- 1Department of Public Health and Infection Management, Gansu Provincial Hospital of Traditional Chinese Medicine, Lanzhou, China
- 2Department of Information Administration, Gansu Provincial Maternity and Child Care Hospital, Lanzhou, China
- 3School of Public Health, Shanghai Jiao Tong University, Shanghai, China
- 4Department of Operation Management, Gansu Provincial Maternity and Child Care Hospital, Lanzhou, China
- 5Department of Human Resource, Gansu Provincial Maternity and Child Care Hospital, Lanzhou, China
Objectives: To investigate the independent and collective effects of maternal folic acid supplementation or dietary folate intake on the risk of low birth weight (LBW), and to further comprehensively examine the joint associations of folic acid supplementation and dietary folate intake with LBW by various clinical subtypes.
Design: Participants were recruited from Gansu Provincial Maternity and Child Care Hospital. A standardized and structured questionnaire was distributed to collect demographic factors, reproductive and medical history, occupational and residential history, physical activity, and diet. Data on pregnancy-related complications and birth outcomes were extracted from medical records. Unconditional logistic regression models were used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for single and joint associations of folic acid supplementation and dietary folate intake with LBW.
Setting: A birth cohort data analysis using the 2010–2012 Gansu Provincial Maternity and Child Care Hospital in Lanzhou, China.
Participants: In total, 9,231 pregnant women and their children were enrolled in the study.
Results: Compared with non-users, folic acid supplementation was associated with a reduced risk of LBW (OR: 0.80, 95% CI: 0.66–0.97), and the reduced risk was mainly seen for term-LBW (OR: 0.59, 95% CI: 0.41–0.85), and multiparous-LBW (OR: 0.72, 95% CI: 0.54–0.94). There were no significant associations between dietary folate intake and LBW, and there was no interaction between folic acid supplement and dietary folate intake on LBW.
Conclusions: Our study results indicated that folic acid supplementation was associated with a reduced risk of LBW, and there was no interaction between folic acid supplements and dietary folate intake on LBW.
Introduction
Low birth weight (LBW) can increase neonatal mortality and is associated with various infant morbidities (1, 2). Furthermore, it can lead to chronic diseases in later life (3, 4), such as metabolic syndrome, diabetes mellitus type 2, cardiovascular diseases, hypertension, or cancer (5–7), which result in large economic costs in terms of immediate neonatal intensive care, ongoing long-term complex health needs, as well as lost economic productivity. Unfortunately, approximately 16% of infants are born weighing <2,500 g worldwide, which represents more than 22 million LBW babies per year (8). Thus, it is an important public health problem that needs to be solved urgently.
Folate is the generic term for compounds that have vitamin activity similar to that of pteroylglutamic acid and is an anti-anemic and growth factor. Folate functions as a co-enzyme in several single carbon transfers leading to the biosynthesis of purine nucleotides and deoxythymidylic acid essential for DNA and RNA synthesis (9).
Conflicting results have been obtained from epidemiological studies that investigated the association between folic acid intake and the risk of LBW. Eight studies found that folic acid supplementation before and/or during pregnancy reduced the risk of LBW (10–17), but three studies reported no association between folic acid supplementation and LBW (18–20). Only two studies were about the relationship between dietary folate intake and LBW (15, 21). Uno et al. found that folate deficiencies are known risk factors for LBW (21), and Rolschau et al. found that the effects of supplementing the diet with folic acid given preconceptionally or in the first half of pregnancy in an affluent northern country were a slight increase of birth weight (15). Scholl et al. investigated that lower concentrations of serum folate at week 28 were also associated with a greater risk of LBW (22), and Bergen et al. found that low folate concentrations (lowest quintile) were associated with birth weight (23). To further comprehensively examine the single and joint associations of folic acid supplementation and dietary folate intake with LBW by various clinical subtypes, we analyzed data from a birth cohort study conducted in Lanzhou, China.
Materials and Methods
Study Population
A birth cohort was conducted in 2010–2012 at the Gansu Provincial Maternity and Child Care Hospital, the largest maternity and child care hospital in Lanzhou, China (24). The study population was described previously (24–28), and 10,542 participants completed in-person interviews. Of those, 323 participants were multiple births, 40 were stillbirths, 253 had birth defects, 30 were data missing on birth weight, 1 was <22 weeks in gestational age, and 664 were more than 4,000 g on birth weight, which yielded 9,231 participants who were included in the current analysis. The in-person interview was conducted by trained study interviewers at the hospital. The questionnaire, which was standardized and structured, collected information on demographic factors, reproductive and medical history, environmental factors, and lifestyle factors. Information on birth outcomes and maternal complications was abstracted from the medical records. This study was approved by the Institutional Review Boards of the Gansu Provincial Maternity and Child Care Hospital. All of the women who took the pills provided oral informed consent.
Low Birth Weight
The gestational age at delivery was calculated in completed weeks from the first day of the last menstrual period. Information on the last menstrual period was extracted from medical records. All self-reported last menstrual period dates were further verified by ultrasound examinations during antenatal care in the hospital. LBW was defined as a birth weight <2,500 g (29), and normal birth weight (NBW) was defined as a birth weight ≥2,500 and ≥4,000 g.
Preterm-LBW infants in our study were defined as infants born LBW between 22 weeks + 0 days and 36 weeks + 6 days of gestation. And the term-LBW infants in our study was defined as infants born LBW between 37 weeks + 0 days and 41 weeks + 6 days of gestation.
Multiparous-LBW infants in our study were defined as infants born LBW whose mothers' parity was more than 1. And nulliparous-LBW infants in our study were defined as infants born LBW whose mothers' parity was 0.
Folic Acid Supplementation and Dietary Folate Intake
Information on folic acid supplementation was collected for the following two time periods: before conception and during pregnancy. For each time period, the duration and frequency of folic acid supplementation alone and folic acid-containing multivitamins were ascertained. Folic acid supplementation users were defined as those who took folic acid supplementation alone or folic acid-containing multivitamins before conception or during pregnancy. Preconception and pregnancy users were defined as those who took folic acid supplementation alone or folic acid-containing multivitamins before conception and during pregnancy. Only preconception users were defined as those who took folic acid supplementation alone or folic acid-containing multivitamins before conception. Only pregnancy users were defined as those who took folic acid supplementation alone or folic acid-containing multivitamins during pregnancy. Non-users were defined as those who never took folic acid supplementation alone or folic acid-containing multivitamins before conception and/or during pregnancy.
Dietary information was collected via a semiquantitative food frequency questionnaire. The daily dietary folate intake was estimated from the frequency of consumption and portion size of food items using the Chinese Standard Tables of Food Consumption (30).
Statistical Analysis
Depending on intake levels among the total study population, we determined the quartile, denoted as Q1 (<143.24), Q2 (143.24–188.55), Q3 (188.55–254.37), Q4 (≥254.37), and folic acid supplementation was classified into no more than 12 weeks and more than 12 weeks.
Pearson's chi-square tests were used to compare selected characteristics between NBW and LBW. Unconditional logistic regression models were used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for single and joint associations of folic acid supplementation and dietary folate intake with LBW and various clinical subtypes. Dose-response relationships (p for trend) were calculated based on those categorical levels. In Tables 2, 3, we adjusted for maternal age, monthly income per capita, maternal education level, smoking, maternal employment, weight gain during pregnancy, preeclampsia, cesarean section, parity, total energy intake, dietary folate intake, or folic acid supplement. In Table 4, we adjusted for maternal age, monthly income per capita, maternal education level, smoking, maternal employ, weight gain during pregnancy, preeclampsia, cesarean section, total energy intake, dietary folate intake, or folic acid supplement. In Table 5, we adjusted for maternal age, monthly income per capita, maternal education level, smoking, maternal employ, weight gain during pregnancy, preeclampsia, cesarean section, parity, and total energy intake. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Of the 9,231 singleton live births, 650 were diagnosed with LBW, and 8,581 were NBW. Table 1 shows the distributions of selected characteristics in participants with LBW and NBW births. Women who had LBW births were more likely to be either younger than 25 years old or older than 30 years old, gain <3,000 monthly income per capita (RMB), have less than a college education, smoke, be unemployed during pregnancy, gain less weight during pregnancy, be diagnosed with preeclampsia, be multipara, and adopt cesarean delivery. Distributions of drinking during pregnancy, prepregnancy BMI, and gender of live birth were similar for LBW and NBW births.
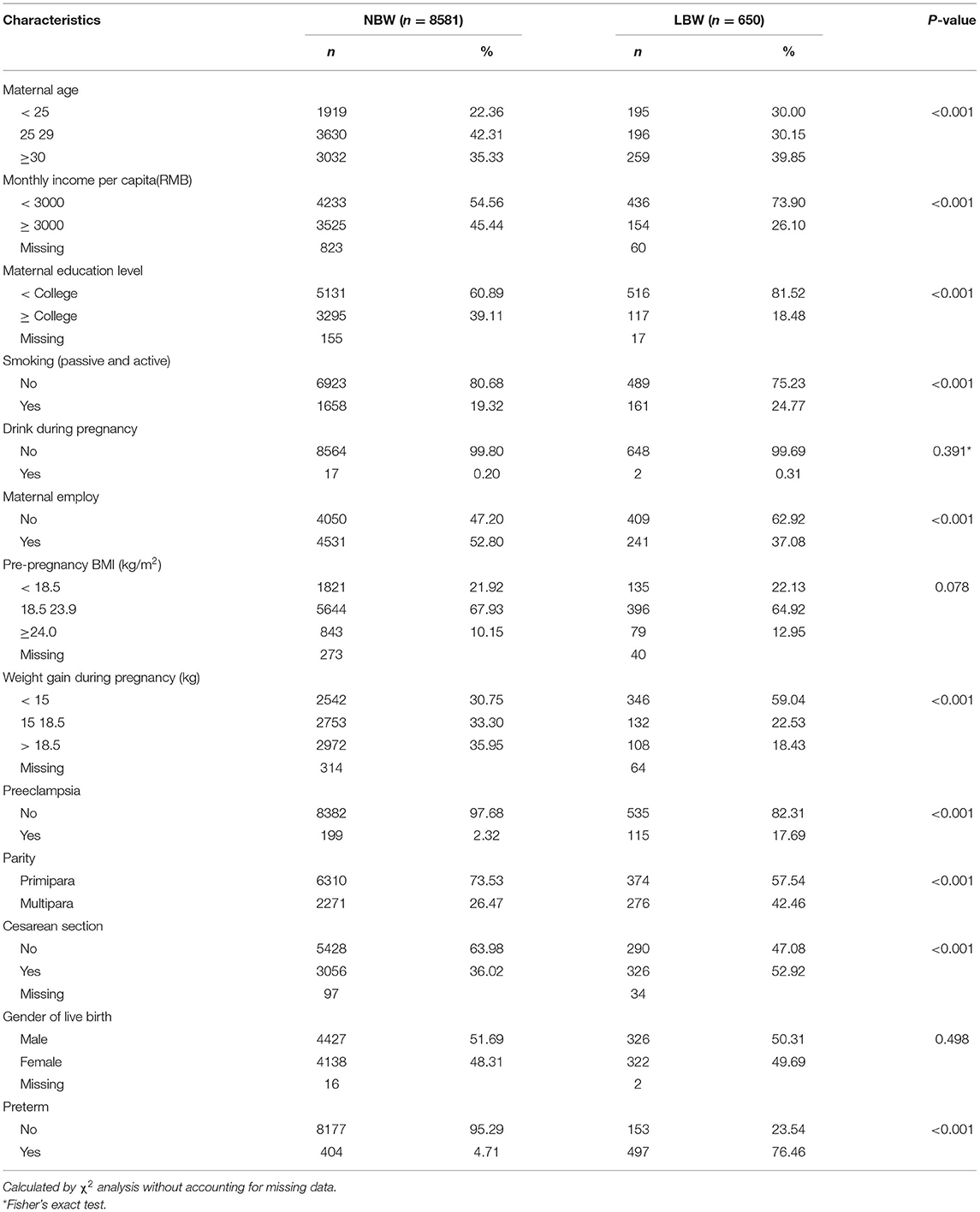
Table 1. Distributions of selected characteristics in participants with normal birth weight (NBW) and low birth weight (LBW).
As shown in Table 2, folic acid supplementation was associated with a reduced risk of LBW overall (OR: 0.80, 95% CI: 0.66–0.97), and the risk of LBW decreased with the increasing duration of folic acid supplementation (p for trend <0.001). After stratifying by periods of folic acid supplementation, slightly significant associations were observed for those who took supplements before conception and during pregnancy (OR: 0.78, 95% CI: 0.59–1.03) or during pregnancy only (OR: 0.80, 95% CI: 0.65–0.99), and significant duration of dose-responses for folic acid supplements were observed for both (p for trend <0.001 and <0.001). However, this pattern is not similar to women who took supplements before conception only at all. Dietary folate intake is not associated with LBW. In addition, there was no interaction between folic acid supplements and dietary folate intake on LBW (Table 3, p = 0.223).
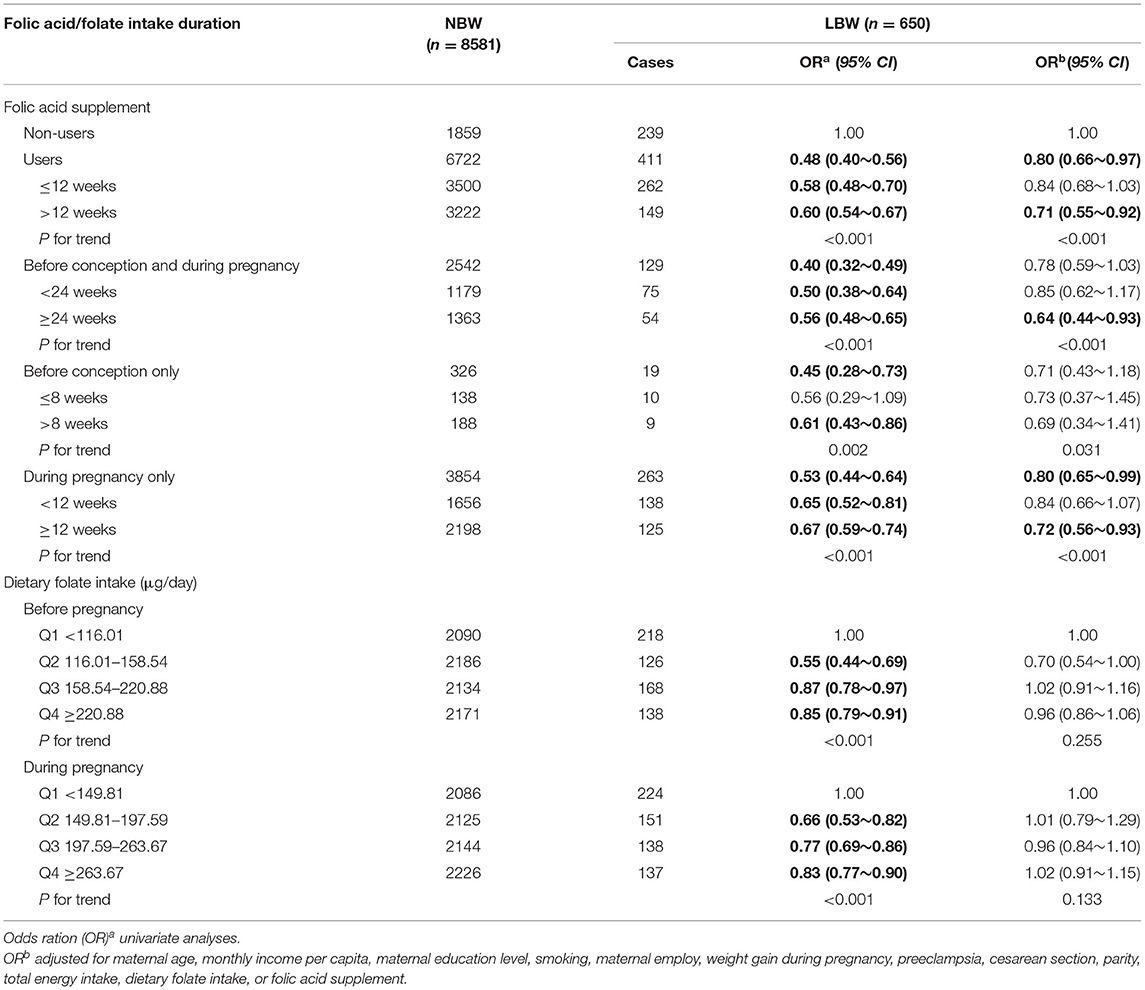
Table 2. Associations of folate acid supplementation and dietary folate intake with the risk of LBW.
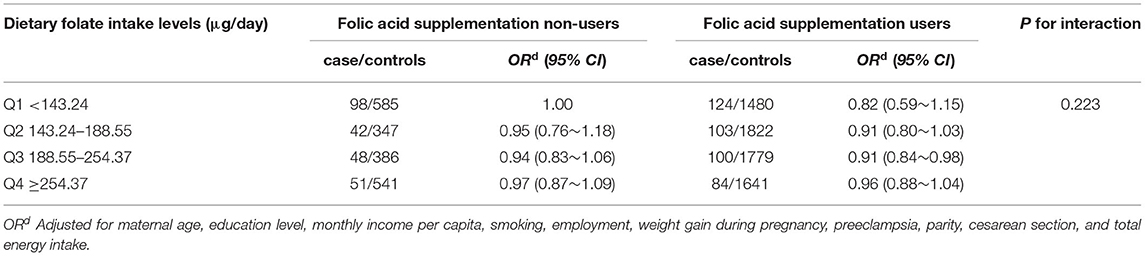
Table 3. Odds ration (95% CI) of LBW by joint effects of folic acid supplement and dietary folate intake.
We then analyzed the data separately for term-LBW and preterm-LBW (Table 4). The protective effect of folic acid supplementation was increased on term-LBW (OR: 0.59, 95% CI: 0.41–0.85), but it was not presented at preterm-LBW (OR: 0.90, 95% CI: 0.72–1.12), and the similar associations were observed for women who took supplements before conception and during pregnancy (OR: 0.40, 95% CI: 0.23–0.70; p for trend <0.001) or during pregnancy only (OR: 0.67, 95% CI: 0.45–0.98; p for trend <0.013). For dietary folate intake, there were no significant associations between term-LBW and preterm-LBW.
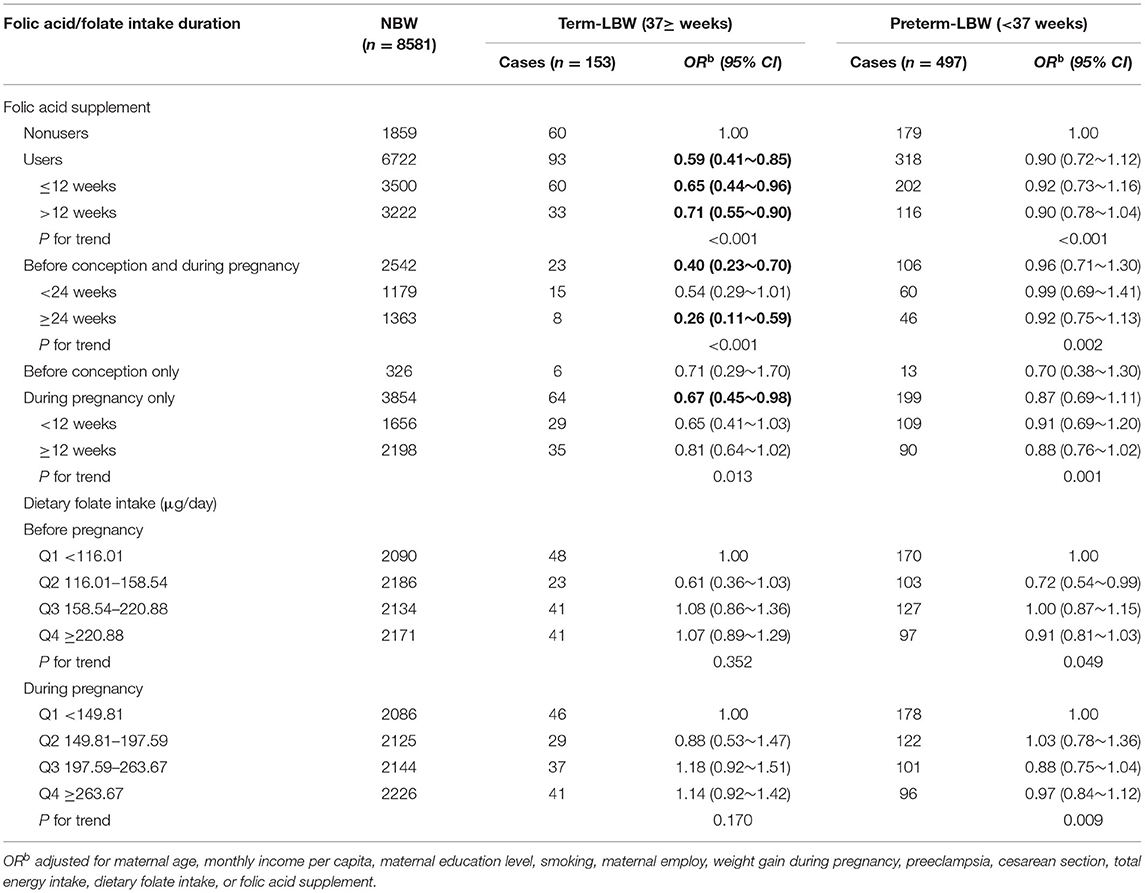
Table 4. Associations of folate acid supplementation and dietary folate intake with the risk of term-LBW and preterm-LBW.
Depending on parity, we divided LBW into nulliparous-LBW and multiparous-LBW (Table 5). A significantly protective effect on multiparous-LBW was observed among folic acid supplementation users (OR: 0.72, 95% CI: 0.54–0.94), but folic acid supplementation was not related to nulliparous-LBW (OR: 0.88, 95% CI: 0.68–1.13). We further analyzed multiparous-LBW by periods of folic acid supplementation, the protective effect was significantly increased before conception and during pregnancy (OR: 0.55, 95% CI: 0.36–0.83), but not during pregnancy only (OR: 0.76, 95% CI: 0.57–1.02) or before conception only (OR: 0.57, 95% CI: 0.26–1.27), and a significant duration of dose-responses for folic acid supplementation was observed before conception and during pregnancy (p for trend < 0.001), and during pregnancy only (p for trend <0.001). For dietary folate intake, there were no significant associations with nulliparous-LBW and multiparous-LBW.
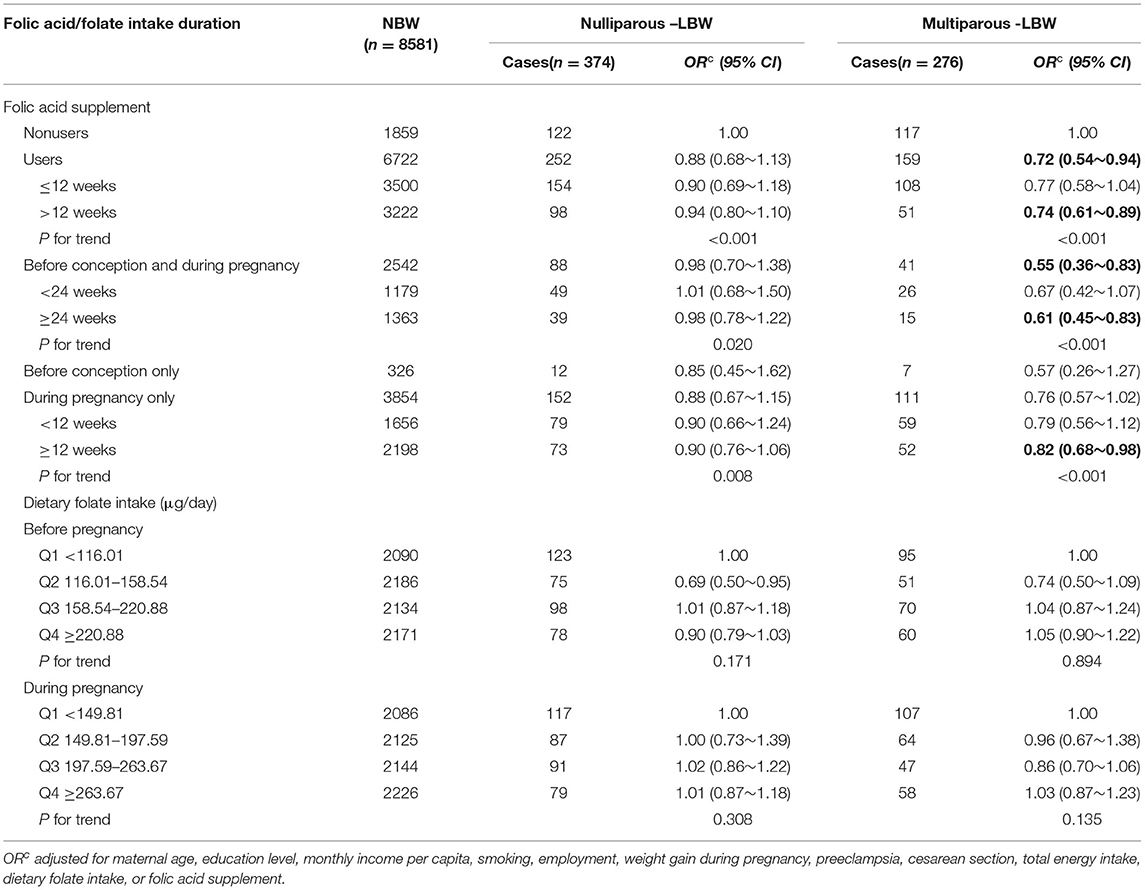
Table 5. Associations of folate acid supplementation and dietary folate intake with the risk of nulliparous-LBW and multiparous-LBW.
Discussion
Our study results indicated that folic acid supplementation was associated with a reduced risk of LBW, term-LBW, and multiparous-LBW, with those risks decreasing with increasing duration of folic acid supplementation. After stratifying by periods of folic acid supplementation, similar patterns were observed for those who took supplements before conception and during pregnancy or pregnancy only. There was no interaction between folic acid supplement and dietary folate intake on LBW.
The occurrence of LBW is a highly complex biologic process, and the precise protective mechanism of folic acid is still unknown. The epigenome is particularly susceptible during the early stages of embryogenesis (31), folate may cause epigenetic modifications resulting in increased placental and fetal growth patterns (32, 33). In addition, folate may indirectly influence fetal growth by modulating placental growth and development (34, 35), and folate plays a critical role in protein and DNA synthesis (36, 37).
Earlier epidemiological research investigating the associations between folic acid supplements and the risk of LBW has provided ambiguous results. In Europe, five studies (10–14) based on cohort studies indicated that folic acid supplementation was associated with birth weight and one cohort study (15) indicated that the effects of supplementing the diet with folic acid given preconceptionally or in the first half of pregnancy were a slight increase of birth weight. Timmermans et al. found that preconception start of folic acid was associated with a decreased risk of LBW, and the start of folic acid supplementation after pregnancy recognition was also associated with a decrease of having a child with LBW (10). Pastor-Valero et al. thought periconceptional use of folic acid supplements > 1 mg/day may entail a risk of decreased birth weight (13), and Papadopoulou et al. indicated that high daily doses of supplementary folic acid in early-to-mid pregnancy may be protective for LBW (12). In addition, Bergen et al. found that low folate concentrations and erythrocyte folic acid were associated with birth weight (23). However, a case control study indicated that there was no significant reduction in the rate of LBW in pregnant women with early or late onset pre-eclampsia after folic acid supplementation (20). In Japan, only one study indicated that lower dietary intake of protein, iron, and folic acid are known risk factors for LBW (21). In the United States, Martinussen et al. found that there were no significant associations between folic acid supplementation and LBW (19), but Scholl et al. indicated that lower concentrations of serum folate at week 28 were also associated with a greater risk of preterm delivery and LBW (22). In China, Li et al. indicated that statistically significant reductions in the risk were evident in women who used folic acid peri- or postconception, but not in those who took folic acid preconception (16). Liu et al. found that the risk of LBW among pregnant women who did not take folic acid during periconception was 1.30 times higher than those who took folic acid (17), but Yang et al. found that folic acid supplementation was not associated with birth weight (18).
The different recommendations about folic acid supplements and dietary pattern among international entities may contribute to the conflicting results. To prevent neural tube defects and other congenital anomalies, more than forty seven countries have recommended taking folic acid supplements in the periconceptional period (38) based on two randomized trials by the British Medical Research Council in 1991 and Hungarian National Institute of Hygiene in 1992 (39, 40). In Europe, no folic acid fortification is required and only voluntary fortification is permitted (41). In North America, folic acid fortification is mandatory in grain products. In China, women were advised to supplement folic acid at least 4 weeks before conception and throughout the pregnancy. Other countries/regions, such as Singapore and Taiwan, emphasize the importance of a healthy diet without the need for supplementation, while Slovenia, Sweden, and Hong Kong published e-leaflets for the general public with detailed information about folate healthy diet during pregnancy (38). In addition to folate, variations in study populations, the time for initiating supplementation of folic acid, and the dosing of use of folic acid may also contribute to the conflicting results.
Our study discovered significant dose-response for supplement duration for those who took supplements before conception and during pregnancy or during pregnancy only, indicating that the risk of LBW, term-LBW, and multiparous-LBW decreased with increasing duration of folic acid supplementation. In China, Li et al. (16) found that the trend relative risks significantly decreased as compliance with folic acid use increased. However, the significant dose-response for the duration of supplementation was not shown, and other previous studies also did not explore this association. This result was important for preventing LBW, and suggested that starting folic acid supplementation should be done earlier in pregnancy and continued for at least 12 weeks.
To our knowledge, this is the first study investigating the associations of term-LBW and preterm-LBW with folic acid supplementation as well as the associations of nulliparous-LBW and multiparous-LBW with folic acid supplementation. Significant associations were observed for term-LBW and multiparous-LBW but not for preterm-LBW or nulliparous-LBW, which indicated that they may have different etiological profiles, and the biologic processes should be further studied.
Actually, there are some limitations to the current study. First, the study participants were predominantly from Lanzhou, so generalizability of our results to other populations with quite different demographic characteristics may not be appropriate. Second, dietary folate in a combination with other micronutrients could potentially confound our results. In models b, c, and d, we have adjusted for total energy, which could control this problem effectively. Third, although we have adjusted for many important confounding factors, we cannot rule out the potential for residual confounding. Since information on folic acid supplementation and dietary folate intake was based on self-reported data, there existed a recall bias. However, during the period of questionnaire design, field investigation, and information input, there were enough professionals who undertook the quality control, ensuring the accuracy of information. In addition, a study has already suggested that there is a strong correlation between self-reported folate intake and serum folate concentrations during pregnancy (22).
Conclusion
Our study results indicated that folic acid supplementation was associated with a reduced risk of LBW, term-LBW, and multiparous-LBW, with those risks decreasing with increasing duration of folic acid supplementation. After stratifying by time periods of folic acid supplementation, similar patterns were observed for those who took supplements before conception and during pregnancy or during pregnancy only. And there was no interaction of folic acid supplement and dietary folate intake on LBW.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Investigation Committees at the GPMCCH. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
QL and JQ designed the birth cohort study. LY, WW, and BM analyzed data and wrote the article. LY, WW, BM, BY, XH, XL, LL, XX, YCh, and YCa conducted this research. YCh and YCa had primary responsibility for the final content. All authors read and approved the final manuscript.
Funding
This study was financially supported by BiosTime Maternal and Child Nutrition Health Research Projects of China CDC Maternal and Child Health Center (No. 2019FYH002), in part supported by Gansu Provincial Science and Technology Department Grant (No. 21JR1RA043), the Health Research Projects (No. GSWSKY-2019-98), and the Key Research and Development Program (No. 20YF8WA095). The funders had no role in the design, analysis, or writing of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the study personnel from the GPMCCH for their exceptional efforts in study subject recruitment.
Abbreviations
LBW, Low birth weight; NBW, Normal birth weight; GPMCCH, Maternity and Child Care Hospital; OR, Odds ratio; CI, Confidence interval.
References
1. Lawn JE, Cousens S, Zupan J. Lancet neonatal survival steering team. 4 million neonatal deaths: when? where? why? Lancet. (2005) 365:891–900. doi: 10.1016/S0140-6736(05)71048-5
2. Bacak SJ, Baptiste-Roberts K, Amon E, Ireland B, Leet T. Risk factors for neonatal mortality among extremely-low-birth-weight infants. Am J Obstet Gynecol. (2005) 192:862–7. doi: 10.1016/j.ajog.2004.07.029
3. Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. (1997) 2:105–12. doi: 10.1530/ror.0.0020105
4. Ahmed S, Hassen K, Wakayo T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: the role of nutritional factors. Nutr J. (2018) 17:103. doi: 10.1186/s12937-018-0409-z
5. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. (2008) 359:61–73. doi: 10.1056/NEJMra0708473
6. Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. (2006) 82:485–91. doi: 10.1016/j.earlhumdev.2006.07.001
7. Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. (2008) 300:2886–97. 2008. 886 doi: 10.1001/jama.2008.886
8. United Nations Children's Fund. Undernourishment in the Womb Can Lead to Diminished Potential Predispose Infants to Early Death. (2014). Availble online at: https://data.unicef.org/topic/nutrition/low-birth- weight (accessed 20 December 2019)
9. Krishnaswamy K, Nair KM. Importance of folate in human nutrition. Br J Nutr. (2001) 85 (Suppl. 2):S115–124. doi: 10.1079/BJN2000303
10. Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RPM. Steegers, EAP. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the generation R study. Br J Nutr. (2009) 102:777–85. doi: 10.1017/S0007114509288994
11. Bakker R, Timmermans S, Steegers EA, Hofman A, Jaddoe VWV. Folic acid supplements modify the adverse effects of maternal smoking on fetal growth and neonatal complications. J Nutr. (2011) 141:2172–9. doi: 10.3945/jn.111.142976
12. Papadopoulou E, Stratakis N, Roumeliotaki T, Sarri K, Merlo DF, Kogevinas M, et al. The effect of high doses of folic acid and iron supplementation in early-to-mid pregnancy on prematurity and fetal growth retardation: the mother-child cohort study in Crete, Greece (Rhea study). Eur J Nutr. (2013) 52:327–36. doi: 10.1007/s00394-012-0339-z
13. Pastor-Valero M, Navarrete-Munoz EM, Rebagliato M, Iñiguez C, Murcia M, Marco M, et al. Periconceptional folic acid supplementation and anthropometric measures at birth in a cohort of pregnant women in Valencia, Spain. Br J Nutr. (2011) 105:1352–60. doi: 10.1017/S0007114510005143
14. Kim MW, Ahn KH Ryu KJ, Hong SH, Lee JS, Nava-Ocampo AA, et al. Preventive effects of folic acid supplementation on adverse maternal and fetal outcomes. PLoS ONE. (2014) 9:e97273. doi: 10.1371/journal.pone.0097273
15. Rolschau J, Kristoffersen K, Ulrich M, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part I. Eur J Obstet Gynecol Reprod Biol. (1999) 87:105–10. doi: 10.1016/S0301-2115(99)00084-6
16. Li N, Li Z, Ye R, Liu JM, Ren A. Impact of periconceptional folic acid supplementation on low birth weight and small-for-gestational-age infants in China: a large prospective cohort study. J Pediatr. (2017) 187:105–10. doi: 10.1016/j.jpeds.2017.04.060
17. Liu A, Zhang R, Li Z, Qu P, Zhao Y, Yan H. Incidence of low birth weight among single live birth neonates and influencing factors in Shaanxi. Zhonghua liu xing bing xue za zhi. (2015) 36:1244–8. doi: 10.3760/cma.j.issn.0254-6450.2015.11.012
18. Yang T, Gu Y, Wei X, Liang X, Chen J, Liu Y, et al. Periconceptional folic acid supplementation and vitamin B12 status in a cohort of Chinese early pregnancy women with the risk of adverse pregnancy outcomes. J Clin Biochem Nutr. (2017) 60:136–42. doi: 10.3164/jcbn.16-45
19. Martinussen MP, Bracken MB, Triche EW, Jacobsen GW, Risnes KR. Folic acid supplementation in early pregnancy and the risk of preeclampsia, small for gestational age offspring and preterm delivery. Eur J Obstet Gynecol Reprod Biol. (2015) 195:94–9. doi: 10.1016/j.ejogrb.2015.09.022
20. Banhidy F, Dakhlaoui A, Dudas I, Czeizel AE. Birth outcomes of newborns after folic Acid supplementation in pregnant women with early and late pre-eclampsia: a population-based study. Adv Prev Med. (2011) 2011:127369. doi: 10.4061/2011/127369
21. Uno K, Takemi Y, Hayashi F, Hosokawa M. Nutritional status and dietary intake among pregnant women in relation to pre-pregnancy body mass index in Japan. Nihon Koshu Eisei Zasshi. (2016) 63:738–49. doi: 10.11236/jph.63.12_738
22. Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr. (1996) 63:520–5. doi: 10.1093/ajcn/63.4.520
23. Bergen NE, Jaddoe VW, Timmermans S, Hofman A, Lindemans J, Russcher H, et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the generation R study. BJOG Int J Obstet Gynaecol. (2012) 119:739–51. doi: 10.1111/j.1471-0528.2012.03321.x
24. Qiu J, He X, Cui H, Zhang C, Zhang HH. Dang Yun, et al. Passive smoking and preterm birth in urban China. Am J Epidemiol. (2014) 180:94–102. doi: 10.1093/aje/kwu092
25. Zhao N, Qiu J, Zhang Y, He X, Zhou M, Li M, et al. Ambient air pollutant PM10 and risk of preterm birth in Lanzhou, China. Environ Int. (2015) 76:71–7. doi: 10.1016/j.envint.2014.12.009
26. Wang Y, Zhao N, Qiu J, He X, Zhou M, Cui H, et al. Folic acid supplementation and dietary folate intake, and risk of preeclampsia. Eur J Clin Nutr. (2015) 69:1145–50. doi: 10.1038/ejcn.2014.295
27. Liu X, Lv L, Zhang H, Zhao N, Qiu J, He X, et al. Folic acid supplementation, dietary folate intake and risk of preterm birth in China. Eur J Nutr. (2016) 55:1411–22. doi: 10.1007/s00394-015-0959-1
28. Mao B, Qiu J, Zhao N, Shao Y, Dai W, He X, et al. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PloS ONE. (2017) 12:e0187996. doi: 10.1371/journal.pone.0187996
29. Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. (2002) 346:731–7. doi: 10.1056/NEJMoa010806
30. Institute of Nutrition and Food Hygiene Chinese Academy of Preventive Medicine. Table of Food Components (national representative values). Beijing: People's Hygiene Press (1999).
31. Timmermans S, Jaddoe VW, Silva LM, Hofman A, Raat H, Steegers-Theunissen RPM, et al. Folic acid is positively associated with uteroplacental vascular resistance: the Generation R study. Nutr Metab Cardiovasc Dis. (2011) 21:54–61. doi: 10.1016/j.numecd.2009.07.002
32. Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition (Burbank, Los Angeles County, Calif). (2004) 20:63–8. doi: 10.1016/j.nut.2003.09.011
33. Pennisi E. Environmental epigenomics meeting. Supplements restore gene function via methylation. Science. (2005) 310:1761. doi: 10.1126/science.310.5755.1761
34. Steegers-Theunissen RP, Steegers EA. Nutrient-gene interactions in early pregnancy: a vascular hypothesis. Eur J Obstet Gynecol Reprod Biol. (2003) 106:115–7. doi: 10.1016/S0301-2115(02)00358-5
35. Bleker OP, Buimer M, van der Post JA, Veen FVD, et al. Ted (G.J.) Kloosterman: on intrauterine growth. the significance of prenatal care. Studies on birth weight, placental weight and placental index. Placenta. (2006) 27:1052–4. doi: 10.1016/j.placenta.2006.01.001
36. Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. (2006) 83:993–1016. doi: 10.1093/ajcn/83.5.993
37. Bailey LB, Gregory JF. 3rd. Folate metabolism and requirements. J Nutr. (1999) 129:779–82. doi: 10.1093/jn/129.4.779
38. Gomes S, Lopes C, Pinto E. Folate and folic acid in the periconceptional period: recommendations from official health organizations in thirty-six countries worldwide and WHO. Public Health Nutr. (2016) 19:176–89. doi: 10.1017/S1368980015000555
39. Wald N. Prevention of neural tube defects: results of the medical research council vitamin study. MRC vitamin study research group. Lancet (London, England). (1991) 338:131–7. doi: 10.1016/0140-6736(91)90133-A
40. Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. (1992) 327:1832–5. doi: 10.1056/NEJM199212243272602
Keywords: folic acid supplementation, dietary folate intake, dietary folate intake, LBW, cohort study
Citation: Yang L, Wang W, Mao B, Qiu J, Guo H, Yi B, He X, Lin X, Lv L, Xu X, Liu Q, Cao Y and Chen Y (2022) Maternal Folic Acid Supplementation, Dietary Folate Intake, and Low Birth Weight: A Birth Cohort Study. Front. Public Health 10:844150. doi: 10.3389/fpubh.2022.844150
Received: 27 December 2021; Accepted: 08 April 2022;
Published: 09 June 2022.
Edited by:
Guodong Ding, Shanghai Children's Hospital, ChinaReviewed by:
Prema Ramachandran, Nutrition Foundation of India, IndiaGuowei Huang, Tianjin Medical University, China
Copyright © 2022 Yang, Wang, Mao, Qiu, Guo, Yi, He, Lin, Lv, Xu, Liu, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchun Cao, Y2FveWNoX2RkQHNpbmEuY29t; Yiming Chen, NTYzNDAzODMxIEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Liping Yang1†
Liping Yang1† Yongchun Cao
Yongchun Cao