
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Public Health, 15 March 2022
Sec. Planetary Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.838949
This article is part of the Research TopicVector Surveillance and Human HealthView all 4 articles
American trypanosomiasis (AT), also known as Chagas disease, is a vector-borne zoonosis of global public health importance caused by the protozoan parasite Trypanosoma cruzi (T. cruzi) that in endemic areas is transmitted mainly by several triatomine bug species (1, 2), although blood transfusion, organ transplantation, oral, sexual, and congenital are other routes to acquire the pathogen (3, 4). Approximately 12,000 AT-related deaths occur annually, up to seven million people are infected by T. cruzi, and around 75 million people are at risk of infection in endemic areas, mostly in regions of Latin American (LA) countries where vulnerable sectors of the population are affected (5–7). Estimates indicate that 184,000–459,000 disability-adjusted life years (DALYs) are lost worldwide due to AT (8). Climate change, international travel, and immigration of humans that are unaware of being infected are among the factors increasing the incidence of AT in endemic and non-endemic parts of the world (9–11). Research conducted since AT was described in 1909 by Carlos Chagas in Brazil documented the complex biology and ecology underlying triatomine vector-host-T. cruzi interactions that influence the risk of human infection (12–15). Cultural practices and anthropogenic environmental influences can result in disturbances of sylvatic and domestic AT cycles that promote T. cruzi transmission to humans (16–19).
Diagnosis and treatment of AT remain challenging (20). The use of a vaccine effective against T. cruzi remains to be realized (21). Early treatment is critical to manage the 28,000 new cases of AT estimated to occur every year (22). Nifurtimox® and Benznidazole® continue to be effective when used in the acute stage of the disease, however in chronic AT their efficacy is limited and their use is under discussion (23, 24). Mexico is one of the American countries endemic for AT where human cases and natural infection of domestic animals and wildlife reservoirs with T. cruzi were reported initially around the middle of the last century (25, 26). Of the more than 30 triatomine species documented in Mexico, around 19 species can be infected with T. cruzi and have domiciliary or intrusive habits, which is conducive to peridomestic transmission (27–29). Following recognition as a national public health problem, AT continues to burden vulnerable sectors of the population in several parts of Mexico, particularly the southern states (30).
Epidemiological data from Mexico's Ministry of Health indicate that AT is prevalent in the 32 states that make up the country (Figure 1) (31). At least two thirds of Mexico provide ecological conditions conducive for T. cruzi transmission by triatomine vectors (32). This was reflected in a 253.5% increase in the number of diagnosed cases that went from 392 to 994 between 2007 and 2016, respectively. Around 60% of the accumulated cases occurred mostly in the southern states of Veracruz, Chiapas, Quintana Roo, Oaxaca and Yucatan, and in the south central state of Morelos. Peak incidence occurred among men aged 25–49 years, largely affecting the rural population engaged in agriculture, which in some cases is an activity providing a secondary source of income (33, 34). Official data for the 2017–2019 period indicate a national upward trend in chronic AT cases (Figure 1). It is estimated that as many as four million people may be infected with T. cruzi in Mexico (35).
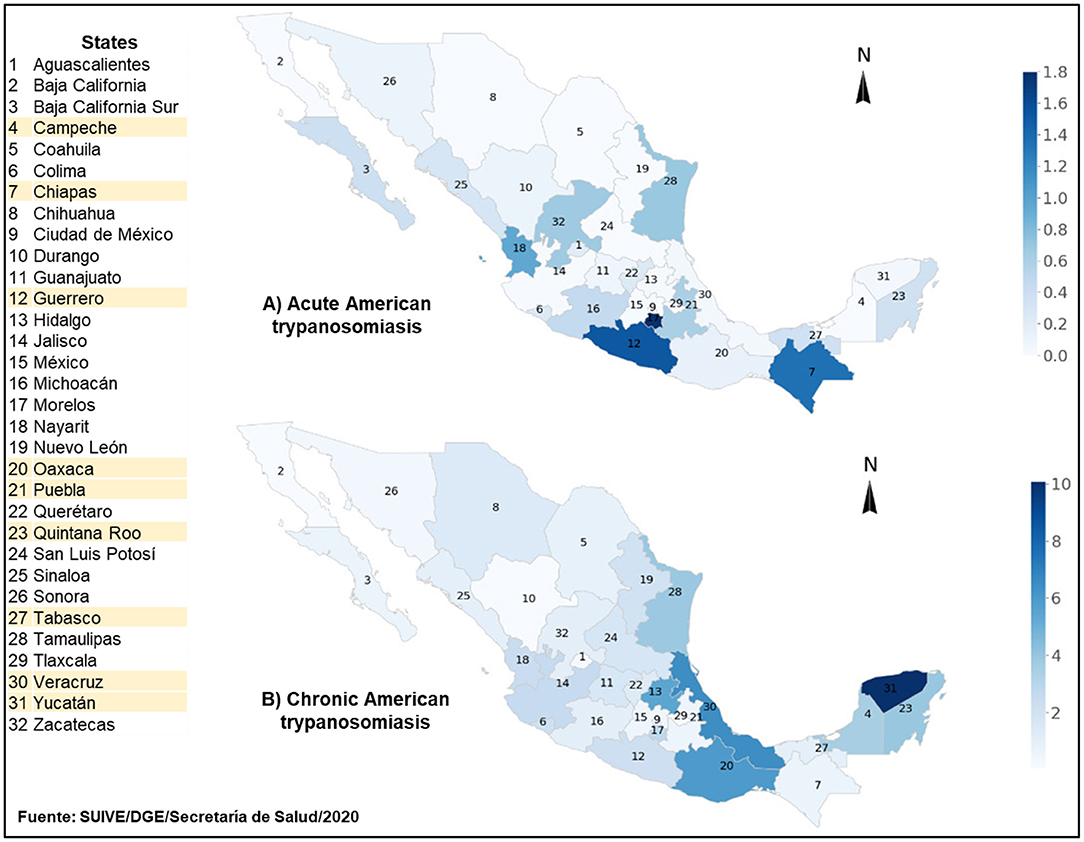
Figure 1. Incidence of diagnosed cases of acute (A) and chronic (B) American trypanosomiasis in Mexico for the 2017–2019 period. American trypanosomiasis (AT) incidence for each by state in Mexico is per 100 thousand inhabitants. Maps A and B depict incidence of diagnosed acute and chronic cases, respectively. The figure is based on AT data reported by the National Epidemiological Surveillance System of Mexico for the aforementioned period. South-Southeastern states are highlighted in yellow. Upper range for AT incidence in maps A and B is 1.8 and 10 per 100 thousand inhabitants, respectively.
Although officially reported data increased in the last decade, the true health burden of AT in the Mexican population remains unclear because the disease remains to be fully reported (36, 37). Filling this knowledge gap is required to understand the epidemiology of AT transmission, vectorial, or non-vectorial, at the national and regional levels (38, 39). Populations in the states of Chiapas and Oaxaca are among the most affected by AT in southern Mexico. Chiapas is second nationally in acute cases of AT where an incidence of 0.43 per 100,000 inhabitants was reported in 2019 (40). The re-emergence of Rhodnius prolixus, one of the most efficient triatomine vectors known, complicates efforts to understand the contribution of vector transmission to the epidemiology of AT in Oaxaca, which is the state with the highest number of fatal cases recorded between 2000 and 2016 (34, 41).
Although AT and triatomine vectors were known to affect native civilizations in pre-Columbian times (42), the pestiferous nature of triatomine bugs to humans was chronicled by expeditionary Europeans in Mexico during the 16th century (43). Triatoma phyllosoma was the first triatome species now known to be a vector described from the country in the 19th century (44), and infection of a triatomine vector with T. cruzi was first reported in 1936 (45). It was until 1990 that AT was made a reportable disease in Mexico (43).
Following the proposal for official efforts based on success to control AT in other LA countries (46, 47), Mexico established the “Programa de Acción Específico para la Vigilancia, Prevención y Control de la Enfermedad de Chagas 2013–2018” (“Specific Action Program: Prevention and Control of Chagas Disease 2013–2018”) (31). Main components of this program include the disruption of vector transmission through integrated triatomine management and the elimination of T. cruzi transmission through the congenital and blood transfusion routes (32). The use of residual insecticides to control vectors in domiciles, structural improvement, and enhancement of domestic hygiene, prevention of invasion, and establishment of vectors indoors using meshed doors and windows and the implementation of bed nets to minimize the risk of human exposure to infected vectors while sleeping are planned to disrupt vector transmission (34). Continued research will yield critical information on the ecoepidemiology of AT that could be used to adapt the program to advance AT surveillance and control in Mexico.
According to the World Health Organization (WHO), One Health is an approach where multiple sectors communicate and work together to achieve better public health outcomes (48). In this context, WHO recognizes the complexity surrounding control of neglected tropical diseases and emphasizes the need for a paradigm shift from disease-specific interventions to holistic cross-cutting approaches coordinating with other disciplines (49). The One Health approach provides the opportunity to realize this shift involving collaborative and transdisciplinary efforts to achieve optimal health outcomes among people, animals, and their shared environment to advance research for sustainable AT surveillance and control (50).
Research in context of the One Health concept was suggested as an alternative that can help understand the complexities of AT as a vector-borne disease system with diverse components under different epidemiological landscapes (51–53). Although the One Health concept resembles the Ecohealth strategy in some regards, there are differences between these approaches to address research on zoonotic vector-borne diseases (54, 55). There is room to integrate the two approaches to advance research on veterinary public health. Although Ecohealth research on AT was conducted (56), studies taking the One Health approach for research on AT remain to be designed and implemented in Mexico.
Several domestic animals and wildlife species are hosts of known triatomine vectors in Mexico (57–59). Wild mammals maintain T. cruzi in nature and facilitate its dispersion (60–62). However, information on the involvement of domestic animals and wildlife as triatomine hosts and T. cruzi reservoirs in the context of ecological and genetic variables is scant for the states of Chiapas and Oaxaca (63, 64). This is a significant epidemiological gap since the first clinical human cases of AT were reported in these states and they are among the most affected (Figure 1).
Applying the One Health concept to surveillance research could generate knowledge to advance AT control efforts in the southern states of Chiapas and Oaxaca through studies that investigate animal, human and environmental health as a unified theme (50). Table 1 exemplifies how consideration of the One Health domains helped identify knowledge gaps in epidemiological aspects of AT related to triatomine vector transmission in the wild, peridomestic, and domestic cycles that could be applied in the design of diverse public-private research partnerships as it was suggested to address the problem with antimicrobial resistance (65). Shared challenges for the effective implementation of One Health research initiatives yielding successful outcomes include overlapping causes and crosscutting causal relations (66, 67). Implementing One Health research on AT in Chiapas and Oaxaca considering the needs in Table 1 will require: (1) identifying common features of the pathogenic landscape between the states to investigate suspected hotspots of AT transmission (68); (2) establishing laboratory network that uses standardized molecular diagnostic tests for improved operations and rapid surveillance reporting (69); (3) developing electronic data reporting linked to information systems that update stakeholders frequently; and (4) adapting the principles of One Health epidemiological reporting of evidence (70).
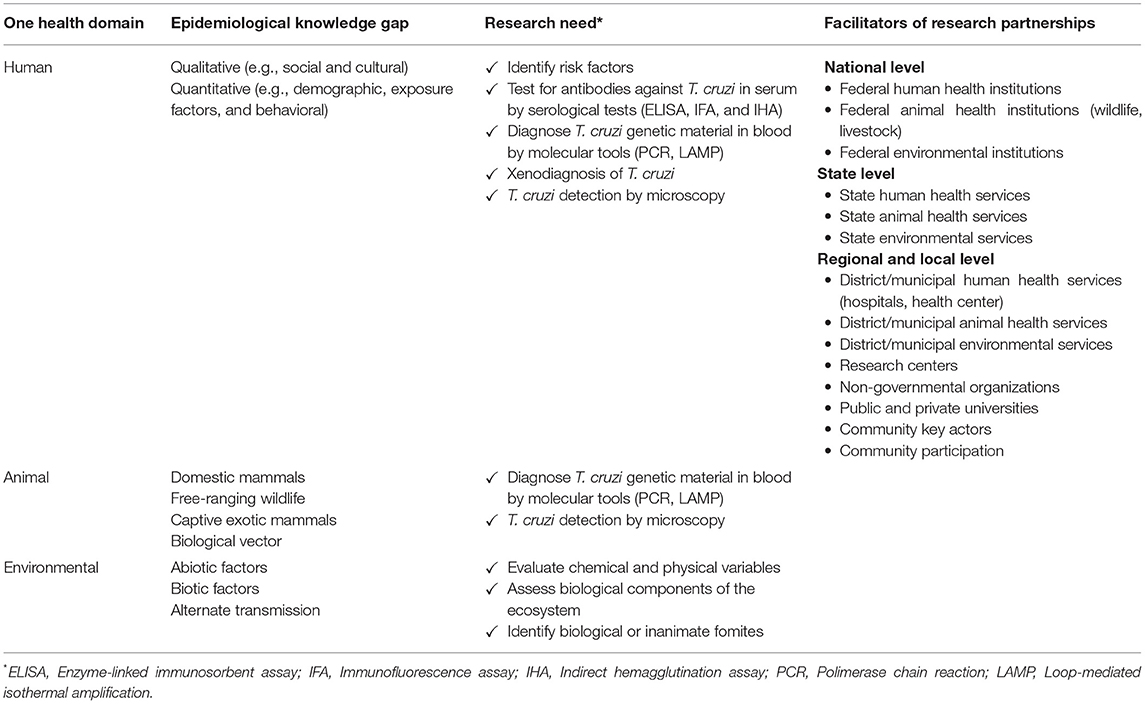
Table 1. Application of One Health approach to identify needs for American trypanosomiasis research emphasizing surveillance in the southern states of Chiapas and Oaxaca presents opportunity to advance disease control through public-private collaboration in Mexico.
Adapting the One Health approach to research on AT is an opportunity to advance surveillance and control efforts of this neglected disease that burdens disproportionately rural and semirural populations in southern Mexico. This may be challenging in the states of Chiapas and Oaxaca where it has been argued the situation reached a crisis point and where other vector-borne diseases affecting urban populations divert attention from AT (71, 72). However, the official action plan to prevent and control AT provides the avenue for transdisciplinary collaboration involving human and animal health care professionals (31), which can facilitate the implementation of One Health research to prevent AT in rural and semirural communities. Adapting the One Health concept will augment the impact of epidemiological studies needed in Oaxaca, Chiapas and other southern states in Mexico to understand the involvement of infected domestic animal and wildlife hosts bitten by triatomine vectors involved in the sylvatic, peridomestic, and domestic cycles. Translating this research could improve molecular tests to characterize and detect T. cruzi across triatomine vector and mammalian host species and develop molecular assays for susceptibility of triatomines to insecticides and T. cruzi to drugs. Community participation could be promoted by sharing with the public a comprehensive perspective of the human-animal-environmental interface based on all this information. Realizing the cultural change required to practice One Health by public health professionals and veterinary clinicians will enable timely diagnosis and treatment of AT.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was funded by National Council of Science and Technology (CONACYT) Project A1-S-70901 to DV-R.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The author thank to the National Council of Science and Technology (CONACYT) for the doctoral level scholarship to DV-R. ECOSUR and USDA are equal opportunity providers and employers.
1. Coura JR, Borges-Pereira J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. (2010) 115:5–13. doi: 10.1016/j.actatropica.2010.03.008
2. Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, et al. Chagas disease: from discovery to a worldwide health problem. Front Public Health. (2019) 7:166. doi: 10.3389/fpubh.2019.00166
3. Carabarin-Lima A, González-Vázquez MC, Rodríguez-Morales O, Baylón-Pacheco L, Rosales-Encina JL, Reyes-López PA, et al. Chagas disease (American trypanosomiasis) in Mexico: an update. Acta Tropica. (2013) 127:126–35. doi: 10.1016/j.actatropica.2013.04.007
4. Gomes C, Almeida AB, Rosa AC, Araujo PF, Teixeira ARL. American trypanosomiasis and Chagas disease: sexual transmission. Int J Infect Dis. (2019) 81:81–4. doi: 10.1016/j.ijid.2019.01.021
5. Chagas Disease. Available online at: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-american-trypanosomiasis (accessed November 4, 2021).
6. Viotti R, Vigliano CA, Alvarez MG, Lococo BE, Petti MA, Bertocchi GL, et al. The impact of socioeconomic conditions on chronic Chagas disease progression. Rev Esp Cardiol. (2009) 62:1224–32. doi: 10.1016/S1885-5857(09)73349-3
7. Fernández M del P, Gaspe MS, Gürtler RE. Inequalities in the social determinants of health and Chagas disease transmission risk in indigenous and creole households in the Argentine Chaco. Parasites Vectors. (2019) 12:184. doi: 10.1186/s13071-019-3444-5
8. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
9. Graves B. Climate Change and Chagas Disease in the Americas: A Qualitative Systematic Review. UT School of Public Health Dissertations (2019). Available online at: https://digitalcommons.library.tmc.edu/uthsph_dissertsopen/87
10. Elkheir N, Carter J, García-Mingo A, Chiodini P. Chagas disease in non-endemic settings. BMJ. (2021) 373:n901. doi: 10.1136/bmj.n901
11. Stigler Granados P, Rohde R. Chagas Disease in the U.S.: What We Do and Don't Know. ASM.org. Available online at: https://asm.org/Articles/2021/April/Chagas-Disease-in-the-U-S-What-We-Know-About-the-K (accessed December 16, 2021).
12. Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. (1909) 1:159–218. doi: 10.1590/S0074-02761909000200008
13. Jansen AM, Xavier SC das C, Roque ALR. Landmarks of the knowledge and Trypanosoma cruzi biology in the wild environment. Front Cell Infect Microbiol. (2020) 10:10. doi: 10.3389/fcimb.2020.00010
14. Jiménez P, Jaimes J, Poveda C, Ramírez JD. A systematic review of the Trypanosoma cruzi genetic heterogeneity, host immune response and genetic factors as plausible drivers of chronic chagasic cardiomyopathy. Parasitology. (2019) 146:269–83. doi: 10.1017/S0031182018001506
15. de Fuentes-Vicente JA, Gutiérrez-Cabrera AE, Flores-Villegas AL, Lowenberger C, Benelli G, Salazar-Schettino PM, et al. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. (2018) 183:23–31. doi: 10.1016/j.actatropica.2018.04.008
16. Ventura-Garcia L, Roura M, Pell C, Posada E, Gascón J, Aldasoro E, et al. Socio-cultural aspects of chagas disease: a systematic review of qualitative research. PLOS Neglect Trop Dis. (2013) 7:e2410. doi: 10.1371/journal.pntd.0002410
17. Stevens L, Monroy MC, Rodas AG, Dorn PL. Hunting, swimming, and worshiping: human cultural practices illuminate the blood meal sources of cave dwelling Chagas vectors (Triatoma dimidiata) in Guatemala and Belize. PLOS Neglect Trop Dis. (2014) 8:e3047. doi: 10.1371/journal.pntd.0003047
18. Valdez-Tah A, Huicochea-Gómez L, Ortega-Canto J, Nazar-Beutelspacher A, Ramsey JM. Social representations and practices towards triatomines and chagas disease in Calakmul, México. PLoS One. (2015) 10:e0132830. doi: 10.1371/journal.pone.0132830
19. Salm A, Gertsch J. Cultural perception of triatomine bugs and Chagas disease in Bolivia: a cross-sectional field study. Parasites Vectors. (2019) 12:291. doi: 10.1186/s13071-019-3546-0
20. Balouz V, Agüero F, Buscaglia CA. Chagas disease diagnostic applications: present knowledge and future steps. Adv Parasitol. (2017) 97:1–45. doi: 10.1016/bs.apar.2016.10.001
21. Bivona AE, Alberti AS, Cerny N, Trinitario SN, Malchiodi EL. Chagas disease vaccine design: the search for an efficient Trypanosoma cruzi immune-mediated control. Biochim Biophys Acta. (2020) 1866:165658. doi: 10.1016/j.bbadis.2019.165658
22. Guidelines for the Diagnosis and Treatment of Chagas Disease. Available online at: https://www.who.int/publications-detail-redirect/9789275120439 (accessed December 16, 2021).
23. Sosa-Estani S, Colantonio L, Segura EL. Therapy of Chagas disease: implications for levels of prevention. J Trop Med. (2012) 2012:e292138. doi: 10.1155/2012/292138
24. Pérez-Molina JA, Molina I. Chagas disease. Lancet. (2018) 391:82–94. doi: 10.1016/S0140-6736(17)31612-4
25. Mazzoti L. Dos casos de enfermedad de Chagas en el estado de Oaxaca. México Gac Med Mex. (1940) 70:417–20.
26. Mazzoti L, Dias E. Resumen de los datos publicados sobre la enfermedad de Chagas en México. Rev Soc Mex Hist Nat. (1949) 10:103–1.
27. Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years–a review. Mem Inst Oswaldo Cruz. (2006) 101:345–54. doi: 10.1590/S0074-02762006000400001
28. Ramsey JM, Peterson AT, Carmona-Castro O, Moo-Llanes DA, Nakazawa Y, Butrick M, et al. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Mem Inst Oswaldo Cruz. (2015) 110:339–52. doi: 10.1590/0074-02760140404
29. Enfermedad de Chagas: Vectores. Available online at: https://www.revistaciencia.amc.edu.mx/index.php/vol-68-numero-1/451-enfermedad-de-chagas-vectores (accessed December 16, 2021).
30. Salazar-Schettino PM, Bucio-Torres MI, Cabrera-Bravo M, Alba-Alvarado MC de, Castillo-Saldaña DR, Zenteno-Galindo EA, et al. Enfermedad de Chagas en México. Rev Facult Med. (2016) 59:6–16.
31. Secretaria de Salud. Programa de Acción Específico Prevención y control de la Enfermedad de Chagas 2013-2018. gob.mx. Available online at: http://www.gob.mx/salud/documentos/programa-de-accion-especifico-prevencion-y-control-de-la-enfermedad-de-chagas-2013-2018 (accessed December 16, 2021).
32. Rojo-Medina J, Ruiz-Matus C, Salazar-Schettino PM, González-Roldán JF. [Enfermedad de Chagas en México]. Gac Med Mex. (2018) 154:605–12. doi: 10.24875/GMM.18004515
33. Ibáñez-Cervantes G, León-García G, Castro-Escarpulli G, Mancilla-Ramírez J, Victoria-Acosta G, Cureño-Díaz MA, et al. Evolution of incidence and geographical distribution of Chagas disease in Mexico during a decade (2007-2016). Epidemiol Infect. (2018) 147:e41. doi: 10.1017/S0950268818002984
34. Centro Nacional de Programas Preventivos y Control de Enfermedades. Manual de Procedimientos para la Enfermedad de Chagas en México. gob.mx. Available online at: http://www.gob.mx/salud|cenaprece/documentos/manual-de-procedimientos-para-la-enfermedad-de-chagas-en-mexico?idiom=es (accessed December 16, 2021).
35. Arnal A, Waleckx E, Rico-Chávez O, Herrera C, Dumonteil E. Estimating the current burden of Chagas disease in Mexico: a systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl Trop Dis. (2019) 13:e0006859. doi: 10.1371/journal.pntd.0006859
36. Shelly EM, Acuna-Soto R, Ernst KC, Sterling CR, Brown HE. A critical assessment of officially reported chagas disease surveillance data in Mexico. Public Health Rep. (2016) 131:59–66. doi: 10.1177/003335491613100112
37. Buekens P, López-Cárdenas J, Dumonteil E, Padilla-Raygoza N. Including unpublished surveys in reviews on Chagas disease in Mexico. Public Health Rev. (2020) 41:24. doi: 10.1186/s40985-020-00140-7
38. Cardoso EJ, Valdéz GC, Campos AC, de la Luz Sanchez R, Mendoza CR, Hernández AP, et al. Maternal fetal transmission of Trypanosoma cruzi: a problem of public health little studied in Mexico. Exp Parasitol. (2012) 131:425–32. doi: 10.1016/j.exppara.2012.05.013
39. González-Zambrano H, Amaya-Tapia G, Franco-Ramos MC, López León-Murguía OJ. Prevalence of Chagas heart disease in dilated cardiomyopathy. Arch Cardiol Mex. (2020) 91:50–7. doi: 10.24875/ACME.M21000188
40. Anuario 1984-2019. Available online at: https://epidemiologia.salud.gob.mx/anuario/html/anuarios.html (accessed November 5, 2021).
41. Antonio-Campos A, Nicolás-Cruz A, Girón-Arias JI, Rivas N, Alejandre-Aguilar R. Presence of Rhodnius prolixus Stäl, 1859 (Hemiptera: Reduviidae) in Oaxaca, Mexico, ten years after the certification of its elimination. J Vector Ecol. (2019) 44:293–5. doi: 10.1111/jvec.12363
42. Guhl F. Chapter 2: Chagas disease in pre-Colombian civilizations**In memoriam to Arthur Aufderheide who opened a new research field in ancient medicine and parasitology. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis Chagas Disease, 2nd Edn. London: Elsevier (2017). p. 23–46.
43. Velasco-Castrejón Ó, Rivas-Sánchez B. Apuntes para la historia de la enfermedad de Chagas en México. Bol Méd Hosp Infant Méx. (2008) 65:57–79.
44. Burmeister H. Handbuch der Entomologie. Berlin: G. Reimer (1835). 416 p. Available online at: https://www.biodiversitylibrary.org/item/80477
45. Mazzoti L. Investigación Sobre la Existencia de la Enfermedad de Chagas en el País. Demostración de tripanosomas en los reduvideos transmisores. Med Rev Mex. (1936) 16:584–5.
46. Salazar Schettino PM, Cravioto QA, Tapia Conver R. Iniciativa México: propuesta para el control y vigilancia epidemiológica de la enfermedad de Chagas en México. Bol Chileno Parasitol. (2001) 56:76–9. doi: 10.4067/S0365-94022001000200008
47. Coura JR, Viñas PA, Junqueira AC. Ecoepidemiology, short history and control of Chagas disease in the endemic countries and the new challenge for non-endemic countries. Mem Inst Oswaldo Cruz. (2014) 109:856–62. doi: 10.1590/0074-0276140236
48. Tripartite UNEP Support OHHLEP's Definition of “One Health.” Available online at: https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed February 7, 2022).
49. Laing G, Vigilato MAN, Cleaveland S, Thumbi SM, Blumberg L, Salahuddin N, et al. One Health for neglected tropical diseases. Trans R Soc Trop Med Hyg. (2021) 115:182–4. doi: 10.1093/trstmh/traa117
50. Lebov J, Grieger K, Womack D, Zaccaro D, Whitehead N, Kowalcyk B, et al. A framework for One Health research. One Health. (2017) 3:44–50. doi: 10.1016/j.onehlt.2017.03.004
51. Odagiu S, Mayer JD. Chagas' Disease in Mexico: Factors, Surveillance, and Recommendations. Cheney, WA: Eastern Washington University (2015).
52. Garcia MN, O'Day S, Fisher-Hoch S, Gorchakov R, Patino R, Feria Arroyo TP, et al. One health interactions of chagas disease vectors, canid hosts, and human residents along the Texas-Mexico border. PLoS Negl Trop Dis. (2016) 10:e0005074. doi: 10.1371/journal.pntd.0005074
53. Jr Z, Balbo I. A One Health Approach to Study the Epidemiology of Trypanosoma cruzi in Humans, Domestic Animals, and Wildlife in the Rio Grande Valley of Texas Along the United States-Mexico Border [Thesis] (2019). Available online at: https://oaktrust.library.tamu.edu/handle/1969.1/186576 (accessed December 17, 2021).
54. Roger F, Caron A, Morand S, Pedrono M, de Garine-Wichatitsky M, Chevalier V, et al. One Health and EcoHealth: the same wine in different bottles? Infect Ecol Epidemiol. (2016) 6. doi: 10.3402/iee.v6.30978
55. Tangkawattana S, Sripa B. Integrative EcoHealth/one health approach for sustainable liver fluke control: the lawa model. Adv Parasitol. (2018) 102:115–39. doi: 10.1016/bs.apar.2018.07.002
56. Waleckx E, Pérez-Carrillo S, Chávez-Lazo S, Pasos-Alquicira R, Cámara-Heredia M, Acuña-Lizama J, et al. Non-randomized controlled trial of the long-term efficacy of an Ecohealth intervention against Chagas disease in Yucatan, Mexico. PLoS Negl Trop Dis. (2018) 12:e0006605. doi: 10.1371/journal.pntd.0006605
57. Ramsey JM, Gutiérrez-Cabrera AE, Salgado-Ramírez L, Peterson AT, Sánchez-Cordero V, Ibarra-Cerdeña CN. Ecological connectivity of Trypanosoma cruzi reservoirs and triatoma pallidipennis hosts in an anthropogenic landscape with endemic Chagas disease. PLoS One. (2012) 7:e46013. doi: 10.1371/journal.pone.0046013
58. López-Cancino SA, Tun-Ku E, De la Cruz-Felix HK, Ibarra-Cerdeña CN, Izeta-Alberdi A, Pech-May A, et al. Landscape ecology of Trypanosoma cruzi in the southern Yucatan Peninsula. Acta Trop. (2015) 151:58–72. doi: 10.1016/j.actatropica.2015.07.021
59. Galaviz-Silva L, Mercado-Hernández R, Zárate-Ramos JJ, Molina-Garza ZJ. Prevalence of Trypanosoma cruzi infection in dogs and small mammals in Nuevo León, Mexico. Rev Argent Microbiol. (2017) 49:216–23. doi: 10.1016/j.ram.2016.11.006
60. Martínez-Hernández F, Rendon-Franco E, Gama-Campillo LM, Villanueva-García C, Romero-Valdovinos M, Maravilla P, et al. Follow up of natural infection with Trypanosoma cruzi in two mammals species, Nasua narica and Procyon lotor (Carnivora: Procyonidae): evidence of infection control? Parasites Vectors. (2014) 7:405. doi: 10.1186/1756-3305-7-405
61. Hernández-Cortazar I, Cecilia Amaya Guardia K, Torres-Castro M, Acosta-Viana K, Guzmán-Marín E, Israel Chan-Pérez J, et al. Frequency of Trypanosoma cruzi infection in synanthropic and wild rodents captured in a rural community in southeast of Mexico. Vet Med Int. (2018) 2018:e8059613. doi: 10.1155/2018/8059613
62. Villalobos G, Muñoz-García CI, Rodríguez-Cabo-Mercado R, Mendoza-Bazán N, Hernández-Ortiz A, Villanueva-García C, et al. Prevalence and epitope recognition of anti-Trypanosoma cruzi antibodies in two procyonid species: implications for host resistance. Pathogens. (2020) 9:E464. doi: 10.3390/pathogens9060464
63. Jimenez-Coello M, Ortega-Pacheco A, Guzman-Marin E, Guiris-Andrade DM, Martinez-Figueroa L, Acosta-Viana KY. Stray dogs as reservoirs of the zoonotic agents Leptospira interrogans, Trypanosoma cruzi, and Aspergillus spp. in an urban area of Chiapas in southern Mexico. Vector Borne Zoonotic Dis. (2010) 10:135–41. doi: 10.1089/vbz.2008.0170
64. Gómez-Sánchez EF, Ochoa-Díaz-López H, Espinoza-Medinilla EE, Velázquez-Ramírez DD, Santos-Hernandez NG, Ruiz-Castillejos C, et al. Mini-exon gene reveals circulation of TcI Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae) in bats and small mammals in an ecological reserve in southeastern Mexico. ZooKeys. (2022) 1084:139–50. doi: 10.3897/zookeys.1084.78664
65. Calore M. The One Health Framework and Its Implementation in Global Health Governance Through Public-Private Partnerships. University of Leeds. 41 p.
66. Garza Ramos J, Arvizu Tovar L. Hacia Una Salud: Propuesta en el marco de la Administración Pública Federal en México. Primera: Yire (2012).
67. dos S, Ribeiro C, van de Burgwal LHM, Regeer BJ. Overcoming challenges for designing and implementing the One Health approach: a systematic review of the literature. One Health. (2019) 7:100085. doi: 10.1016/j.onehlt.2019.100085
68. Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr. (2010) 9:54. doi: 10.1186/1476-072X-9-54
69. Carter CN, Smith JL. A proposal to leverage high-quality veterinary diagnostic laboratory large data streams for animal health, public health, and One Health. J Vet Diagn Invest. (2021) 33:399–409. doi: 10.1177/10406387211003088
70. Davis MF, Rankin SC, Schurer JM, Cole S, Conti L, Rabinowitz P, et al. Checklist for One Health Epidemiological Reporting of Evidence (COHERE). One Health. (2017) 4:14–21. doi: 10.1016/j.onehlt.2017.07.001
71. Willoquet JMR. Simposio XIV. Chagas disease transmission in Mexico: a case for translational research, while waiting to take disease burden seriously. Salud Públ Méx. (2007) 49:291–5.
Keywords: American trypanosomiasis, surveillance, southern Mexico, One Health research, epidemiology
Citation: Velázquez-Ramírez DD, Pérez de Léon AA and Ochoa-Díaz-López H (2022) Review of American Trypanosomiasis in Southern Mexico Highlights Opportunity for Surveillance Research to Advance Control Through the One Health Approach. Front. Public Health 10:838949. doi: 10.3389/fpubh.2022.838949
Received: 18 December 2021; Accepted: 14 February 2022;
Published: 15 March 2022.
Edited by:
Roxanne Connelly, Centers for Disease Control and Prevention, United StatesReviewed by:
Saul Lozano, Division of Vector Borne Diseases–Centers for Disease Control and Prevention, United StatesCopyright © 2022 Velázquez-Ramírez, Pérez de Léon and Ochoa-Díaz-López. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Héctor Ochoa-Díaz-López, aG9jaG9hQGVjb3N1ci5teA==; Adalberto A. Pérez de Léon, YmV0by5wZXJlemRlbGVvbkB1c2RhLmdvdg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.