- 1Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
- 2Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, China
- 3Institute for Global Health and Development, Peking University, Beijing, China
- 4National Health Commission Key Laboratory of Reproductive Health, Peking University, Beijing, China
Background: At present, the widespread variants and the weakened immunity provided by vaccines over time have further emphasized the importance of vaccination, boosters, and prevention efforts against COVID-19. Here, this study intends to investigate the acceptability of a booster dose of COVID-19 vaccine among child caregivers, aiming to explore the association between risk perception and child vaccine acceptance.
Methods: This anonymous, national, cross-sectional survey was conducted for one week from November 12, 2021 in mainland China. The risk perception among child caregivers was assessed based on the Health Belief Model (HBM) and the individuals was equally divided into three levels according to the total preset scores of each perception dimension. Pearson χ2 test was used to compare the differences among participants stratified by sociodemographic characteristics, health status, knowledge factors and risk perception. Univariate and multivariate logistic regression models were performed to explore the associations between risk perception and the acceptance of a booster dose of COVID-19 vaccine.
Results: A total of 88.46% of 1,724 participants were willing to accept the booster dose of the COVID-19 vaccine for their children. People who lived in central China (91.93%), had a high school or polytechnic school level education (93.98%), and had a history of COVID-19 vaccination (88.80%) were more likely to accept a booster dose of the COVID-19 vaccine for their children. The complicated vaccination process (24.5%) and uncertainty about the safety (16.5%) and efficacy (21.3%) of vaccines were the three main reasons for vaccine hesitancy among child caregivers. The acceptance of the booster dose of the COVID-19 vaccine was closely related to a higher level of perceived susceptibility (moderate: aOR = 1.56, 95% CI: 1.07–2.29, P = 0.022; high: aOR = 1.75, 95% CI: 1.06–2.89, P = 0.029) and high perceived benefit (high: aOR = 7.22, 95% CI: 2.63–19.79, P < 0.001). The results were stable in the sensitivity analysis.
Conclusions: 88.46% of child caregivers were willing to have a booster dose of COVID-19 vaccine to children, and the acceptance was closely associated with a higher level of perceived susceptibility and perceived benefit. The complicated vaccination process, uncertainty about the safety and effectiveness of COVID-19 vaccines were the main reasons for their hesitancy. Therefore, targeted public health measures to increase perceived susceptibility and benefit are still needed to meet the requirements of higher-level immunization coverage.
Introduction
As a major public health concern, coronavirus disease 2019 (COVID-19) has raged around the world for nearly 2 years, with more than 260 million people struggling in this disease (1, 2). The invention of vaccines has proven to be a landmark in human history. Undoubtedly, vaccination is the most economical public health intervention to prevent and control infectious diseases, especially during the time without specific medicine (3). The recent emergence of the new variants (e.g., Delta variant and Omicron variant) and the weakened immunity provided by vaccines over time further emphasize the importance of vaccination, boosters, and prevention efforts against COVID-19 (4–7). According to the statistics of Our World in Data, approximately 326.22 million people in 21 countries have administered a booster dose of the COVID-19 vaccine as of December 9, 2021 (8).
Surveillance data from the WHO indicated that the proportion of confirmed cases in the 5 and 5–14 year age groups has increased from 1.0 and 2.5% in January 2020 to 2.9 and 19.9% in December 2021, respectively (9). Despite the observed milder illness and disease course of COVID-19 in children compared to adults, severe illness, death, and severe complications caused by COVID-19 among children are still worrying (10, 11). Additionally, Delta variate has led to a surge of infection in children, and the incidence rate of severe cases was 1%, and the case fatality rate was 1/10,000 (12). The increasing rate of children's severe illness and mortality has seriously threatened children's lives and could increase the risk of community transmission. The severe illness or death of children caused by COVID-19 will be even more damaging to families and societies (11). Therefore, the vaccination of children and adolescents is of great significance, and it is urgent to establish an immune barrier against this pandemic.
Children have very active and sensitive immune systems, which has led researchers to be more careful about the vaccination (13). Typically, every vaccine candidate, even for other conditions, would be evaluated first in adult patients and then in progressively younger ages (13). As a result, children received the initial COVID-19 vaccination much later than adults. Studies have shown the safety and effectiveness of child vaccination (14), most countries have now approved the initial COVID-19 vaccines for children aged 12 or older, and a few have already included some children under 12 (15–19). The United States already began vaccinating children and adolescents over the age of 5 in May 2021 (16). People aged 3–17 years were approved the emergency use of inactivated vaccines by Chinese government in July 2021 (17). Consent form for children and young people or parents and caregivers was also released by the UK government on September 21, 2021 (15). Previous studies have only assessed children's acceptance of the initial COVID-19 vaccine. Of 3,011 reproductive women, 8.44% were hesitant about the initial child vaccination against COVID-19 in China (20). In a cross-sectional survey consisting of 16 countries, only 69.2% of the respondents intended to accept the COVID-19 vaccines for their children. India, the Philippines, and Latin America generally have the highest vaccine acceptance to children, while the lowest acceptance was in Russia, the United States and Australia (21). Risk perception is people's subjective judgment of the characteristics and severity of a particular risk, which can ultimately influence people's behavior (22, 23). Children and adolescents under the age of 18, who make up more than a quarter of the global population, often rely on parental decision-making and guidance for their vaccination behavior (22).
At present, multiple variants have emerged globally, including five variants of concern that have been identified, and many countries have seen another outbreak with breakthrough cases reported from time to time (24). Due to the late start of childhood vaccination, few studies have linked risk perception of child caregivers with their willingness to give their children a booster dose of COVID-19 vaccines. The possible influencing factors of children's vaccination of a booster dose, especially parents' risk perceptions, are crucial for the subsequent formulation of policies to promote booster vaccination among children. Here, we intend to investigate the acceptability of a booster dose of COVID-19 vaccine among children's guardians, aiming to explore the adjusted association between risk perception and the acceptance of a booster dose of COVID-19 vaccines for children.
Methods and Materials
Study Design and Participants
This anonymous study was a national cross-sectional survey conducted for 1 week from November 12, 2021 in China. Our study relied on an online survey platform called Wen Juan Xing (25). As a professional data collection platform (Changsha Ranxing Information Technology Co., Ltd., Hunan, China), it contains more than 2.6 million fixed members (stratified by different regions, gender, age, occupation, etc.), and can provide authentic and reliable samples that meet the needs of scientific research (25). Our inclusion criteria were as follows: (1) Chinese citizens; (2) Having child aged under 18 years old; and (3) agreement to participate in this survey.
Since 87% of Chinese parents were willing to vaccinate their children with the initial COVID-19 vaccines (26), we calculated the sample size with α as 0.05 and confidence interval width as 0.087 (0.1P) by the exact (Clopper-Pearson) method using PASS 15 (NCSS LLC., Kaysville, UT, USA). The authors randomly allocated the questionnaires to be collected in 31 provinces according to the population proportion of 31 provinces in the Statistical Yearbook 2021 (Supplementary File 1). A total of 1,724 valid questionnaires were collected, fully meeting the minimum sample size requirements.
Assessment of Risk Perception
This research assessed the risk perception of COVID-19 among child caregivers using a survey tool based on the Health Belief Model (HBM), which was widely used to estimate vaccination intention in previous literature (27). The HBM is an important tool for people to carry out health behavior intervention programs, which can be used to study the factors affecting vaccination intention and promote the expected changes in vaccination behavior. This theoretical framework is constructed by five dimensions, including perceived susceptibility, perceived severity, perceived barriers, and perceived benefits, and cues to action (27). Here, the first four dimensions matched to a total of ten questions were we mainly used to determine the risk perception of the child caregivers. All questions were answered based on a three-point Likert scale (“very concerned or agree”, “concerned or not sure,” and “not concerned or disagree”), which were assigned scores of 3, 2, and 1, respectively. After summing up the individual's total preset score for each dimension, the individuals were equally divided into three levels.
Acceptance for a Booster Dose COVID-19 Vaccine to Children Among Child Caregivers
All eligible participants answered the question “Are you willing to give your child a booster dose of COVID-19 vaccination if available?” The acceptance rate of a booster dose of COVID-19 vaccine to children was defined as the proportion of participants who answered “yes” of all child caregivers in this study. In this study, a child was defined as a minor under the age of 18.
Covariates
In addition to the primary variates related to risk perception and attitudes toward a booster dose of COVID-19 vaccine for children, this research also included three groups of covariates that might influence the acceptability. Sociodemographic characteristics contained region, age group, sex, education, monthly household income per capita. history of chronic disease and history of COVID-19 vaccination were collected as information reflecting participants' health status. According to the previous studies (20), the knowledge of COVID-19 and COVID-19 vaccines of was child caregivers also investigated, including the sources of infection, common symptoms, prevention measures, susceptible population, vaccine safety and effectiveness, etc. For knowledge related questions, the correct answer was set in advance and represented 1 score, and the rest of the answers were given a score of 0. Finally, the score was divided into three classes by tertiles as “low”, “moderate” and “high”.
Data Analysis
Frequencies and percentages were used to summarize the characteristics of categorical variables. The Pearson χ2 test was used to compare the differences among participants stratified by sociodemographic characteristics, health status, knowledge factors and risk perception. Univariate and multivariate logistic regression models were performed to explore the associations between risk perception and the acceptance of a booster dose of COVID-19 vaccine. To examine the robustness, the authors finally constructed four different models in sensitivity analyses. Model A is a univariate logistic regression model using crude odds ratios (cORs) to explain vaccine acceptance in different risk perception groups, and sociodemographic characteristics mentioned above were adjusted in model B. In model C, the authors controlled the remaining covariates based on model B—health status, knowledge factors and the other three aspects of risk perceptions. Furthermore, model D contained only the significant covariates in the Pearson χ2 test and the other three risk perceptions.
Based on model C, there were subgroup analyses that included different regions, age groups, sex, education, income, history of chronic disease, history of COVID-19 vaccination, knowledge score on COVID-19 and COVID-19 vaccination. P for interaction was calculated to test the possible interactions between risk perception related variates and covariates. All analyses in this study were performed by SPSS 26.0, and a p-value of >0.05 was indicated statistically significant.
Results
Characteristics of 1,724 Participants
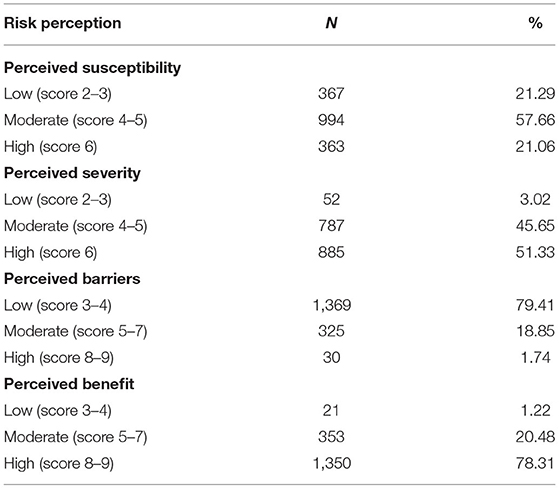
Finally, 1,724 child caregivers were eligible for recruitment. Among them, 46.23% lived in eastern China, 50.29% were male, and 77.26% had at least a bachelor's degree. Participants under 40 years old accounted for a large proportion (Table 1). Of the 1,724 child caregivers, 57.66% had moderate perceived susceptibility, 51.33% and 78.31% had high perceived severity and high perceived severity, respectively. Regarding perceived barriers, nearly 80% of the participants perceived only a low degree of barriers to COVID-19 vaccination (Table 2).
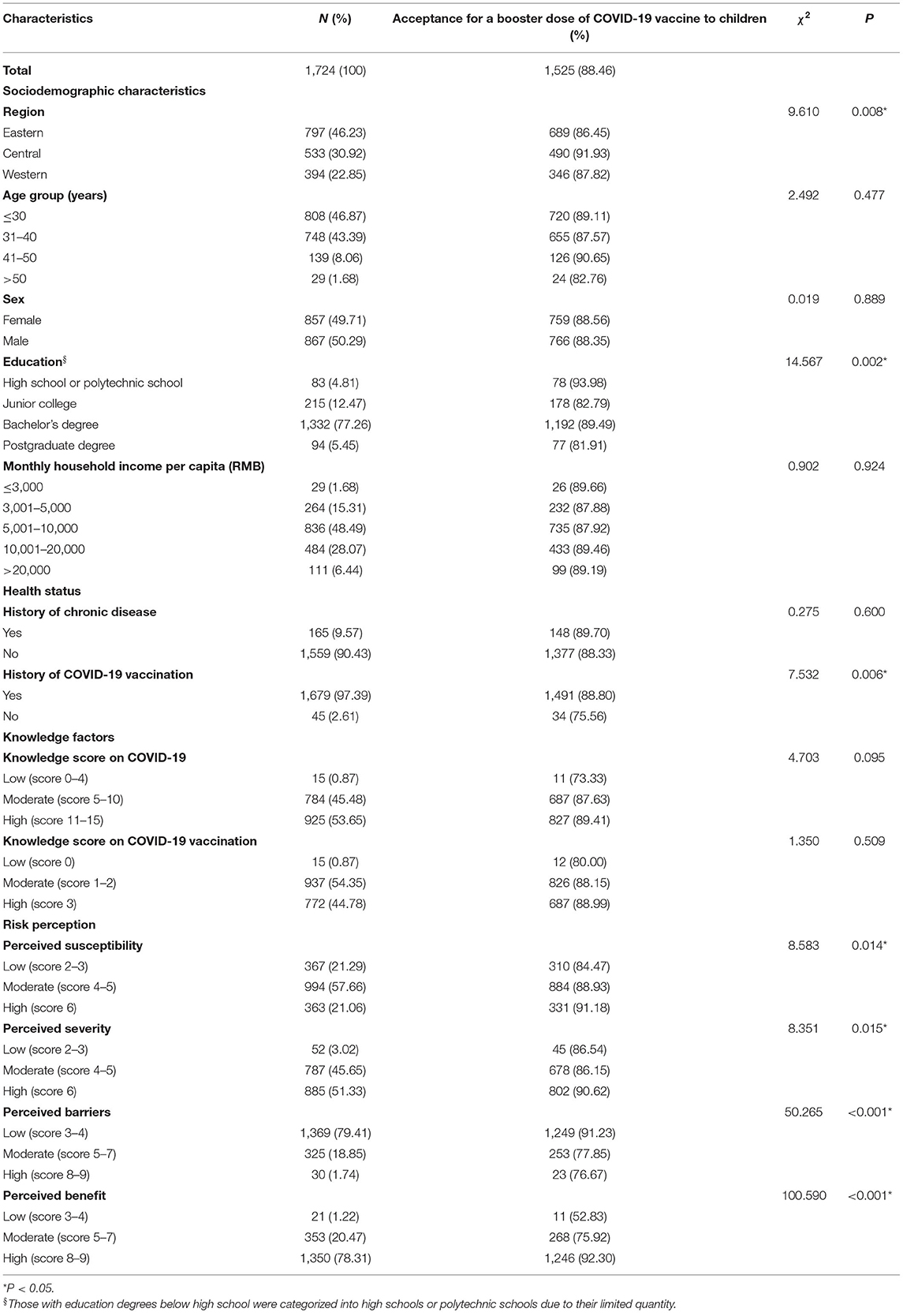
Table 1. Acceptance for a booster dose of COVID-19 vaccine to children among 1,724 child caregivers in China by characteristics.
Of all participants, 88.46% (95% CI, 86.95–89.97%) were willing to accept the booster dose of COVID-19 vaccine for their children. The acceptance differences for a booster dose of COVID-19 vaccine to children were not statistically significant among different groups of age, sex, monthly household income per capita (RMB), history of chronic disease, and knowledge factors. People who lived in central China (91.93%), had a high school or polytechnic school level education (93.98%), and had a history of COVID-19 vaccination (88.80%) were more likely to accept a booster dose of the COVID-19 vaccine for their children (Table 1). Notably, there were 188 child caregivers who had a history of initial COVID-19 vaccination but did not want to give their children a booster dose. The complicated vaccination process and uncertainty about the safety and efficacy of vaccines were the main reasons for vaccine hesitancy (Figure 1). Furthermore, child caregivers with higher perceptions of susceptibility (91.18%), severity (90.62%) and benefit (92.30%) had a stronger willingness to receive a booster dose of vaccination for children. Additionally, vaccine acceptance was more likely to be observed in participants with low perceived barriers (91.23%) (Table 1).
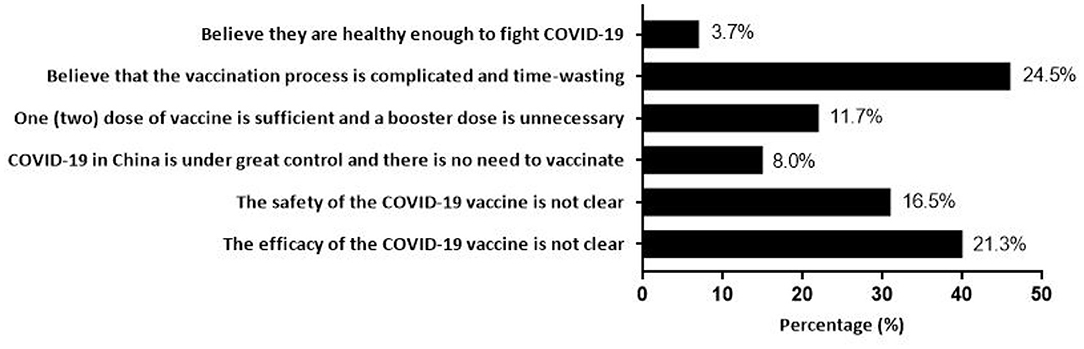
Figure 1. Reasons for hesitating to give a booster dose of COVID-19 vaccine to children among 188 child caregivers with COVID-19 vaccination history.
Association Between Risk Perception and Acceptance for a Booster Dose of COVID-19 Vaccine to Children
The association between risk perception and acceptance of a booster dose of COVID-19 vaccine to children is shown in Table 3. Without controlling for any confounding factor in model A, booster dose acceptance was associated with a higher level of perceived susceptibility (moderate: cOR = 1.48, 95% CI: 1.05–2.09; high: cOR = 1.90, 95% CI: 1.20–3.01), higher perceived benefit (moderate: cOR = 2.87, 95% CI: 1.18–6.98; high: cOR = 10.89, 95% CI: 4.52–26.24), and a low level of perceived barriers (low: cOR = 3.17, 95% CI: 1.33–7.54). The associations above remained stable in model B after controlling for sociodemographic characteristics, including region, age, sex, etc. Multivariate logistic regression of model C with all covariates adjusted displayed that the major factors related to the acceptance of the booster dose of COVID-19 vaccine were higher level of perceived susceptibility (moderate: aOR = 1.56, 95% CI: 1.07–2.29; high: aOR = 1.75, 95% CI: 1.06–2.89) and high perceived benefit (high: aOR = 7.22, 95% CI: 2.63–19.79). Model D showed similar association results when only covariates with statistical significance were included.
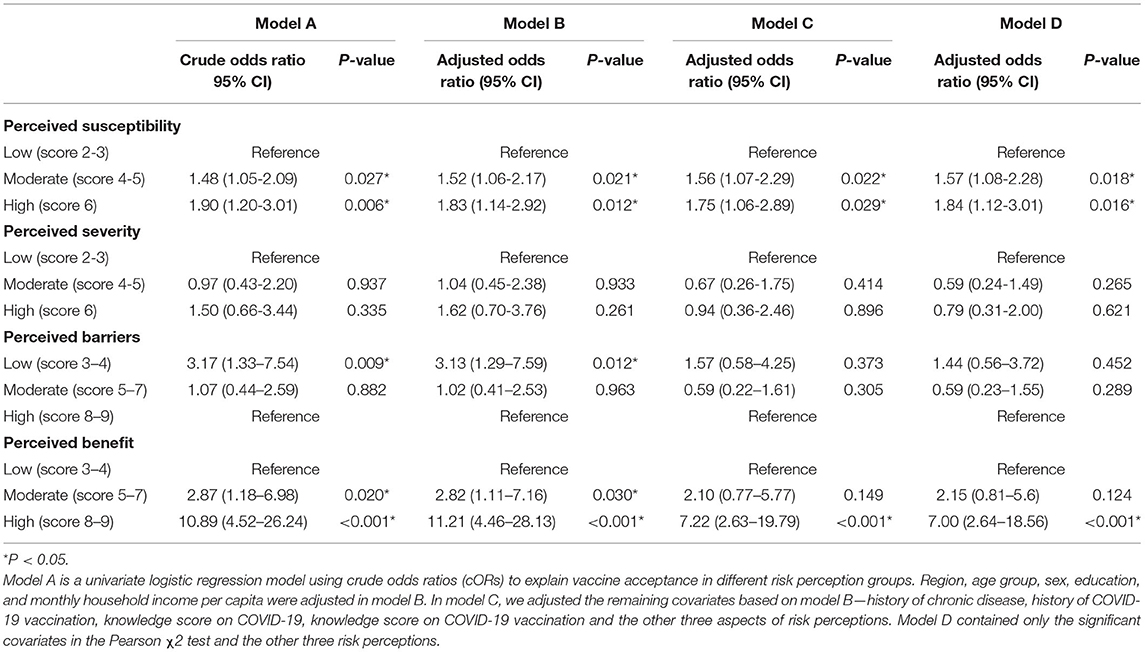
Table 3. The association between risk perception and the acceptance of a booster dose of COVID-19 vaccine to children among 1,724 child caregivers in China.
Subgroup analysis was performed, and no interactions were found in most subgroups (Supplementary File 2). Child caregivers with moderate perceived susceptibility in western China were more likely to accept a booster dose of COVID-19 vaccine for their children than caregivers in the other two regions (P for interaction = 0.004, Figure 2). Similar results could also be observed in participants with high perceived susceptibility in western China (P for interaction = 0.002, Figure 3).
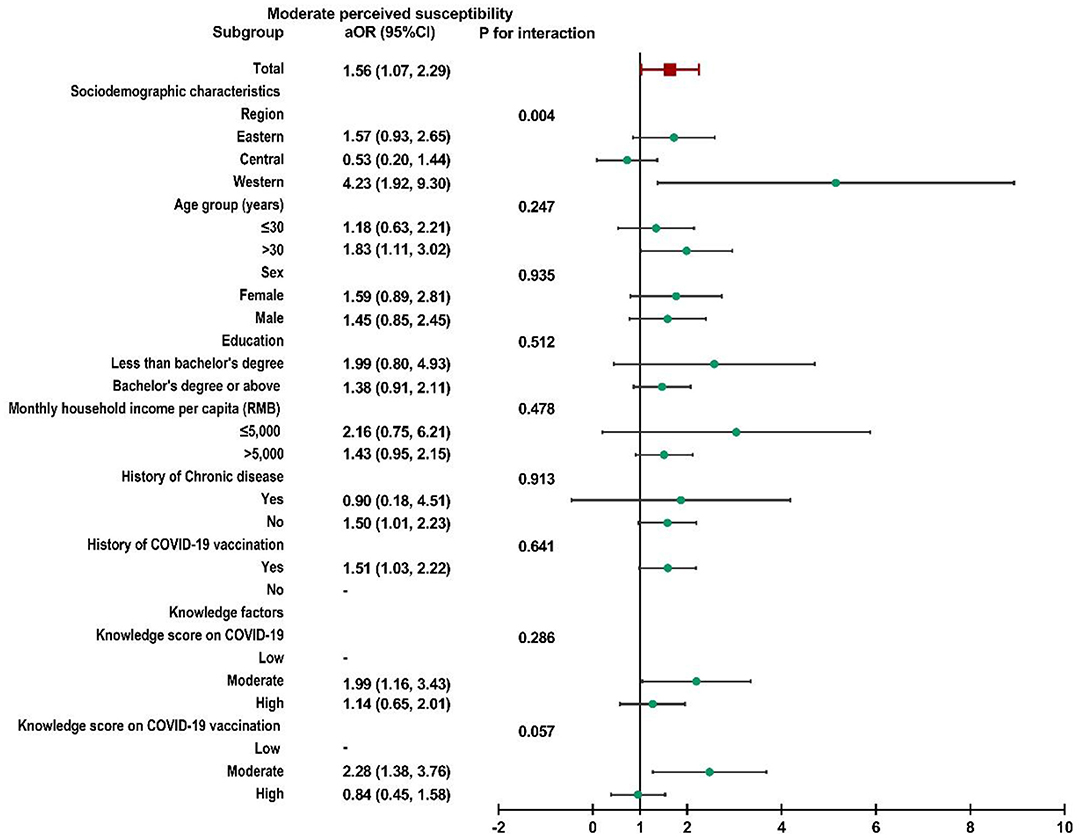
Figure 2. Subgroup analysis of the association between risk perception and acceptance of a booster dose of COVID-19 vaccine to children among caregivers with moderate perceived susceptibility.
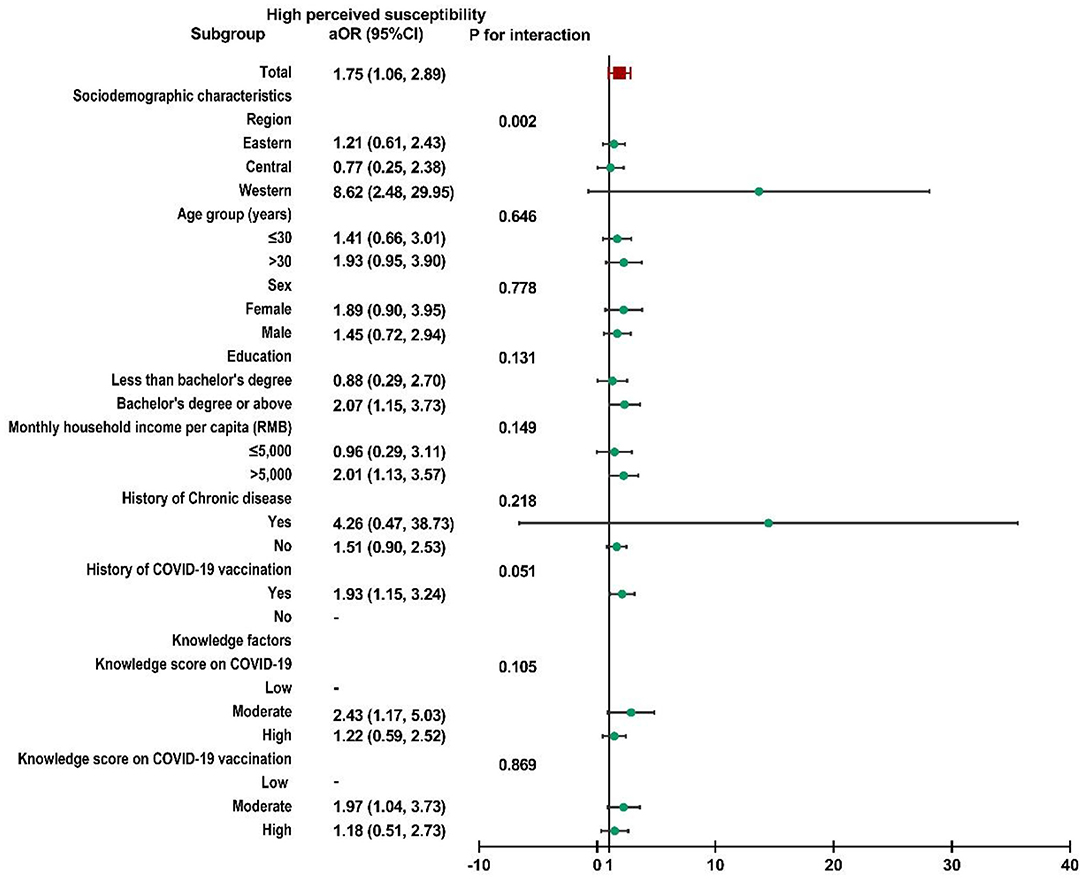
Figure 3. Subgroup analysis of the association between risk perception and acceptance of a booster dose of COVID-19 vaccine to children among caregivers with high perceived susceptibility.
Discussion
Currently, hundreds of countries around the world are still reeling from the COVID-19 pandemic. China, the United States, the United Kingdom, Japan, South Korea, Israel, and many other countries all have started to implement conditional vaccination policies for children (16–18, 28). Due to the particularity of children, initial COVID-19 vaccination is given much later than adults, and there are few studies on booster shots for children (13, 16–18, 28). Our research showed that 1,525 (88.46%) child caregivers were willing to give the booster dose of the COVID-19 vaccine for their children. But for the participants with COVID-19 vaccination history, complicated vaccination process and uncertainty about the safety and efficacy of vaccines were the main reasons they hesitated to vaccinate their kids. With all covariates adjusted, we found that the higher level of perceived susceptibility and perceived benefit, the more willing parents were to give booster shots to their children. To the best of our knowledge, this is the first nationwide study to explore the association between risk perception and acceptance of a booster dose of COVID-19 vaccine to children among child caregivers in China. A global survey demonstrated that the vaccine hesitancy changed over time because of the ever-changing public's risk perception of COVID-19 and information related to the vaccine safety and effectiveness (29). As a result, our findings have important theoretical implications for the subsequent development of strategies for the booster dose of COVID-19 vaccine in children.
According to our research, 88.46% of 1,724 participants were willing to accept the booster dose of the COVID-19 vaccine for their children. Due to its good safety and efficacy in adults (14), 55.3% of the world population has received at least one dose of a COVID-19 vaccine, and 8.28 billion doses have been administered globally as of December 9, 2021 (30). Evidence on the safety and effectiveness of COVID-19 vaccination for children and adolescents is gradually improving, and many countries and regions around the world have gradually implemented childhood vaccination programs (31–34). Multiple studies have reported mild to moderate adverse events in children vaccinated with COVID-19 vaccines, and vaccine-related severe adverse events were extremely rare (32–36). The results of a randomized, double-blind, controlled clinical trial for phase I/II in China suggest that inactivated COVID-19 vaccines BBIBP-CorV (32) and CoronaVac (37) could induce a strong humoral immune response against SARS-COV-2 in people under 18 years old. Ali et al. found that the mRNA-1273 vaccine had acceptable safety and was feasible and effective in preventing COVID-19 in adolescents aged 3–17 years, but it was currently difficult to assess the efficacy of the vaccine 14 days after completion of two doses of the vaccine accurately (33). In addition, two doses of the Pfizer/BioNTech vaccine were 93% (95% CI, 83–97%) effective in preventing COVID-19-related hospitalizations in children and adolescents aged 12–18 (31). However, studies on the safety and effectiveness of the booster dose of the COVID-19 vaccine in children and adolescents are lacking, and further time is needed to verify them.
Our results showed that the acceptance of a booster dose of vaccine in children was closely associated with a higher level of perceived susceptibility and perceived benefit, but not with perceived severity and perceived barriers. Parents' vaccination decisions are complex and multidimensional. Factors such as experience, emotion, risk perception and trust affect parents' attitudes and decision-making processes (38). Higher risk of infection, severe disease consequences, higher levels of perceived benefit, and lower perceived barriers were associated with higher vaccine acceptance among adults (39–42). And the concern about COVID-19 outbreaks and close attention to media coverage usually predicted a higher willingness to vaccinate (43). Min et al. found that women with lower perceived susceptibility (aOR = 2.44, 95%CI: 1.60–3.70 and lower perceived benefit (aOR = 4.59, 95%CI: 2.98–7.07) were more likely to be hesitant to vaccinate their kids. Association between parental acceptance of child vaccination and perceived severity was also not statistically significant (20). Another study showed that children with high perceived severity and susceptibility were much more likely to be vaccinated than those with low perceived threat (OR = 1.82, 95% CI: 1.21–2.72) (44). For young children, evidence suggested an association between vaccination and perceived disease susceptibility, but the evidence for an association between perceived disease severity and vaccination was weak (45). This may be because parents usually considered whether their child was susceptible to a disease before considering its severity (45). The association between perceived severity, perceived severity barriers and COVID-19 acceptance in children remains unclear. In conclusion, our findings suggested that increasing perceived risk to COVID-19 and the benefits of vaccination against COVID-19 at this stage is an effective way to increase children's willingness to be vaccinated against COVID-19.
Findings stratified by characteristics suggested that people who lived in central China, had a high school or polytechnic school level education, and had a history of COVID-19 vaccination were more likely to accept a booster dose of COVID-19 vaccine for their children. However, other studies found higher vaccine hesitancy for initial vaccination among child caregivers with lower education levels (46, 47). And the vaccination rates of initial COVID-19 vaccines have risen steadily with higher levels of education in the USA (48). 21.3% of 1,724 guardians of children in this research still have inadequate risk perception of vulnerability to COVID-19, which may be one of the reasons that prevents children from getting booster shots in the future. Targeted public health measures should be designated in accordance with local conditions to increase awareness of susceptibility to COVID-19 and the benefits to be gained from vaccination in children. While the global vaccination of children against COVID-19 has just begun, an early survey on children's willingness to receive booster shots can help identify potential barriers and remove barriers.
There are some limitations in our study. First, our risk perception is based on the HBM, so that the association between vaccination intention and risk perceptions among different theoretical models may not be comparable. Second, as our study was an online survey conducted in China, the results need to be interpreted with caution due to the limitations of the survey area and Internet users. Moreover, these findings may represent the attitudes of all children's guardians across the country toward the booster dose of COVID-19 vaccine, as only participants who already had kids were included. Also, this study didn't specifically investigate the age of each child.
Conclusion
A total of 88.46% of 1,724 participants were willing to accept the booster dose of the COVID-19 vaccine for their children, and it was closely associated with a higher level of perceived susceptibility and perceived benefit. The complicated vaccination process, uncertainty about the safety and effectiveness of COVID-19 vaccines were the main reasons for their hesitancy. To promote vaccination, targeted public health measures should be designated to increase awareness of susceptibility to COVID-19 and the benefits to be gained from vaccination in children. Therefore, our findings have important theoretical implications for the subsequent development of strategies for the booster dose of COVID-19 vaccine in children.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Ethics Committee of Peking University Third Hospital. All participants signed informed consent forms and agreed to provide relevant data.
Author Contributions
CQ, RW, and LT: conceptualization. CQ and JL: methodology and analysis. CQ: visualization and writing—original draft preparation. CQ, RW, ML, and LT: review and editing. JL: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major Infectious Disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the coauthors for the preparation of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our sincere gratitude to all the participants who enrolled in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.834572/full#supplementary-material
Supplementary File 1. A questionnaire about the acceptance of a booster dose of COVID-19 vaccine among child caregivers.
Supplementary File 2. Subgroup analysis of the association between risk perception and acceptance of a booster dose of COVID-19 vaccine to children for child caregivers.
References
1. World Health Organization. COVID-19 has Assumed Global Pandemic Characteristics. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed December 10, 2021).
2. University JH. COVID-19 Map. Available at: https://coronavirus.jhu.edu/map.html (accessed December 10, 2021).
3. Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. (2018) 9:1963. doi: 10.3389/fimmu.2018.01963
4. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. (2021) 596:276–80. doi: 10.1038/s41586-021-03777-9
5. Hwang H, Lim JS, Song SA, Achangwa C, Sim W, Kim G, et al. Transmission dynamics of the Delta variant of SARS-CoV-2 infections in South Korea. J Infect Dis. (2021). doi: 10.21203/rs.3.rs-934350/v1
6. Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. (2021) 600:21. doi: 10.1038/d41586-021-03552-w
7. Centers for Disease control and Prevention. Data Supporting Need for a Booster Shot. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed December 10, 2021).
8. data, Owi. COVID-19 Vaccine Booster Doses Administered. Available at: https://ourworldindata.org/covid-vaccinations (accessed December 10, 2021).
9. World Health Organization. Confirmed and Probable COVID-19 Cases and Deaths by Age. Available at: https://app.powerbi.com/view?r=eyJrIjoiYWRiZWVkNWUtNmM0Ni00MDAwLTljYWMtN2EwNTM3YjQzYmRmIiwidCI6ImY2MTBjMGI3LWJkMj QtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9 (accessed December 10, 2021).
10. Rajapakse N, Dixit D. Human and novel coronavirus infections in children: a review. Paediatr Int Child Health. (2021) 41:36–55. doi: 10.1080/20469047.2020.1781356
11. Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease. (2019 (COVID-19). J Med Virol. (2021) 93:1057–69. doi: 10.1002/jmv.26398
12. American Academy of Pediatrics. Children and COVID-19: State-Level Data Report. Available at: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/ (accessed December 10, 2021).
13. Christensen J. Why a Covid-19 vaccine for Younger Children is Taking Longer than a Vaccine for Adults(2021). Available online at:. Available at: https://edition.cnn.com/2021/09/02/health/kids-covid-vaccine-timeline-wellness/index.html (accessed February 15, 2022).
14. Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. (2021) 10:132. doi: 10.1186/s40249-021-00915-3
15. COVID-19 vaccination consent form for children and young people or parents and carers. Available at: https://www.gov.uk/government/publications/covid-19-vaccination-consent-form-for-children-and-young-people-or-parents (accessed December 10, 2021).
16. Centers for Disease control Prevention. COVID-19 Vaccines for Children and Teens. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Frecommendations%2Fadolescents.html (accessed December 10, 2021).
17. China, NHCotPsRo. http://www.nhc.gov.cn/xcs/yqfkdt/202106/e28487f08ad745c5952356e448a87f13.shtml (accessed December 10, 2021).
18. Ministry of Health. Ministry of Health's Position Regarding Vaccination of Teens. Available at: https://govextra.gov.il/ministry-of-health/covid19-vaccine/en-covid19-vaccines-for-teens/ (accessed December 10, 2021).
19. NMDOH. COVID-19 Shots Available for Children 5-11 in Lea County. Available at: https://www.nmhealth.org/news/vaccine/2021/11/?view=1715 (accessed December 10, 2021).
20. Du M, Tao L, Liu J. The association between risk perception and COVID-19 vaccine hesitancy for children among reproductive women in China: an online survey. Front Med (Lausanne). (2021) 8:741298. doi: 10.3389/fmed.2021.741298
21. Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. (2021) 36:197–211. doi: 10.1007/s10654-021-00728-6
22. Goldman RD, McGregor S, Marneni SR, Katsuta T, Griffiths MA, Hall JE, et al. Willingness to vaccinate children against influenza after the coronavirus disease 2019 Pandemic. J Pediatr. (2021) 228:87–93.e2. doi: 10.1016/j.jpeds.2020.08.005
23. Liu C, Huang N, Fu M, Zhang H, Feng XL, Guo J. Relationship between risk perception, social support, and mental health among general Chinese population during the COVID-19 pandemic. Risk Manag Healthc Policy. (2021) 14:1843–53. doi: 10.2147/RMHP.S302521
24. Variants of Concern (VOC). Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed December 10, 2021).
25. Sample service of Wen Juanxing. Available at: https://www.wjx.cn/sample/service.aspx (accessed December 10, 2021).
26. Wan X, Huang H, Shang J, Xie Z, Jia R, Lu G, et al. Willingness and influential factors of parents of 3-6-year-old children to vaccinate their children with the COVID-19 vaccine in China. Hum Vaccin Immunother. (2021) 1–6. doi: 10.1080/21645515.2021.1955606
27. Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. (1988) 15:175–83. doi: 10.1177/109019818801500203
28. Agency, UHS. COVID-19 Vaccination: A Guide for Eligible Children and Young People Aged 12 to 17. Available at: https://www.gov.uk/government/publications/covid-19-vaccination-resources-for-children-and-young-people/covid-19-vaccination-a-guide-for-eligible-children-and-young-people-aged-12-to-17 (accessed December 10, 2021).
29. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
30. Our World in Data. Available at: https://ourworldindata.org/covid-vaccinations (accessed December 10, 2021).
31. Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12-18 Years—United States, June-September. (2021). MMWR Morb Mortal Wkly Rep. (2021) 70:1483–8.
32. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. (2021). doi: 10.1016/S1473-3099(21)00462-X
33. Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. New Engl J Med. (2021). doi: 10.1056/NEJMoa2109522
34. Frenck RW. Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. (2021) 385:239–50. doi: 10.1056/NEJMoa2107456
35. Snapiri O, Rosenberg Danziger C, Shirman N, Weissbach A, Lowenthal A, Ayalon I, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. (2021) 40:e360–e3. doi: 10.1097/INF.0000000000003235
36. Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomised, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis (. (2021). doi: 10.1093/cid/ciab845
37. Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. (2021) 21:1645–53. doi: 10.1016/S1473-3099(21)00319-4
38. Dubé E, Gagnon D, MacDonald N, Bocquier A, Peretti-Watel P, Verger P. Underlying factors impacting vaccine hesitancy in high income countries: a review of qualitative studies. Expert Rev Vaccines. (2018) 17:989–1004. doi: 10.1080/14760584.2018.1541406
39. Wang J, Lu X, Lai X, Lyu Y, Zhang H, Fenghuang Y, et al. The changing acceptance of covid-19 vaccination in different epidemic phases in China: a longitudinal study. Vaccines (Basel). (2021) 9. doi: 10.3390/vaccines9030191
40. Ikiişik H, Akif Sezerol M, Taşçi Y, Maral I. COVID-19 vaccine hesitancy: A community-based research in Turkey. Int J Clin Pract. (2021) 75:e14336. doi: 10.1111/ijcp.14336
41. Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. (2020) 14:e0008961. doi: 10.1371/journal.pntd.0008961
42. Li L, Jing R, Guo J, Song Y, Geng S, Wang J, et al. The associations of geographic location and perceived risk of infection with the intentions to get vaccinated against COVID-19 in China. Expert Rev Vaccines. (2021) 20:1351–60. doi: 10.1080/14760584.2021.1969917
43. Faasse K, Newby J. Public Perceptions of COVID-19 in Australia: Perceived Risk, Knowledge, Health-Protective Behaviors, and Vaccine Intentions. Front Psychol. (2020) 11:551004. doi: 10.3389/fpsyg.2020.551004
44. Kelly BJ, Southwell BG, McCormack LA, Bann CM, MacDonald PDM, Frasier AM, et al. Predictors of willingness to get a COVID-19 vaccine in the US. BMC Infect Dis. (2021) 21:338. doi: 10.1186/s12879-021-06023-9
45. Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ A. systematic review of factors affecting vaccine uptake in young children. Vaccine. (2017) 35:6059–69. doi: 10.1016/j.vaccine.2017.09.046
46. Montalti M, Rallo F, Guaraldi F, Bartoli L, Po G, Stillo M, et al. Would parents get their children vaccinated against SARS-CoV-2? rate and predictors of vaccine hesitancy according to a survey over 5000 families from Bologna, Italy. Vaccines (Basel). (2021) 9. doi: 10.3390/vaccines9040366
47. Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health. (2021) 46:270–7. doi: 10.1007/s10900-020-00958-x
Keywords: COVID-19 vaccine, booster dose, children, risk perception, China
Citation: Qin C, Wang R, Tao L, Liu M and Liu J (2022) Association Between Risk Perception and Acceptance for a Booster Dose of COVID-19 Vaccine to Children Among Child Caregivers in China. Front. Public Health 10:834572. doi: 10.3389/fpubh.2022.834572
Received: 14 December 2021; Accepted: 21 February 2022;
Published: 16 March 2022.
Edited by:
Bijaya Kumar Padhi, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Pritish Mondal, Penn State Milton S. Hershey Medical Center, United StatesBirhan Tsegaw Taye, Debre Berhan University, Ethiopia
Copyright © 2022 Qin, Wang, Tao, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jue Liu, anVlbGl1QGJqbXUuZWR1LmNu
 Chenyuan Qin
Chenyuan Qin Ruitong Wang
Ruitong Wang Liyuan Tao
Liyuan Tao Min Liu
Min Liu Jue Liu
Jue Liu