
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 11 April 2022
Sec. Environmental Health and Exposome
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.814927
Triclosan (2,4,4′-trichloro-2′-hydroxy-diphenyl ether, TCS) is widely used in personal care and household products. It is ubiquitous across the ecosystem nowadays. Several in vitro and in vivo studies have suggested the possible adverse effects of TCS on male reproductive health. However, little research has been done on human beings, especially in eastern countries. To assess the effects of TCS exposure on male fecundity, we recruited couples who planned to conceive and went to the preconception care clinics for physical examination in Shanghai, China. TCS was quantified in male urine samples collected at enrollment. For this study, 443 couples were included in the cohort, and 74.7% of couples (n = 331) were prospectively followed 12 months later. The outcomes of interest included the pregnancy status of their wives and time to pregnancy. Elevated male urinary TCS concentrations were found to be associated with diminished fecundability (fecundability odds ratio (FOR) 0.77; 95% CI, 0.62–0.97). The risk of infertility significantly increased (OR = 1.6; 95% CI, 1–2.6) as TCS levels elevated. Besides, we divided TCS concentration into tertiles a priori, and there tended to be a dose-response pattern in both analyses. Our findings suggest that environmental exposure to TCS may have an adverse impact on male fecundity.
Triclosan (2,4,4′-trichloro-2′-hydroxy-diphenyl ether, TCS) is a broad spectrum biocide added routinely in a wide array of antiseptic wash products, laundry detergents, cosmetics, and household products (1). Many antimicrobial consumer products are washed down the drain after being used. Despite sewage treatment, a small portion of TCS is still discharged into the environment (2). With the development of the economy, the usage of antiseptic products increases worldwide, and more and more TCS is released into the environment. It is reported that TCS has reached surface water, groundwater, soil, and even drinking water. Moreover, it can accumulate in sediment and aquatic organisms (3).
The uptake of TCS in humans occurs via two main routes, namely dermal contact and oral route (ingestion of contaminated drinking water and food) (4, 5). Besides the direct use of TCS products, long-term environmental exposure to TCS also leads to TCS uptake. The majority of TCS is eliminated in the urine in the form of glucuronide conjugate (1).
As it is obviously ubiquitous across the ecosystem, the effects of TCS on human health have attracted attention. Nowadays, TCS has also been detected in diverse human body fluids and tissues (e.g., urine, plasma, milk, and fingernail) (6–9). Research in animals shows that TCS is suspected to be an endocrine-disrupting chemical (EDC) and may adversely affect male sperm quality. In vitro study by Kumar et al. (10) demonstrated that TCS disrupted intermediate steroidogenic cascade and depressed testosterone synthesis. Kumar et al. (11) found that TCS decreased the production of androgens and reduced semen production in treated male rats. Lan et al. (12) found TCS induced sperm toxicity and epididymal damage in rats due to the epididymal accumulation tendency of TCS. The study of Bruno GM et al. even indicated maternal (placenta and lactation) exposure to TCS compromised sperm parameters in rats of the F1 generation (13).
Several studies of TCS exposure in men take semen quality as the primary outcome. Many reported a significant statistical association between TCS exposure and semen parameters. Nassan et al. (14) reported lower percent morphologically normal sperm in men with urinary triclosan in the second or third quartile than those undetectable concentrations. The findings of Zhu et al. (15) suggested the adverse effect of TCS on semen quality at the environment-relevant dose. Jurewicz et al. (16) found that triclosan exposure is associated with poorer semen quality. However, the absolute value difference among semen parameters of different TCS exposure groups was moderate, and neither dose-response effect was pronounced (14, 15). Therefore, we wonder whether TCS exposure in men adversely affects male fecundity (his biological capacity to reproduce). The evidence of TCS's potential effects on male fecundity is still limited to date, especially in Asian countries. The current study aimed to fill this void by evaluating the association of urinary concentrations of TCS in men and male fecundity.
This study recruited couples who went to male preconception care clinics in a university affiliated teaching hospital for pre-pregnancy eugenic check from November 2013 to March 2014. Enrolled couples were married (men ≥ 22 years old and women ≥ 20 years old) and planned to conceive recently. Couples who had continuously tried to conceive over 12 months but failed were excluded. All couples signed written informed consent. A trained interviewer obtained information on couples' demographic characteristics, living and working environment, sexual and reproductive status, health-related behaviors, and medical history that may affect reproductive health through a face-to-face interview. The male participants were asked to provide urine and semen samples. The study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China (XHEC-C-2013-001).
The couples were then prospectively followed via a telephone interview after 12 months. Information collected included contraceptive use, pregnancy status, hospital visit (if any), and time to pregnancy (TTP). TTP is an epidemiologic metric widely used for studies of human fecundity (17). TTP was defined as the time length required to achieve spontaneous pregnancy. It was a combination of self-reported months of attempts without contraception usage. If the couples did not conceive with TTP over 12 months, they were labeled as infertile. Fecundability odds ratios (FORs) were calculated to estimate the odds of becoming pregnant each month. A FOR <1 denotes a reduction in fecundity or longer TTP, and a FOR > 1 denotes a shorter TTP.
A single spot urine sample (100 ml) was collected in a sterile polypropylene cup from each male participant, dispensed into polypropylene tubes (15 ml, 430791 Corning CentriStar), and stored at −80°C until measurement. Total urinary TCS concentration (free and conjugated) was measured using the method reported previously (18). After thawed at 4°C, 10 μl internal standard TCS-D3 (2μg/mL, Dr. Ehrenstorfer GmbH, Augsburg, Germany) was added into a 4 ml urine sample. Firstly, the urine samples were incubated with 20,000 IU/ml β-glucuronidase (Type H-1 from Helix pomatia, Sigma-Aldrich, St. Louis, MO, USA) to hydrolyze the conjugated TCS, then concentrated on a solid-phase extraction column (500 mg/3 ml; Supelco, Bellefonte, PA, USA). Thirdly, the concentrated TCS was vaporized by speed vacuum concentrator and dissolved in 200 μL 90% methanol. The final extract was analyzed by liquid chromatography-electrospray ionization tandem mass spectrometry (Agilent 1290-6490, Agilent Technologies, USA). The limit of detection (LOD) was 0.1 μg/L. Linearity was valid over the range of 0.1–50 ng/ml (r2 = 0.998). All the intra- and inter-batch precisions were <15%. The recovery was 91.1%. We also measured each urine sample's creatinine level using an enzymatic method on an automatic chemical analyzer (7100 Automatic Analyzer, Hitachi, Japan) to correct the fluctuations of TCS concentrations caused by urine concentration or dilution. Analysts were blinded to all information during the tests.
All data collected were doubly entered into the EpiData database. Urine TCS values less than the LOD were imputed as LOD/sqrt2 (19, 20). Taking the fluctuations of TCS concentration caused by urine concentration or dilution into consideration, TCS concentration was corrected by dividing the creatinine level of each urine sample. Given that the distribution of TCS concentration was skewed, the corrected TCS concentration was included in the models after natural logarithm transformation.
The student's t-test was used for continuous variables with normal distribution and the Wilcoxon rank-sum test for non-normally distributed continuous variables. The chi-square test was used to examine differences in categorical variables. The association between the urinary TCS level and infertility was examined by Logistic regression, while the association between TCS level and fecundability was examined by the Cox model modified for discrete-time data.
Factors that may impact the relationship between TCS exposure and the interest outcomes were considered as confounders (21). Age, smoking status, education, and household income were factors for fecundity and were found to be associated with TCS exposure (22). These confounders were adjusted for in the analysis. Body mass index (BMI) and excessive consumption of alcohol were also risk factors for fecundity (23, 24). These variables were adjusted to improve precision. We also considered male and female reproductive history, given its uncertain relation with fecundity. Although semen quality is an important factor of male fecundity, it was not included in the model to avoid over adjustment (25, 26). Data analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Figure 1 illustrates the inclusion and exclusion criteria of the study population. Among the 526 couples recruited, those with male or female factors that may influence fecundity were excluded. Those with female partners aged 40 years older or male partners aged 45 years older were also excluded, as well as those with male partners who did not provide urine samples or infertile couples (couples who had tried to conceive over 12 months continuously but failed). Male factors included varicocele and cryptorchidism. Female factors included endometriosis, leiomyoma, intrauterine adhesion, uterine malformation, ovarian insufficiency, fallopian tube abnormality, and hyperprolactinemia. Among 443 qualified couples, 96.7% of male urine samples had TCS concentrations above LOD. The median (25th and 75th) of TCS exposure levels in our study was 1.12 (0.5, 3.48) ng/ml. The geometric mean (GM) (25th and 75th) of TCS exposure levels was 1.4 (1.31, 1.62) ng/ml. In total, 331 couples were prospectively followed after 12 months (follow-up rate = 74.7%). Two other couples were also excluded for data analysis, whereas one conceived with the help of medical assistance and the other delayed their pregnancy plan until 2 years later. Among the remaining 329 couples, 208 (63.2%) wives became pregnant within 12 months, 50 (15.2%) wives did not conceive spontaneously despite continuous attempts for 12 months, and 71 (21.6%) wives were still not pregnant at the end of the follow-up since they tried to conceive <12 months.
The baseline characteristics were comparable among pregnant, infertile, and not pregnant couples, except for male reproductive history and corrected TCS concentration (Table 1). Compared with the pregnant group, the men in the infertile group seemed less likely to make their partners pregnant before (66 vs. 42.3%), and corrected TCS concentrations were significantly higher in the infertile group (median 1.29 vs.0.89 ng/mg creatinine). The baseline characteristics of the participants who completed following-up or missing subjects are shown in Supplementary Table 1. Among the missing couples, male partners are relatively older and are less likely to answer family income, while the female partners have fewer college education opportunities and are less likely to get pregnant before.
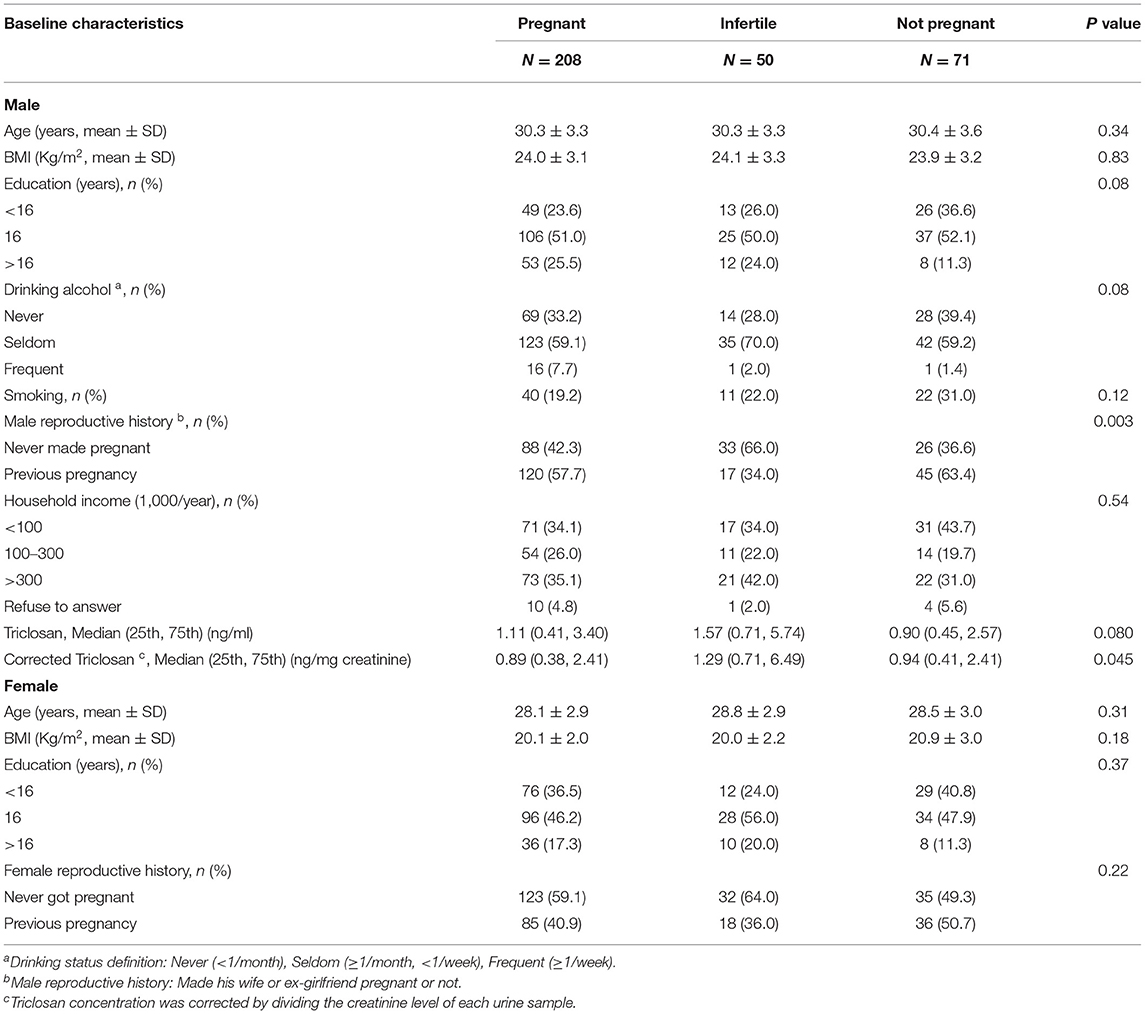
Table 1. The baseline characteristics of couples who are pregnant, infertile, and not pregnant in this study.
Table 2 presents the results of the Cox model modified for discrete-time data for the association between TCS concentrations and male fecundability. The remaining 329 couples were all included in the Cox model. Since couples' ages were correlated (r = 0.66), only male ages were adjusted in the following analyses. Higher TCS concentrations were positively associated with reduction in fecundability (adjusted OR = 0.77; 95% CI [0.62, 0.97]). Moreover, a significant (P = 0.02) trend was observed when we divided the TCS concentrations into tertiles.
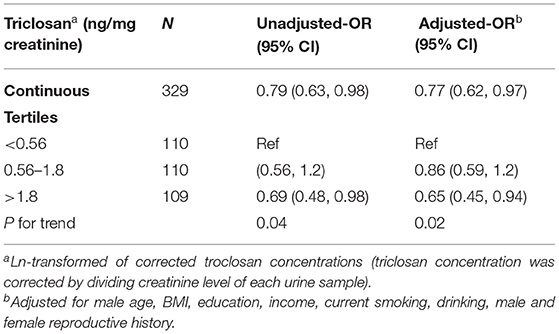
Table 2. Fecundability odds ratios for triclosan exposure using the Cox model modified for discrete time data.
Multivariable logistic regression models were used to study the effect of pre-pregnancy urinary concentrations of TCS on male infertility. Among the remaining 329 couples, 71 tried to conceive for <12 months and the wives were still not pregnant at the end of the follow-up. Therefore, they were excluded from this analysis. As shown in Table 3, increased TCS concentrations were associated with a higher rate of infertility (adjusted OR = 1.6; 95% CI [1, 2.6]). Furthermore, we divided the TCS concentrations into tertiles. Compared with the lowest tertile (<0.57 ng/mg creatinine), the middle TCS levels (0.57–1.8 ng/mg creatinine) were nearly significantly associated with increased infertility, while the highest TCS levels (> 1.8 ng/mg creatinine) were significantly associated with increased infertility. Moreover, there seemed to be a dose-response pattern (P = 0.06).
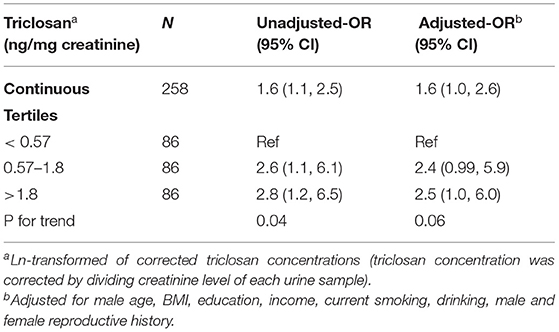
Table 3. The association between triclosan and infertility using a multivariable logistic regression model.
We found a positive association between TCS concentration and infertility as well as a negative association between TCS concentration and fecundability in this study. The findings suggest that environmental exposure to TCS may have an adverse impact on male fecundity.
Male fecundity refers to the male component in the biological ability for reproduction, which could be evaluated by the time it takes for the female partner to conceive (27). To our best knowledge, this is the first report showing a negative association between male TCS exposure and TTP.
Recently, TCS has been suspected to be a potential reproductive toxicant. The reproductive endocrine-disrupting effects of TCS were demonstrated in vivo and in vitro studies through three perspectives. Firstly, TCS was revealed to bind to estrogen and androgen receptors exhibiting estrogen, and anti-androgenic activities (28–31). Secondly, with a chemical structure similar to thyroid hormones, TCS has been shown to disrupt thyroid hormone homeostasis. Finally, TCS may also suppress testicular steroidogenesis and adversely affect male reproduction (32).
The biological mechanism of the impact of TCS on male fecundity is unclear yet. Nevertheless, in vivo studies have demonstrated that TCS has anti-androgenic properties and could adversely affect male reproduction and fertility. Ha et al. (32) gave male Sprague-Dawley Rats TCS daily by oral gavage for 31 days and found the inhibition of testicular steroidogenesis through the miR-6321/Map3k1-regulated JNK/c-Jun/Nur77 cascade. Ena et al. (33) found that daily sperm production was significantly diminished with marked inhibition of androgen receptor protein expression with subchronic exposure to high doses of TCS (750 mg/kg) in prepubertal male rats. Subchronic treatment with TCS in weanling male rats was also showed significantly decreased testosterone, luteinizing hormone, follicle-stimulating hormone levels, and a state of testicular oxidative stress, which play a role in testicular DNA damage and endocrine disruption (34). Furthermore, some other cell-based assays revealed that TCS bound to estrogen and androgen receptors exhibit estrogen, and anti-androgenic activities (29, 35). With a similar chemical structure of TCS to thyroid hormones, TCS has been shown to disrupt thyroid hormone homeostasis (36), which is essential for maintaining male reproduction (37).
These findings suggest that TCS may disrupt hormone homeostasis, reduce semen quality, and then affect fecundity in humans. However, population studies on this topic are still in the preliminary stage due to a lack of research. A case-control study conducted by Chen et al. (38) in China found no relationship between TCS exposure and idiopathic male infertility. In another cohort study, Smarr et al. (39) prospectively assessed couples' urinary concentrations of TCS in the context of fecundity, measured as TTP. Nevertheless, no associations were observed when TCS concentrations were modeled continuously. In our previous study, TCS exposure was related to a decrease in sperm concentration, sperm count, the number of forward-moving sperms, and the number of normally morphologic sperms (15). The results are in agreement with the study performed by Jurewicz et al. (16). These findings suggest TCS may be a risk factor of impaired male fecundity to some extent.
Triclosan exposure levels (GM = 1.4 ng/ml) in our study seem to be similar to Asian levels (GM = 1.29 ng/ml) in Japan; GM = 0.99 ng/ml in Korea (40), but lower than American levels (GM = 12.3 μg/L) in Canada (22); GM = 13μg/L in US (7) and European levels (GM = 2–3 μg/L) in Belgium (41); GM = 6.1 μg/L in Spain (42). This may be due to different lifestyles and products used between the western and eastern countries.
Several limitations of our study need to be kept in mind. First, due to the variability of TCS levels in urine, a spot urine sample may not be representative of the usual environmental exposure level of an individual. However, it was reported that TCS concentration in a single urine sample could represent the six-month average exposure to TCS in children 6–10 years old (43). This finding cannot be generalized to adults directly, but adults usually have much more stable environmental exposure with a comparatively stable habitual lifestyle and living environment. Bodin et al. (44) reported that most men did not adjust their lifestyles in the preparation period for pregnancy. Besides, Lassen et al. (45) found modest consistency in repeated measurements of TCS in spot urine samples (intra-class correlation coefficients (ICC):0.55–0.9). Second, we collected self-reported data on TTP, which is subject to recall bias. Nevertheless, the degree of error might be more minor in China. Under the long-term influence of the one-child family policy, couples usually pay much more attention to the process of trying to conceive. Furthermore, since none of the men were aware of their TCS levels, differential misclassification of infertility by TCS levels is unlikely in our study. Third, we did not take TCS exposure in women into consideration in this study. As a successful pregnancy is a couple-dependent outcome, it is possible that TCS affects female fecundity and results in a long TTP and even infertility (18). Couples' TCS exposure was found significantly correlated but modest (r = 0.31) (39), which might not be able to explain the total association between reduced male fecundity and TCS exposure. Last but not least, information on the frequency of intercourse was not collected in this study. Infrequent intercourse or intercourse outside of the fertile window can also result in prolonged TTP or infertility. However, these couples who desired to achieve natural conception were given pre-pregnancy eugenic check and preconception education at the reproductive clinic. Thus, we do not think the frequency of intercourse should be a vital issue for the vast majority of couples recruited.
Our prospective study suggests that environmental exposure to TCS adversely impacts male fecundity. But in light of the limitations of this work, our results await corroboration by more large-scale prospective cohort studies of repeated preconception urinary measures of couple's TCS exposure in the context of couple fecundity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China (XHEC-C-2013-001). The patients/participants provided their written informed consent to participate in this study.
WZ designed the study, analyzed and interpreted the clinical data, and drafted the manuscript. CX collected clinical data and managed the database. SZ measured the level of TCS creatinine concentration in urine. DZ and HZ supervised the project and critically revised the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (82003470 and 81401253), the China Postdoctoral Science Foundation (2012M520910), Projects of Shanghai Municipal Health Commission (201840140), and Projects of Shanghai Stomatological Hospital (SSDCE-2016-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to express their thanks to all the participants of this prospective study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.814927/full#supplementary-material
1. Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. (2010) 40:422–84. doi: 10.3109/10408441003667514
2. Chalew TE, Halden RU. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Triclocarban. J Am Water Works Assoc. (2009) 45:4–13. doi: 10.1111/j.1752-1688.2008.00284.x
3. Milanovic M, Duric L, Milosevic N, Milic N. Comprehensive insight into triclosan-from widespread occurrence to health outcomes. Environ Sci Pollut Res Int. (2021). doi: 10.1007/s11356-021-17273-0. [Epub ahead of print].
4. Moss T, Howes D, Williams FM. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4′-trichloro-2′-hydroxydiphenyl ether). Food Chem Toxicol. (2000) 38:361–70. doi: 10.1016/S0278-6915(99)00164-7
5. Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. (2006) 69:1861–73. doi: 10.1080/15287390600631706
6. Allmyr M, Adolfsson-Erici M, Mclachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. (2006) 372:87–93. doi: 10.1016/j.scitotenv.2006.08.007
7. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environ Health Perspect. (2008) 116:303–7. doi: 10.1289/ehp.10768
8. Geens T, Neels H, Covaci A. Sensitive and selective method for the determination of bisphenol-A and triclosan in serum and urine as pentafluorobenzoate-derivatives using GC-ECNI/MS. J Chromatogr B Analyt Technol Biomed Life Sci. (2009) 877:4042–6. doi: 10.1016/j.jchromb.2009.10.017
9. Yin J, Wei L, Shi Y, Zhang J, Wu Q, Shao B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ Geochem Health. (2016) 38:1125–35. doi: 10.1007/s10653-015-9777-x
10. Kumar V, Balomajumder C, Roy P. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. (2008) 250:124–31. doi: 10.1016/j.tox.2008.06.012
11. Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol. (2009) 27:177–85. doi: 10.1016/j.reprotox.2008.12.002
12. Lan Z, Hyung Kim T, Shun Bi K, Hui Chen X, Sik Kim H. Triclosan exhibits a tendency to accumulate in the epididymis and shows sperm toxicity in male Sprague-Dawley rats. Environ Toxicol. (2015) 30:83–91. doi: 10.1002/tox.21897
13. Montagnini BG, Forcato S, Pernoncine KV, Monteiro MC, Pereira MRF, Costa NO, et al. Developmental and Reproductive Outcomes in Male Rats Exposed to Triclosan: Two-Generation Study. Front Endocrinol (Lausanne). (2021) 12:738980. doi: 10.3389/fendo.2021.738980
14. Nassan FL, Minguez-Alarcon L, Williams PL, Dadd R, Petrozza JC, Ford JB, et al. Urinary triclosan concentrations and semen quality among men from a fertility clinic. Environ Res. (2019) 177:108633. doi: 10.1016/j.envres.2019.108633
15. Zhu W, Zhang H, Tong C, Xie C, Fan G, Zhao S, et al. Environmental Exposure to Triclosan and Semen Quality. Int J Environ Res Public Health. (2016) 13:224. doi: 10.3390/ijerph13020224
16. Jurewicz J, Radwan M, Wielgomas B, Kaluzny P, Klimowska A, Radwan P, et al. Environmental levels of triclosan and male fertility. Environ Sci Pollut Res Int. (2018) 25:5484–90. doi: 10.1007/s11356-017-0866-5
17. Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. (1986) 124:470–80. doi: 10.1093/oxfordjournals.aje.a114417
18. Zhu W, Zhou W, Huo X, Zhao S, Gan Y, Wang B, et al. Triclosan and Female Reproductive Health: A Preconceptional Cohort Study. Epidemiology. (2019) 30 (Suppl 1):S24–31. doi: 10.1097/EDE.0000000000001011
19. Mendiola J, Jorgensen N, Andersson AM, Calafat AM, Ye X, Redmon JB, et al. Are environmental levels of bisphenol a associated with reproductive function in fertile men? Environ Health Perspect. (2010) 118:1286–91. doi: 10.1289/ehp.1002037
20. Li DK, Zhou Z, Miao M, He Y, Wang J, Ferber J, et al. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil Steril. (2011) 95:625–30 e621–624. doi: 10.1016/j.fertnstert.2010.09.026
21. Kleinbaum Dg KL, Muller K, Nizam A. Selecting the best regression equation. In: Applied Regression Analysis and Other Multivariate Methods. 3rd ed. Pacific Grove, CA: Brooks/Cole (1998).
22. Arbuckle TE, Marro L, Davis K, Fisher M, Ayotte P, Belanger P, et al. Exposure to free and conjugated forms of bisphenol A and triclosan among pregnant women in the MIREC cohort. Environ Health Perspect. (2015) 123:277–84. doi: 10.1289/ehp.1408187
23. Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril. (2017) 107:848–59. doi: 10.1016/j.fertnstert.2017.02.115
24. Katz DJ, Teloken P, Shoshany O. Male infertility - The other side of the equation. Aust Fam Physician. (2017) 46:641–6.
25. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. (2009) 20:488–95. doi: 10.1097/EDE.0b013e3181a819a1
26. Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, et al. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril. (2014) 101:453–62. doi: 10.1016/j.fertnstert.2013.10.022
27. Olsen J, Ramlau-Hansen CH. Epidemiologic methods for investigating male fecundity. Asian J Androl. (2014) 16:17–22. doi: 10.4103/1008-682X.122198
28. Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, et al. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. (2004) 67:167–79. doi: 10.1016/j.aquatox.2003.12.005
29. Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. (2008) 28:78–91. doi: 10.1002/jat.1316
30. Raut SA, Angus RA. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem. (2010) 29:1287–91. doi: 10.1002/etc.150
31. Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci. (2010) 117:45–53. doi: 10.1093/toxsci/kfq180
32. Ha M, Zhang P, Li L, Liu C. Triclosan Suppresses Testicular Steroidogenesis via the miR-6321/JNK/ Nur77 Cascade. Cell Physiol Biochem. (2018) 50:2029–45. doi: 10.1159/000495049
33. Ena L, Lim JS, Son JY, Park YJ, Lee YH, Kim JY, et al. Evaluation of subchronic exposure to triclosan on hepatorenal and reproductive toxicities in prepubertal male rats. J Toxicol Environ Health A. (2018) 81:421–31. doi: 10.1080/15287394.2018.1451188
34. Riad MA, Abd-Rabo MM, Abd El Aziz SA, El Behairy AM, Badawy MM. Reproductive toxic impact of subchronic treatment with combined butylparaben and triclosan in weanling male rats. J Biochem Mol Toxicol. (2018) 32:e22037. doi: 10.1002/jbt.22037
35. Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. (2008) 116:1203–10. doi: 10.1289/ehp.11200
36. Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, et al. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. (2009) 107:56–64. doi: 10.1093/toxsci/kfn225
37. Patel N, Kashanian JA. Thyroid Dysfunction and Male Reproductive Physiology. Semin Reprod Med. (2016) 34:356–60. doi: 10.1055/s-0036-1593491
38. Chen M, Tang R, Fu G, Xu B, Zhu P, Qiao S, et al. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater. (2013) 250–1:115–21. doi: 10.1016/j.jhazmat.2013.01.061
39. Smarr MM, Sundaram R, Honda M, Kannan K, Louis GM. Urinary Concentrations of Parabens and Other Antimicrobial Chemicals and Their Association with Couples' Fecundity. Environ Health Perspect. (2017) 125:730–6. doi: 10.1289/EHP189
40. Iyer AP, Xue J, Honda M, Robinson M, Kumosani TA, Abulnaja K, et al. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ Res. (2018) 160:91–6. doi: 10.1016/j.envres.2017.09.021
41. Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, et al. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environ Int. (2015) 84:154–60. doi: 10.1016/j.envint.2015.07.017
42. Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. (2011) 37:858–66. doi: 10.1016/j.envint.2011.02.012
43. Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. (2008) 106:257–69. doi: 10.1016/j.envres.2007.09.010
44. Bodin M, Kall L, Tyden T, Stern J, Drevin J, Larsson M. Exploring men's pregnancy-planning behaviour and fertility knowledge:a survey among fathers in Sweden. Ups J Med Sci. (2017) 122:127–35. doi: 10.1080/03009734.2017.1316531
Keywords: endocrine disrupting chemical (EDC), triclosan, male fecundity, infertility, prospective study
Citation: Zhu W, Xie C, Zhao S, Zhang D and Zhang H (2022) Environmental Exposure to Triclosan and Male Fecundity: A Prospective Study in China. Front. Public Health 10:814927. doi: 10.3389/fpubh.2022.814927
Received: 17 November 2021; Accepted: 09 March 2022;
Published: 11 April 2022.
Edited by:
Sonia Dagnino, Imperial College London, United KingdomReviewed by:
Han Li, Guangxi Medical University, ChinaCopyright © 2022 Zhu, Xie, Zhao, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Zhang, emhhbmdkYW5Aemp1LmVkdS5jbg==; Hao Zhang, aGFvemhhbmdfOTlAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.