
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 11 April 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.870065
This article is part of the Research Topic Infections in the Intensive Care Unit View all 17 articles
Aim: Syndecan-1 (SDC-1) has been shown to have a high predictive value for sepsis development, though uncertainty around these results exists. The aim of this meta-analysis was to assess the prognostic ability of SDC-1 in predicting sepsis-related complications and mortality.
Methods: We searched PubMed, EMBASE, Cochrane Library, and Google Scholar databases from January 01, 1990, to March 17, 2021, to identify eligible studies. The search terms used were “SDC-1,” “sepsis,” “severe sepsis,” and “septic shock,” and a meta-analysis was performed using the RevMan 5.4 software.
Results: Eleven studies with a total of 2,318 enrolled patients were included. SDC-1 concentrations were significantly higher in the composite poor outcome group [standardized mean difference (SMD) = 0.55; 95% CI: 0.38–0.72; P < 0.001] as well as in deceased patients (SMD = 0.53; 95% CI: 0.40–0.67; P < 0.001), patients with septic shock (SMD = 0.81; 95% CI: 0.36–1.25; P < 0.001), and patients with acute kidney injury (SMD = 0.48; 95% CI: 0.33–0.62; P < 0.001). Statistical significance was also found in the subgroup analysis when stratified by different sepsis diagnostic criteria.
Conclusion: Baseline SDC-1 levels may be a useful predictor of sepsis-related complications and mortality.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021246344, PROSPERO, identifier: CRD42021246344.
Sepsis is a life-threatening condition characterized by a dysregulated response to infection and is associated with organ dysfunction and high mortality rates (1, 2). Early identification of sepsis patients with a high risk of poor outcomes is vital and can reduce mortality and improve prognosis.
Glycocalyx degradation is a critical driver of organ failure in sepsis due to a combination of pathophysiologic insults (3, 4). It is associated with the development of shock (5, 6), acute kidney injury (AKI) (7), coagulopathy (8), acute respiratory distress syndrome (ARDS)/respiratory failure (9, 10), and mortality (9, 11). Identifying biological markers of glycocalyx degradation may be an essential step in improving outcomes in patients with sepsis.
Syndecan-1 (SDC-1) has been identified as one such biomarker (12, 13), with levels of SDC-1, being elevated in some studies (14, 15). Moreover, multiple studies have shown that SDC-1 levels increased in patients with sepsis, including those with severe sepsis and septic shock. However, only a few studies have demonstrated SDC-1 as a prognostic tool and predictive marker of poor outcomes in patients with sepsis (5, 11, 16, 17). Some previous studies have also included patients with severe sepsis and septic shock. The diagnostic criteria for sepsis have changed three times from 1991 to 2016, which complicates generalization across these studies. Moreover, SDC-1 levels are variable across the longitudinal course of sepsis (11, 18, 19).
The aim of this meta-analysis was to examine the prognostic value of SDC-1 levels upon admission as a predictor of sepsis-related complications and mortality.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered with PROSPERO (CRD42021246344).
A systematic search of the literature across the PubMed, EMBASE, Cochrane Library, and Google Scholar databases from January 01, 1990, to March 17, 2021, was performed using the following keywords: “sepsis,” “severe sepsis,” “septic shock,” and “SDC-1.” We excluded review articles, letters, communications, case reports, and articles published in languages other than English. The reference lists of articles were also reviewed to identify additional relevant studies.
Studies containing the following were included: (1) a prospective study method, (2) patient cohorts aged >18 years, (3) an SDC-1 assessment of serum or plasma within 24 h after admission, and (4) clear diagnostic criteria for sepsis. Moreover, the following reports were excluded: (1) duplicated publications, (2) studies with data not reported or data that could not be transformed into a mean with the standard deviation (SD), and (3) studies which included patients without sepsis. Two investigators (TS and YW) independently extracted studies that complied with the criteria.
A standardized form containing first author, year of publication, admission setting, study design, age, sex, number of participants, serum or plasma concentrations, outcomes, and the standards used to define sepsis was recorded. The mean difference and SD were used to pool data, while other forms of data were transformed and described as the mean ± SD (20, 21). For this meta-analysis, “poor outcome” was a composite measure, incorporating mortality and sepsis-associated complications, including septic shock, AKI, disseminated intravascular coagulation (DIC), and ARDS.
Two authors (YW and XW) performed the data extraction independently, using the Newcastle-Ottawa Scale (NOS) to assess the quality of the observational studies. The NOS assigns studies a score of up to nine points based on subject, comparability, and the outcome of interest assessed, with a score of ≥6 indicating a high-quality study.
Sepsis and septic shock definitions were based on three criteria: sepsis 1 (ACCP/SCCM 1991) (22), sepsis 2 (SCCM/ACCP/ATS/SIS 2001) (23), or sepsis 3 (SCCM/ESICM 2016) (24). The diagnosis of AKI was based on either the Acute Kidney Injury Network (AKIN) (25) or Kidney Disease Improving Global Outcomes (KDIGO) (26) criteria. Diagnoses of DIC and ARDS were based on the criteria specified by the International Society of Thrombosis and Hemostasis (27) and Berlin ARDS definition 2012 (28), respectively.
For this meta-analysis, we used the Review Manager 5.4 (Cochrane Collaboration) software to investigate the association between SDC-1 and poor outcome. Heterogeneity between studies was assessed using the χ2 test and inconsistency index (I2). An I2 > 50% with P < 0.05 was considered indicative of significant heterogeneity. In such cases, a random effect model was chosen, where each measure for poor outcome was then sub-analyzed to explore the source of heterogeneity. Otherwise, a fixed effect model was used. We evaluated publication bias by examining funnel plots when the number of studies reporting the primary clinical outcomes was 10 or more. All tests were two-tailed, and p < 0.05 was defined as statistically significant.
Our initial search of the databases led to the identification of 628 reports, of which 208 were duplicates and subsequently discarded. The titles and abstracts of the remaining 420 reports were then screened, after which, 380 reports were discarded. The full-text articles for 40 studies were read. In total, 11 studies conducted in Asia, Europe, and North America met our inclusion criteria. The procedures used for study selection are described in Figure 1.
The eligible studies had a total of 2,318 enrolled patients, 1,375 of whom were male (59.3%). The measures of poor outcome examined included mortality in six studies (11, 29–33), septic shock in five studies (11, 33–36), AKI in three studies (29, 30, 37), and DIC in two studies (36, 38). The study characteristics (i.e., country, year, study design, sepsis definition, age, gender, sampling to analysis, and outcome), Sequential Organ Failure Assessment (SOFA) scores of patients in each study, and NOS scores of the 11 studies (range = 6–7) are shown in Table 1.
SDC-1 levels were significantly higher in the poor outcome group (standardized mean difference [SMD] 0.55; 95% confidence interval [CI] 0.38–0.72; I2 = 57%; p < 0.001), indicating their potential use for early prediction of poor outcome (Figure 2).
Results from the subgroup analysis are presented in Figure 3A. SDC-1 levels were significantly higher in patients who died (SMD = 0.53; 95% CI: 0.40–0.67; I2 = 0%; p < 0.001), as well as in those who developed septic shock (SMD = 0.81; 95% CI: 0.36–1.25; I2 = 79%; p < 0.001) or AKI (SMD = 0.48; 95% CI: 0.33–0.62; I2 = 0%; p < 0.001). Similar results were found in a subgroup analysis when patients were stratified according to the different diagnostic criteria of sepsis 1, sepsis 2, and sepsis 3, as shown in Figure 3B (p < 0.001, p < 0.001, and p = 0.01, respectively). When combining studies which used the same diagnostic criteria, similar results were found.
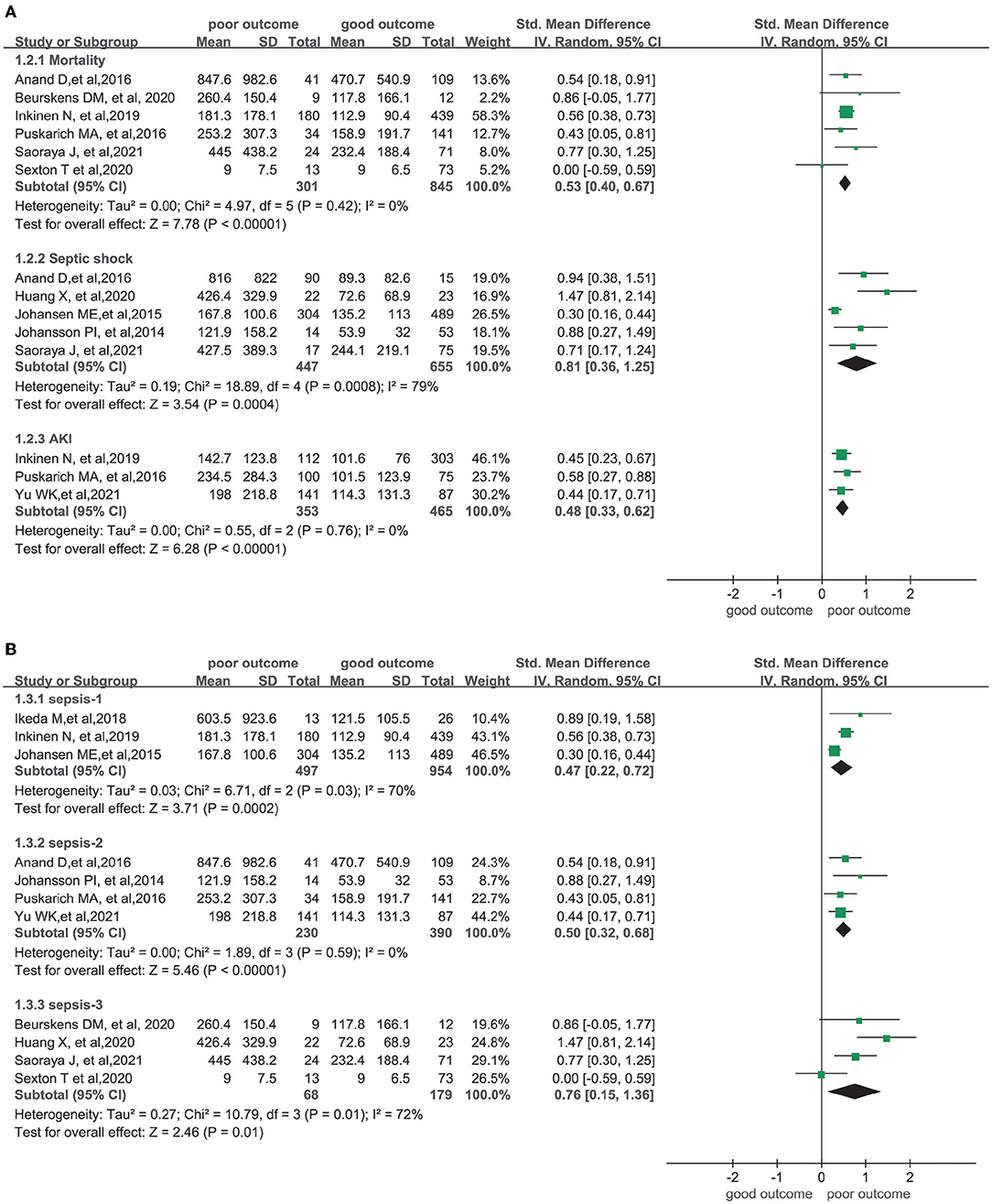
Figure 3. Forest plots of subgroups. (A) Subgroup of complications and mortality; (B) Subgroup of three diagnostic criterias for sepsis.
Among the studies which used the sepsis 1 and 2 diagnostic criteria, five reported SOFA scores (11, 29, 30, 34, 38) ranging from 5 to 9. Another study by Yu et al. (37) only included patients with severe sepsis. According to the sepsis 3 definition (24), patients in these six combined studies, with SOFA scores above 2 or with severe sepsis noted, could be categorized as having sepsis. A meta-analysis including 10 of the studies was also conducted, and a significant difference in SDC-1 levels was noted between patients with poor and good outcomes (SMD = 0.57; 95% CI: 0.45–0.68; I2 = 40%; p < 0.001), as illustrated in Figure 4.
A leave-one-out meta-analysis was performed to detect the influence of heterogeneity on SMD. Sensitivity analysis revealed that heterogeneity decreased when the studies conducted by Huang et al. (from 55 to 36%) and Johansen et al. (from 55 to 40%) were individually removed. When both were removed, heterogeneity was further reduced (from 55 to 0%), and higher SDC-1 levels were noted in the poor outcome group (SMD = 0.54; 95% CI: 0.42–0.66, p < 0.001).
To evaluate publication bias, the included studies were examined using a funnel plot. A qualitatively symmetrical funnel plot was noted, indicating that no significant publication bias existed in this meta-analysis (Figure 5).
This is the first meta-analysis to examine the prognostic value of baseline SDC-1 levels to predict sepsis-related complications and mortality. SDC-1 levels were higher in the poor outcome group compared with the good outcome group. In a subgroup analysis, SDC-1 levels were significantly higher in deceased patients as well as in those with septic shock or AKI. These results suggest that sepsis patients with higher baseline SDC-1 levels may be at a higher risk of poor outcomes.
A common factor in organ failure is endothelial dysfunction. Degradation of the endothelial glycocalyx occurs in inflammatory states and quickly alters the physiological function of the endothelium, which is implicated in the pathogenesis of critically ill (39), ARDS (40), coronavirus disease 2019 (COVID-19) (41), and pneumonia patients (42). The Sidestream Dark Field (SDF) imaging of the sublingual area is a direct method to assess the thickness of glycocalyx in sepsis patients, but its application is limited by specialized equipment and software. Donati et al. (43) used SDF imaging and found more severe glycocalyx alterations in sepsis patients than in intensive care unit (ICU) patients without sepsis. Beurskens et al. (31) also found significantly lower endothelial glycocalyx thickness in non-survivors than in survivors with sepsis. The most common method for measuring glycocalyx breakdown products is through plasma/serum measurements. SDC-1, a biomarker of glycocalyx degradation, increases with disease severity and is related to poor prognosis in sepsis patients (10, 16, 17, 44). In our meta-analysis, only one of the included studies did not support the prognostic role of SDC-1 in patients with sepsis (32).
However, SDC-1 levels vary over the longitudinal course and relative progression of sepsis (11, 15). Anand et al. (11) found that SDC-1 levels increased over the first week of ICU admission in non-surviving patients with sepsis, compared with those who survived. In the surviving group, SDC-1 levels tended to decrease after the first week. Fraser et al. (45) found a persistent elevation in SDC-1 levels over the first 3 days of ICU admission in patients with COVID-19. In our meta-analysis, the unified selection criteria included prospective studies where SDC-1 levels were measured within the first 24 h after admission, which allowed us to further confirm the prognostic value of SDC-1 for the outcome prediction in patients with sepsis.
Although, there was significant heterogeneity across the 11 studies included in this review, sensitivity analyses indicated that the pooled results were robust. In sensitivity analysis testing, similar results were found when the two studies by Johansen et al. (35) and Huang et al. (36) were removed. The present meta-analysis suggests that SDC-1 may be a useful biological marker for the prediction of sepsis-related complications and mortality.
In the subgroup analysis, we found considerably higher heterogeneity in the septic shock and sepsis 3 subgroups. In the septic shock group, two studies used sepsis 2 criteria, two used sepsis 3 criteria, and only one used sepsis 1 criteria. Therefore, we speculated that the heterogeneity may have been due to the different diagnostic criteria of sepsis, as the diagnosis of septic shock varied considerably across the three criteria. A subgroup analysis, which included 10 studies that all met the sepsis 3 criteria, was also performed. Significantly higher concentrations of SDC-1 were observed in this subgroup compared with patients with good outcomes.
Despite the results of our meta-analysis, the use of a single biomarker to predict sepsis may not always be reliable. We hope that ongoing randomized trials (NCT 04718623 and NCT 04644302) will include a more in-depth analysis of the predictive markers for patients with sepsis.
This meta-analysis had several limitations. First, SDC-1 levels had a high SD, indicating a high level of variability. SDC-1 levels were reported using medians and interquartile range, which were then used to calculate the means and SDs in this meta-analysis. Second, the sample sizes of the included publications were small. Although we pooled the results of these publications, it may still have been possible to miss the effectiveness of the meta-analysis. Third, the included studies used different definitions of sepsis, which may have affected our results. In particular, the definition of septic shock was different, which could partially explain the substantial heterogeneity noted in the septic shock subgroup. However, subgroup and sensitivity analyses indicated that the pooled results were robust. Finally, prospective cohort trials were most qualified for our study objective, as the intervention could not be randomized. Therefore, our meta-analysis of the observational studies, and not of randomized control trials, could only support the potential association between increased SDC-1 and poor outcome in patients with sepsis.
This meta-analysis supported the prognostic value of SDC-1 as a predictor of mortality and sepsis-related complications.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
TS and YW extracted studies from the eligible papers. YW and XW performed the data extraction. TZ and YC analyzed the data. TS and QZ wrote the paper. QZ reviewed and revised the paper. All authors contributed to the conception and design of the work.
This work was funded by National Natural Science Foundation of China (NO. 81870072); Horizontal subject (NO. 2019-HX-77).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.870065/full#supplementary-material
1. Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. (2019) 23:196. doi: 10.1186/s13054-019-2478-6
2. Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O'Brien C, et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. (2019) 2:e187571. doi: 10.1001/jamanetworkopen.2018.7571
3. Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. (2019) 23:16. doi: 10.1186/s13054-018-2292-6
4. Cerny V, Astapenko D, Brettner F, Benes J, Hyspler R, Lehmann C, et al. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit Rev Clin Lab Sci. (2017) 54:343–57. doi: 10.1080/10408363.2017.1379943
5. Nelson A, Berkestedt I, Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand. (2014) 58:36–43. doi: 10.1111/aas.12223
6. Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettila V. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand. (2012) 56:316–22. doi: 10.1111/j.1399-6576.2011.02578.x
7. Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. (2016) 194:439–49. doi: 10.1164/rccm.201511-2281OC
8. Ostrowski SR, Haase N, Muller RB, Moller MH, Pott FC, Perner A, et al. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care. (2015) 19:191. doi: 10.1186/s13054-015-0918-5
9. Murphy LS, Wickersham N, McNeil JB, Shaver CM, May AK, Bastarache JA, et al. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann Intensive Care. (2017) 7:102. doi: 10.1186/s13613-017-0325-y
10. Smart L, Bosio E, Macdonald SPJ, Dull R, Fatovich DM, Neil C, et al. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J Crit Care. (2018) 47:93–8. doi: 10.1016/j.jcrc.2018.06.015
11. Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. (2016) 49:768–76. doi: 10.1016/j.clinbiochem.2016.02.014
12. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. (2015) 80:389–402. doi: 10.1111/bcp.12629
13. Jedlicka J, Becker BF, Chappell D. Endothelial glycocalyx. Crit Care Clin. (2020) 36:217–32. doi: 10.1016/j.ccc.2019.12.007
14. Suwarto S, Sasmono RT, Sinto R, Ibrahim E, Suryamin M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J Infect Dis. (2017) 215:992–9. doi: 10.1093/infdis/jix041
15. Smart L, Macdonald SPJ, Burrows S, Bosio E, Arendts G, Fatovich DM. Endothelial glycocalyx biomarkers increase in patients with infection during Emergency Department treatment. J Crit Care. (2017) 42:304–9. doi: 10.1016/j.jcrc.2017.07.001
16. Wei S, Gonzalez Rodriguez E, Chang R, Holcomb JB, Kao LS, Wade CE. Elevated syndecan-1 after trauma and risk of sepsis: a secondary analysis of patients from the pragmatic, randomized optimal platelet and plasma ratios (PROPPR) trial. J Am Coll Surg. (2018) 227:587–95. doi: 10.1016/j.jamcollsurg.2018.09.003
17. Holzmann MS, Winkler MS, Strunden MS, Izbicki JR, Schoen G, Greiwe G, et al. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomark Med. (2018) 12:119–27. doi: 10.2217/bmm-2017-0231
18. Orbegozo D, Rahmania L, Irazabal M, Mendoza M, Annoni F, De Backer D, et al. Endocan as an early biomarker of severity in patients with acute respiratory distress syndrome. Ann Intensive Care. (2017) 7:93. doi: 10.1186/s13613-017-0311-4
19. Naumann DN, Hazeldine J, Davies DJ, Bishop J, Midwinter MJ, Belli A, et al. Endotheliopathy of trauma is an on-scene phenomenon, and is associated with multiple organ dysfunction syndrome: a prospective observational study. Shock. (2018) 49:420–428. doi: 10.1097/SHK.0000000000000999
20. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
21. Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. (2020) 11:641–54. doi: 10.1002/jrsm.1429
22. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
23. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. (2003) 29:530–8. doi: 10.1007/s00134-003-1662-x
24. Deutschman CS, Singer M. Definitions for sepsis and septic shock–reply. JAMA. (2016) 316:458–9. doi: 10.1001/jama.2016.6389
25. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. (2007) 11:R31. doi: 10.1186/cc5713
26. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
27. Taylor FB Jr., Toh CH, Hoots WK, Wada H, Levi M, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. (2001) 86:1327–30. doi: 10.1055/s-0037-1616068
28. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
29. Puskarich MA, Cornelius DC, Tharp J, Nandi U, Jones AE. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at a high risk of intubation following large volume intravenous fluid resuscitation. Ann Emerg Med. (2016) 68:S147. doi: 10.1016/j.annemergmed.2016.08.402
30. Inkinen N, Pettilä V, Lakkisto P, Kuitunen A, Jukarainen S, Bendel S, et al. Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. (2019) 9:103. doi: 10.1186/s13613-019-0575-y
31. Beurskens DM, Bol ME, Delhaas T, van de Poll MC, Reutelingsperger CP, Nicolaes GA, et al. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth Intensive Care. (2020) 48:221–8. doi: 10.1177/0310057X20916471
32. Sexton T, Chalhoub G, Ye S, Morris W, Annabathula R, Dugan A, et al. Autotaxin activity predicts 30-day mortality in sepsis patients and correlates with platelet count and vascular dysfunction. Shock. (2020) 54:738–43. doi: 10.1097/SHK.0000000000001569
33. Saoraya J, Wongsamita L, Srisawat N, Musikatavorn K. Plasma syndecan-1 is associated with fluid requirements and clinical outcomes in emergency department patients with sepsis. Am J Emerg Med. (2021) 42:83–9. doi: 10.1016/j.ajem.2021.01.019
34. Johansson PI, Haase N, Perner A, Ostrowski SR. Association between sympathoadrenal activation, fibrinolysis, and endothelial damage in septic patients: a prospective study. J Crit Care. (2014) 29:327–33. doi: 10.1016/j.jcrc.2013.10.028
35. Johansen ME, Johansson PI, Ostrowski SR, Bestle MH, Hein L, Jensen AL, et al. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost. (2015) 41:16–25. doi: 10.1055/s-0034-1398377
36. Huang X, Hu H, Sun T, Zhu W, Tian H, Hao D, et al. Plasma endothelial glycocalyx components as a potential biomarker for predicting the development of disseminated intravascular coagulation in patients with sepsis. J Intensive Care Med. (2020) 36:1286. doi: 10.1177/0885066620949131
37. Yu WK, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, Ware LB. Angiopoietin-2 outperforms other endothelial biomarkers associated with severe acute kidney injury in patients with severe sepsis and respiratory failure. Crit Care. (2021) 25:48. doi: 10.1186/s13054-021-03474-z
38. Ikeda M, Matsumoto H, Ogura H, Hirose T, Shimizu K, Yamamoto K, et al. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care. (2018) 43:48–53. doi: 10.1016/j.jcrc.2017.07.049
39. Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. (2014) 289:8194–202. doi: 10.1074/jbc.M113.539452
40. Benatti MN, Fabro AT, Miranda CH. Endothelial glycocalyx shedding in the acute respiratory distress syndrome after flu syndrome. J Intensive Care. (2020) 8:72. doi: 10.1186/s40560-020-00488-7
41. Rauch A, Dupont A, Goutay J, Caplan M, Staessens S, Moussa M, et al. Endotheliopathy is induced by plasma from critically ill patients and associated with organ failure in severe COVID-19. Circulation. (2020) 142:1881–4. doi: 10.1161/CIRCULATIONAHA.120.050907
42. Nikaido T, Tanino Y, Wang X, Sato S, Misa K, Fukuhara N, et al. Serum syndecan-4 as a possible biomarker in patients with acute pneumonia. J Infect Dis. (2015) 212:1500–8. doi: 10.1093/infdis/jiv234
43. Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, et al. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res. (2013) 90:86–9. doi: 10.1016/j.mvr.2013.08.007
44. Ostrowski SR, Gaini S, Pedersen C, Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. (2015) 30:90–6. doi: 10.1016/j.jcrc.2014.10.006
Keywords: syndecan-1, sepsis, septic shock, mortality, acute kidney injury, meta-analysis
Citation: Sun T, Wang Y, Wu X, Cai Y, Zhai T and Zhan Q (2022) Prognostic Value of Syndecan-1 in the Prediction of Sepsis-Related Complications and Mortality: A Meta-Analysis. Front. Public Health 10:870065. doi: 10.3389/fpubh.2022.870065
Received: 05 February 2022; Accepted: 22 February 2022;
Published: 11 April 2022.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Qi Wu, Xuzhou Medical University, ChinaCopyright © 2022 Sun, Wang, Wu, Cai, Zhai and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyuan Zhan, ZHJ6aGFucXlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.