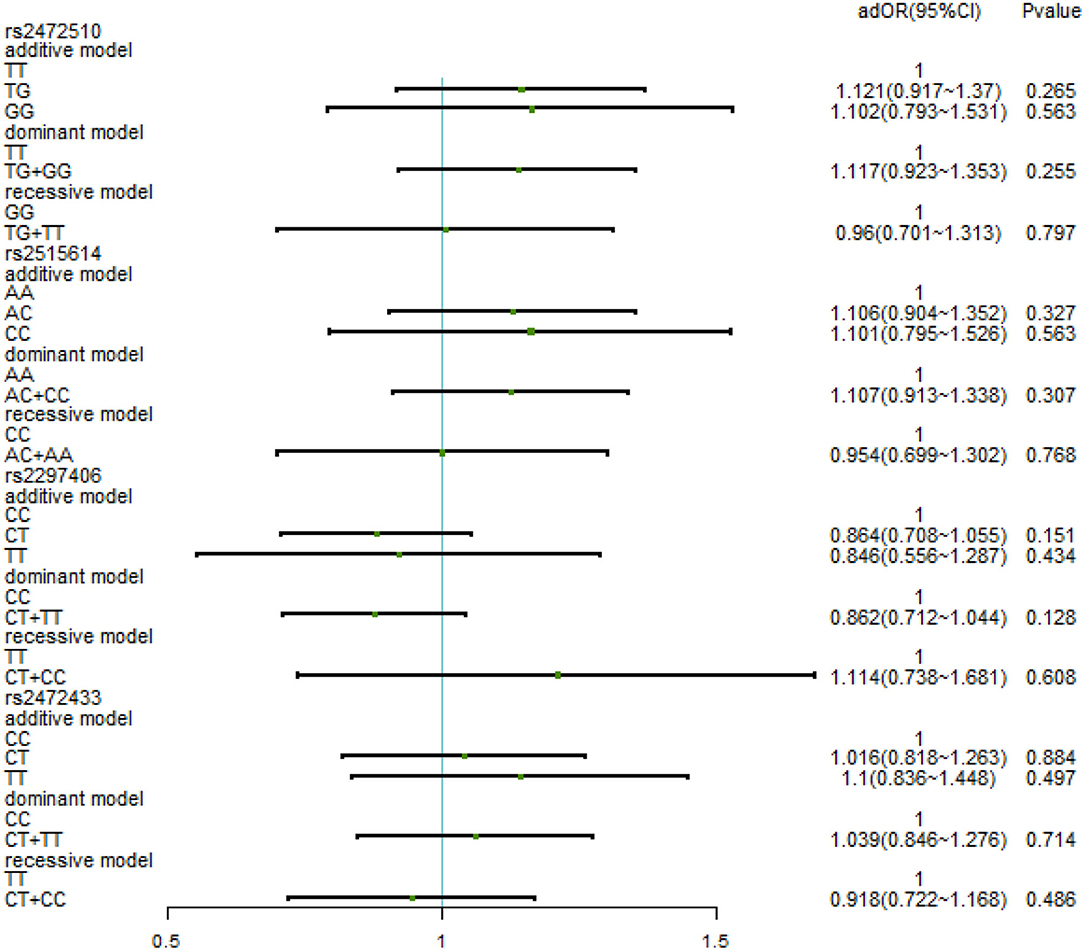
Figure 3. Binary logistic regression analysis for association between genotypes of rs2472510, rs2515614, rs2297406, rs2472433, and high SBP. Adjusted some covariates, such as age, BMI, waist circumference, TC (total cholesterol)/TG (triglyceride)/LDL-C (low-density lipoprotein)/HDL-C (high-density lipoprotein cholesterol), family history, education level, daily iodized salt consumption, and smoking history.
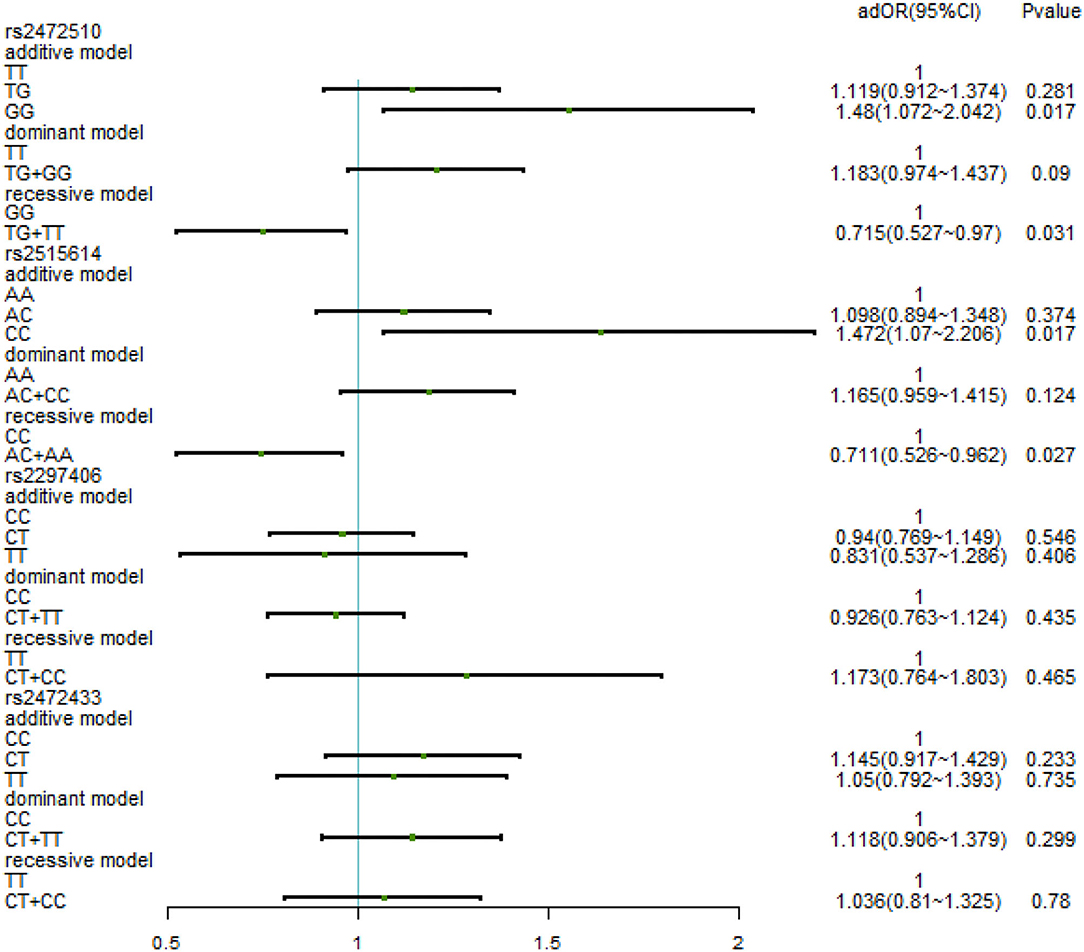
Figure 4. Binary logistic regression analysis for association between genotypes of rs2472510, rs2515614, rs2297406, rs2472433, and high DBP. Adjusted some covariates, gender, age, BMI, waist circumference, TC/TG/LDL-C, family history of the disease, education level, times of fried foods per week, times of desserts per week, and daily iodized salt consumption.
Association Between ABCA1 Haplotypes and Hypertension
The frequencies of the four haplotypes were compared between the hypertensive cases and controls, and the allelic arrangement of the haplotypes was rs2297406-rs2472433-rs2472510-rs2515614. Eight common haplotypes (frequency > 1%) that were derived from the four SNPs accounting for 99.6% of the haplotype variation were selected, and the remaining haplotypes were CCGA, TTTC, TCGA, TTGA, TCTC, CCTC, CTTC, and CTGA (the frequencies of the all were less than 1%). Table 4 shows the relationship between haplotypes and high SBP. Taking CTTA as a reference, only the TCTA (OR = 0.791, 95% CI = 0.659–0.951, p = 0.006) type had a 0.791 times higher risk of high SBP, and other haplotypes were not associated with high SBP. In the relationship between haplotype and high DBP (Table 5), compared with CTTA, CCTA had a 0.872 times higher risk of DBP, TCTA was 0.801 times, and TTTA was 0.68 times (OR = 0.872, 95% CI = 0.751–1.013, p = 0.037; OR = 0.801, 95% CI = 0.656–0.979, p = 0.015; OR = 0.68, 95% CI = 0.491–0.943, p = 0.011), and other haplotypes had no statistically significant effect on high DBP.
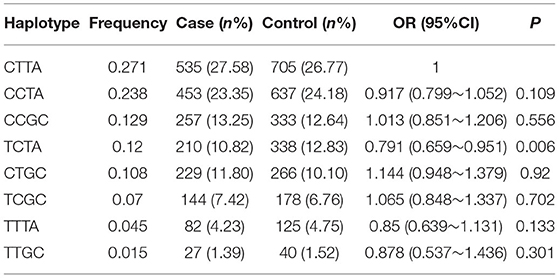
Table 4. Frequencies of haplotypes (rs2297406-rs2472433-rs2472510-rs2515614) among cases and controls and association with the risk of SBP.
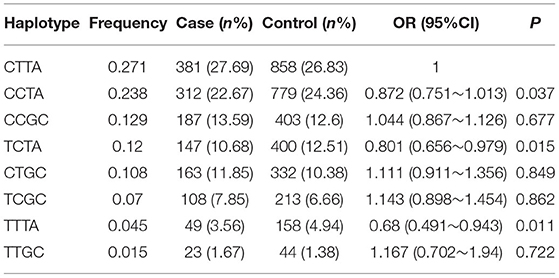
Table 5. Frequencies of haplotypes (rs2297406-rs2472433-rs2472510-rs2515614) among cases and controls and association with the risk of DBP.
Discussion
This study explored the significant relationship between four SNPs (rs2297406, rs2472433, rs2472510, and rs2515614) in ABCA1 with hypertension in the southeast Chinese Han population. The evidence suggested that mutations in the ABCA1 gene were associated with extremely low levels of HDL in humans (20).
High-density lipoproteins (HDL) have a mean size of 8–10 nm and a density of 1.063–1.21 g/ml (21). The biogenesis of HDL and synthesis and secretion of apolipoproteinsA1 (ApoA1) mainly happen in the liver and intestine. ApoA1 interacts with the ABCA1 expressed by many cell types (including hepatocytes, enterocytes, and macrophages) to exchange lipids producing a nascent HDL particle (22, 23). Therefore, ABCA1 performs a necessary role in mediating cellular cholesterol, phospholipid efflux, and HDL biosynthesis (24). ABCA1 was an integral membrane protein, which transported excess cholesterol from peripheral tissues to the liver for excretion, by mediating the first step of reverse cholesterol transport (RCT) (25, 26). Xu et al. verified that cholesterol efflux from monocyte-derived macrophages to autologous serum, HDL was impaired in hypertensive patients, and that ABCA1 expression was associated with this impairment (17).
Recent studies have reported that ABCA1 plays a key role in the biogenesis of HDL (17). ABCA1 influenced the secretion of cellular inflammatory cytokine by modulating cholesterol content in the plasma membrane and within intracellular compartments. In humans, the mutations of ABCA1 can lead to cholesterol deposition in tissue macrophages, resulting in a severe family HDL-deficiency syndrome (20, 27). In addition, ABCA1 was identified to be the causative gene for Tangier disease (TD), a rare genetic disorder that exhibits severe HDL reduction and a high incidence of premature cardiovascular disease. Therefore, ABCA1 plays an important function in the development of atherosclerosis and hypertension (28).
It was shown by a mouse model that a specific deletion of ABCA1 in principal cells would elevate the intracellular cholesterol levels and increase SBP (29). Wang et al. have also demonstrated that inhibitors of ABCA1 stimulate ENaC in distal renal cells, through a pathway associated with inhibition of the ABCA1 transporter, increases intracellular cholesterol (30). ENaC plays an important role in regulating sodium reabsorption and controlling BP levels. Therefore, mutations in ABCA1 lead to intracellular cholesterol accumulation and stimulate ENaC activity, increasing BP levels finally (31).
Furthermore, a study supported that the impairment of ABC transporters might not only potentially influence the cholesterol deposition in monocyte/macrophages to form foam cells but also indicate vascular injury in hypertensive patients (17). Cholesterol accumulation was considered to be associated with the vascular endothelium that actively regulates vascular tone, lipid breakdown, thrombogenesis, inflammation, and vessel growth (32). Hypertension was considered to be related to the inflammatory and immune processes. Therefore, our study suggests that the ABCA1 gene polymorphisms may contribute to the inflammatory responses, vascular injury processes, cholesterol efflux dysfunction, and the activation of the ENaC, ultimately resulting in increased BP levels. These require further research and exploration for us.
The environmental factors of hypertension were also analyzed in this study. We found that age, BMI, waist, dyslipidemia, family history, daily iodized salt consumption, and educational level were associated with hypertension. Aging was a vital contributor to the development of hypertension. It was predicted that the elderly will account for 30% of the whole population of China by 2050. Therefore, regularly monitoring BP levels in middle-aged and elderly people was very significant (33). BMI and waist-to-hip ratio had been a comprehensive indicator of the outcome of acquired lifestyle and are closely associated with the occurrence of hypertension (34, 35). Family history of hypertension was a crucial marker of genetic factors, it was used as an alternative indicator to study the relationship between genetic factors and diseases frequently (36). The speedy economic growth, especially with a higher dietary salt intake, was another new and crucial factor related to the increased prevalence of hypertension in China, and quite a few lines of proof that include epidemiological observations, animal studies, and clinical trials have constantly proven a causal relationship between dietary salt consumption and hypertension (37, 38). Excess dietary salt consumption was also an essential risk factor for the development and progression of cardiovascular disease. Educational degree is an important socioeconomic factor, there was developing proof that in addition to these widespread risk factors, income level and social status were factors that may associated be with BP control (39). As a result, stopping unhealthy lifestyles may additionally make a contribution to patients' health. Interactions between environmental and epigenetic elements need to be further investigated. Thus, healthcare providers were cautioned to provide furnish extra support and health education for those patients to achieve proposed BP goals and thereby decrease future cardiovascular risk.
There were some limitations to this study. First of all, this study only mentioned the association of ABCA1 gene polymorphisms with hypertension. There were still many unmeasured genetic factors and environmental factors that were not taken into consideration. We could not estimate all the potential risk factors for hypertension completely. Secondly, this study was performed in eastern China, and a massive number of samples were needed to investigate the relationship and confirm our findings. Finally, this study only included Han Chinese people, future research should also encompass different nations or races due to genetic heterogeneity among different human species, this could enable more accurate results.
Despite these limitations, these results still have practical implications for the prevention and treatment of hypertension. Hypertension is one of the most prevalent diseases and public health problems that threaten human health, genetic and genomic research studies have provided important viewpoints into the etiology and pathogenesis of hypertension. Since 2007, the sample size of a series of sequential genome-wide association studies (GWAS) for BP and hypertension has grown exponentially, with the latest recent study involving 1 million subjects and having identified over 1,477 SNPs associated with BP traits, explaining approximately 27% of the 30–50% estimates heritability of BP (40, 41). Because of the complexity of BP regulation mechanisms, further genomic studies may be able to provide genetic tools in the etiology of hypertension and provide a new treatment option for hypertension patients. In addition, genomics can provide a deeper understanding of molecular pathways that regulate BP, which can inform precision medicine and provide the basis for new drug development and personalized treatment.
Conclusion
In conclusion, the present study has identified that hypertension probability was associated with rs2472510 and rs2515614. Considering these relevant factors can also show informative for predicting hypertension.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Hangzhou Normal University (No. 2013020). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YR, ET, XT, and LY designed the study and with all co-authors carrying out the study design. CD, YZ, and LX recruited and followed up the study participants. YR and ET drafted the manuscript and which was revised by all authors. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Zhejiang Province (Grant Nos. LQ18H190003 and LY12H16028) and the National Natural Science Foundation of China (Grant No. 81772168).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the individuals who participated in this study. We also thank the investigators that worked on this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.878610/full#supplementary-material
References
1. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. doi: 10.1016/s0140-6736(21)01330-1
2. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. (2007) 370:591–603. doi: 10.1016/S0140-6736(07)61299-9
3. Phillips RA, Arnold RM, Peterson LE. Hypertension guidelines: the threads that bind them. J Am Coll Cardiol. (2018) 72:1246–51. doi: 10.1016/j.jacc.2018.07.014
4. Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL. Effects of intensive BP control in CKD. J Am Soc Nephrol. (2017) 28:2812–23. doi: 10.1681/ASN.2017020148
5. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. (2017) 120:472–95. doi: 10.1161/CIRCRESAHA.116.308398
6. Malhotra R, Nguyen HA, Benavente O, Mete M, Howard BV, Mant J. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: a systematic review and meta-analysis. JAMA Intern Med. (2017) 177:1498–505. doi: 10.1001/jamainternmed.2017.4377
7. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. (2017) 5:543–51. doi: 10.1016/j.jchf.2017.04.012
8. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC Health Promotion Series. J Am Coll Cardiol. (2018) 72:1278–93. doi: 10.1016/j.jacc.2018.07.008
9. Joseph G, Marott JL, Biering-Sørensen T, Johansen MN, Saevereid HA, Nielsen G. Level of physical activity, left ventricular mass, hypertension, and prognosis. Hypertension. (2020) 75:693–701. doi: 10.1161/HYPERTENSIONAHA.119.14287
10. Zhou B, Bentham J, Cesare Di, Bixby M, Danaei H, Cowan GMJ, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. (2017) 389:37–55. doi: 10.1016/s0140-6736(16)31919-5
11. Li AL, Fang X, Zhang YY, Peng Q, Yin XH. Familial aggregation and heritability of hypertension in Han population in Shanghai China: a case-control study. Clin Hypertens. (2019) 25:17. doi: 10.1186/s40885-019-0122-z
12. Yamada Y, Matsui K, Takeuchi I, Oguri M, Fujimaki T. Association of genetic variants with hypertension in a longitudinal population-based genetic epidemiological study. Int J Mol Med. (2015) 35:1189–98. doi: 10.3892/ijmm.2015.2151
13. Frambach S, de Haas R, Smeitink JAM, Rongen GA, Russel FGM, Schirris TJJ. Brothers in arms: ABCA1- and ABCG1-mediated cholesterol efflux as promising targets in cardiovascular disease treatment. Pharmacol Rev. (2020) 72:152–190. doi: 10.1124/pr.119.017897
14. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochimica et Biophysica Acta (BBA) - Rev Cancer. (2020) 1874:188394. doi: 10.1016/j.bbcan.2020.188394
15. Zhai YJ, Wu MM, Linck VA, Zou L, Yue Q, Wei SP. Intracellular cholesterol stimulates ENaC by interacting with phosphatidylinositol-4,5-bisphosphate and mediates cyclosporine A-induced hypertension. Biochimica et Biophysica Acta (BBA) - Molec Basis Dis. (2019) 1865:1915–1924. doi: 10.1016/j.bbadis.2018.08.027
16. Wang ZR, Liu HB, Sun YY, Hu QQ, Li YX, Zheng WW, et al. Dietary salt blunts vasodilation by stimulating epithelial sodium channels in endothelial cells from salt-sensitive Dahl rats. Br J Pharmacol. (2018) 175:1305–1317. doi: 10.1111/bph.13817
17. Xu M, Zhou H, Gu Q, Li C. The expression of ATP-binding cassette transporters in hypertensive patients. Hypertens Res. (2009) 32:455–461. doi: 10.1038/hr.2009.46
18. Yamada Y, Kato K, Yoshida T, Yokoi K, Matsuo H, Watanabe S. Association of polymorphisms of ABCA1 and ROS1 with hypertension in Japanese individuals. Int J Mol Med. (2008) 21:83–9. doi: 10.3892/ijmm.21.1.83
19. Prevention CH. Guidelines for the prevention and treatment of hypertension in China (2018 Revised Edition). Chin Cardiov J. (2019) 24:24–56. doi: 10.3969/j.issn.1007-5410.2019.01.002
20. Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. (1999) 22:336–345. doi: 10.1038/11905
21. Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. (1955) 34:1345–53. doi: 10.1172/JCI103182
22. Parks JS, Chung S, Shelness GS. Hepatic ABC transporters and triglyceride metabolism. Curr Opin Lipidol. (2012) 23:196–200. doi: 10.1097/MOL.0b013e328352dd1a
23. Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: much more than lipid transporters. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21030732
24. Wang D, Hiebl V, Xu T, Ladurner A, Atanasov AG, Heiss EH. Impact of natural products on the cholesterol transporter ABCA1. J Ethnopharmacol. (2020) 249:112444. doi: 10.1016/j.jep.2019.112444
25. Oram JF, Vaughan AM, Stocker R. ATP-binding Cassette Transporter A1 Mediates Cellular Secretion of α-Tocopherol. J Biol Chem. (2001) 276:39898–902. doi: 10.1074/jbc.M106984200
26. Huesca-Gómez C, Torres-Paz YE, Martínez-Alvarado R, Fuentevilla-Álvarez G, Del Valle-Mondragón L, Torres-Tamayo M. Association between the transporters ABCA1/G1 and the expression of miR-33a/144 and the carotid intima media thickness in patients with arterial hypertension. Mol Biol Rep. (2020) 47:1321–9. doi: 10.1007/s11033-019-05229-0
27. Wang F, Wang X, Ye P, Cao R, Zhang Y, Qi Y, et al. High-density lipoprotein 3 cholesterol is a predictive factor for arterial stiffness: a community-based 4.8-year prospective study. Lipids Health Dis. (2018) 5. doi: 10.1186/s12944-017-0650-z
28. Fan Q, Zhu Y, Zhao F. Association of rs2230806 in ABCA1 with coronary artery disease: An updated meta-analysis based on 43 research studies. Medicine. (2020) 99:e18662–e18662. doi: 10.1097/MD.0000000000018662
29. Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X. Structure of the Human Lipid Exporter ABCA1. Cell. (2017) 169:1228–1239.e10. doi: 10.1016/j.cell.2017.05.020
30. Wu MM, Liang C, Yu XD, Song BL, Yue Q, Zhai YJ, et al. Lovastatin attenuates hypertension induced by renal tubule-specific knockout of ATP-binding cassette transporter A1, by inhibiting epithelial sodium channels. Br J Pharmacol. (2019) 176:3695–3711. doi: 10.1111/bph.14775
31. Wang J, Zhang ZR, Chou CF, Liang YY, Gu Y, Ma HP. Cyclosporine stimulates the renal epithelial sodium channel by elevating cholesterol. Am J Physiol Renal Physiol. (2009) 296:F284–90. doi: 10.1152/ajprenal.90647.2008
32. Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. (2007) 32:172–9. doi: 10.1016/j.tibs.2007.02.001
33. Vogel RA. Cholesterol lowering and endothelial function. Am J Med. (1999) 107:479–87. doi: 10.1016/S0002-9343(99)00261-2
34. Chen Z, Song YJ, Chui Y. Aging Beijing: challenges and strategies of health care for the elderly. Age Res Rev. (2010) 9:S2–S5. doi: 10.1016/j.arr.2010.07.001
35. Li AL, Peng Q, Shao YQ, Fang X, Zhang YY. The effect of body mass index and its interaction with family history on hypertension: a case-control study. Clin Hypertens. (2019) 25:6. doi: 10.1186/s40885-019-0111-2
36. Song J, Zhao Y, Nie S, Chen X, Wu X, Mi J. The effect of lipid accumulation product and its interaction with other factors on hypertension risk in Chinese Han population: a cross-sectional study. PLoS ONE. (2018) 13:e0198105. doi: 10.1371/journal.pone.0198105
37. Li AL, Peng Q, Shao YQ, Fang X, Zhang YY. The interaction on hypertension between family history and diabetes and other risk factors. Sci Rep. (2021) 11:4716. doi: 10.1038/s41598-021-83589-z
38. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation. (2018) 137:2344–56. doi: 10.1161/CIRCULATIONAHA.117.032380
39. Ma Z, Hummel SL, Sun N, Chen Y. From salt to hypertension, what is missed? J Clin Hypertens (Greenwich). (2021) 23:2033–41. doi: 10.1111/jch.14402
40. Mourtzinis G, Manhem K, Kahan T, Schiöler L, Isufi J, Ljungman C. Socioeconomic status affects achievement of blood pressure target in hypertension: contemporary results from the Swedish primary care cardiovascular database. Scand J Prim Health Care. (2021) 39:519–26. doi: 10.1080/02813432.2021.2004841
Keywords: hypertension, ABCA1, association, gene polymorphisms, Chinese
Citation: Ren Y, Tong E, Di C, Zhang Y, Xu L, Tan X and Yang L (2022) Association Between ABCA1 Gene Polymorphisms and the Risk of Hypertension in the Chinese Han Population. Front. Public Health 10:878610. doi: 10.3389/fpubh.2022.878610
Received: 18 February 2022; Accepted: 25 March 2022;
Published: 20 May 2022.
Edited by:
Lianping He, Taizhou University, ChinaReviewed by:
Kexue Wang, Guangzhou Jianli Medical and Beauty Outpatient Department, ChinaBaohong Xue, Anhui Polytechnic University, China
Copyright © 2022 Ren, Tong, Di, Zhang, Xu, Tan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Tan, eGlhb2h1YXRhbkBoem51LmVkdS5jbg==; Lei Yang, eWFuZ2xlaTYyQGh6bnUuZWR1LmNu
†These authors have contributed equally to this work
 Yanli Ren
Yanli Ren Enyu Tong
Enyu Tong Chunhong Di
Chunhong Di Yunheng Zhang1
Yunheng Zhang1 Xiaohua Tan
Xiaohua Tan Lei Yang
Lei Yang