
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 12 May 2022
Sec. Environmental Health and Exposome
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.821905
 Li-Li Li1
Li-Li Li1 Yan-Hong Huang2
Yan-Hong Huang2 Jing Li3
Jing Li3 Shu Liu4
Shu Liu4 Yan-Ling Chen5
Yan-Ling Chen5 Cheng-Zhi Jiang6
Cheng-Zhi Jiang6 Zong-Jiao Chen4
Zong-Jiao Chen4 Yan-Yan Zhuang7*
Yan-Yan Zhuang7*Evidence of the association between maternal sulfur dioxide (SO2) exposure and the risk of omphalocele is limited and equivocal. We aimed to assess the aforementioned topic during the first trimester of pregnancy. A population-based case-control study was carried out in infants consisting of 292 cases of omphalocele and 7,950 healthy infant controls. Exposure to SO2, particulate matter with aerodynamic diameters ≤ 10 μm, and nitrogen dioxide was assessed by averaging the concentration from all stations in the mother's residential city. SO2 exposure was categorized into three groups, with the lowest tertile defined as the reference category. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression models. Higher SO2 exposure during the first trimester was significantly associated with omphalocele risk [per standard deviation (42 ug/m3) increment: OR = 1.39, 95% CI = 1.22–1.65]. When focusing on shorter exposure windows, similar positive associations were observed for SO2 exposure in the first and third months of pregnancy. In addition, compared with the lowest tertile, high SO2 exposure in the second month of pregnancy increased the risk of omphalocele (OR = 2.80, 95% CI = 1.61–4.97). Maternal exposure to SO2 during the first trimester may increase the risk of omphalocele in offspring.
Omphalocele is one of the most frequently observed congenital abdominal wall defects, with an incidence rate of ~1–3.8 per 10,000 live births (1–4). In China, the incidence of omphalocele in 2019 was 0.82 per 10,000 births, with no significant changes during recent years (5). In omphalocele, organs protrude through a midline defect in the abdominal wall along with the umbilical cord (6). This can affect not only the small intestine and liver, but also other organs such as the bladder, spleen, and ovaries (7). The survival rate of isolated omphalocele can reach 90%, but is significantly reduced with other malformations (8). Due to lack of a clear consensus to explain the precise embryological mechanisms leading to the occurrence of omphalocele (7), prevention is best achieved by exploring environmental teratogens and providing recommendations to pregnant women at the optimal time window.
Growing epidemiological evidence has shown that maternal exposure to ambient air pollution may have adverse effects on a developing fetus or newborn (9, 10). Several studies from the most recent 5 years have found significant associations between maternal air pollution exposure and increased risk of congenital cardiac defects, neural tube defects, and limb malformations (9–11). Furthermore, findings from our colleagues also suggested that maternal air pollution exposure is positively associated with neural tube defects, oral clefts, polydactyly, and syndactyly (12–17). However, only a few studies focused on the association between ambient air pollution and the risk of congenital abdominal wall defects (18, 19). For example, a population-based case-control study in Barcelona reported that exposure to ambient particulate matter with aerodynamic diameters ≤ 10 μm (PM10), PMcoarse, and PM2.5 increased the risk of abdominal wall defects, but exposure to nitrogen dioxide (NO2) and nitrous oxide had no effect (18). However, previous studies have found that maternal exposure to sulfur dioxide (SO2) is not associated with the risk of multiple birth defects (19, 20). A previous geographical study in England failed to observe a significant relationship between maternal SO2 exposure and omphalocele risk (19). In addition, no significant association of maternal SO2 exposure during the first trimester was observed among 75 omphalocele cases in Xi'an from 2010 to 2015 (10).
In light of the inadequate evidence on the risk of omphalocele in the different stages of pregnancy as well as the lack of studies in China, and based on data from the Liaoning Province, we conducted a population-based case-control study to explore whether exposure to ambient SO2 during the first 3 months of pregnancy was associated with omphalocele risk.
Data on live births with omphalocele were obtained between 1 January 2010 and 31 December 2015 from the Maternal and Child Health Certificate Registry of Liaoning Province, which was managed by Liaoning Women and Children's Health Hospital, Shenyang, China. Several previous studies have described this registry in detail (21). In short, the registry is a hospital-based active monitoring system for monitoring live births. The hospital is a large obstetrics and gynecology hospital and a comprehensive nursing institution. Liaoning Maternal and Child Health Care Guidance Center has provided comprehensive health care services for pregnant women since 1986. A total of 14 cities (Shenyang, Dalian, Anshan, Fushun, Benxi, Dandong, Jinzhou, Yingkou, Fuxin, Liaoyang, Panjin, Tieling, Chaoyang, and Huludao) in Liaoning Province have been registered. During the study period, there were ~6,000 cases of birth defects across all obstetric units each year (22). Liaoning is one of 31 provinces that provides data to the national birth defects monitoring database, which is maintained by the Chinese Birth Defects Monitoring Network. Fourteen maternal and child health care institutions in Liaoning Province provide birth defect data to the Liaoning Women and Children's Health Hospital each month (23). We have previously described the geographical divisions of Liaoning Province and the source of the control group (14). The control group was constructed to represent the general population of births in Liaoning Province, from where the case groups were recruited. Therefore, 1.5% of live births without birth defects were randomly selected from five cities (Shenyang, Dalian, Fuxin, Chaoyang, and Huludao) as a control of random birth year sampling unrelated to cases from 2010 to 2015. If the subject did not have a permanent address or if key covariates were missing, they were excluded from the current analysis. The study protocol has been approved by the Institutional Review Board of Liaoning Women and Children's Health Hospital and carried out in accordance with local and national regulations.
Several previous studies had described the data collection procedure (21). In short, provincial and municipal monitoring networks and clinical expert groups have been established to collect data. An experienced obstetrician or pediatrician examined each newborn (or terminated fetus) immediately after birth. Suspected cases of omphalocele that were diagnosed by prenatal ultrasonography were confirmed after termination or postnatal examination. Omphalocele (ICD-10-CM code, Q79.2) were registered and coded according to the International Classification of Diseases 10th edition. After identifying and confirming omphalocele cases in the hospital, an experienced obstetric or pediatric expert interviewed the mother of the infant and completed the Birth Defects Registration Form, which was easily used to collect demographic characteristics, clinical features, and obstetric factors. Subsequently, forms were submitted to the local maternal and child healthcare institution, and then to the Liaoning Women and Children's Health Hospital. The data were reviewed and analyzed by a team of national grade experts in medical genetics and pediatrics (24). We have previously reported quality control data (24); in short, experts at all levels identified the disease diagnosis, data collection, data checking, as well as medical records according to the procedure manual to ensure high quality of data. Additionally, an independent retrospective investigation was conducted by experts to identify inadequacy and inaccuracies in the data (24).
The data from 77 air quality monitoring stations in 14 cities of Liaoning Province (two of which served as controls) were used to assess the ambient air pollution exposure during the period of pregnancy (Figure 1). The 77 air pollutant monitoring stations across Liaoning Province were located primarily in urban areas, covering residential areas to represent the ambient air pollution levels of the whole area. We used the above mentioned monitoring stations to measure the daily SO2, NO2, and PM10 levels from 2010 to 2015. The hourly concentration of the monitoring station was tested and reported in strict accordance with the ambient air quality standards of the Chinese government (25). To assess maternal exposure level, we used the average concentrations of SO2, NO2, and PM10 at all sites in the city where the mother lived, and then used these values to calculate an average monthly exposure (Supplementary Figure S1). Environmental SO2, NO2, and PM10 concentrations were available from the Environment Protection Bureau of each city. The monthly averages were linked to birth records according to the city where the mother lived, as indicated on the Birth Defects Registration Form. Most congenital malformations occur in the first trimester of pregnancy, which is a very important stage of development for the baby's organogenesis (17). And we choose the first trimester of pregnancy and their individual month. In order to investigate the exposure window during the period of pregnancy, average SO2 air pollution concentrations for every participant were calculated at a time point 3 months after conception. As with previous studies (14, 17), we assumed that the pregnancy date occurred on the first day of the last menstruation, and we calculated the pregnancy date according to the birth date and gestational age information on the form. If the date of pregnancy occurred in the first half of the month, then the month was regarded as the first month after conception; otherwise, it was regarded as the first month before conception.
We identified potential confounders based on a previous causal knowledge of the existing literature (26, 27), including: season of conception [four categories: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February)]; maternal age (three categories: <30 and ≥30 years), parity (≤1 and ≥2), gravidity (≤1 and ≥2); maternal education level (four categories: elementary school or less, middle school, high school, and college or above); and maternal NO2 and PM10 exposure.
The characteristics of cases and controls were compared using the chi-square test. The monthly distributions of SO2 concentrations in Liaoning Province during the study period were described by mean ± standard deviation and median (25th−75th percentile) to provide an overall view of the characteristics of ambient air pollution in the study area. Omphalocele was treated as a dichotomous dependent variable in the analysis. The exposure concentration of SO2 acted as the principal independent variable that was calculated as the categorical variable based on tertiles of distribution in the control groups. We used the lowest tertile as the reference category. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated through crude and multivariable logistic regression analysis. The models were: without any adjustment (crude); adjusted for maternal age, season of conception, gravidity, parity, and maternal education (model 1); additionally adjusted for NO2 on the basis of model 1 (model 2); additionally adjusted for PM10 on the basis of model 1 (model 3); and additionally adjusted for NO2 and PM10 on the basis of model 1 (model 4). In addition, we completed subgroup analysis to assess potential effect modification by maternal age (<30 and ≥30 years). Potential interactions between the concentration of SO2 and maternal age were assessed by adding cross-product terms to the multivariable logistic models. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P < 0.05, based on the two-sided test.
The basic characteristics of cases and controls in Liaoning Province are shown in Table 1. Cases of omphalocele (n = 292) and controls (n = 7,950) were included in our analysis. Compared with controls, omphalocele cases had younger gestational age, lower birth weight, and more births occurring during fall and winter (P < 0.001). There were no significant differences between the two groups with respect to infant sex or maternal age. Table 2 shows the distribution characteristics of ambient SO2 concentration (μg/m3) in the 14 cities in Liaoning Province from 2010 to 2015. The SO2 concentration in Panjin was the lowest during the study period; Shenyang, the capital of Liaoning Province, had the highest SO2 concentration and exceeded the recommended annual SO2 concentration limit (60 μg/m3).
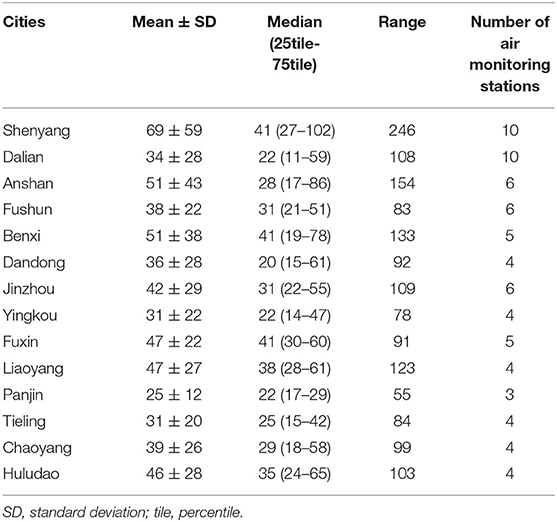
Table 2. Ambient SO2 levels (μg/m3) and the number of air monitoring stations in 14 cities in Liaoning Province, China, between 2010 and 2015.
Findings of the main analyses are displayed in Table 3. In model 4, which adjusted for maternal age, season of conception, gravidity, parity, nitrogen dioxide, and PM10, we found that higher SO2 exposure increased the risk of omphalocele [per standard deviation (42 ug/m3) increment: OR = 1.39, 95% CI = 1.22–1.65]. Further evaluation by single month showed similar results for the first and third months of pregnancy. In addition, we found that the highest tertile of maternal SO2 exposure was associated with an increased risk of omphalocele during the second month of pregnancy (OR T3vs.T1 = 2.80, 95% CI = 1.61–4.97).
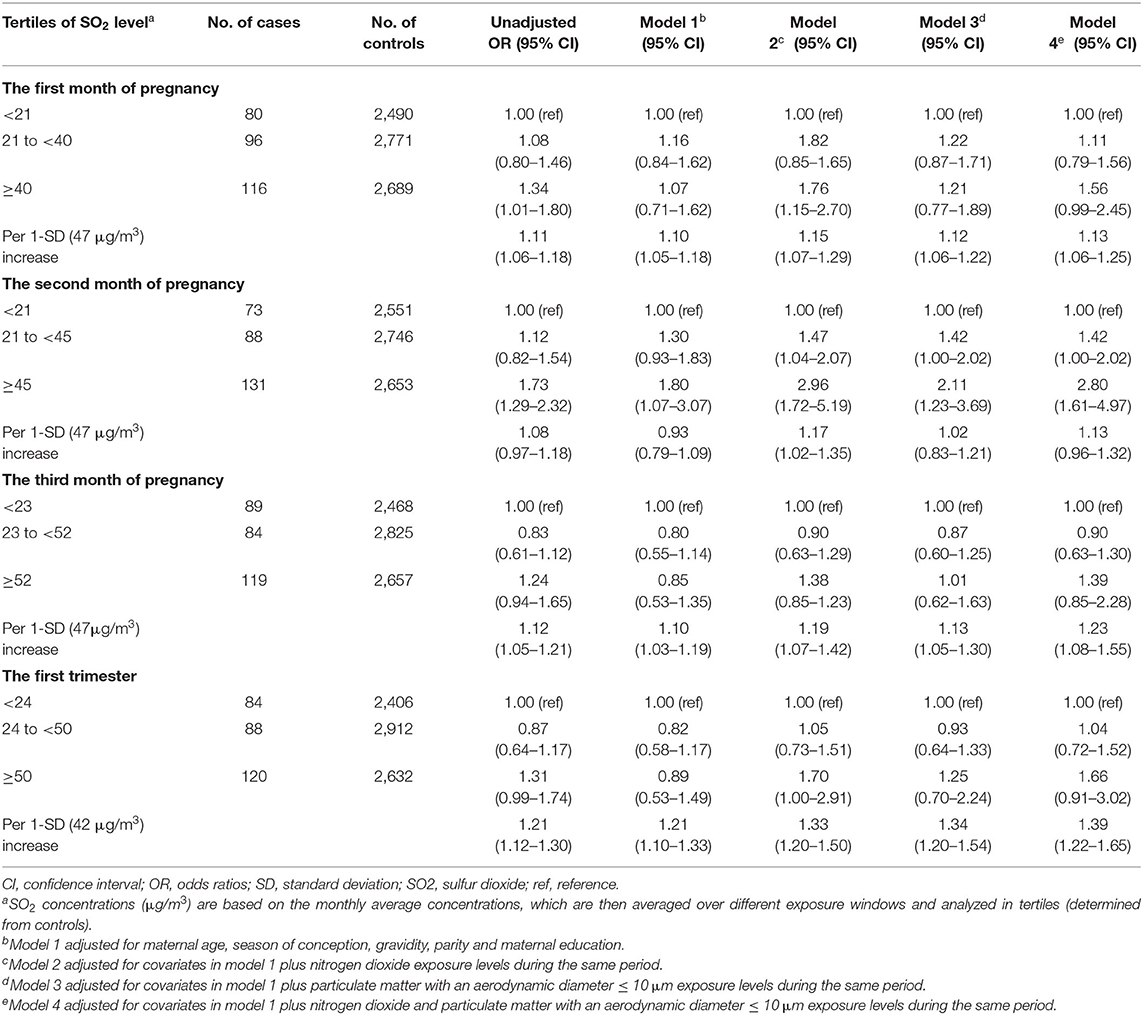
Table 3. Associations between maternal exposure to ambient SO2 during various exposure windows and the risk of omphalocele in offspring.
Supplementary Table 1 shows the adjusted risk estimates between maternal SO2 exposure and omphalocele risk, stratified by maternal age. A significant association between the highest tertile of maternal SO2 exposure and omphalocele risk was found during the first trimester in the younger age group. Additionally, no interaction was observed between maternal SO2 exposure and maternal age.
In this population-based case-control study, we estimated the association between maternal exposure to air pollutants and the risk of omphalocele in offspring using the database of the Maternal and Child Health Certificate Registry of Liaoning Province from 2010 to 2015. We observed a significant positive association between ambient SO2 exposure during the first trimester and omphalocele risk.
The mechanism by which maternal SO2 exposure during pregnancy causes omphalocele remains unclear, and further mechanistic research and animal experiments are needed. However, several possible mechanisms involving placental inflammation (28), oxidative stress (39), epigenetic changes (29), and microRNA (30) have been proposed in many epidemiological studies to explain the observed effects birth defects following maternal exposure to environmental pollutants. Specifically, SO2 may disrupt the structure of DNA and induce epigenetic changes, such as DNA methylation and histone modifications, which can be passed on to offspring (31). In addition, SO2 absorbed into the human body can produce toxic effects on embryonic development, and destroy the function and microstructure of germ cells (32).
To our knowledge, limited air pollution studies (10, 19, 33, 34) have focused on omphalocele as the primary or secondary study outcome. For example, in a previous exploratory investigation in Texas, Vinikoor-Imler et al. found that high maternal PM2.5 and O3 exposures during the first trimester were not associated with an increased risk of omphalocele in offspring (33). As a rare but serious birth defect, only two published studies (10, 19) have examined the association between maternal SO2 exposure during pregnancy and the risk of omphalocele in offspring. Contrary to our results, these two studies did not find a positive association between maternal SO2 exposure during the first trimester and omphalocele risk. Inconsistencies may be attributed to differences in study design (time-series study, ecological study, or case-control study), statistical analysis methods (generalized additive model, Poisson regression model, or logistic regression model), exposure assessment methods, sample size, and adjustments for confounding factors. For example, Dolk et al. (19) performed a geographical study analyzing a population-based active surveillance database of birth defects across four regions of England from 1991 to 1999 to estimate associations between average annual exposure to SO2, NO2, and PM10 and broad groups of birth defects. A total of 183 cases with omphalocele were included for final poisson regression analysis, and the results reported a significant positive association between maternal PM10 exposure during the first trimester and omphalocele risk. However, the same multivariable logistic regression model showed no association between maternal SO2 or NO2 exposures during the first trimester and the risk of omphalocele. Wang et al. (10) used a time-series design to investigate the associations between various types of birth defects and maternal SO2, NO2, and PM10 exposures during early pregnancy in Xi'an city, China, from 2010 to 2015. However, this study did not observe any significant positive association between the risk of omphalocele (n = 75) and SO2 exposure during the first 3 months of pregnancy.
Our study has several advantages. First, the sample size included in the final analysis was larger than in the two previous studies, which enabled us to examine the association between SO2 exposure during the first trimester and the risk of omphalocele in a more statistically precise manner. Second, our provincial birth registry database recruited not only live births with omphalocele, but also stillbirths and aborted fetuses with omphalocele, which increased the number of cases and reduced possible selection bias.
Our findings should be interpreted carefully and are not without limitation. First, the method of exposure assessment may have masked the true associations. In our study, we assigned to each subject average air pollutant levels for all air quality monitoring stations of maternal residential areas, which may have created exposure misclassification. Due to lack of data on land use and transportation, we were not able to evaluate maternal SO2 exposures using the land-use regression model. Future air pollution studies with birth defects as the primary or secondary health outcome should emphasize the application of accurate exposure assessments to reduce measurement errors that may arise from exposure assessments. Second, the possible movement or relocation of a pregnant woman during pregnancy also affects the concentration of exposure assessed from fixed air quality monitoring stations. Investigators typically survey mothers after birth and may overlook differences in residence changes between early pregnancy and delivery. A review (35) of 14 air pollution studies with maternal residential mobility data available for the entire pregnancy reported that 9–32% gravidae change residence between conception and delivery, and pregnant women generally maintain a low mobility rate during the early stages of pregnancy. Notably, the results of two large air pollution studies conducted in China (36, 37) showed that the mobility rate of Chinese women during pregnancy is about 3%. Therefore, errors in exposure assessments due to changes in residence are unlikely to have a significant effect on the associations of maternal air pollutants exposures with birth defects. Third, our data were collected from the birth registrations. Although patients with birth defects were recorded by active surveillance and rigorous quality control, unreported and misclassified omphalocele cases are inevitable, especially in areas with limited medical resources (38). Fourth, although we adjusted some important confounding factors based on our experience and previous studies, the influence of residual confounding factors on our results cannot be completely excluded. Due to the availability of data, we were unable to adjust for some maternal environmental exposure factors, including maternal illness, maternal smoking or alcohol intake, medication exposures, and maternal nutritional intake during early pregnancy. However, it is unlikely that such factors were associated with exposure to air pollutants and were partially controlled by adjusting for family income or maternal education level in the multivariable logistic regression model (15). Fifth, since PM2.5 monitoring began in 2013 in Liaoning Province and there were insufficient data available, we cannot assess the effects of PM2.5 in our models. Future studies should attention smaller particles.
In conclusion, our study found that high maternal SO2 exposure during the first trimester is associated with an increased risk of omphalocele in offspring. We recommend that women in early pregnancy should avoid or reduce exposure to air pollutants; further mechanistic studies are necessary to confirm the associations identified in this population study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Liaoning Women and Children's Health Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-YZ and Y-HH: study conceptualization and design. JL and SL: data collection. Y-YZ: data cleaning and discrepancy checks. Y-LC and C-ZJ: analytic strategy. L-LL and Z-JC: analysis and interpretation of data. L-LL, JL, and SL: manuscript preparation. All authors read and approved the final manuscript.
This study was supported by the Liaoning Providence Science and Technology Project (2015225025 for Y-HH), the Shenyang science and technology project (F15-139-9-09 for Y-HH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.821905/full#supplementary-material
Supplementary Figure S1. Monthly mean ambient SO2 concentrations (ugm3) in 14 cities in Liaoning Province, China.
Supplementary Table 1. The maternal age stratification (<30,≥30 years) subgroups were analyzed for the associations between ambient SO2 exposure(μg/m3) with controls and cases.
CIs, confidence intervals; NO2, nitrogen dioxide; ORs, odds ratios; O3, ozone; PM10, particulate matter with aerodynamic diameter ≤ 10 μm; PM2.5, particulate matter with aerodynamic diameters ≤ 2.5 μm; SD, standard deviation; SO2, sulfur dioxide; Tile, percentile.
1. Marshall J, Salemi JL, Tanner JP, Ramakrishnan R, Feldkamp ML, Marengo LK, et al. Prevalence, correlates, and outcomes of omphalocele in the United States, 1995-2005. Obstet Gynecol. (2015) 126:284–93. doi: 10.1097/AOG.0000000000000920
2. Springett A, Draper ES, Rankin J, Rounding C, Tucker D, Stoianova S, et al. Birth prevalence and survival of omphalocele in england and wales: 2005 to 2011. Birth Defects Res A Clin Mol Teratol. (2014) 100:721–5. doi: 10.1002/bdra.23301
3. Raitio A, Tauriainen A, Syvänen J, Kemppainen T, Löyttyniemi E, Sankilampi U, et al. Omphalocele in Finland from 1993 to 2014: trends, prevalence, mortality, and associated malformations-a population-based study. Eur J Pediatr Surg. (2021) 31:172–6. doi: 10.1055/s-0040-1703012
4. Benjamin B, Wilson GN. Anomalies associated with gastroschisis and omphalocele: analysis of 2825 cases from the Texas Birth Defects Registry. J Pediatr Surg. (2014) 49:514–9. doi: 10.1016/j.jpedsurg.2013.11.052
5. Ministry of Health, People's Republic of China. Report on Women and Children's Health Development in China (2021).
6. Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. (2019) 28:84–8. doi: 10.1053/j.sempedsurg.2019.04.007
7. Khan FA, Hashmi A, Islam S. Insights into embryology and development of omphalocele. Semin Pediatr Surg. (2019) 28:80–3. doi: 10.1053/j.sempedsurg.2019.04.003
8. Fogelström A, Caldeman C, Oddsberg J, Löf Granström A, Mesas Burgos C. Omphalocele: national current birth prevalence and survival. Pediatr Surg Int. (2021) 37:1515–20. doi: 10.1007/s00383-021-04978-z
9. Ren Z, Zhu J, Gao Y, Yin Q, Hu M, Dai L, et al. Maternal exposure to ambient PM10 during pregnancy increases the risk of congenital heart defects: Evidence from machine learning models. Sci Total Environ. (2018) 630:1–10. doi: 10.1016/j.scitotenv.2018.02.181
10. Wang L, Xiang X, Mi B, Song H, Dong M, Zhang S, et al. Association between early prenatal exposure to ambient air pollution and birth defects: evidence from newborns in Xi'an, China. J Public Health. (2019) 41:494–501. doi: 10.1093/pubmed/fdy137
11. Padula AM, Tager IB, Carmichael SL, Hammond SK, Lurmann F, Shaw GM. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. (2013) 177:1074–85. doi: 10.1093/aje/kws367
12. Liu FH, Dai HX, Gong TT, Zhang JY, Li J, Chen ZJ, et al. Maternal preconception and first trimester exposure to PM10 and the risk of oral clefts in offspring: a population-based, case-control study. Occup Environ Med. (2020) 77:721–7. doi: 10.1136/oemed-2020-106434
13. Liu FH, Xing Z, Gong TT, Zhang JY, Huang YH, Li J, et al. Maternal exposure to sulfur dioxide and the risk of oral clefts in Liaoning Province, China: a population-based case-control study. Environ Sci Pollut Res Int. (2021) 28:39101–9. doi: 10.1007/s11356-021-13461-0
14. Zhang JY, Gong TT, Huang YH, Li J, Liu S, Chen YL, et al. Association between maternal exposure to PM10 and polydactyly and syndactyly: a population-based case-control study in Liaoning province, China. Environ Res. (2020) 187:109643. doi: 10.1016/j.envres.2020.109643
15. Zhang JY, Dai HX, Wu QJ, Li J, Huang YH, Chen ZJ, et al. Maternal exposure to ambient levels of sulfur dioxide and risk of neural tube defects in 14 cities in Liaoning province, China: a population-based case-control study. J Expo Sci Environ Epidemiol. (2021) 31:266–75. doi: 10.1038/s41370-020-00273-6
16. Jiang YT, Gong TT, Zhang JY, Huang YH, Li J, Liu S, et al. Maternal exposure to ambient SO2 and risk of polydactyly and syndactyly: a population-based case-control study in Liaoning Province, China. Environ Sci Pollut Res Int. (2021) 28:11289–301. doi: 10.1007/s11356-020-11351-5
17. Zhang JY, Wu QJ, Huang YH, Li J, Liu S, Chen YL, et al. Association between maternal exposure to ambient PM10 and neural tube defects: A case-control study in Liaoning Province, China. Int J Hyg Environ Health. (2020) 225:113453. doi: 10.1016/j.ijheh.2020.113453
18. Schembari A, Nieuwenhuijsen MJ, Salvador J, de Nazelle A, Cirach M, Dadvand P, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. (2014) 122:317–23. doi: 10.1289/ehp.1306802
19. Dolk H, Armstrong B, Lachowycz K, Vrijheid M, Rankin J, Abramsky L, et al. Ambient air pollution and risk of congenital anomalies in England, 1991-1999. Occup Environ Med. (2010) 67:223–7. doi: 10.1136/oem.2009.045997
20. Xiong L, Xu Z, Wang H, Liu Z, Xie D, Wang A, et al. The association between ambient air pollution and birth defects in four cities in Hunan province, China, from 2014 to 2016. Medicine. (2019) 98:e14253. doi: 10.1097/MD.0000000000014253
21. Gong TT, Wu QJ, Chen YL, Jiang CZ, Li J, Li LL, et al. Evaluating the time trends in prevalence of omphalocele in 14 cities of Liaoning province, 2006 to 2015. Sci Rep. (2016) 6:32901. doi: 10.1038/srep32901
22. Chen YL, Li CH, Huang YH. Incidence of birth defects in Liaoning Province, 2006-2015. Chin J Public Health. (2018) 34:1662–4. doi: 10.11847/gggws1119905
23. Huang YH, Wu QJ, Chen YL, Jiang CZ, Gong TT, Li J, et al. Trends in the prevalence of congenital hydrocephalus in 14 cities in Liaoning province, China from 2006 to 2015 in a population-based birth defect registry from the Liaoning Women and Children's Health Hospital. Oncotarget. (2018) 9:14472–80. doi: 10.18632/oncotarget.24239
24. Xu L, Li X, Dai L, Yuan X, Liang J, Zhou G, et al. Assessing the trend of gastroschisis prevalence in China from 1996 to 2007 using two analytical methods. Birth Defects Res A Clin Mol Teratol. (2011) 91:177–84. doi: 10.1002/bdra.20753
25. China's Ambient Air Quality Standard. China's Ambient Air Quality Standard GB3095–2012 China (2012).
26. Ji X, Meng X, Liu C, Chen R, Ge Y, Kan L, et al. Nitrogen dioxide air pollution and preterm birth in Shanghai, China. Environ Res. (2019) 169:79–85. doi: 10.1016/j.envres.2018.11.007
27. Tu J, Tu W, Tedders SH. Spatial variations in the associations of term birth weight with ambient air pollution in Georgia, USA. Environ Int. (2016) 92–93:146–56. doi: 10.1016/j.envint.2016.04.005
28. Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. (2006) 114:1636–42. doi: 10.1289/ehp.9081
29. Stein RA. Epigenetics and environmental exposures. J Epidemiol Commun Health. (2012) 66:8–13. doi: 10.1136/jech.2010.130690
30. Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat Res. (2011) 714:105–12. doi: 10.1016/j.mrfmmm.2011.05.004
31. Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health Glob Access Sci Source. (2017) 16:82. doi: 10.1186/s12940-017-0291-8
32. Ha S, Hu H, Roussos-Ross D, Haidong K, Roth J, Xu X. The effects of air pollution on adverse birth outcomes. Environ Res. (2014) 134:198–204. doi: 10.1016/j.envres.2014.08.002
33. Vinikoor-Imler LC, Stewart TG, Luben TJ, Davis JA, Langlois PH. An exploratory analysis of the relationship between ambient ozone and particulate matter concentrations during early pregnancy and selected birth defects in Texas. Environ Pollut. (2015) 202:1–6. doi: 10.1016/j.envpol.2015.03.001
34. Vinikoor-Imler LC, Davis JA, Meyer RE, Luben TJ. Early prenatal exposure to air pollution and its associations with birth defects in a state-wide birth cohort from North Carolina. Birth Defects Res A Clin Mol Teratol. (2013) 97:696–701. doi: 10.1002/bdra.23159
35. Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. (2012) 22:429–38. doi: 10.1038/jes.2012.42
36. Huang CC, Wen HJ, Chen PC, Chiang TL, Lin SJ, Guo YL. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. (2015) 173:981–8. doi: 10.1111/bjd.14039
37. Jin L, Qiu J, Zhang Y, Qiu W, He X, Wang Y, et al. Ambient air pollution and congenital heart defects in Lanzhou, China. Environ Res Lett. (2015) 10:074005. doi: 10.1088/1748-9326/10/7/074005
38. Zhang TN, Gong TT, Chen YL, Wu QJ, Zhang Y, Jiang CZ, et al. Time trends in the prevalence and epidemiological characteristics of neural tube defects in Liaoning Province, China, 2006-2015: a population-based study. Oncotarget. (2017) 8:17092–104. doi: 10.18632/oncotarget.15060
Keywords: air pollution, case-control study, omphalocele, risk, sulfur dioxide
Citation: Li L-L, Huang Y-H, Li J, Liu S, Chen Y-L, Jiang C-Z, Chen Z-J and Zhuang Y-Y (2022) Maternal Exposure to Sulfur Dioxide and Risk of Omphalocele in Liaoning Province, China: A Population-Based Case-Control Study. Front. Public Health 10:821905. doi: 10.3389/fpubh.2022.821905
Received: 27 November 2021; Accepted: 31 March 2022;
Published: 12 May 2022.
Edited by:
Fabrizio Bianchi, Italian National Research Council, ItalyCopyright © 2022 Li, Huang, Li, Liu, Chen, Jiang, Chen and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Yan Zhuang, MTk4NjAxMDl6eXlAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.