- 1Department of Anesthesiology and Critical Care Medicine, Peking University First Hospital, Beijing, China
- 2Department of Anesthesiology, Beijing Obstetrics and Gynecology Hospital, Beijing, China
- 3Department of Anesthesiology, Haidian Maternal & Child Health Hospital, Beijing, China
- 4Department of Pediatrics, Peking University First Hospital, Xicheng District, Beijing, China
- 5Department of Biostatistics, Peking University First Hospital, Beijing, China
- 6Section of Anesthetics, Pain Management and Intensive Care, Department of Surgery and Cancer, Imperial College London, Chelsea and Westminster Hospital, London, United Kingdom
- 7Outcomes Research Consortium, Cleveland, OH, United States
Background: Neuraxial analgesia is widely used to relieve labor pain; its effects on long-term neurodevelopment of offspring remain unclear. This study was designed to investigate the influence of maternal neuraxial labor analgesia on offspring mental development.
Methods: This was a predefined secondary analysis of a 2-year prospective longitudinal study. Nulliparous women with single-term cephalic pregnancy preparing for vaginal delivery self-selected neuraxial analgesia or not during labor. Mothers and their offspring were followed up 2 years later. children's mental development was assessed with the bayley scales of infant development. A multivariable logistic model was used to identify factors associated with below-average mental development (Mental Development Index <90).
Results: A Total of 508 pairs of mothers and children completed a 2-year follow-up. after propensity score matching, 387 pairs were included in the analysis. In both cohorts, the proportions with below-average mental development were slightly lower in children whose mothers received neuraxial labor analgesia, although not statistically significant [in the full cohort: 9.8 % (36/368) vs. 15.7% (22/140), P = 0.060; In the matched cohort: 8.3% (21/254) vs. 14.3% (19/133), P = 0.065]. A higher 2-year depression score (in the full cohort: Odds Ratio 1.15, 95% CI 1.08–1.22, P < 0.001; In the matched cohort: Odds Ratio 1.09, 95% CI 1.01–1.18, P = 0.037), but not neuraxial analgesia exposure, was associated with an increased risk of below-average mental development.
Conclusions: Maternal depression at 2 years was associated with the risk of below-average mental development, whereas maternal exposure to neuraxial labor analgesia was not.
Clinical Trial Registration: The study was registered with www.chictr.org.cn (ChiCTR-OCH-14004888) and ClinicalTrials.gov (NCT02823418).
Introduction
How perinatal factors affect neurodevelopment of children has always been an issue of much concern. Prenatal maternal stress has been reported to be associated with impaired cognitive development, poor intellectual performance, as well as behavioral and emotional problems during childhood (1, 2). As one of the most painful events during a woman's lifetime, the intense labor pain provokes serious maternal stress responses, which are related to impaired uterine contraction (3), neonatal hypoxia and metabolic acidosis (4), and even maternal postpartum depression (5). It is also possible that the maternal and fetal stress during labor may produce long-lasting effects on children (6), which has been verified in animal models (7). Indeed, numerous studies suggest that postpartum depression may affect long-term physical and neurodevelopment of children (8, 9).
Neuraxial labor analgesia, including epidural analgesia and combined spinal-epidural analgesia, is a well-established technique to relieve labor pain. It can help to reduce the maternal stress response during labor (10) and might be associated with a lower risk of maternal postpartum depression (11, 12); all these may be beneficial to the long-term development in offspring. On the other hand, neuraxial labor analgesia increases intrapartum maternal fever and instrumental delivery, which may potentially worsen neonatal outcomes and increase birth trauma (13, 14). In addition, anesthetic exposure during neuraxial analgesia may lead to fetal-neonatal depression (15) and even neurotoxic effects of less mature neonatal brain (16). Taking all these into account, the potential long-term effects of neuraxial analgesia on offspring neurodevelopment are still controversial and deserve further study. The objective of this analysis was to investigate if there is any association between maternal exposure to neuraxial analgesia during labor and neurocognitive development in offspring at 2 years of age.
Materials and methods
Study design
This was a predefined secondary analysis of a 2-year prospective longitudinal study. The study was conducted in Peking University First Hospital (a tertiary general hospital), Beijing Obstetrics and Gynecology Hospital (a tertiary specialized hospital) and Haidian Maternal & Child Health Hospital (a secondary specialized hospital) in Beijing, China. Results of the underlying study have been published elsewhere (11, 17).
Participant recruitment
We enrolled nulliparae with a single-term cephalic pregnancy preparing for vaginal delivery. Women who met any of the following criteria were excluded: age <18 years or >34 years; a history of psychiatric disease (schizophrenia); contraindications to neuraxial analgesia, such as diseases of the central nervous system (e.g., poliomyelitis, cerebrospinal meningitis, encephalitis), spinal or intraspinal diseases (e.g., trauma or surgery of spinal column, intraspinal canal mass, lumbar disc herniation), systematic infectious diseases (e.g., sepsis, bacteremia), infection of skin or soft tissue at the site of puncture, and coagulopathy; or delivery room admission outside daytime working hours.
Conduct of neuraxial labor analgesia
After being informed about the benefits and potential risks of neuraxial labor analgesia, parturients self-decided to receive neuraxial labor analgesia or not. Epidural analgesia or combined spinal-epidural analgesia was performed for those who requested analgesia. Neuraxial analgesia was initiated when the cervix was dilated to 1 cm or more. A patient-controlled epidural analgesia (PCEA) pump established with a mixture of 0.1% ropivacaine plus 0.5 μg/ml sufentanil was attached to the epidural catheter to maintain analgesia until end of the third stage of labor. Routine care was provided for those who did not request neuraxial analgesia, including intramuscular meperidine prescribed by the obstetricians. Detailed procedures of neuraxial analgesia were described previously (11, 17).
Baseline and perinatal data collection
Baseline data of mothers and fathers were collected. Prenatal assessments were completed by parturients themselves at admission to the delivery ward. Depressive symptoms were assessed with the Edinburgh Postnatal Depression Scale [EPDS, a 10-item self-report questionnaire; the total score ranges from 0 to 30, with higher score indicating more severe depressive symptoms (18)]. Marriage satisfaction was assessed with the ENRICH Marital Satisfaction Scale [total score ranges from 10 to 50, with higher score indicating better marital satisfaction (19)]. Anxiety level was assessed with the Zung Self-Rating Anxiety Scale [total score ranges from 25 to 100, with higher score indicating more severe anxiety (20)]. Social support was assessed with the Social Support Rating Scale [total score ranges from 11 to 62, with higher score indicating better social support (21)]. The Chinese versions of the above instruments had been validated (22–25).
Intrapartum data included use of neuraxial analgesia, duration of labor, the highest body temperature, mode of delivery, lateral episiotomy, and estimated blood loss. Neonatal data included sex, birth weight, Apgar scores at 1 and 5 min after birth, and postnatal management (admission to neonatal ward or intensive care unit). A telephone follow-up was performed at 6 weeks (42–49 days) postpartum. The severity of maternal depression was assessed with the EPDS; and an EPDS score ≥10 was defined as the threshold of postpartum depression (25). The condition of neonates, the mode of baby feeding, the existence of persistent pain and the NRS pain score were recorded.
Two-year follow-up of mothers and children
A face-to-face interview was completed between 23 and 24 months after childbirth. Maternal data were collected and included body weight and height, duration of breast-feeding, new-onset diseases requiring therapy or surgical procedures, and another childbirth. Social support was assessed with the Social Support Rating Scale. Depressive symptoms were assessed with the EPDS, and those with a 2-year EPDS score ≥10 were defined as having 2-year depression (25). Infant data was collected and included month age, body weight and height, time to start complementary feeding, pediatric diseases requiring therapy or surgical procedures. Physical development was evaluated according to the Reference Standard for Growth and Development of Children under 7 Years of Age in China which was released by the Ministry of Health of China on 2 June 2009 (26). A height or weight of < -2 standard deviation (SD) was defined as physical development delay.
Neurocognitive development of infants was assessed with the Chinese Revision of Bayley Scales of Infant Development, which has been validated in Chinese urban children aged from 2 to 30 months (27) and widely used in related studies (28). It has two primary subtests, i.e., the mental scale, which includes 163 items and evaluates children's cognition, language and social development, and the psychomotor scale, which includes 81 items and assesses gross and fine motor development. The Mental Development Index (MDI) and Psychomotor Development Index (PDI) were converted from the age-adjusted raw scores of mental and psychomotor scales, respectively. The average scores of MDI and PDI in normal urban children are both 100 with a SD of 15, with higher scores indicating better neurocognitive development. The MDI and PDI scores were classified into seven levels, i.e., developmental delay (<70), borderline (70–79), below average (80–89), middle level (90–109), above average (110–119), good (120–129), and outstanding (≥130) (27). In the present study, a MDI score <90 was defined as below-average mental development and a PDI <90 as below-average psychomotor development. Investigators who performed neurocognitive assessment were trained to use the Bayley Scales of Infant Development and were blinded to the exposure to neuraxial analgesia during labor. The primary endpoint was occurrence of below-average mental development at 2 years of age.
Statistical analysis
Mothers and their offspring were divided into two groups according to neuraxial analgesia exposure during labor. Between-group differences of baseline variables were compared using the absolute standardized differences (ASDs), which are defined as the absolute difference in means, mean ranks or proportions divided by the pooled standard deviation and calculated with the formula published by Austin (29). An ASD ≥ 0.195 (i.e., 1.96) was considered unbalanced between the two groups. For intrapartum and postpartum variables, continuous variables were compared with independent samples t-test or Mann-Whitney U test, and categorical variables were compared with χ2 test or Fisher's exact test.
Baseline variables that were considered clinically relevant were used for propensity score matching in order to balance the potential bias in selecting neuraxial analgesia. These variables were selected a priori and included sociodemographic characteristics, medical history before last pregnancy, history of last pregnancy, prenatal hemoglobin, as well as prenatal assessment results of depression, marital satisfaction, anxiety, and social support (30, 31). A logistic regression model was used to calculate propensity scores predicting the probability of receiving neuraxial labor analgesia. In the present study, we carried out a 1:2 matching without replacement using the nearest-neighbor matching algorithm with caliper widths equal to 0.2.
For both the full cohort and the matched cohort, univariable logistic regression analyses were performed to screen variables that might be associated with below-average mental development in 2-year-old offspring. After testing for collinearity, factors with P < 0.15 in univariable analyses or were considered clinically important were included in a multivariable logistic regression model to identify independent factors associated with below-average mental development using a backward procedure. To further explore the impact of neuraxial analgesia duration on children's neurocognitive development, we divided neuraxial analgesia exposure time into 4 levels, i.e., no analgesia, <4 h, 4 to 8 h, and more than 8 h, and adjusted with the same aforementioned covariates in multivariable logistic regression models. An exploratory analysis was performed to further clarify the association between neuraxial analgesia and cognitive development delay in 2-year-old offspring with the MDI cutoff score set at 80. A two-tailed P value of <0.05 was considered statistically significant. Statistical analyses were performed with the SPSS 25.0 software (IBM SPSS Inc., Chicago, IL, USA) and the free software package “R” version 2.15.3 including the “Matchit” and the “ROC” plugin.
Results
Participants
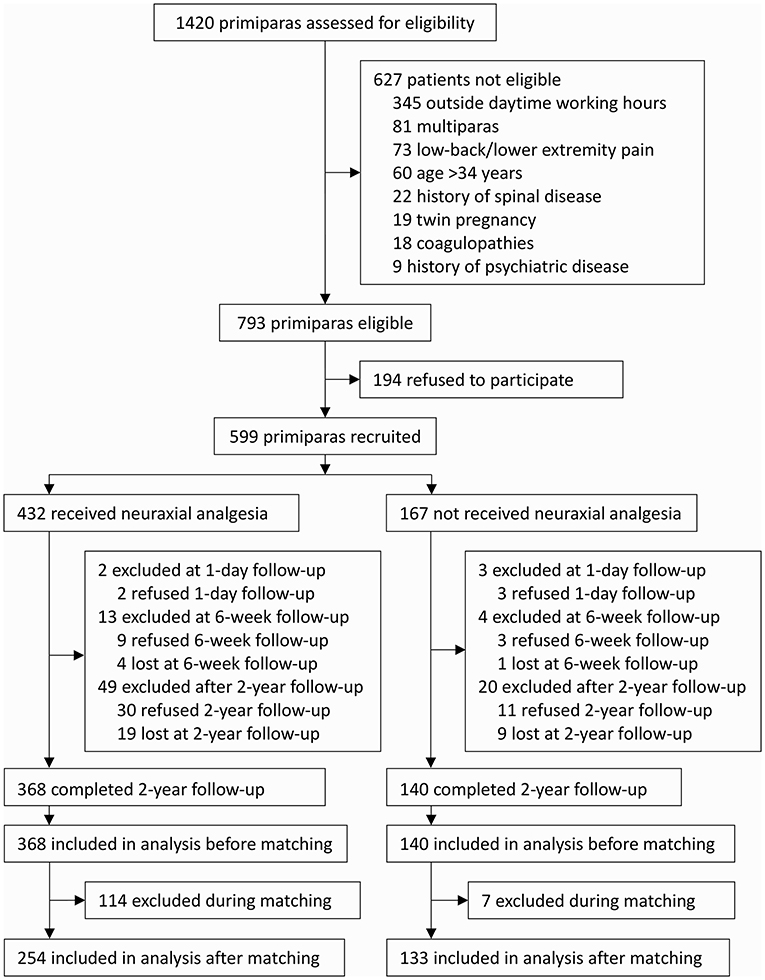
From 1 August 2014 to 29 May 2015, 599 nulliparae were enrolled after obtaining written informed consents. Among these, 577 mothers and their neonates completed the 1-day and 6-week follow-ups (17 refused and 5 were lost to follow-up). From 9 July 2016 to 25 April 2017, 508 mothers and their offspring completed the 2-year follow-up (41 refused follow-up and 28 were lost to follow-up) and were included in the final analysis. There were no significant differences regarding demographic and baseline data between parturients who completed the 2-year followed-up and those who did not (Supplementary Table S1). Of the included 508 mothers, 368 (72.4%) were given neuraxial analgesia and 140 (27.6%) were not. After propensity score matching, 387 mothers and their offspring remained in the analysis, of whom 254 mothers (65.6%) were given neuraxial analgesia and 133 (34.4%) were not (Figure 1).
Baseline and perinatal data
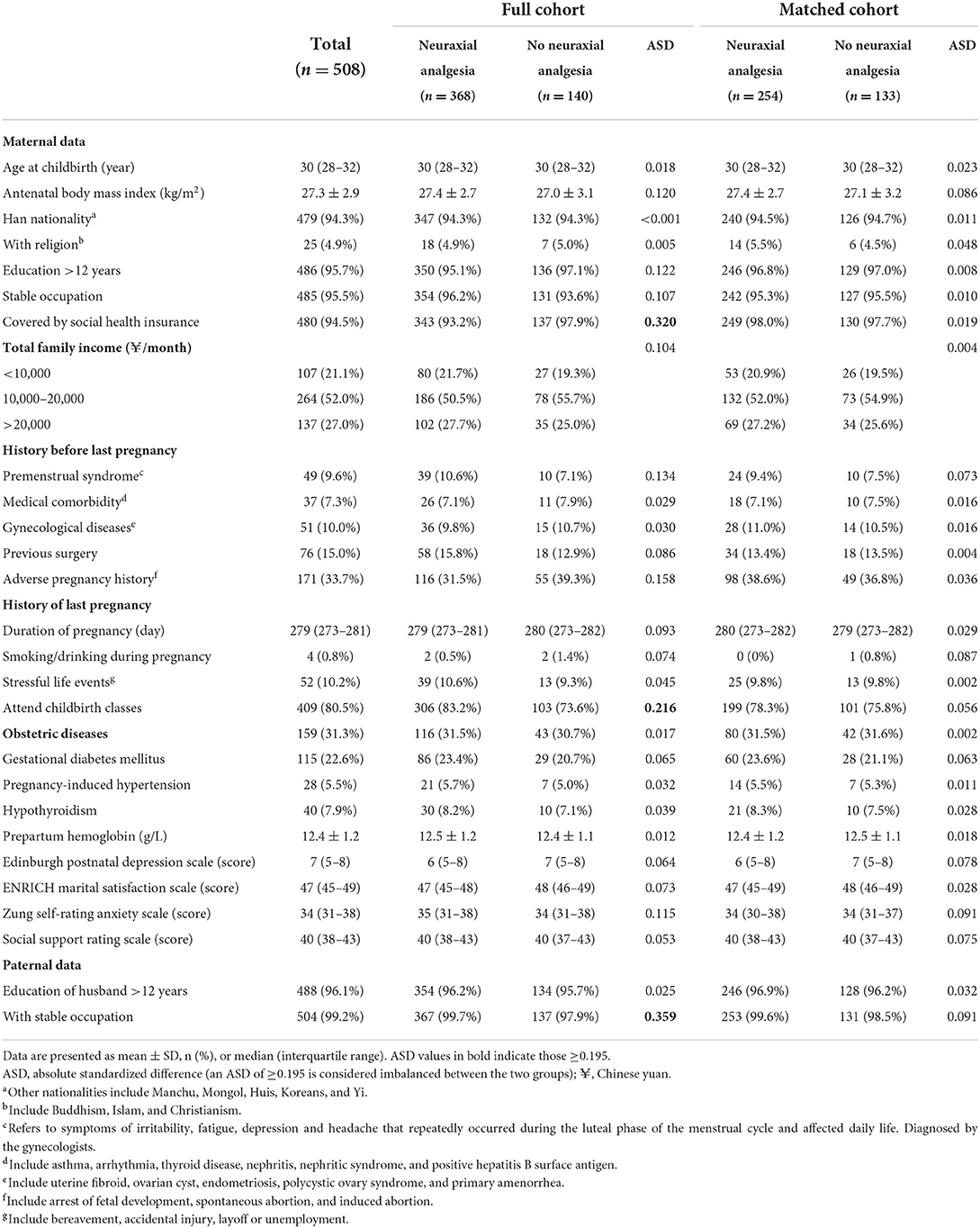
Of the 508 mothers who completed the study, the proportions of attending childbirth classes and husband with stable occupation were higher, whereas the proportion of covered by social health insurance was lower in those who received neuraxial analgesia than in those who did not. Twenty-four variables collected at the baseline were used for propensity score matching. In the matched cohort, all baseline variables were well balanced (Table 1).
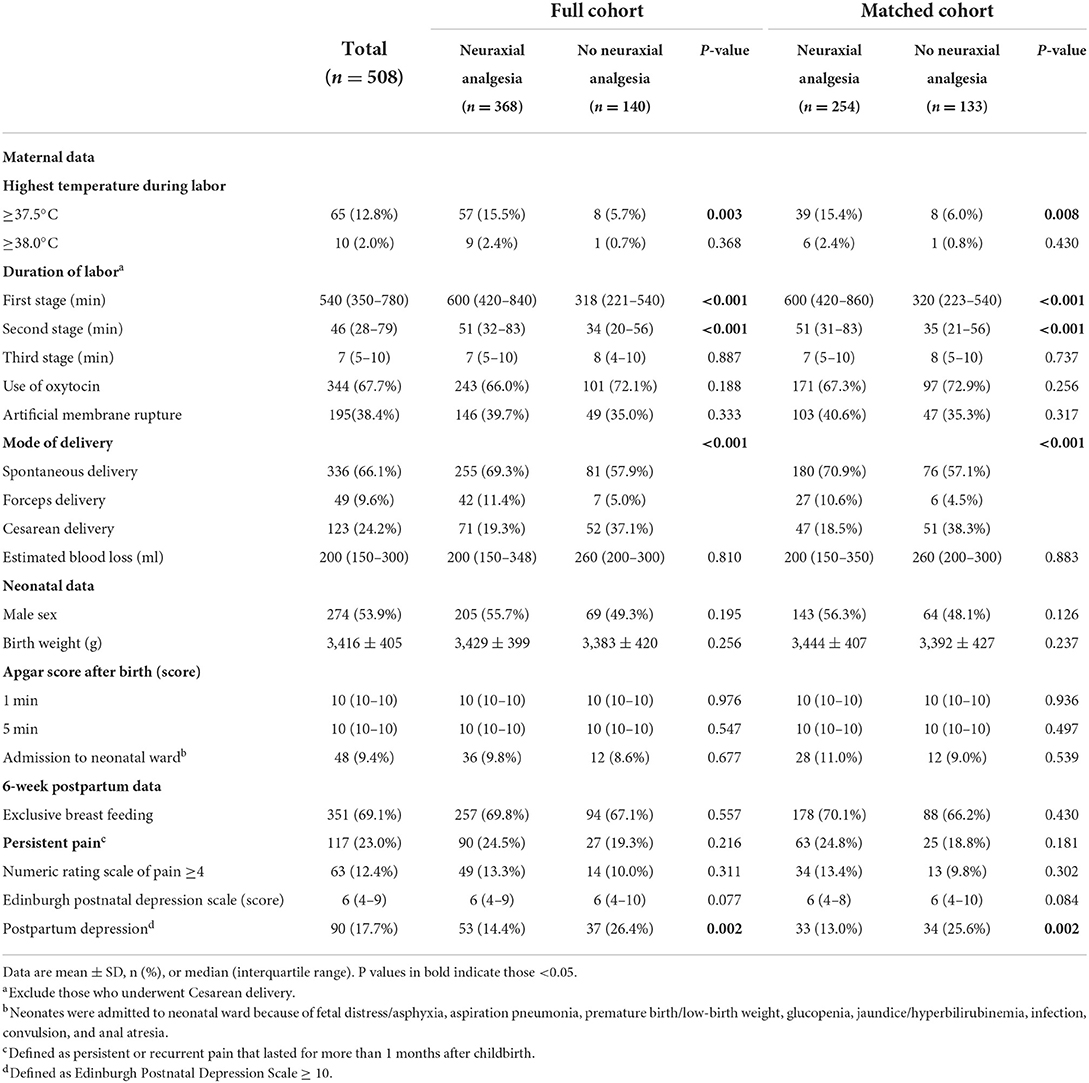
In the full cohort, mothers with neuraxial labor analgesia suffered from more intrapartum fever (≥37.5 °C), had longer durations of the first and second labor stages, underwent less cesarean delivery (but gave more spontaneous and instrumental delivery), and experienced less postpartum depression at 6 weeks when compared with those without (Table 2). In the matched cohort, the above differences were also present between the two groups (Table 2).
Outcomes of 2-year follow-up
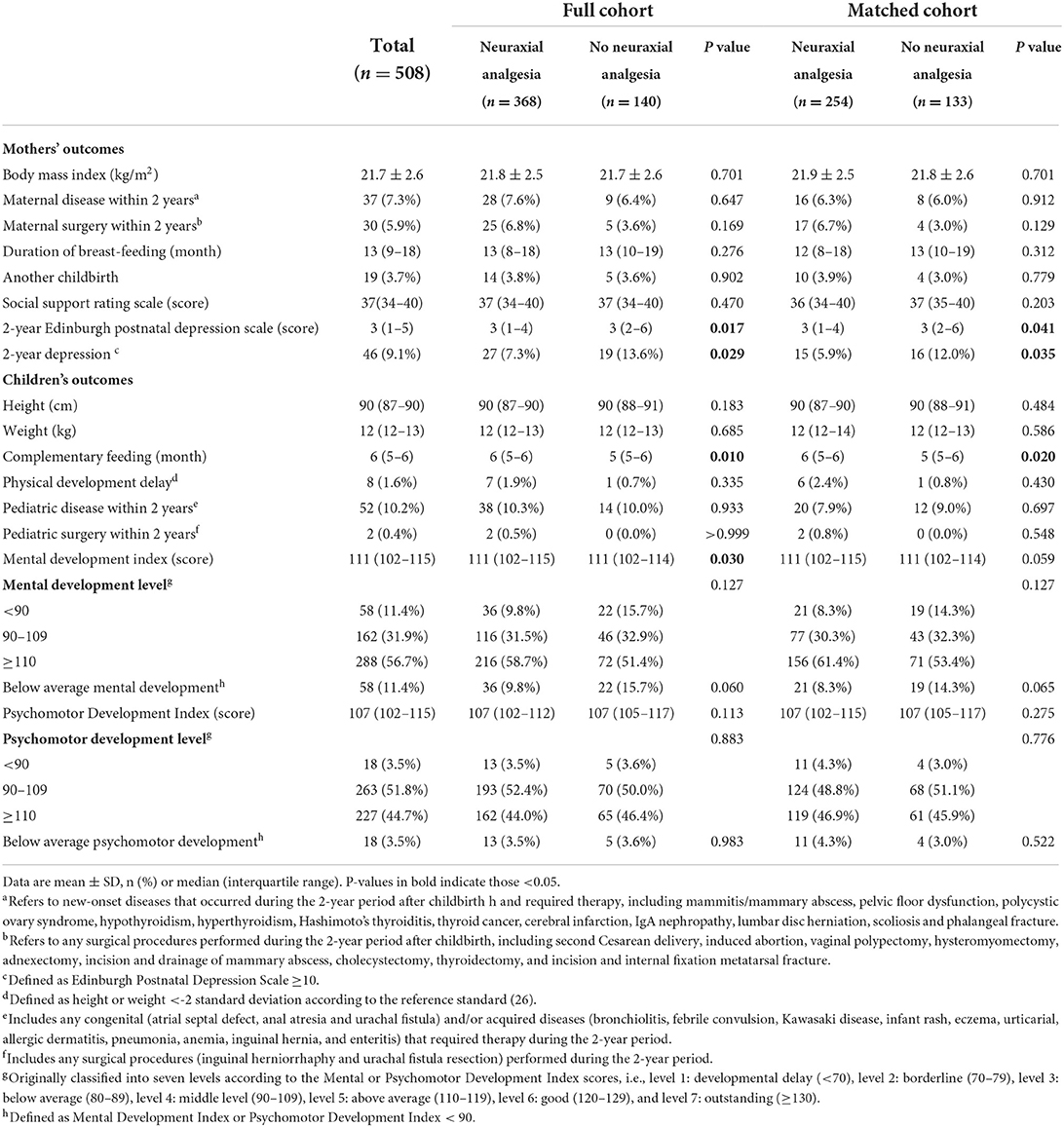
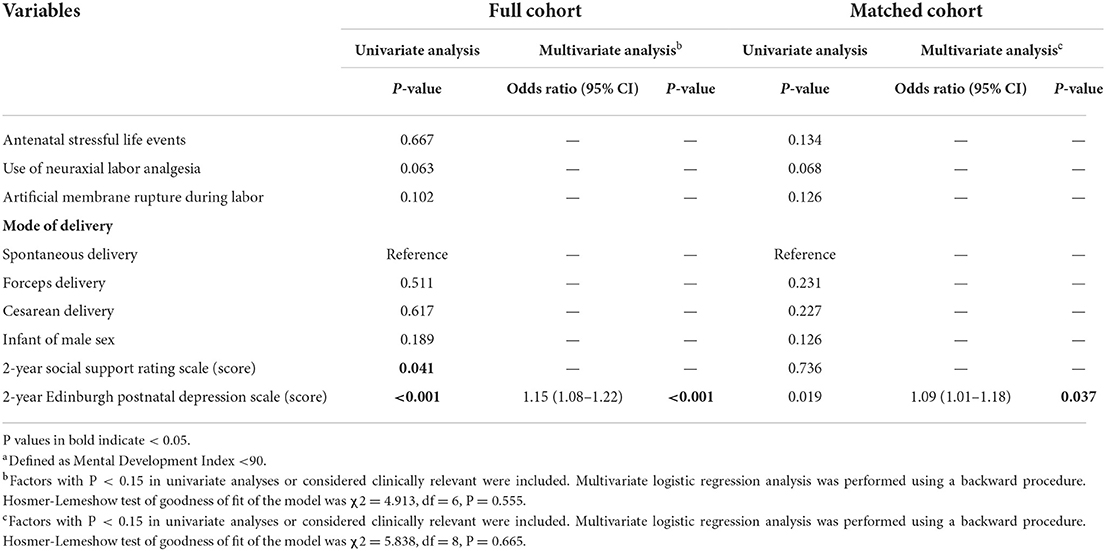
In the full cohort, mothers with neuraxial labor analgesia had a lower 2-year EPDS score [median 3 (IQR 1–4) vs. 3 (2–6), P = 0.017] and a lower prevalence of 2-year depression [7.3% (27/368) vs. 13.6% (19/140), P = 0.029] when compared with those without. The offspring of mothers with neuraxial analgesia had a later start of complementary feeding [6 months (5–6) vs. 5 months (5–6), P = 0.010] and a higher Mental Development Index score at 2 years [111 (102–115) vs. 111 (102–114), P = 0.030] when compared with those of mothers without; they had a slightly lower rate of below-average MDI [9.8% (36/368) vs. 15.7% (22/140), P = 0.060] but not statistically significantly (Table 3, Supplementary Table S2, Supplementary Figure S1).
In the matched cohort, mothers with neuraxial labor analgesia had a lower 2-year EPDS score [3 (1–4) vs. 3 (2–6), P = 0.041] and a lower prevalence of 2-year depression [5.9% (15/254) vs. 12.0% (16/133), P = 0.035] when compared with those without. The offspring of mothers with neuraxial analgesia had a later start of complementary feeding [6 months (5–6) vs. 5 months (5–6), P = 0.020] when compared with those of mothers without; they had a slightly higher Mental Development Index score at 2 years [111 (102–115) vs. 111 (102–114), P = 0.059] and a slightly lower rate of below-average mental development [8.3% (21/254) vs. 14.3% (19/133), P = 0.065] but not statistically significant (Table 3, Supplementary Table S2).
Factors associated with below-average mental development at 2 years of age
In both cohorts, six factors with P < 0.15 in univariable analyses (Supplementary Table S3) or considered clinically important were included in the multivariate logistic regression model. After correction for confounding factors, a higher 2-year EPDS score of mothers was the only independent factor that significantly associated with an increased risk of below-average mental development in their 2-year-old offspring (in full cohort: OR 1.15, 95% CI 1.08–1.22, P < 0.001; in matched cohort: OR 1.09, 95% CI 1.01–1.18, P = 0.037; Table 4), whereas maternal exposure to neuraxial analgesia during labor or duration of neuraxial analgesia was not (Supplementary Table S4). Similar results were observed when the cutoff point of MDI was set at 80, i.e., there was no significant association between maternal exposure of neuraxial labor analgesia and cognitive development delay in 2-year-old offspring (Supplementary Table S5).
Discussion
In this prospective longitudinal study, we found that in offspring born to nulliparous women with single cephalic term pregnancy and planned vaginal delivery, 11.4% had below-average mental development at 2 years of age. Maternal depression at 2 years was associated with an increased risk of below-average mental development in their 2-year-old offspring, whereas maternal exposure to neuraxial labor analgesia was not.
Neuraxial analgesia is recognized as the most effective method to relieve labor pain (4). However, despite well-established benefits, concerns exist regarding the potential impact on the outcomes of offspring. Available studies suggest that neuraxial labor analgesia may produce both favorable and unfavorable effects on neonates and children, but evidences are still lacking (32). The fear of the potential unfavorable effects might have impeded some parturients and even health care professionals from accepting neuraxial labor analgesia, especially in China (33). Further studies on this topic will help mothers and professionals to consider neuraxial labor analgesia from a more rational perspective.
Low-concentration local anesthetic and opioid combinations are currently a common practice used for neuraxial labor analgesia, in order to provide effective analgesia while minimizing potential unfavorable effects (34). Over decades, concerns exist regarding the potential influence of labor analgesia on infant brain development (35). In the participating centers of the present study, parturients who did not request neuraxial analgesia were rarely given pharmacological analgesia. Therefore, it is not proper to perform a randomized controlled trial to explore the long-term effects of maternal exposure to epidural analgesia during labor. We therefore performed this observational follow-up study. We collected various sociodemographic and baseline data of parents and performed propensity score matching, in order to balance the effects of potential confounding factors due to the non-random exposure.
Neurodevelopment of children is a complex process and affected by multiple factors (36). However, due to lack of evidences, the long-term effects of neuraxial analgesia on offspring development remain unclear. A few human studies investigated the association between maternal exposure to epidural labor analgesia and offspring risk of autism spectrum disorders and reported heterogenous results (37, 38). In a study of rhesus monkeys, Golub and colleagues found that epidurally administered bupivacaine did not produce neonatal abnormalities or specific cognitive deficits, but altered the normal course of behavioral development (39). In a population-based cohort study, Randall and colleagues revealed that the use of neuraxial analgesia during labor and vaginal delivery was not associated with the presence of learning disabilities before the age of 19 years (40). In the present study, we did not find significant association between maternal exposure to neuraxial labor analgesia and offspring mental development outcome at 2 years of age; this is consistent with previous reports.
As the most important and closest person during early life stages, mothers play a critical role in the growth and development of children. Perinatal mental disorders of mothers may produce harmful effects on the risk of psychological and developmental disturbances in their offspring (8). Indeed, studies showed that infants of mothers with depression and personality disorder had higher levels of dysregulated behavior at 18 months (41), and children whose mothers had postnatal depression had more intellectual problems at 11 years of age (42). In a prospective longitudinal study, Sutter-Dallay and colleagues reported that maternal depression at 6 weeks was associated with poor cognitive performance of children at the age of 2 years, and part of this association could be attributed to chronic depressive symptoms (43). As a matter of fact, multiple studies revealed that persistent maternal depression is associated with a higher risk of negative outcomes in children (8, 44). In line with these, our results also confirmed that a higher maternal depressive score at 2 years was significantly associated with an increased risk of below-average mental development in the offspring. We cannot exclude the possibility that maternal depressive mood was a consequence of poor neurodevelopment of their children. However, this is less likely to be the case in our patients because both physical and psychomotor developments were similar in children of the two groups, and even the difference of mental development was not clinically important.
Although controversial, several studies including ours showed that neuraxial labor analgesia is associated with a decreased risk of postpartum depression (45, 46). Our underlying study found that neuraxial labor analgesia is also associated with a reduced risk of maternal depression at 2 years after childbirth (17). In our results of both the full and the matched cohort, the proportion with below-average MDI was slightly lower in children whose mothers received neuraxial analgesia during labor, but the difference was not statistically significant. It is possible that neuraxial labor analgesia may produce favorable effects on children's mental development by relieving early and late maternal depressive symptoms after childbirth. However, sample size of the present study was too small to reveal this effect and further studies are needed to test this hypothesis.
Despite strengths including a prospective design and use of propensity score matching to balance baseline variables, our study has some limitations. Firstly, this was a predefined secondary analysis of a 2-year longitudinal study and the sample size was not calculated for the current primary endpoint. Secondly, the distributions of MDI and PDI scores in the present study were skewed, indicating potential sampling or information biases. This is likely due to the limited sample size and the metropolitan medical centers participated in the study, which also limited the generalizability of our results. Besides, as an observational cohort study, we cannot establish the causal relationship between exposure to neuraxial labor analgesia and 2-year outcomes. Further studies with larger sample sizes and more participating centers are required to verify the findings.
Conclusions
Our study did not find a significant association between maternal exposure to neuraxial labor analgesia and the risk of below-average mental developmental in 2-year-old children. High maternal depression score at 2 years was associated with an increased risk of below-average mental development in children. Further studies are warranted to clarify the effect of maternal neuraxial analgesia exposure on offspring neurocognitive outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University First Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
C-MD, TD, M-JX, LW, and D-XW conceived and designed the study. C-MD, TD, Z-HL, and S-TH collected data. C-MD, TD, Z-HL, J-HM, ML, W-LL, X-YL, DM, and D-XW analyzed and interpreted data. C-MD, TD, and Z-HL drafted the manuscript. D-XW critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Interdisciplinary Clinical Research Project of Peking University First Hospital (2019CR36) and the National High Level Hospital Clinical Research Funding (Multi-center Clinical Research Project of Peking University First Hospital) (2022CR56). The funders had no roles in the study design, data collection, data analysis, data interpretation, report writing, or decision to submit.
Acknowledgments
We thank Xin-Yu Sun (MD, Department of Psychiatrics, Peking University Sixth Hospital, Beijing, China) for her help in psychiatric consultation and Si-Chao Xu (MD, Department of Anesthesiology and Critical Care Medicine, Peking University First Hospital, Beijing, China), Shu Li (MD, Department of Anesthesiology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China) and Bo Lei (MD, Department of Anesthesiology, Haidian Maternal & Child Health Hospital, Beijing, China) for their help in collecting data. The authors also thank all the children and their parents who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.831538/full#supplementary-material
References
1. Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. a review. Neurosci Biobehav Rev. (2005) 29:237–58. doi: 10.1016/j.neubiorev.2004.10.007
2. Karam F, Sheehy O, Huneau MC, Chambers C, Fraser WD, Johnson D, et al. Impact of maternal prenatal and parental postnatal stress on 1-year-old child development: results from the OTIS antidepressants in pregnancy study. Arch Womens Ment Health. (2016) 19:835–43. doi: 10.1007/s00737-016-0624-6
3. Brownridge P. The nature and consequences of childbirth pain. Eur J Obstet Gynecol Reprod Biol. (1995) 59:S9–15. doi: 10.1016/0028-2243(95)02058-Z
4. Wong CA. Advances in labor analgesia. Int J Womens Health. (2010) 1:139–54. doi: 10.2147/IJWH.S4553
5. Lim G, LaSorda KR, Farrell LM, McCarthy AM, Facco F, Wasan AD. Obstetric pain correlates with postpartum depression symptoms: a pilot prospective observational study. BMC Pregnancy Childbirth. (2020) 20:240. doi: 10.1186/s12884-020-02943-7
6. Taylor A, Fisk NM, Glover V. Mode of delivery and subsequent stress response. Lancet. (2000) 355:120. doi: 10.1016/S0140-6736(99)02549-0
7. Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. (2005) 566:967–77. doi: 10.1113/jphysiol.2005.090191
8. Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. (2014) 384:1800–19. doi: 10.1016/S0140-6736(14)61277-0
9. Aoyagi SS, Tsuchiya KJ. Does maternal postpartum depression affect children's developmental outcomes. J Obstet Gynaecol Res. (2019) 45:1809–20. doi: 10.1111/jog.14064
10. Scull TJ, Hemmings GT, Carli F, Weeks SK, Mazza L, Zingg HH. Epidural analgesia in early labour blocks the stress response but uterine contractions remain unchanged. Can J Anaesth. (1998) 45:626–30. doi: 10.1007/BF03012090
11. Deng CM, Ding T, Li S, Lei B, Xu MJ, Wang L, et al. Neuraxial labor analgesia is associated with a reduced risk of postpartum depression: a multicenter prospective cohort study with propensity score matching. J Affect Disord. (2020) 281:342–50. doi: 10.1016/j.jad.2020.12.027
12. Suhitharan T, Pham TP, Chen H, Assam PN, Sultana R, Han NL, et al. Investigating analgesic and psychological factors associated with risk of postpartum depression development: a case-control study. Neuropsychiatr Dis Treat. (2016) 12:1333–9. doi: 10.2147/NDT.S105918
13. Linder N, Linder I, Fridman E, Kouadio F, Lubin D, Merlob P, et al. Birth trauma–risk factors and short-term neonatal outcome. J Matern Fetal Neonatal Med. (2013) 26:1491–5. doi: 10.3109/14767058.2013.789850
14. Morton S, Kua J, Mullington CJ. Epidural analgesia, intrapartum hyperthermia, and neonatal brain injury: a systematic review and meta-analysis. Br J Anaesth. (2021) 126:500–15. doi: 10.1016/j.bja.2020.09.046
15. Reynolds F. The effects of maternal labour analgesia on the fetus. Best Pract Res Clin Obstet Gynaecol. (2010) 24:289–302. doi: 10.1016/j.bpobgyn.2009.11.003
16. Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. (2000) 108:511–33. doi: 10.1289/ehp.00108s3511
17. Liu ZH, He ST, Deng CM, Ding T, Xu MJ, Wang L, et al. Neuraxial labour analgesia is associated with a reduced risk of maternal depression at 2 years after childbirth: a multicentre, prospective, longitudinal study. Eur J Anaesthesiol. (2019) 36:745–54. doi: 10.1097/EJA.0000000000001058
18. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
19. Fowers BJ, Olson DH. ENRICH Marital Satisfaction Scale: a brief research and clinical tool. J Fam Psychol. (1993) 7:176–85. doi: 10.1037/0893-3200.7.2.176
20. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
21. Xiao SY. A theoretical research and practical application of Social Support Rating Scale. Lin Chuang Jing Shen Yi Xue Za Zhi. (1994) 4:98–100.
22. Duan QQ, Sheng L. Differential validity of SAS and SDS among psychiatric non-psychotic outpatients and their partners. Chinese Ment Health J. (2012) 26:676–9. doi: 10.3969/j.issn.1000-6729.2012.09.007
23. Liu JW, Li FY, Lian YL. Investigation of reliability and validity of the social support scale. J Xinjiang Medical Univ. (2008) 31:1–3. doi: 10.3969/j.issn.1009-5551.2008.01.001
24. Cheng ZH, Tan LX, Yang Y, Lin XH, Zhou D, Jiang XJ, et al. The Chinese marital quality inventory: development, reliability and validity. Chin J Clin Psychol. (2004) 12:226–30. doi: 10.2174/978160805186111001010061
25. Lee DT, Yip SK, Chiu HF, Leung TY, Chan KP, Chau IO, et al. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh postnatal depression scale. Br J Psychiatry. (1998) 172:433–7. doi: 10.1192/bjp.172.5.433
26. Ministry of Health of China. Reference Standards for Growth and Development of Children Under 7 Years of Age in China. (2009). Available online at: www.nhc.gov.cn/fys/s7906/200910/994a7f6e1bd1491a9e8efa8e762a313f.shtml (assessed July 07, 2022).
27. Yi SR, Luo XR, Yang ZW, Wan GB. Bayley infant development scale revised in China (urban version). Chin J Clin Psychol. (1993) 1:71–5.
28. Liu W, Luo D, Xia W, Tao Y, Wang L, Yu M, et al. Prenatal exposure to halogenated, aryl, and alkyl organophosphate esters and child neurodevelopment at two years of age. J Hazard Mater. (2021) 408:124856. doi: 10.1016/j.jhazmat.2020.124856
29. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
30. Nguyen LD, Nguyen AD, Farber MK, Phan CT, Khuat LT, Nguyen HT, et al. Sociodemographic factors associated with request for labor epidural analgesia in a tertiary obstetric hospital in Vietnam. Biomed Res Int. (2021) 2021:8843390. doi: 10.1155/2021/8843390
31. Ochroch EA, Troxel AB, Frogel JK, Farrar JT. The influence of race and socioeconomic factors on patient acceptance of perioperative epidural analgesia. Anesth Analg. (2007) 105:1787–92. doi: 10.1213/01.ane.0000290339.76513.e3
32. Liu ZH, Wang DX. Potential impact of epidural labor analgesia on the outcomes of neonates and children. Chin Med J. (2020) 133:2353–8. doi: 10.1097/CM9.0000000000000900
33. Wu J, Ling K, Song WT, Yao SL. Perspective on the low labor analgesia rate and practical solutions for improvement in China. Chin Med J. (2020) 133:606–8. doi: 10.1097/CM9.0000000000000660
34. Lim G, Facco FL, Nathan N, Waters JH, Wong CA, Eltzschig HK, et al. Review of the impact of obstetric anesthesia on maternal and neonatal outcomes. Anesthesiology. (2018) 129:192–215. doi: 10.1097/ALN.0000000000002182
35. Golub MS. Labor analgesia and infant brain development. Pharmacol Biochem Behav. (1996) 55:619–28. doi: 10.1016/S0091-3057(96)00254-7
36. Walkden GJ, Pickering AE, Gill H. Assessing long-term neurodevelopmental outcome following general anesthesia in early childhood: challenges and opportunities. Anesth Analg. (2019) 128:681–94. doi: 10.1213/ANE.0000000000004052
37. Qiu C, Lin JC, Shi JM, Chow T, Desai VN, Nguyen VT, et al. Association between epidural analgesia during labor and risk of autism spectrum disorders in offspring. JAMA Pediatr. (2020) 174:1168–75. doi: 10.1001/jamapediatrics.2020.3231
38. Wall-Wieler E, Bateman BT, Hanlon-Dearman A, Roos LL, Butwick AJ. Association of epidural labor analgesia with offspring risk of autism spectrum disorders. JAMA Pediatr. (2021) 175:698–705. doi: 10.1001/jamapediatrics.2021.0376
39. Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol Teratol. (1998) 20:29–41. doi: 10.1016/S0892-0362(97)00068-8
40. Flick RP, Lee K, Hofer RE, Beinborn CW, Hambel EM, Klein MK, et al. Neuraxial labor analgesia for vaginal delivery and its effects on childhood learning disabilities. Anesth Analg. (2011) 112:1424–31. doi: 10.1213/ANE.0b013e3181f2ecdd
41. Conroy S, Pariante CM, Marks MN, Davies HA, Farrelly S, Schacht R, et al. Maternal psychopathology and infant development at 18 months: the impact of maternal personality disorder and depression. J Am Acad Child Adolesc Psychiatry. (2012) 51:51–61. doi: 10.1016/j.jaac.2011.10.007
42. Hay DF, Pawlby S, Sharp D, Asten P, Mills A, Kumar R. Intellectual problems shown by 11-year-old children whose mothers had postnatal depression. J Child Psychol Psychiatry. (2001) 42:871–89. doi: 10.1111/1469-7610.00784
43. Sutter-Dallay AL, Murray L, Dequae-Merchadou L, Glatigny-Dallay E, Bourgeois ML, Verdoux H, et al. prospective longitudinal study of the impact of early postnatal vs. chronic maternal depressive symptoms on child development. Eur Psychiatry. (2011) 26:484–9. doi: 10.1016/j.eurpsy.2010.05.004
44. Oyetunji A, Chandra P. Postpartum stress and infant outcome: a review of current literature. Psychiatry Res. (2020) 284:112769. doi: 10.1016/j.psychres.2020.112769
45. Ding T, Wang DX, Qu Y, Chen Q, Zhu SN. Epidural labor analgesia is associated with a decreased risk of postpartum depression: a prospective cohort study. Anesth Analg. (2014) 119:383–92. doi: 10.1213/ANE.0000000000000107
Keywords: labor, obstetric [MeSH], analgesia obstetric, depression postpartum, child development, cognition
Citation: Deng CM, Ding T, Liu ZH, He ST, Ma JH, Xu MJ, Wang L, Li M, Liang WL, Li XY, Ma D and Wang DX (2022) Impact of maternal neuraxial labor analgesia exposure on offspring's neurodevelopment: A longitudinal prospective cohort study with propensity score matching. Front. Public Health 10:831538. doi: 10.3389/fpubh.2022.831538
Received: 08 December 2021; Accepted: 12 July 2022;
Published: 29 July 2022.
Edited by:
Michal Kovo, Wolfson Medical Center, IsraelReviewed by:
Juliet Richetto, University of Zurich, SwitzerlandZifeng Xu, International Peace Maternity and Child Health Hospital, China
Han Huang, Sichuan University, China
Copyright © 2022 Deng, Ding, Liu, He, Ma, Xu, Wang, Li, Liang, Li, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Xin Wang, d2FuZ2Rvbmd4aW4mI3gwMDA0MDtob3RtYWlsLmNvbQ==; ZHh3YW5nNjUmI3gwMDA0MDtiam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Chun-Mei Deng
Chun-Mei Deng Ting Ding
Ting Ding Zhi-Hua Liu1
Zhi-Hua Liu1 Shu-Ting He
Shu-Ting He Jia-Hui Ma
Jia-Hui Ma Daqing Ma
Daqing Ma Dong-Xin Wang
Dong-Xin Wang