- 1Department of Epidemiology, College of Public Health, Harbin Medical University, Harbin, China
- 2Heilongjiang Provincial Center for Disease Control and Prevention, Harbin, China
- 3Chinese Center for Disease Control and Prevention, Beijing, China
- 4Jixi Municipal Center for Disease Control and Prevention, Harbin, China
- 5Harbin Municipal Center for Disease Control and Prevention, Harbin, China
Background: This study aimed to evaluate HIV incidence, factors associated with HIV incidence and transmitted drug resistance (TDR) among newly infected men who have sex with men (MSM) in Harbin, P.R. China.
Methods: A cohort study was conducted among MSM in Harbin during 2013 and 2018, with a follow-up frequency of every 6 months. Blood samples from MSM were tested for HIV antibodies, RNA was extracted from plasma, and the pol gene was sequenced, and genotypic drug-resistance analyses were performed.
Results: From 2013 to 2018, the overall rate of HIV incidence was 3.55/100 PY. Syphilis infection, unprotected sex with men in the past 6 months, and unawareness of HIV/AIDS knowledge were risk factors for HIV seroconversion. The distribution of HIV genotypes was as follows: CRF01_AE, 57.1%; CRF07_BC, 28.5%; CRF55_01B, 2.0%; B, 8.2%. The prevalence of transmitted drug resistance was 4.08%.
Conclusion: HIV incidence in MSM in Harbin is moderately high, and transmitted drug resistance exists in the population.
Introduction
In 2019, there were 38.0 million people living with HIV and 1.7 million new HIV infections worldwide (1). The proportion of men who have sex with men (MSM) accounted for 12% in 2015 and increased to 23% in 2019 globally (2, 3). In China, the estimated number of people living with HIV was 1.25 million at the end of 2018. The number of MSM accounted for 25.5% of newly identified HIV/AIDS cases in China in 2017 (4). HIV prevalence in MSM increased from 5.73% in 2010 to 6.9% in 2018 in China (3, 5). As a potential bridge population for HIV transmission, HIV epidemic in MSM is of particular concern in China (6, 7).
Longitudinal research is important for tracking the leading edge of the epidemic, monitoring the trend of new infections, and targeting prevention programs (8). However, longitudinal research to estimate HIV incidence, as well as factors associated with HIV infection in MSM in Heilongjiang has not been undertaken. Heilongjiang lies in northeast China. Harbin is the capital city of Heilongjiang province, and the center of MSM gathering. The number of MSM in Harbin accounted for over a half of cumulative identified HIV/AIDS cases across the whole province (9). Most previous studies are cross-sectional studies and focused on the prevalence of HIV in MSM in Heilongjiang province (10). Longitudinal research in MSM is urgently needed to determine the incidence of HIV infection and factors associated with HIV infection.
Antiretroviral therapy (ART) has a prominent effect on reducing mortality in people living with HIV (11). Drug resistance can be transmitted to others, resulting in ART failure (12), which is a challenge for controlling HIV epidemic. TDR is a public health issue as it can affect ART at individual and population level (13). The expansion of ART and pre-exposure prophylaxis should arouse great attention for the issue of TDR. TDR surveillance is of importance to evaluate the spread of HIV drug resistance mutations, and develop antiretroviral therapy strategy (14). Prevalence of TDR in patients of acute HIV infection (AHI) is higher than patients of chronic HIV infections (15). Due to high levels of viremia, the rate of HIV transmission is high during early HIV infection (16). Attention should be paid to the transmission of drug-resistant HIV among MSM because of high-risk behaviors, and high levels of viremia in MSM during the recent infection stage (17). Previous studies have shown that prevalence of HIV TDR in China was low during the last two decades (18–20). Many studies have reported the prevalence of TDR among MSM in China, ranging from 2.4% to 6.3% (21–27). Some articles have reported an increase of TDR among MSM, such as Shanghai and Tianjin (22, 27), some reported a stable TDR, such as Jiangsu and Guangzhou (21, 26). Several studies have been published on the genetic characterization and HIV drug resistance among MSM in Harbin (28–30), although these studies are cross-sectional study. Transmitted drug resistance among newly infected MSM has not been characterized by longitudinal research.
To evaluate the trend of HIV incidence in MSM in Harbin and its risk factors, HIV subtypes, and transmitted drug resistance in newly infected MSM, a prospective survey was conducted in MSM in Harbin, the capital city of Heilongjiang province.
Materials and Methods
Study Design and Participant Enrollment
This study was conducted in Harbin between April, 2013 and December, 2018. Men who were 18 years and above, had sex with men in the past 12 months and tested HIV negative at baseline were recruited through snowball sampling at various venues, including the internet, bathing room, sauna, and park, etc.. The participants were followed up every 6 months by trained staff of non-government organizations (NGOs) and collected information on socio-demographic characteristics, history of drug use, sexually transmitted infections (STIs), HIV/AIDS knowledge awareness, and sexual behavior. We defined HIV/AIDS knowledge awareness as correctly responding six or above questions out of eight questions, otherwise defined as unawareness.
Laboratory Tests
At every follow-up, participants were tested for HIV and syphilis. For HIV testing, we used an enzyme-linked immunosorbent assay (ELISA; Wantai Biotech Inc., China and Lizhu Biotech Inc., Beijing, China) or rapid test (SD; Kyonggi-do Biotech Co. Ltd, Korea and Yingkexinchuang Biotech Inc., Xiamen, China) for screening, and western blotting for confirmation (HIV Blot 2.2; MP Diagnostics, Singapore). For syphilis testing, we used an enzyme-linked immunosorbent assay (ELISA; Yingke-xinchuang Biotech Inc., China) or rapid test (Wantai Biotech Inc., China). Toluidine red unheated serum test (TRUST; Wantai Biotech Inc., China) was used for syphilis confirmation. Positive for both ELISA and TRUST, or positive for both rapid test and TRUST were defined as being infected with syphilis.
RNA was extracted (QIAamp Viral RNA Mini Kit, Germany). Sequences were assembled and edited using Sequencher version 5.0, and aligned using Bioedit, following the manual editing according to HIV-1 reference subtypes downloaded from the Los Alamos HIV Sequence Database. HIV-1 subtypes were classified according to phylogenetic analysis of the pol sequence. Phylogenetic analysis was performed using the neighbor-joining method in MEGA version 6.0 for subtyping analysis. Mutations involved with drug resistance were analyzed using the Stanford HIV Drug Resistance Database (hivdb.stanford.edu) (31). Resistance mutations were defined according to the WHO surveillance drug resistance mutation (SDRM) list updated in 2009 (32). Pairwise genetic distances were calculated using the neighbor-joining method based on the Tamura-Nei 93 model, and transmission clusters were defined as pairwise genetic distances of <1.5%.
Statistical Analysis
The date of HIV incidence was estimated at the midpoint between the date of last HIV negative test and the first HIV positive test. Cox regression model was used to analyze risk factors associated with HIV incidence in univariate and multivariate analyses. Logistic regression model was used to identify risk factors of lost-to-follow-up. Variables with a p-value < 0.1 in the univariate analysis were entered into the multivariate models. Cox regression analysis was performed to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) of interactions between factors on HIV incidence. The effects of combination between factors on HIV incidence were assessed by crossover analysis. All analyses were performed using SPSS 23.0.
Results
Baseline Characteristics of the Participants
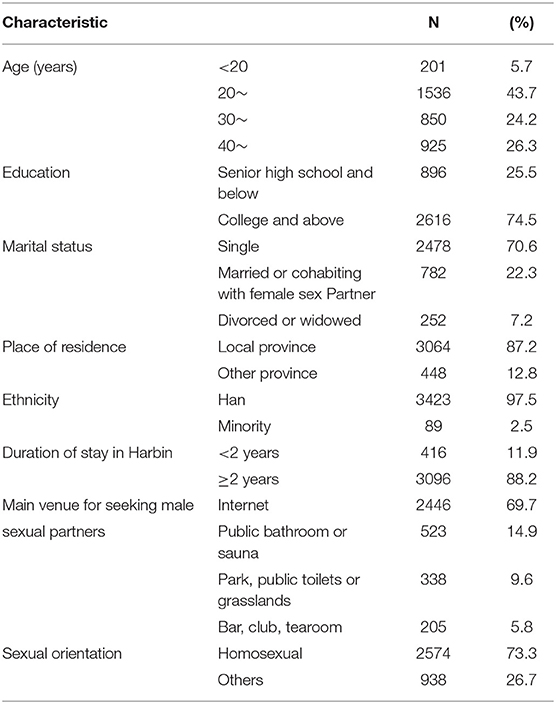
The median age of the participants was 30 years, 70.6% were single, 22.3% were married or cohabiting with female sex partners, 7.2% were divorced or widowed, 97.5% were of Han ethnicity, 74.5% had received a college education or above, 88.2% had lived in Harbin for more than 2 years, 69.7% found their sex partners through the internet, and 73.3% identified themselves as exclusively homosexual (Table 1).
Incidence of HIV, Factors Associated With HIV Seroconversion
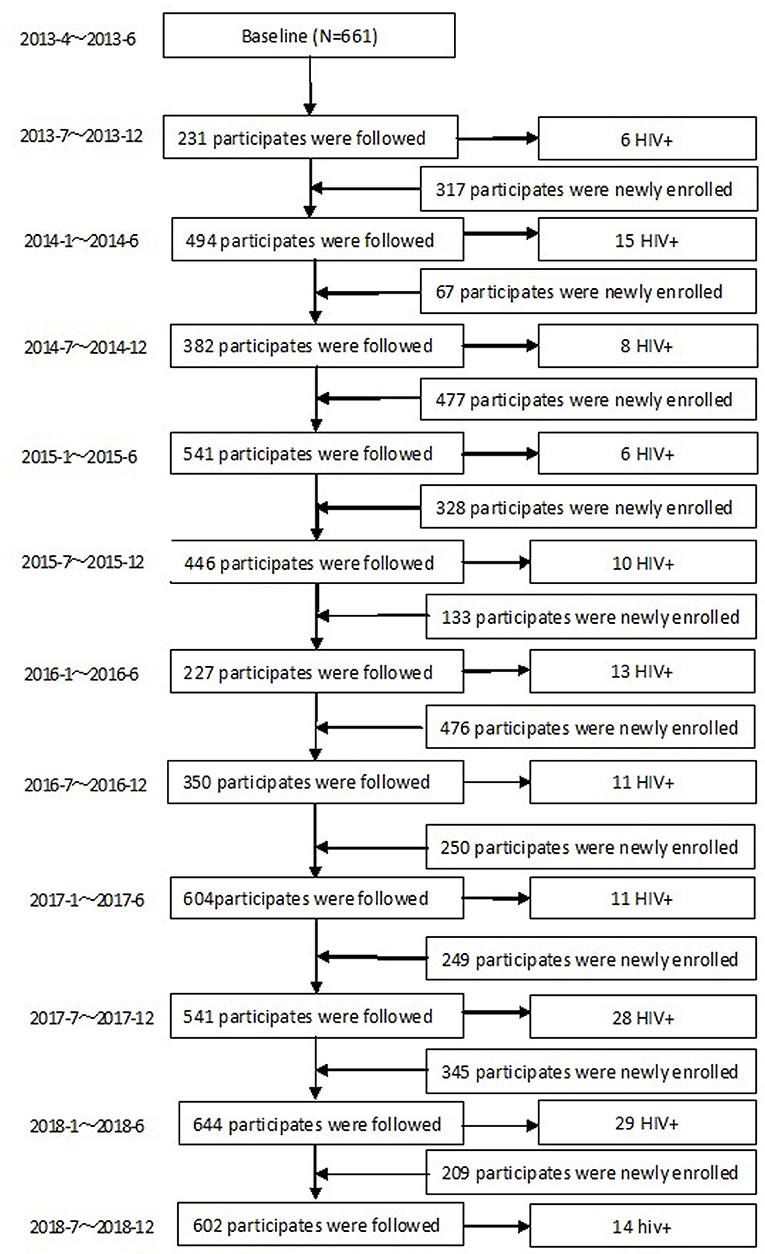
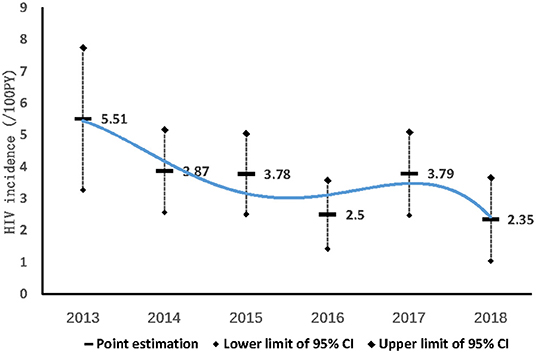
During the 68 months of follow-up, 3,512 MSM were enrolled into the dynamic prospective cohort (Figure 1). 80.5% (2,826/3,512) were followed more than once, with a total time of 4,250 person-years (PY). The median follow-up time was 1.31 PY (IQR: 0.63–2.13); 36.9% (1,296/3,512) were followed once, 19.2% (673/3,512) were followed twice, and 24.4% (857/3,512) were followed more than twice. In total, 151 (5.34%) MSM converted to HIV–positive, HIV incidence density was 3.55/100 PY (95% CI: 2.99–4.11/100). HIV incidence in MSM in Harbin decreased from 5.51/100PY (95%CI:3.27–7.75/100PY) in 2013, 3.87/100PY (95%CI:2.57–5.17/100 PY) in 2014, 3.78/100PY (95%CI:2.51–5.05/100PY) in 2015, 2.5/100PY (95%CI: 1.42–3.58/100PY) in 2016, 3.79/100PY (95%CI:2.48–5.1/100PY) in 2017, to 2.35/ 100PY (95%CI:1.04–3.66/100PY) in 2018 (χ2 = 5.041, Ptrend= 0.025) (Figure 3).
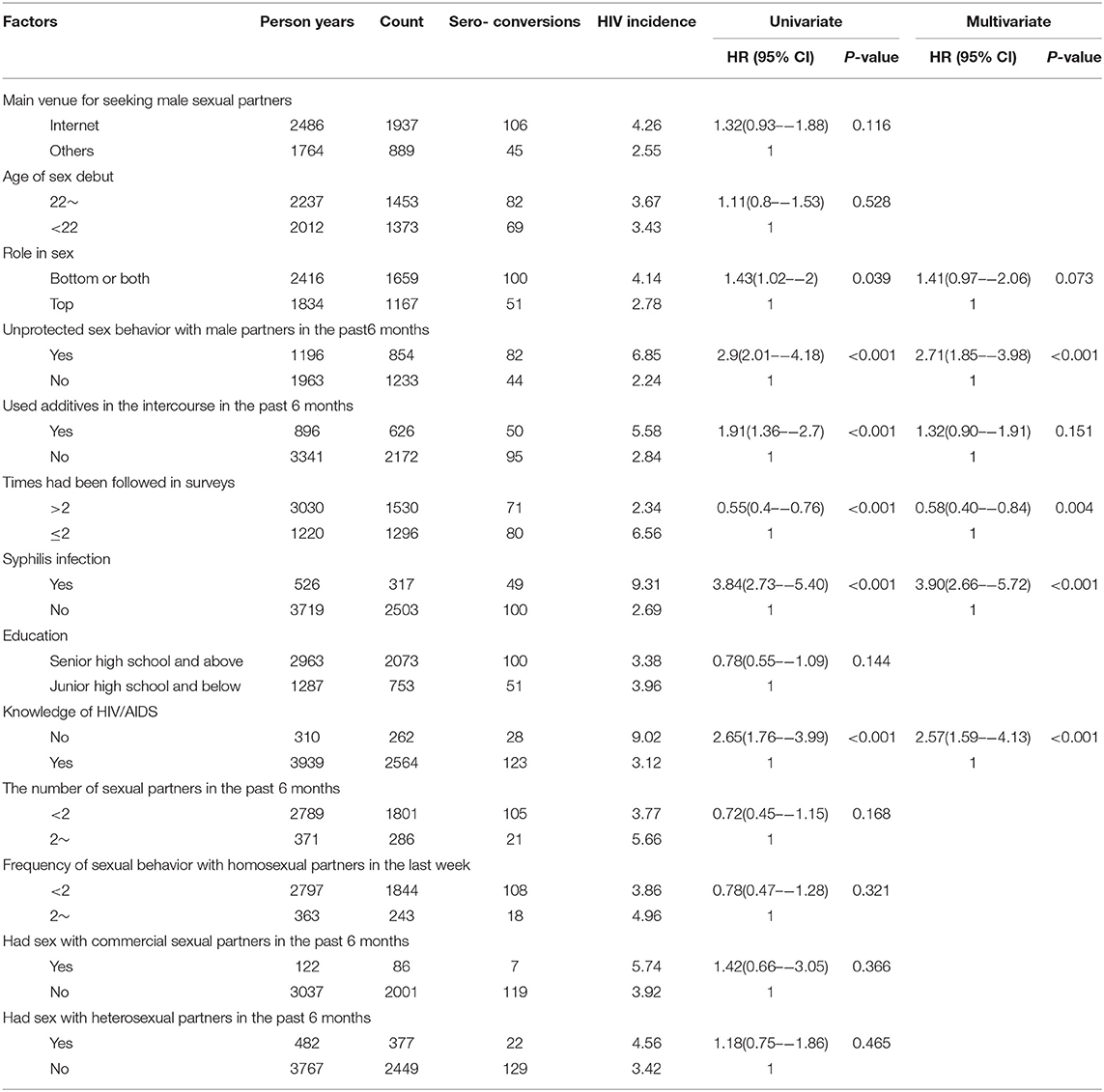
Multivariate Cox regression analysis indicated that syphilis infection (HR: 3.90, 95% CI: 2.66–5.72), unprotected sex with males in the past 6 months (HR: 2.71, 95% CI: 1.85–3.98), and unawareness of HIV/AIDS knowledge (HR: 2.57, 95% CI: 1.59–4.13), were independent risk factors for HIV incidence. Having been involved in surveys more than twice was a protective factor (HR: 0.58, 95% CI 0.40–0.84) (Table 2).
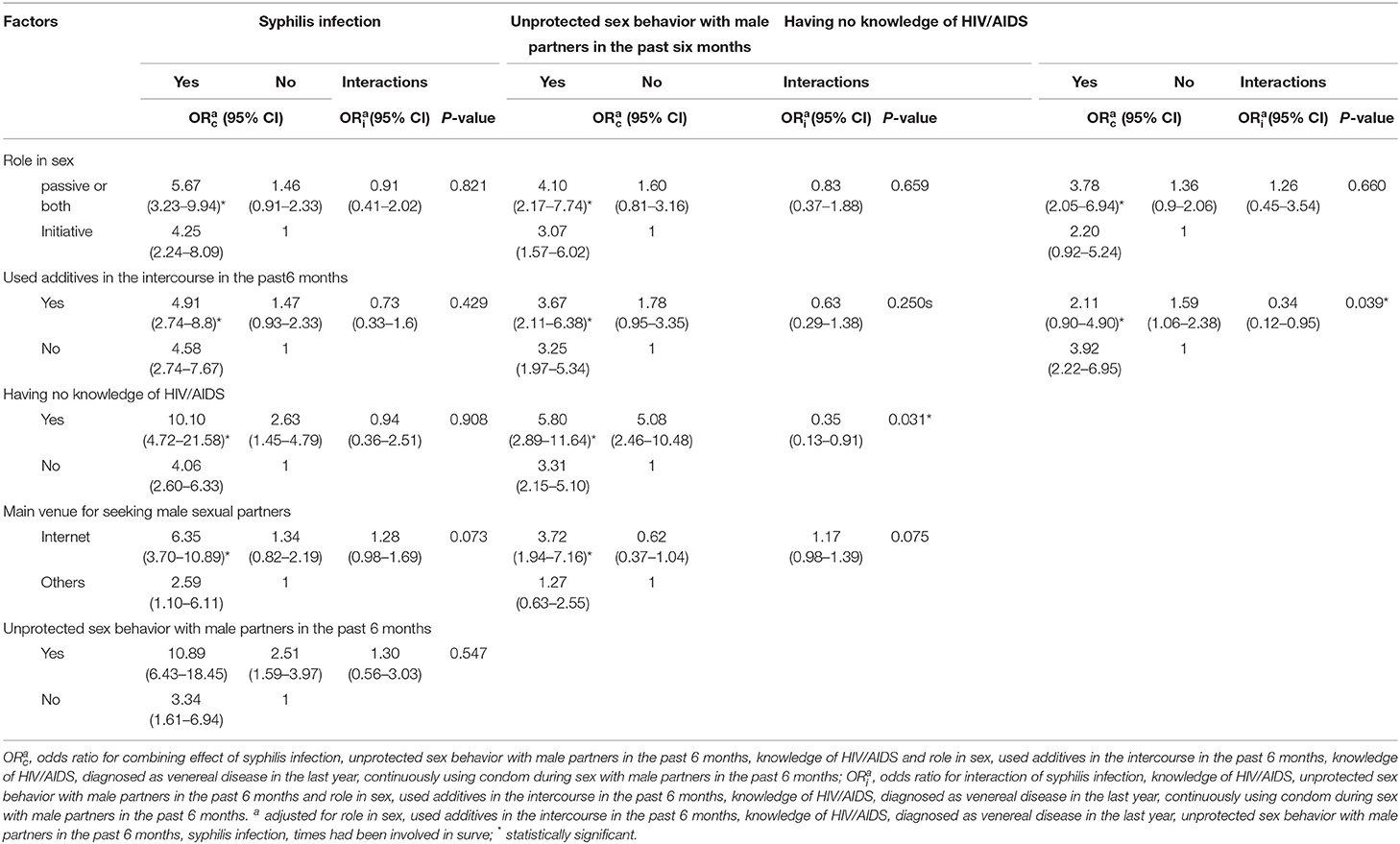
There was a significant combination effect between seeking sex partners through the internet and syphilis infection on the risk of HIV incidence (ORc: 6.35, 95% CI: 3.70–10.89). There was a significant combination effect between unprotected sex with male partners in the past 6 months and unawareness of HIV/AIDS knowledge (ORc: 5.80, 95% CI: 2.89–11.64). A significant combination effect of unprotected sex with male partners in the past 6 months and syphilis infection on the risk of HIV incidence was observed (ORc: 10.89, 95% CI: 6.43–18.45). A significant combination effect between unawareness of HIV/AIDS knowledge and syphilis infection on the risk of HIV seroconversion was observed (ORc: 10.10, 95% CI: 4.72–21.58) (Table 3).
Factors Associated With Loss to Follow-up
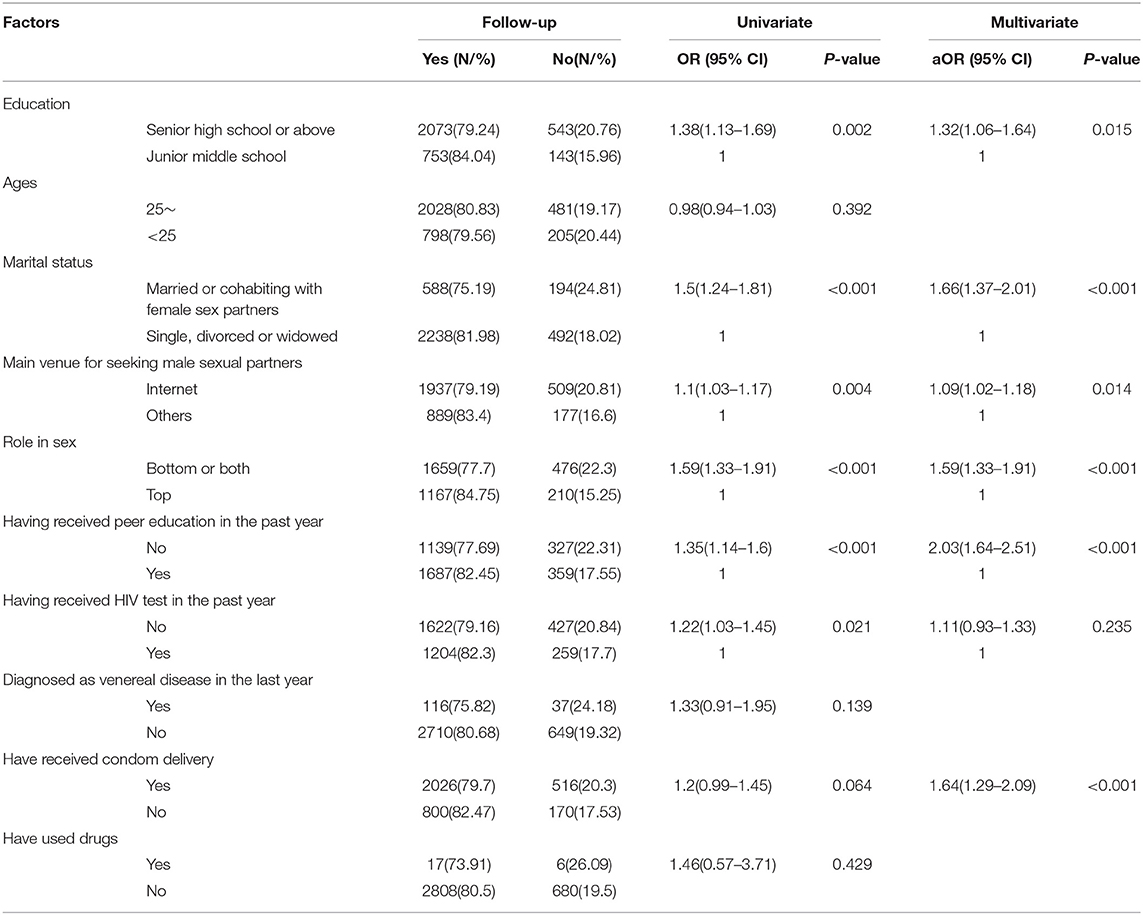
Statistically significant variables in the univariate analysis were entered into a multiple logistic regression model. Having an education of senior high school or above (aOR: 1.32, 95% CI: 1.06–1.64), being married or cohabiting with female sex partners (aOR: 1.66, 95% CI:1.37–2.01), seeking sex partners through the internet (aOR: 1.09, 95% CI: 1.02–1.18), passive role in sex (aOR: 1.59, 95% CI: 1.33–1.91), having not received peer education regarding HIV/AIDS in the past year (aOR: 2.03, 95% CI: 1.64–2.51) were independent risk factors associated with loss to follow-up in the cohort (Table 4).
HIV Subtype and Transmitted Drug Resistance (TDR)
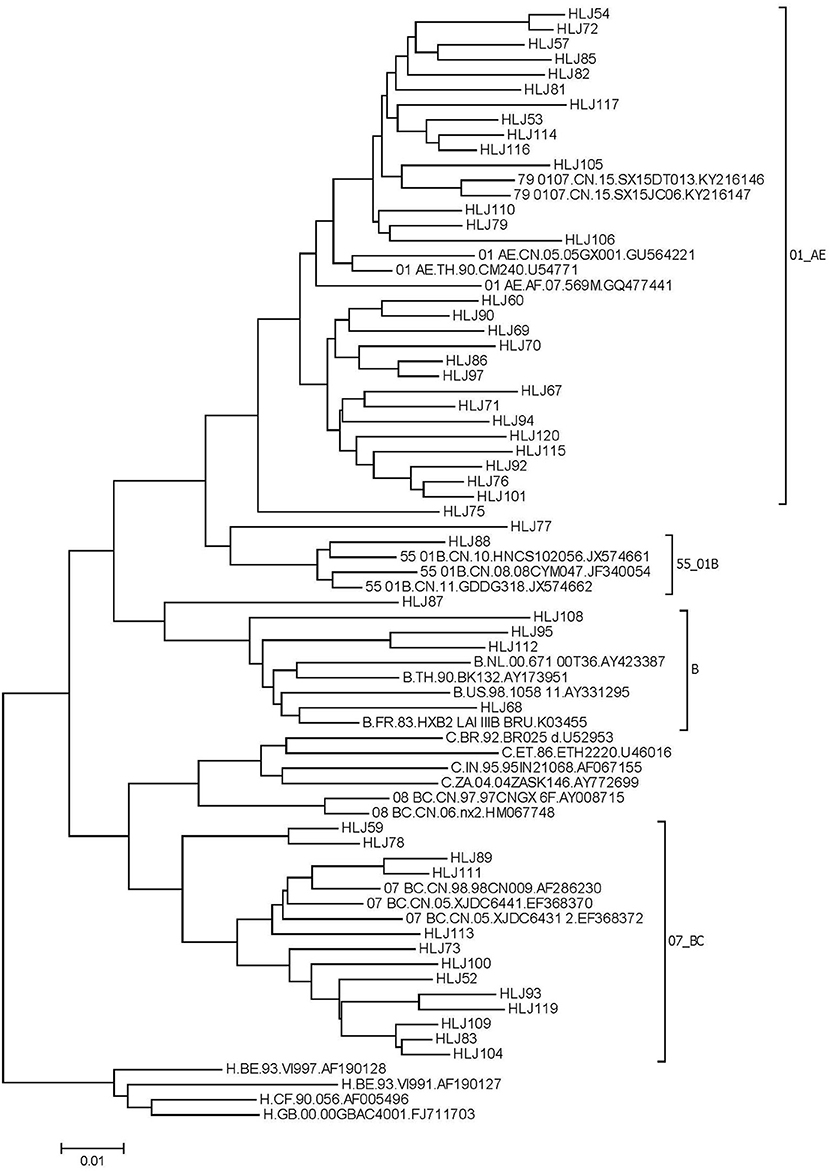
From July, 2016 to June, 2018, 79 new HIV infections were observed, 26 blood samples were not collected, and a total of 53 samples were amplified. Of these, 49 HIV−1 nucleotide sequences were successfully amplified and sequenced. There was no significant difference in age group (χ2 = 0.561, P=0.454), marital status (χ2 = 0.434, P = 0.51), education (χ2 = 0.434, P = 0.51), sexual orientation (χ2 = 2.736, P = 0.098), or cruising venue (χ2 = 0.172, P = 0.678) between samples successfully amplified and not. The distribution of HIV-1 genotypes was as follows: CRF01_AE, 57.1% (28/49); CRF07_BC, 28.5% (13/49); CRF55_01B, 2.0% (1/49); B, 8.2% (4/49); and URF 6.2% (3/49) (Figure 2).
The prevalence of drug resistance was 10.2% (5/49) according to the Stanford HIV Drug Resistance Database, and four mutations in non–nucleoside reverse transcriptase inhibitor (NNRTI) were detected. Two were observed in the subtype CRF01_AE sequence, one was observed in the subtype CRF55_01B sequence, and one was observed in the subtype B sequence. One mutation associated with NNRTI and nucleoside reverse transcriptase inhibitor (NRTI) was observed in the CRF01_AE subtype. Ten mutations, D67G, K70E, Y115F, M184V, V179T, V106M, E138G, K103N, V179E, and V179D, were detected. According to the WHO SDRM list, the prevalence of TDR was 4.08% (2/49). The prevalence of TDR was 2.04% (1/49) for NRTI resistance and 2.04% (1/49) for NNRTI and NRTI resistance.
Nine sequences with genetic distances less than 1.5% were segregated into four clusters. Among them, three clusters were comprised of two individuals, and the other cluster was comprised of three individuals. The clustering rate of CRF07_BC was 23.08%, and that of CRF01_AE was 21.43%. Among the nine individuals, one drug–resistant sample was detected in the molecular clusters. Nine samples in the molecular clusters sought partners through the internet. Four of them had used additive drugs in the past 6 months.
Discussion
This is the first longitudinal study evaluating HIV incidence and its risk factors, HIV subtype, and TDR in MSM in Heilongjiang province. HIV incidence in MSM in Harbin is 3.55/100 PY, which is consistent with the HIV incidence in MSM in Beijing and Thailand (6, 33), lower than HIV incidence in MSM in Chengdu (7).
In our study, syphilis infection and unprotected sex with men in the past 6 months were independent predictors of HIV incidence, which is similar to factors associated with HIV incidence in Beijing (6). Syphilis facilitates HIV transmission (34), syphilis screening and treatment could be an effective HIV prevention strategy in MSM in Harbin. MSM who were lack of knowledge of HIV/AIDS showed higher HIV incidence, that may because they have less awareness of the dangers of HIV and do not use protection during sexual activities. In addition, there was a significant combination effect between unprotected sex with male partners in the past 6 months and unawareness of HIV/AIDS knowledge (ORc: 5.80, 95%CI 2.89–11.64). According to our survey, most MSM sought their partners through the internet. There was a significant combination effect between seeking sex partners through the internet and syphilis infection on the risk of HIV incidence (ORc: 6.35, 95% CI (3.70–10.89). In China, along with the continuous development and growing popularity of information technology, an increasing number of MSM seek their partners through the internet. The traditional communication mode of MSM is changing; considering that there are many unknown factors on the internet, behavior intervention strategies in MSM should be adjusted to adapt to this new situation.
MSM who had not received peer education in the past year showed a higher risk of loss to follow–up; to some extent, MSM who have received HIV/AIDS education may be aware of the dangers of HIV and the necessity of regular HIV testing, thus more likely to take HIV testing than those who did not. Participants who were married or cohabiting with female sex partners showed a higher risk of loss to follow–up, which may be a result of the fact that MSM receive more pressure from their family or wife, are more secretive, and have fewer chances for HIV testing.
Genotypic analysis found that 57.1% of samples were CRF01_AE and 28.5% were CRF07_BC among newly HIV–infected MSM in Harbin, which is consistent with the genotypic analysis in Kunming MSM in China and the distribution of HIV genotypes in a cross–sectional study of HIV subtypes in Heilongjiang province (29, 30).
The TDR of HIV in MSM in Harbin was 4.08%, consistent with the studies among MSM in China (3.1%), Kunming (5%), Guangxi (4.8%), Jiangsu (4.0%), Guangdong (3.32%) (18, 21, 24–26, 35), lower than the TDR among MSM in Tianjin (6.3%) (27), but higher than the TDR among MSM in Xi'an (2.4%) (23). The prevalence of HIV−1 TDR was relatively low among MSM in Heilongjiang province, which suggests that current strategies of treatment are effective in MSM in Heilongjiang province. Prevalence of mutations related to NNRTI resistance were higher in the study, which might be attributed to lower genetic barriers to NNRTI resistance, or the use of first–line regimen (18, 36). In our survey, the major HIV genotype in MSM in Harbin was CRF01_AE, accounting for half of the samples. It has been reported that CRF01_AE infection is associated with rapid CD4+ T–cell decline and more likely progression to AIDS than infections of other types (37, 38). TDR in CRF01_AE infections should be considered. In our study, the TDR rate of HIV new infections in the MSM cohort from Harbin was low, although samples with drug–resistant mutations were present in the molecular clusters with a high risk of transmission, which suggests that TDR surveillance in MSM is necessary.
This study had some limitations. First, the retention rate of our survey was relatively low, and the incidence of HIV may be biased because it is not clear whether the HIV incidence of the participants retained in the survey were the same as those lost to follow–up, the representativeness of MSM may be limited. Second, 79 MSM seroconverted to HIV positive during the follow–up period from 2016 to 2018, while only 49 HIV−1 nucleotide sequences were successfully amplified. Although there was no significant difference in demographic characteristics between samples successfully amplified and not, the results may be biased in evaluating the distribution of HIV−1 genotypes among newly HIV–infected MSM in Harbin. The third limitation was the use of Sanger sequencing for genotypic drug resistance genes, which is unable to detect variants at levels below approximately 20% (39, 40). Next–generation sequencing for the detection of transmitted drug resistance mutations should be performed in future research. The fourth limitation was that pooled nucleic acid amplification testing (NAAT) was not used for the screening of HIV–negative participants and samples in the HIV acute infection stage could not be detected (41). If acutely infected participants who cannot be detected by ELISA are lost to follow–up, HIV incidence may be underestimated. The fourth limitation was, we could not analyze the relationship between socioeconomic characteristics such as occupation and income and HIV incidence for lack of information which need to be further explored.
Conclusion
Above all, HIV incidence of MSM in Harbin is moderately high. Syphilis infection, unprotected sex with males in the past 6 months, and lack of knowledge of HIV/AIDS were independent predictors of HIV incidence. The transmitted drug resistance rate of HIV new infections in the MSM cohort in Harbin was low, although samples with drug-resistant mutations were present in the molecular clusters with a high risk of transmission. Surveillance of TDR should be conducted. In our survey, most MSM sought their partners through the Internet. Considering that there are many unknown factors on the internet, behavioral intervention strategies in MSM should be adjusted to adapt to this new situation, and early identification and effective interventions are urgently needed to break the transmission of HIV in MSM in Harbin.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by this study was approved by Institutional Review Board of National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (Project NO: X130419285). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YZ, FL, SH, and FC conceived and designed. SH, and JZ performed the experiments. SH, FC, and YL analyzed the data. YZ, FL, SH, and FC wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
Project of research on new HIV infection in MSM, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. UNAIDS. UNAIDS Data 2020. (2020). Available online at: https://www.unaids.org/en/resources/documents/2020/unaids-data.2020 (accessed September 9, 2021).
2. UNAIDS. UNAIDS Data 2017. (2017). Available online at: https://www.unaids.org/en/resources/documents/2017/2017_data_book.2017. (accessed July 4, 2018)
3. UNAIDS. UNAIDS Data 2019. (2019). Available online at: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data.2019 (accessed September 21, 2020).
4. National Health Commission of the People's Republic of China. Progress in Aids Control and Prevention in China. Available online at: http://www.nhc.gov.cn/wjw/xwdt/201811/5fe377b577d04d369a057970c0f816d1.shtml.2018 (accessed July 25, 2019).
5. Lin GE, LD–m, Pei–long L, Wei G, Yan C. Population specific sentinel surveillance for HIV infection, syphilis and HCV infection in China, during 2010–2015. Disease Surveillance. (2017) 32:111–7.
6. Mi GD, Zhang BB, Yu F, Ren XL, Ong JJ, Fan YQ, et al. HIV incidence among men who have sex with men using geosocial networking smartphone application in Beijing, China: an open cohort study. Infect Dis Poverty. (2021) 10:27. doi: 10.1186/s40249–021–00814–7
7. You X, Gilmour S, Cao W, Lau JT, Hao C, Gu J, et al. HIV incidence and sexual behavioral correlates among 4578 men who have sex with men (MSM) in Chengdu, China: a retrospective cohort study. BMC Public Health. (2021) 21:802. doi: 10.1186/s12889–021–10835–4
8. Brookmeyer R, Konikoff J, Laeyendecker O, Eshleman SH. Estimation of HIV incidence using multiple biomarkers. Am J Epidemiol. (2013) 177:264–72. doi: 10.1093/aje/kws436
9. Ma J, Tong X, Hui S, Yan H, Yu L, LI Y. Analysis on HIV/AIDS epidemic situiation in Heilongjiang province from 2012 to 2017. Chin Med J. (2018) 38:222–4.
10. Hui S, Li Y, Zhang J, Wang KL, Meng B, Tong X. Application of BED – CEIA to estimate incidence of human immunodeficiency virus among men who have sex with men in Heilongjiang Province in 2011. Chin J Public Health. (2012) 28:399–400. doi: 10.19568/j.cnki.23-1318.2012.04.005
11. Bradley H, Mattson CL, Beer L, Huang P, Shouse RL, Medical Monitoring P. Increased antiretroviral therapy prescription and HIV viral suppression among persons receiving clinical care for HIV infection. AIDS. (2016) 30:2117–24. doi: 10.1097/QAD.0000000000001164
12. Blassel L, Zhukova A, Villabona–Arenas CJ, Atkins KE, Hué S, Gascuel O. Drug resistance mutations in HIV: new bioinformatics approaches and challenges. Curr Opin Virol. (2021) 51:56–64. doi: 10.1016/j.coviro.2021.09.009
13. Wittkop L, Günthard HF, de Wolf F, Dunn D, Cozzi–Lepri A, de Luca A, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord–CHAIN joint project): a European multicohort study. Lancet Infect Dis. (2011) 11:363–71. doi: 10.1016/S1473–3099(11)70032–9
14. Guo C, Wu Y, Zhang Y, Liu X, Li A, Gao M, et al. Transmitted Drug Resistance in Antiretroviral Therapy–Naive Persons With Acute/Early/Primary HIV Infection: A Systematic Review and Meta–Analysis. Front Pharmacol. (2021) 12:718763. doi: 10.3389/fphar.2021.718763
15. Yanik EL, Napravnik S, Hurt CB, Dennis A, Quinlivan EB, Sebastian J, et al. Prevalence of transmitted antiretroviral drug resistance differs between acutely and chronically HIV–infected patients. J Acquir Immune Defic Syndr. (2012) 61:258–62. doi: 10.1097/QAI.0b013e3182618f05
16. Castor D, Low A, Evering T, Karmon S, Davis B, Figueroa A, et al. Transmitted drug resistance and phylogenetic relationships among acute and early HIV−1–infected individuals in New York City. J Acquir Immune Defic Syndr. (2012) 61:1–8. doi: 10.1097/QAI.0b013e31825a289b
17. Pines HA. Wertheim Jo Fau – Liu L, Liu L Fau – Garfein RS, Garfein Rs Fau – Little SJ, Little Sj Fau – Karris MY, Karris MY. Concurrency and HIV transmission network characteristics among MSM with recent HIV infection. AIDS. (2016) 30:2875–83. doi: 10.1097/QAD.0000000000001256
18. Yuan H, Liu Z, Wu X, Wu M, Fang Q, Zhang X, et al. Prevalence of transmitted HIV−1 drug resistance among treatment–naive individuals in China, 2000–2016. Arch Virol. (2021) 166:2451–60. doi: 10.1007/s00705–021–05140–9
19. Pang X, Tang K, He Q, Huang J, Fang N, Zhou X, et al. HIV drug resistance and HIV transmission risk factors among newly diagnosed individuals in Southwest China. BMC Infect Dis. (2021) 21:160. doi: 10.1186/s12879–021–05854–w
20. Lan Y, Li L, He X, Hu F, Deng X, Cai W, et al. Transmitted drug resistance and transmission clusters among HIV−1 treatment–naïve patients in Guangdong, China: a cross–sectional study. Virol J. (2021) 18:181. doi: 10.1186/s12985–021–01653–6
21. Yin Y, Zhou Y, Lu J, Liu X, Shi L, Fu G, et al. Molecular epidemiology of HIV−1 in Jiangsu Province, Southeast China: genotypes and HIV−1 transmission networks among newly diagnosed men having sex with men in 2017. AIDS Res Hum Retroviruses. (2021) 37:62–9. doi: 10.1089/aid.2020.0165
22. Wang XQ, Yu XL, Lin Y, Gai J, Zheng M, Zhou YQ, et al. Prevalence of primary drug–resistance among newly diagnosed HIV−1 infected men who have sex with men in Shanghai,2012–2014. Chin J AIDS & STD. (2017) 12:1119–23. doi: 10.13419/j.cnki.aids.2017.12.12
23. Chang W, Zhang M, Ren Q, Zou Y, Dong L, Jia H, et al. HIV−1 genetic diversity and recombinant forms among men who have sex with men at a sentinel surveillance site in Xi'an City, China. Infect Genet Evol. (2020) 81:104257. doi: 10.1016/j.meegid.2020.104257
24. Chen M, Ma Y, Chen H, Dai J, Dong L, Yang C, et al. HIV−1 genetic transmission networks among men who have sex with men in Kunming, China. PLoS ONE. (2018) 13:e0196548. doi: 10.1371/journal.pone.0196548
25. Pang X, Wei H, Huang J, He Q, Tang K, Fang N, et al. Patterns and risk of HIV−1 transmission network among men who have sex with men in Guangxi, China. Sci Rep. (2021) 11:513. doi: 10.1038/s41598–020–79951–2
26. Han ZG, Zhang YL, Wu H, Gao K, Zhao YT, Gu YZ, et al. Prevalence of drug resistance in treatment–naive HIV infected men who have sex with men in Guangzhou, 2008–2015. Zhonghua Liu Xing Bing Xue Za Zhi. (2018) 39:977–82.
27. Zheng MN, Ning TL, Zhou N, Zhao X, Li L, Zhu JJ, et al. Transmitted drug resistance among HIV infected men who have sex with men in Tianjin, 2014–2017. Zhonghua Liu Xing Bing Xue Za Zhi. (2018) 39:619–24.
28. Li QH, Wang FX, Yue C, Wang JY, Jin G, Zhang CL, et al. Molecular genotyping of HIV−1 strains from newly infected men who have sex with men in Harbin, China. AIDS Res Hum Retroviruses. (2016) 32:595–600. doi: 10.1089/aid.2016.0028
29. Shao B, Song B, Cao L, Du J, Sun D, Lin Y, et al. Molecular epidemiology is becoming complex under the dynamic HIV prevalence: The perspective from Harbin, China. J Med Virol. (2016) 88:807–14. doi: 10.1002/jmv.24407
30. Li Y, Hui S, Zhang J, Liu F, Li T, Zhang X, et al. Analysis on HIV subtypes, molecular transmission cluster and drug resistance among newly reported infections in Heilongjiang Province in 2018. Chin J AIDS & STD. (2021) 27:680–4.
31. WHO. HIV Drug Resistance Laboratory Operational Framework. Available online at: https://appswhoint/iris/handle/10665/259731. 2017.
32. Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. Drug resistance mutations for surveillance of transmitted HIV−1 drug–resistance: 2009 update. PLoS One. (2009) 4:e4724. doi: 10.1371/journal.pone.0004724
33. Kritsanavarin U, Bloss E, Manopaiboon C, Khawcharoenporn T, Harnlakon P, Vasanti–Uppapokakorn M, et al. HIV incidence among men who have sex with men and transgender women in four provinces in Thailand. Int J STD AIDS. (2020) 31:1154–60. doi: 10.1177/0956462420921068
34. Chun HM, Carpenter RJ, Macalino GE, Crum–Cianflone NF. The role of sexually transmitted infections in HIV-1 Progression: a comprehensive review of the literature. J Sexu Transm Dis. (2013) 2013:176459. doi: 10.1155/2013/176459
35. Wang X, Liu X, Li F, Zhou H, Li J, Wang Y, et al. Epidemiological surveillance of HIV-1 transmitted drug resistance among newly diagnosed individuals in Shijiazhuang, northern China, 2014–2015. PLoS ONE. (2018) 13:e0198005. doi: 10.1371/journal.pone.0198005
36. Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. (2016) 46:292–307. doi: 10.1016/j.meegid.2016.08.031
37. Song H, Ou W, Feng Y, Zhang J, Li F, Hu J, et al. Disparate impact on CD4 T cell count by two distinct HIV-1 phylogenetic clusters from the same clade. Proc Natl Acad Sci U S A. (2019) 116:239–44. doi: 10.1073/pnas.1814714116
38. Cui H, Geng W, Sun H, Han X, An M, Jiang Y, et al. Rapid CD4+ T–cell decline is associated with coreceptor switch among MSM primarily infected with HIV−1 CRF01_AE in Northeast China. AIDS. (2019) 33:13–22. doi: 10.1097/QAD.0000000000001981
39. Arias A, López P, Sánchez R, Yamamura Y, Rivera–Amill V. Sanger and next generation sequencing approaches to evaluate HIV−1 virus in blood compartments. Int J Environ Res Public Health. (2018) 15:1697. doi: 10.3390/ijerph15081697
40. Parikh UM, McCormick K, van Zyl G, Mellors JW. Future technologies for monitoring HIV drug resistance and cure. Curr Opin HIV AIDS. (2017) 12:182–9. doi: 10.1097/COH.0000000000000344
Keywords: HIV, MSM, incidence, drug resistance, seroconversion
Citation: Hui S, Chen F, Li Y, Cui Y, Zhang J, Zhang L, Yang Y, Liu Y, Zhao Y and Lv F (2022) Factors Associated With Newly HIV Infection and Transmitted Drug Resistance Among Men Who Have Sex With Men in Harbin, P.R. China. Front. Public Health 10:860171. doi: 10.3389/fpubh.2022.860171
Received: 22 January 2022; Accepted: 26 April 2022;
Published: 02 June 2022.
Edited by:
Zining Zhang, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Qinghai Hu, Key Laboratory of AIDS Immunology of National Health and Family Planning Commission, ChinaBin Su, Capital Medical University, China
Copyright © 2022 Hui, Chen, Li, Cui, Zhang, Zhang, Yang, Liu, Zhao and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yashuang Zhao, emhhb195YXNodWFuZ0AyNjMubmV0; Fan Lv, ZmFubHZAY2hpbmFhaWRzLmNu
†These authors have contributed equally to this work
 Shan Hui
Shan Hui Fangfang Chen3†
Fangfang Chen3† Yashuang Zhao
Yashuang Zhao