- 1Department of Dermatology and Venereology, First Affiliated Hospital of Kunming Medical University, Kunming, China
- 2NHC Key Laboratory of Drug Addiction Medicine, First Affiliated Hospital of Kunming Medical University, Kunming Medical University, Kunming, China
- 3Scientific Research Laboratory Center, First Affiliated Hospital of Kunming Medical University, Kunming, China
Background: Deep fungal infection is a type of life-threatening opportunistic infection. Its incidence has been increasing in recent years. This infection can affect the prognosis of patients, prolong hospital stays and raise costs for patients and their families.
Objective: We aimed to understand the current situation of deep fungal infections in the First Affiliated Hospital of Kunming Medical University and to provide a basis for the clinical diagnosis and treatment of deep fungal infections.
Methods: This was a retrospective analysis of 528,743 cases in the hospital from 2015 to 2019, including the epidemiological characteristics, treatment and prognosis of deep fungal infections.
Results: A total of 274 cases (0.05%) with deep fungal infections were identified, accounting for 0.05% of the total number of hospitalizations. The incidence of deep fungal infections in the hospital showed an increasing trend from 2015 to 2019. The most commonly infected site was the respiratory tract (93.07%). Among patients with deep fungal infections, 266 specimens were positive for fungal culture, by which 161 cultured Candida albicans (C. albicans), accounting for 60.53%, the main pathogen causing deep fungal infection. From 2015 to 2019, the percentage of C. albicans cases showed a downward trend, while that of non-C. albicans showed an opposite trend. Antibiotics were the most common predisposing factor for deep fungal infections (97.45%). Among the underlying diseases of patients with deep fungal infections, infectious diseases (59.49%) were the most common. Those with underlying diseases such as renal insufficiency and neurological diseases had a worse prognosis. Indwelling catheters, nervous system disease and tumors were risk factors for a poor prognosis.
Conclusions: We report for the first time the epidemiological data of deep fungal infections in a general hospital in southwestern China from 2015 to 2019. In the past 5 years, the number of patients with deep fungal infections in the First Affiliated Hospital of Kunming Medical University has been increasing. Although the clinical data are limited, these results can provide references for the diagnosis and treatment of deep fungal infections.
Introduction
Deep fungal infections can involve subcutaneous tissue, mucosa, and internal organs, including limited single-organ infections and systemic infections involving multiple organs. Deep fungal infection is a life-threatening opportunistic infection that commonly occurs in immunocompromised individuals, and its incidence has been increasing in recent years (1). The occurrence of deep fungal infections is related to the widespread use of broad-spectrum antibacterial drugs, immunosuppressive agents, catheter technology, and organ transplantation, and the increase in patients with AIDS, malignant tumors, and the elderly (2).
Epidemiological research reports showed that among patients who died of deep fungal infection in general hospitals, the mortality rate caused by candidiasis was 50–71% (1). Candida is the most common pathogen of deep fungal infections (3). It is widely found in human skin and mucous membranes and is usually maintained as a benign colonization state.
At least 15 different Candida species have been identified to be associated with diseases in humans. More than 90% of invasive candidiasis cases are caused by the five most common pathogens: Candida albicans (C. albicans), Candida glabrata (C. glabrata), Candida tropicalis (C. tropicalis), Candida parapsilosis (C. parapsilosis), and Candida krusei (C. krusei) (4). Although C. albicans is still the most common species, the infection rate of non-C. albicans spp. has increased significantly in recent decades, especially in ICU patients (5–7). According to an epidemiological study conducted in Europe, the mortality rate of patients with non-C.albicans infections is higher than that of patients with C. albicans infections (47.3 and 32.4%, respectively) (8, 9). Bloodstream infections caused by non-C. albicans spp. are difficult to treat due to antifungal drug resistance and have a high mortality rate (10–14).
The common pathogens of deep fungal infections are Aspergillus and Cryptococcus rank after Candida (3). In recent years, some rare filamentous fungi such as Mucor and Fusarium have been reported from time to time in patients with hematological malignancies and bone marrow transplantation (11). In this study, we conducted a retrospective analysis of the status of deep fungal infections in the First Affiliated Hospital of Kunming Medical University from January 2015 to December 2019.
Materials and Methods
Patient Selection
We screened inpatients from January 2015 to December 2019 from the medical record room of the First Affiliated Hospital of Kunming Medical University, and then screened out cases of fungal infection by their laboratory tests. Each hospitalization represented a case, and if the patient was hospitalized again and received another round of treatment, it was considered a new independent case. The same patient was considered to be a case if the same fungus was cultured multiple times from specimens from the same source during the same hospitalization period. Case exclusion criteria: cases with incomplete data on epidemiology, fungal microscopy, and culture examinations.
Criteria for Study Inclusion
A retrospective investigation method was used to retrospectively analyse 528,743 medical records from the inpatient department of the First Affiliated Hospital of Kunming Medical University from January 2015 to December 2019 and perform statistical analysis on the cases of fungal infection by laboratory tests. The diagnostic criteria for patients with deep fungal infections included in this study were based on the diagnostic criteria for nosocomial infections, EORTC/MSGERC (12), and the diagnostic criteria for deep fungal infections formulated by the Chinese Journal of Internal Medicine (13). The information analyzed included general information about the patient, related information about the underlying disease, fungal infection, and information about the use of antifungal drugs.
Methods for Identifying Fungal Agents
Fungal identification was done by fungal direct microscopy and mycologic culture. The samples such as sputum, cerebrospinal fluid, blood, urine, exudates were inoculated on Sabouraud's dextrose agar with chloramphenicol at 35°C for 5–7 days. Positive samples were sub-cultured on Sabouraud chloramphenicol agar or blood agar at 35°C for 24–48 h. The yeasts were identified by API 20 C AUX, other filamentous fungi were identified by biochemical and morphological features.
Statistical Analysis
Statistical analysis was performed using SPSS 25.0. This study is based on the count data described by the number of cases and the percentage. The test chi-square test was used for comparisons between groups of enumeration data. A two-tailed t-test was used to determine significant differences between the means in age and the length of stay. Binary logistic regression was used to compare multivariate associations. P < 0.05 indicates that the difference is statistically significant.
Results
Incidence and Annual Distribution of Deep Fungal Infections
From January 2015 to December 2019, there were 528,743 inpatients in the First Affiliated Hospital of Kunming Medical University, including 86,551, 88,393, 105,890, 119,575 and 128,334, respectively. According to the above diagnostic criteria, 274 (0.05%) cases of deep fungal infections were diagnosed. The diagnosed deep fungal infection case numbers and the rates (the number of diagnosed cases of deep fungal infection/the total number of hospitalized cases in the year × 100%) were 13 (0.015%), 40 (0.045%), 43 (0.041%), 86 (0.072%), and 92 (0.072%) from 2015 to 2019, respectively. Retrospective analysis of 528,743 cases demonstrated that the incidence of deep fungal infections showed an increasing trend from January 2015 to December 2019, during which 2018 and 2019 had the highest incidences, whose highest values were the same (0.072%) (Figure 1A).

Figure 1. Distribution of selected characteristics of deep fungal infections. (A) Annual distribution of the rate of deep fungal infections. (B) Distribution of patients with deep fungal infections in different age groups. (C) Distribution diagram of departments with patients with deep fungal infections. (D) Distribution of deep fungal infection sites.
General Situation of Patients With Deep Fungal Infections
The sex statistics of patients diagnosed with deep fungal infections were 187 men, accounting for 68.25%, and 87 women, accounting for 31.75% (Table 1). The age of the patients with deep fungal infections ranged from 4 years old to 93 years old, with an average age of 61.04 ± 16.89. Most of them were distributed in the three age groups of 51–60 years old (16.79%), 61–70 years old (25.91%), and 71–80 years old (19.71%). Patients over 60 years old accounted for 57.66% of the total number of deep fungal infections (Figure 1B).
The top three departments diagnosing patients with deep fungal infections were the respiratory department (94 cases, 34.31%), the transplantation department (34 cases, 12.41%), and the cardiology department (30 cases, 10.95%) (Figure 1C).
The main site of deep fungal infection was the respiratory system with 255 cases (93.07%), followed by central nervous system with 11 cases(4.01%), the abdominal cavity with 4 cases (1.46%) and the thoracic cavity and blood with 2 cases each (0.73%) (Figure 1D).
Pathogens of Deep Fungal Infections
Among the patients with deep fungal infections, 266 specimens were positive on fungal culture. The results were as follows: 161 cases (60.53%) of C. albicans, 56 cases (21.05%) of C. glabrata, 22 cases (8.27%) of C. krusei, 13 cases (4.89%) of C. tropicalis, 3 cases (1.13%) of other Candida, 8 cases (3.01%) of Aspergillus, 1 case (0.38%) each of Cryptococcus, Fusarium, and Geotrichum (Figure 2A). From 2015 to 2019, the percentage of Candida albicans showed a downwards trend, while the percentage of non-C. albicans spp. showed an upwards trend.
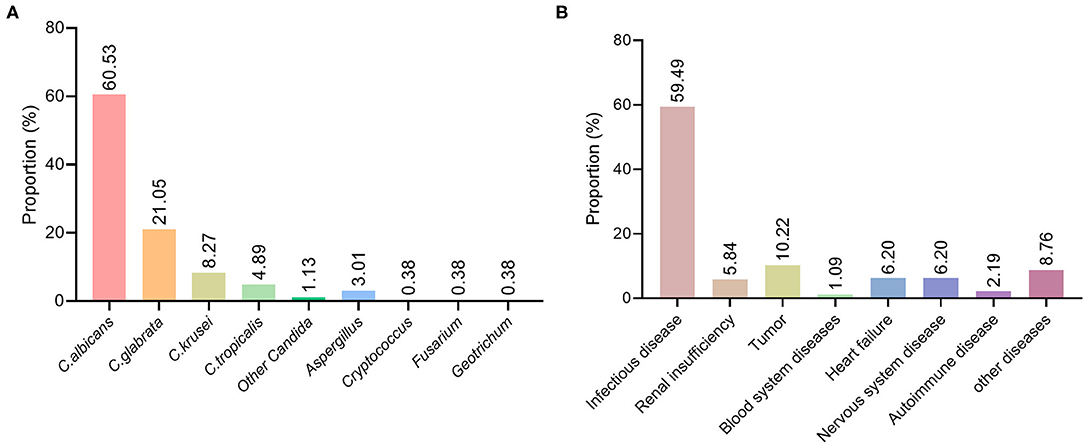
Figure 2. Distribution of pathogenic fungi and basic diseases in patients with deep fungal infections. (A) Distribution of various fungal species in patients with deep fungal infections. (B) Distribution of underlying diseases in patients with deep fungal infections.
Predisposing Factors of Patients With Deep Fungal Infections
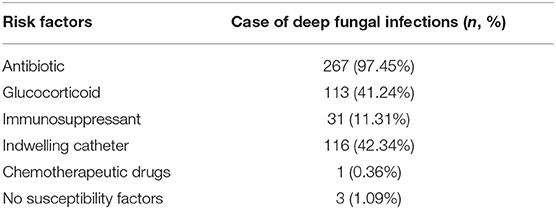
Among the 274 patients diagnosed with deep fungal infections, 267 (97.45%) had history of using antibiotics, 116 (42.34%) had history of using indwelling catheters, 113 (41.24%) had history of using glucocorticoids, 31 (11.31%) had history of using immunosuppressive agents, 1 (0.36%) had history of using chemotherapy drugs, and 3 (1.09%) had no specific predisposing factors (Table 2). Indwelling catheters were a risk factor for a poor prognosis (OR = 3.525, 2.041-6.089).
Among the 274 patients diagnosed with a deep fungal infection, 90 patients (32.85%) had 1 predisposing factor, 122 patients (44.53%) had 2 predisposing factors, and 42 patients had 3 predisposing factors. (15.33%), while 17 cases (6.20%) had 4 predisposing factors.
Analysis of Underlying Diseases in Patients With Deep Fungal Infections
Among the 274 patients diagnosed with deep fungal infections, 163 had an underlying infectious disease, including an acute exacerbation of chronic obstructive pulmonary disease, pyelonephritis, central nervous system infection, pneumonia, AIDS, tuberculosis, and cholecystitis, accounting for 59.49%;16 cases of renal insufficiency, accounting for 5.84%; 28 cases of tumors, accounting for 10.22%; 3 cases of hematological diseases, accounting for 1.09%; 17 cases of cardiac insufficiency, accounting for 6.20%; 17 cases of neurological diseases, accounting for 6.20%; 6 cases of autoimmune diseases, accounting for 2.19%; and 24 cases of other diseases, accounting for 8.76% (Figure 2B).
The Use of Antifungal Drugs for Deep Fungal Infections
Of the 274 patients with deep fungal infections, 220 were treated with antifungal drugs, accounting for 80.29%: 97 cases of voriconazole (35.40%), 11 cases of itraconazole (4.01%), 22 cases of caspofungin (8.03%), 22 cases of micafungin (8.03%), 105 cases of fluconazole (38.32%), 14 cases of amphotericin B (5.11%) and 2 cases of posaconazole (0.73%) (Figure 3A).
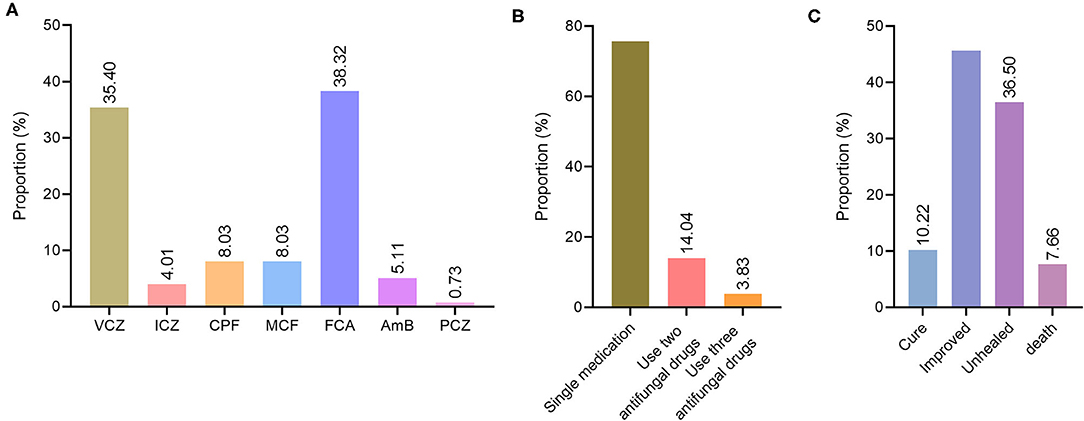
Figure 3. Treatment and prognosis of deep fungal infections. (A) Distribution diagram of antifungal drug species for deep fungal infections. VCZ, voriconazole; ICZ, itraconazole; CPF, caspofungin; MCF, micafungin; FCA, fluconazole; AmB, amphotericin B; PCZ, posaconazole. (B) Distribution of antifungal drug use patterns in patients with deep fungal infections. (C) The distribution of prognostic types for deep fungal infections.
Among the 220 patients with deep fungal infections who used antifungal drugs, 178 were single drugs, accounting for 75.74%, and 42 (17.87%) were used in combination, alternate use, or combined/alternative use. Among them, two antifungal drugs were used in 33 cases (14.04%), and three antifungal drugs- were used in 9 cases (3.83%) (Figure 3B).
The prognosis of 153 (55.84%) patients among the 274 with deep fungal infections was good (cured + improved), including 28 cured (10.22%) and 125 improved (45.62%). A poor prognosis (unhealed + death) was observed for 121 (44.16%) of the deep fungal infections, including unhealed 100 cases (36.50%) and 21 died cases (7.66%) (Figure 3C).
Analysis of Factors Related to a Poor Prognosis of Patients With Fungal Infections
There was no statistically significant difference in age, sex or length of hospital stay between patients with deep fungal infections with a good prognosis and those with a poor prognosis (P > 0.05). Among the basic diseases, patients with deep fungal infections with renal insufficiency, tumors, and neurological diseases had a poor prognosis (P < 0.05). A tumor was a risk factor for a poor prognosis (OR = 2.558, 1.127–5.804). A nervous system disease was also a risk factor for a poor prognosis (OR = 5.288, 1.620–17.265). See Table 3 for details.
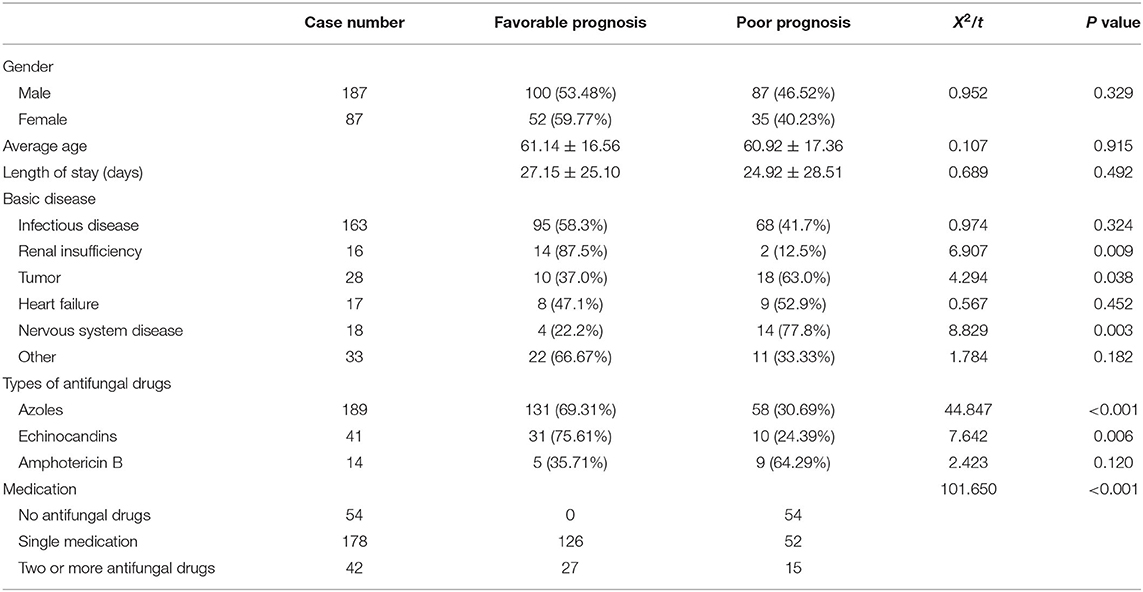
Table 3. Comparison of different prognostic related factors in patients with deep fungal infections.
Discussion
As a life-threatening opportunistic infection, the incidence of deep fungal infection has increased in recent years (1, 14, 15). Recent studies have shown that 1.5 million people die from fungal infections worldwide each year, which is similar to the number of deaths caused by tuberculosis each year (16). The lack of specific symptoms makes the diagnosis of deep fungal infections more difficult. Once inpatients develop a deep fungal infection, it will increase the patient's hospital stay and economic costs. Therefore, research and analysis into deep fungal infections in general hospitals are of significance for the clinical diagnosis and treatment of deep fungal infections and for improving the prognosis of the patients.
This study demonstrated that from 2015 to 2019, deep fungal infections in the First Affiliated Hospital of Kunming Medical University showed an upward trend in prevalence from year to year, and reaching a peak in 2018 and 2019. According to the literature, fungal diseases are becoming more predominant in the United States, with 1,047,422 deaths in 2018 (17). Our research results are consistent with those reported in the previous literature. In recent years, the incidence of deep fungal infections has been increasing. In addition to the increased use of antibiotics, immunosuppressants, and indwelling catheters, this may also be related to the improvement of diagnostics. Improved fungal tests helps to identify patients with fungal infections earlier and enables timely intervention and treatment so that patients can obtain a better outcome (18).
Many studies have shown that age is a risk factor for deep fungal infections (1). Most elderly patients have a variety of underlying diseases, low immune function, and long hospital stays, and they are prone to multisystem infections. At the same time, their defense ability of the skin and mucous membranes is reduced, so their risk of fungal infection is greatly increased. In this study, elderly patients with deep fungal infections accounted for 57.66% of patients diagnosed with deep fungal infections. With the aging of the population in China and the increase in elderly patients, clinicians should pay attention to the higher risk of deep fungal infections in elderly patients and strive to improve the immunity of elderly patients.
Previous studies have demonstrated that fungal infections mainly occur in the respiratory system and urinary system (19). The analysis of this study showed that patients with deep fungal infections mainly came from the respiratory department and transplantation department, accounting for 34.31 and 12.41% respectively. The main sites of infection were the respiratory system, central nervous system and abdominal cavity, which accounted for 93.07, 4.01, and 1.46% respectively. During clinical treatment, special attention should be given to patients in departments with a high incidence of respiratory fungal infections, especially respiratory and transplantation departments. The reason why deep fungal infections occurred more frequently in the respiratory department may be that patients in the respiratory department were hospitalized for a long time. Prolonged hospitalizations may increase the exposure of fungal pathogens. Fungal pathogens are very abundant in hospitals settings and difficult to eradicate (20). In addition, most of the patients in the respiratory department were elderly patients with serious underlying diseases. Most of them needed to use broad-spectrum antibiotics. For elderly patients, the ability to clear cilia of respiratory tract mucosa is weak, and there is more hyperplasia and secretion by bronchial glands (21), and reduced pathogen clearance ability. There are many normal microorganisms colonizing the respiratory tract. Only when the immune function of the body is weakened can these opportunistic pathogens cause infection in the corresponding site. In addition, the large number of patients with deep fungal infections in the respiratory department may be correlated with the importance attached to fungal infections by respiratory doctors in our hospital so that relevant examinations are performed in a timely fashion to confirm the diagnosis. At the same time, due to the development of transplantation technology in recent years, the increase in transplant patients, invasive surgery and the widespread use of antibiotics, hormones or immunosuppressive agents, there is an increased susceptibility to deep fungal diseases, so the transplantation department has become a high incidence department of deep fungal infections.
Candida infections are ranked first in the species distribution of deep fungal infections. Among them, C. albicans was the main pathogen causing deep fungal infections (60.53%), followed by C. glabrata (21.05%). This result is consistent with the distribution of fungal species reported in the literature (22). The proportion of C. glabrata in Candida infections have been increasing in recent years. According to international reports, C. glabrata accounts for 17%-26% of patients with invasive fungal infections (23, 24). Our results are consistent with this finding. C. glabrata is resistant to a variety of antifungal drugs, and its invasive infection has a fatality rate of up to 50%, which brings great difficulties to the treatment of patients with invasive fungal infections (25). Most C. glabrata are naturally resistant to azole antifungal drugs (26). The mortality rate of patients with C. glabrata candidaemia who used voriconazole was significantly higher than that of patients who used echinocandin (27). The wrong choice of azole antifungal drugs by clinicians may lead to a further decrease in the patient's immunity, which means they are more likely to have multiple Candida infections. Other studies have suggested that invasive C. glabrata infection is likely to occur in patients with weakened immunity and neutropenia (25), which may be the reason why patients with invasive C. glabrata infection are more likely to have multiple Candida infections. According to previous reports, in general hospitals in northeastern China, the most common pathogen of deep infections is C. parapsilosis (34.8%), followed by C. gillimonella (26.7%), C. albicans (18.5%), and C. glabrata (8.1%) (20). This is different from the findings of this study, which may be due to differences in geographic regions, climate and temperature. The literature reports that 69.2% of aspergillosis occurs in the ICU (28). Our research identified aspergillus as accounting for 3.01% of deep fungal infections. This relatively low incidence may be correlated with the relatively dry climate in this region.
The use of broad-spectrum antibiotics, glucocorticoids, immunosuppressants, indwelling catheters, and chemotherapy drugs are common predisposing factors for deep fungal infections. Our study found that the use of antibiotics was the most prominent predisposing factor for deep fungal infections (97.45%), which is consistent with previous report (29). This was followed by indwelling catheters (42.34%)and the long-term use of glucocorticoids (41.24%). For the application of broad-spectrum antibiotics, the wider the scope of the antibiotic, the stronger its antibacterial ability, and the greater the risk that it will cause fungal infections, especially carbapenems, third-generation cephalosporins and fourth-generation quinolones. Furthermore, our study found that the most commonly used antifungal drugs in our hospital for the treatment of deep fungal infections were voriconazole, itraconazole, caspofungin, micafungin, fluconazole, amphotericin B, and posaconazole, among which the top two ranking drugs were fluconazole (38.32%) and voriconazole (35.40%) both of which are triazoles. Most of the deep fungal infections in our hospital were treated with single medications, which is consistent with the trend of previous research results. Clinically, patients with mild symptoms are mostly treated with a single medication, while severe deep fungal infections are often treated with combination medications.
Indwelling catheters can increase the risk of fungal infections. Candida is currently the leading cause of bloodstream infections with high mortality and high morbidity (30). Candidemia is highly related to the formation of biofilms on a central venous catheter. Catheters can provide an attachment surface for Candida, allowing it to settle and form a biofilm, while enhancing its resistance to antifungal drugs, especially azoles and polyenes, thereby promoting the formation of infections (30, 31). The biofilm formed by Candida is more resistant to antifungal drugs (31). Therefore, the formation and development of biofilms on medical indwelling catheters is an ongoing problem during clinical treatment (32). Clinically, patients with indwelling catheters should be alert to increased risk of fungal infections.
Infectious diseases (59.49%) and tumors (10.22%) are the most important underlying diseases in patients with deep fungal infections. Infections and tumors damage the local or overall immune function of the body so that the fungus can break through the immune defense of the body and cause deep tissue infection or fungal bacteremia. This study found that among the underlying diseases, renal insufficiency, tumors, and neurological diseases can influence the prognosis of deep fungal infections (P < 0.05). Due to the lack of specific clinical manifestations of deep fungal infection, infectious diseases such as HIV, tuberculosis, lung bacterial infection, bacteremia, etc. not only increase the difficulty of diagnosis of a deep fungal infection but also aggravate the patient's condition (33).
Most patients with deep fungal infections had a good prognosis (55.84%), and their age did not affect their prognosis, while underlying diseases such as renal insufficiency, tumors, and neurological diseases affected the prognosis of deep fungal infection (P < 0.05). Therefore, for patients with underlying diseases, especially renal insufficiency, tumors, and neurological diseases, attention should be given to the possibility of deep fungal infections, and the use of antifungal drugs should be emphasized to improve their prognosis and avoid deterioration of their disease.
Conclusions
From 2015 to 2019, the deep fungal infection rate and Candida glabrata infections in the First Affiliated Hospital of Kunming Medical University both showed an upwards trend. Deep fungal infections mostly occurred in patients over 60 years old, affecting more men than women. The respiratory and transplantation department had higher incidences of deep fungal infections, and the most common sites were the respiratory and central nervous system. Candida is the main pathogen, with Candida albicans accounting for 60.53%, followed by Candida glabrata (21.05%). Patients with deep fungal infections with renal insufficiency and neurological diseases have a worse prognosis. These findings will provide references for the diagnosis and treatment of deep fungal infections.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
S-RW, Y-KC, T-XD, and Y-YL collected and filtered the data. Z-HY and S-RW analyzed the data and wrote the initial draft of the article. Z-HY, S-RW, Y-QK, and H-BL interpreted the data. Y-QK and H-BL conceived and designed the study and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Yunnan Provincial Department of Education Science Research Fund Project, Yunnan, China (2018JS211), the Joint Special Fund of Science and Technology Department of Yunnan Province - Kunming Medical University, Yunnan, China (202001AY0700001-302), and the Key Plan Project of Science and Technology from the Department of Science and Technology of Yunnan Province (202101AY070001-022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang SP, Chen YY, Hsu HS, Wang FD, Chen LY, Fung CP. A risk factor analysis of healthcare-associated fungal infections in an intensive care unit: a retrospective cohort study. BMC Infect Dis. (2013) 13:10. doi: 10.1186/1471-2334-13-10
2. Sai Saran PV, Azim A. Risk factors for early invasive fungal disease in critically ill patients. Indian J Crit Care Med. (2016) 20:750. doi: 10.4103/0972-5229.195723
3. Colombo AL, de Almeida Júnior JN, Slavin MA, Chen SC, Sorrell TC. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis. (2017) 17:e344–56. doi: 10.1016/S1473-3099(17)30304-3
4. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. (2016) 62:e1–50. doi: 10.1093/cid/civ933
5. Borjian Boroujeni Z, Shamsaei S, Yarahmadi M, Getso MI, Salimi Khorashad A, Haghighi L, et al. Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: a 3-year experience with 490 patients under intensive care. Microb Pathog. (2021) 152:104616. doi: 10.1016/j.micpath.2020.104616
6. Zarrinfar H, Makimura K, Satoh K, Khodadadi H, Mirhendi H. Incidence of pulmonary aspergillosis and correlation of conventional diagnostic methods with nested PCR and real-time PCR assay using BAL fluid in intensive care unit patients. J Clin Lab Anal. (2013) 27:181–5. doi: 10.1002/jcla.21580
7. Hosseinikargar N, Basiri R, Asadzadeh M, Najafzadeh MJ, Zarrinfar H. First report of invasive Aspergillus rhinosinusitis in a critically ill COVID-19 patient affected by acute myeloid leukemia, northeastern Iran. Clin Case Rep. (2021) 9:e04889. doi: 10.1002/ccr3.4889
8. Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJGT, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. (2019) 25:1200–12. doi: 10.1016/j.cmi.2019.04.024
9. Montagna MT, Lovero G, Borghi E, Amato G, Andreoni S, Campion L, et al. Candidemia in intensive care unit: a nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Sci. (2014) 18:661–74.
10. Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg. (2008) 106:523–9. doi: 10.1213/ane.0b013e3181607262
11. Larcher R, Platon L, Amalric M, Brunot V, Besnard N, Benomar R, et al. Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study. J Fungi (Basel). (2021) 7:330. doi: 10.3390/jof7050330
12. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. (2016) 71:1367–76. doi: 10.1093/cid/ciz1008
13. Chinese Association Hematologists; Chinese Invasive Fungal Infection Working Group. The Chinese guidelines for the diagnosis and treatment of invasive fungal disease in patients with hematological disorders and cancers (the 6th revision). Zhonghua Nei Ke Za Zhi. (2020) 59:754–63. doi: 10.3760/cma.j.cn112138-20200627-00624
14. Layegh P, Tayyebi Meibodi N, Razmyar M, Taghizadeh-Armaki M, Zarrinfar H. Disseminated phaeohyphomycosis caused by Cyphellophora ludoviensis: A novel case report from Iran. Australas J Dermatol. (2020) 61:250–2. doi: 10.1111/ajd.13262
15. Kashefi E, Seyedi S, Zarrinfar H, Fata A, Mehrad-Majd H, Najafzadeh M. Molecular Identification of Candida Species in Bronchoalveolar Lavage Specimens of Hospitalized Children with Pulmonary Disorders. JBUMS. (2021) 23:331–6.
16. Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). (2017) 3:57. doi: 10.3390/jof3040057
17. Rayens E, Norris KA, Cordero JF. Mortality Trends in Risk Conditions and Invasive Mycotic Disease in the United States, 1999-2018. Clin Infect Dis. (2022) 74:309–18. doi: 10.1093/cid/ciab336
18. Zarrinfar H, Kaboli S, Dolatabadi S, Mohammadi R. Rapid detection of Candida species in bronchoalveolar lavage fluid from patients with pulmonary symptoms. Braz J Microbiol. (2016) 47:172–6. doi: 10.1016/j.bjm.2015.02.001
19. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. (2012) 17:913–26. doi: 10.1111/j.1440-1843.2012.02150.x
20. Li Y, Gao Y, Niu X, Wu Y, Du Y, Yang Y, et al. A 5-Year Review of Invasive Fungal Infection at an Academic Medical Center. Front Cell Infect Microbiol. (2020) 10:553648. doi: 10.3389/fcimb.2020.553648
21. Zeng X, Peng M, Liu G, Huang Y, Zhang T, Wen J, et al. Strain Distribution and Drug Susceptibility of Invasive Fungal Infection in Clinical Patients With Systemic Internal Diseases. Front Bioeng Biotechnol. (2021) 8:625024. doi: 10.3389/fbioe.2020.625024
22. Ostrosky-Zeichner L, Al-Obaidi M. Invasive Fungal Infections in the Intensive Care Unit. Infect Dis Clin North Am. (2017) 31:475–87. doi: 10.1016/j.idc.2017.05.005
23. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. (2009) 48:1695–703. doi: 10.1086/599039
24. Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, et al. French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010). Intensive Care Med. (2014) 40:1303–12. doi: 10.1007/s00134-014-3408-3
25. Andes DR, Safdar N, Baddley JW, Alexander B, Brumble L, Freifeld A, et al. TRANSNET Investigators. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. (2016) 18:921–31. doi: 10.1111/tid.12613
26. Kołaczkowska A, Kołaczkowski M. Drug resistance mechanisms and their regulation in non-albicans Candida species. J Antimicrob Chemother. (2016) 71:1438–50. doi: 10.1093/jac/dkv445
27. Eschenauer GA, Carver PL, Lin SW, Klinker KP, Chen YC, Potoski BA, et al. Fluconazole versus an echinocandin for Candida glabrata fungaemia: a retrospective cohort study. J Antimicrob Chemother. (2013) 68:922–6. doi: 10.1093/jac/dks482
28. Corcione S, Lupia T, Raviolo S, Montrucchio G, Trentalange A, Curtoni A, et al. Putative invasive pulmonary aspergillosis within medical wards and intensive care units: a 4-year retrospective, observational, single-centre study. Intern Emerg Med. (2021) 16:1619–27. doi: 10.1007/s11739-021-02705-z
29. Cheng L, Hong-Bin L I, Gong L H, et al. Retrospctive Analysis of Deep Fungus Infection of Inpatients in General Hospital. J Kunming Med Univ. (2015).
30. Zuo XS, Liu Y, Cai X, Zhan L, Hu K. Association of different Candida species with catheter-related candidemia, and the potential antifungal treatments against their adhesion properties and biofilm-forming capabilities. J Clin Lab Anal. (2021) 35:e23738. doi: 10.1002/jcla.23738
31. Uppuluri P, Srinivasan A, Ramasubramanian A, Lopez-Ribot JL. Effects of fluconazole, amphotericin B, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob Agents Chemother. (2011) 55:3591–3. doi: 10.1128/AAC.01701-10
32. Nourizadeh N, Adabizadeh A, Zarrinfar H, Majidi M, Jafarian A, Najafzadeh M. Fungal biofilms in sinonasal polyposis: The role of fungal agents is notable? J Oral Maxillofac Surg Med Pathol. (2019) 31:295–8. doi: 10.1016/j.ajoms.2019.01.007
Keywords: deep fungal infections, epidemiology, China, general hospital, retrospective study
Citation: Wen S-R, Yang Z-H, Dong T-X, Li Y-Y, Cao Y-K, Kuang Y-Q and Li H-B (2022) Deep Fungal Infections Among General Hospital Inpatients in Southwestern China: A 5-Year Retrospective Study. Front. Public Health 10:842434. doi: 10.3389/fpubh.2022.842434
Received: 23 December 2021; Accepted: 01 March 2022;
Published: 28 March 2022.
Edited by:
Marwan Osman, Cornell University, United StatesReviewed by:
Guanzhao Liang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaHossein Zarrinfar, Mashhad University of Medical Sciences, Iran
Timothy Kudinha, Charles Sturt University, Australia
Copyright © 2022 Wen, Yang, Dong, Li, Cao, Kuang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Qun Kuang, eXE2MTA0MzNAaG90bWFpbC5jb20=; Hong-Bin Li, a211aG9uZ2JpbmxpQDE2My5jb20=
†These authors have contributed equally to this work
 Shu-Ran Wen
Shu-Ran Wen Zheng-Hui Yang1†
Zheng-Hui Yang1† Tian-Xiang Dong
Tian-Xiang Dong Yu-Ye Li
Yu-Ye Li Yi-Qun Kuang
Yi-Qun Kuang