
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 23 March 2022
Sec. Public Health Education and Promotion
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.797794
This article is part of the Research Topic Trends and Challenges of Medical Education in the Changing Academic and Public Health Environment of the 21st Century View all 37 articles
Global cancer statistics suggest that breast cancer (BC) is the most diagnosed cancer in women, with an estimated 2. 3 million new cases reported in 2020. Observational evidence shows a clear link between prevention and development of invasive BC and lifestyle-based interventions such as a healthy diet and physical activity. The recent findings reveal that even minimal amounts of daily exercise and a healthy diet reduced the risk of BC, mitigated the side effects of cancer treatment, and stopped the recurrence of cancer in the survivors. Despite the myriad benefits, the implementation of these lifestyle interventions in at-risk and survivor populations has been limited to date. Given the need to disseminate information about the role of physical activity and nutrition in BC reduction, the review aimed to present the recent scientific outreach and update on associations between the lifestyle interventions and BC outcomes to narrow the gap and strengthen the understanding more clearly. This review covers more direct, detailed, and updated scientific literature to respond to frequently asked questions related to the daily lifestyle-based interventions and their impact on BC risk and survivors. This review also highlights the importance of the oncology provider's job and how oncology education can reduce the BC burden.
Breast cancer (BC) is a growing global public health concern. Although many high-income countries have reported decreases in BC mortality due to the advances in treatment, diagnosis, and implantation mammographic screening (1), BC mortality is steadily increasing in most developing countries due to low health knowledge and limited medical facilities (2–4). According to the recent reports, ~2.3 million new cases of BC are diagnosed each year, with a mortality rate of roughly 4,50,000 per year (5, 6). The leading risk factors for BC include age, genetic mutations (BRCA1 and BRCA2) (7), lifestyle-based (non-genetic) risk factors (8), early menarche, nulliparity, first pregnancy after the age of 30 years, older age at menopause, dense breast tissue (9), hormone replacement therapy (10), use of oral contraceptives (11), personal and family history of BC, and other clinical complaints (12, 13).
In cancer education, it is challenging but fundamentally important to accurately evaluate the role of modifiable risk factors, such as diet, physical activity, and other non-genetic factors, to estimate BC risk for individual women—the first essential step toward precision prevention.
Evaluating the complex interaction between diet and physical activity and better BC outcomes resulted in a significant reduction in BC incidence and enhanced survival rates (14, 15). Substantial evidence underlines the importance of a balanced diet and increased physical activity to prevent BC and reduce the chances of recurrence and mortality in both pre- and post-menopausal women (16, 17). Currently, exercise and nutrition are considered as two integrative therapy components that play a crucial role in relieving the side effects of active cancer treatment and especially cancer-related fatigue (18). The World Cancer Research Fund recommends specific guidelines of moderate aerobic exercise for muscle strengthening and nutrition, that is, eating more plant-based foods, limiting red and processed meat, limiting energy-dense foods, salt, sugary drinks, and alcohol, and not relying on dietary supplements (19). The published literature has elaborated on the role of physical activity in improving fitness, reducing psychological distress, and enhancing cognitive abilities (20–24), whereas nutritional consultations may help manage challenges associated with anemia, diarrhea, nausea, and vomiting which contribute to cancer-related fatigue (25–27).
Strategies are needed for disseminating literature on the association between BC and physical activity and nutrition. Thus, this review summarizes and reviews the evidence linking lifestyle factors—especially diet and exercise—to BC risks and outcomes. The purpose of this narrative review is to present recent scientific outreach and update on associations between the lifestyle interventions and BC outcomes to narrow the gap and strengthen the understandings more clearly. In comparison with the published studies on the subject, the current review covers more direct, detailed, and updated scientific literature to respond to frequently asked questions related to the daily life style-based interventions and their impact on BC risk and survivors. This review also highlights the importance of the oncology provider job and how oncology education can reduce the BC burden.
We performed a literature search using the different search databases, PubMed, Google, Google Scholar, and Research Gate with the key words of BC,physical activity, exercise, diet, food menu, and other related terms. In addition to the relevancy of the title and abstracts, for strong conceptual and logical understanding, more recent studies were selected for inclusion based on the year of publication between 2016 and 2021. Though minor older publications and some news agencies, government data reports were also cited to strengthen the subject's background. All selected articles have been cited accordingly.
Adhering to a healthy lifestyle in terms of high-quality food intake influences the risk of BC onset and post-diagnosis outcomes. Epidemiological and preclinical data suggest that some food and nutrients (e.g., saturated fats, red and processed meat) increase the circulating levels of endogenous estrogens, insulin-like growth factors, and pro-inflammatory cytokines, thus supporting BC development. In contrast, polyunsaturated fatty acids, vitamins C and E, fresh fruits, and vegetables have protective effects against BC onset or progression (28, 29). Several studies report an inverse relationship between BC and the Mediterranean diet. However, there is insufficient information to date to confirm the interplay of diet and BC incidence and mortality (30–33).
Fresh fruits and vegetables are known as natural antioxidants. Their routine consumption increases the consumer polyphenols and fiber level in the body, which provides resistance against tumorigenesis (34–36). Polyphenols have the potential to modulate the proliferation and metastatic activity of BC by regulating the cellular signaling pathways and inhibiting the enzymatic activity of tumor-supportive proteins such as the transcriptional factor NF-B (Figure 1) (37–39). Polyphenols can also antagonize the estrogen-signaling pathway by inhibiting aromatase and the production of estrogen or blocking the estrogen receptors (ERs) (40–42). A similar mechanism has been observed in fibers to prevent carcinogenesis by binding estrogens and reducing their serum levels or by improving insulin sensitivity and reducing weight gain (43, 44). Furthermore, the European Prospective Investigation into Cancer and Nutrition (Italian) study showed an inverse association between high consumption of fruits and leafy vegetables, which include raw tomatoes, and BC risk (45). However, available evidence suggests that further exploration is needed.
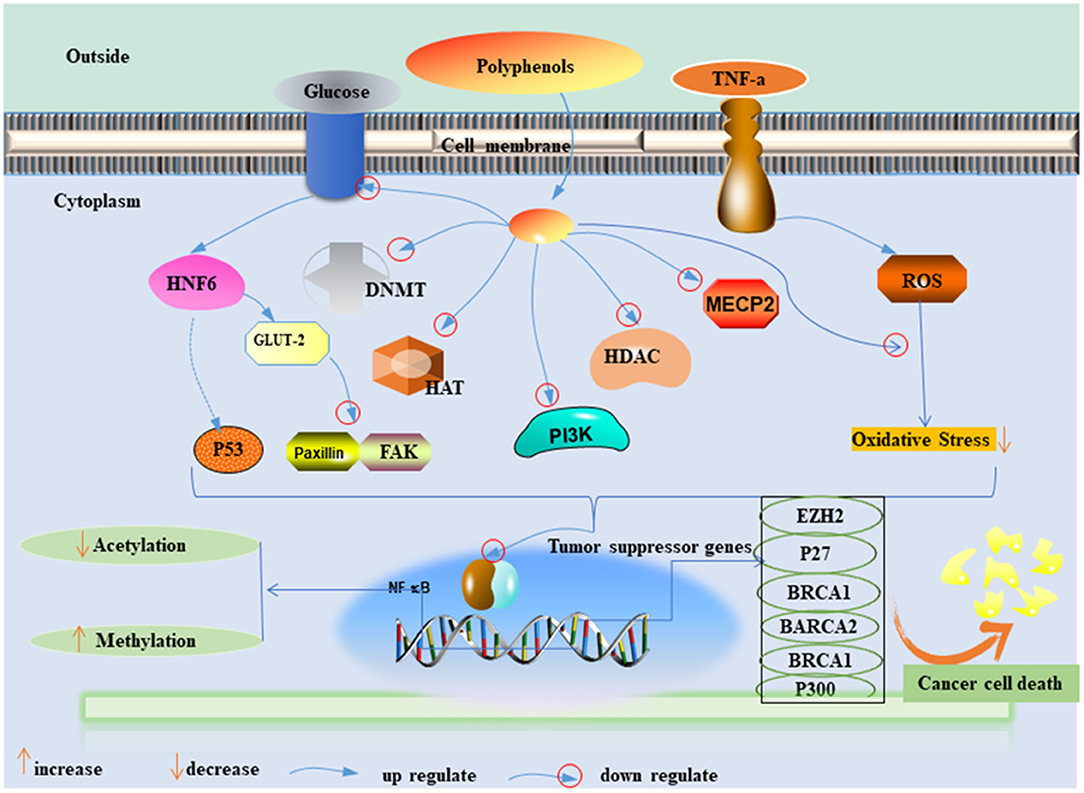
Figure 1. Possible mechanisms of polyphenol effect on the inhibition of BC cell proliferation. PI3K, phosphoinositide-3–kinase; TNF a, tumor necrosis alpha; ROS, radioactive oxide species; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; DNMT, DNA methyltransferase; HAT, histone acetyltransferases; HMT, histone methyltransferases; HDAC, histone deacetylases; GLUT-2, glucose transporter-2; MeCP, methyl CpG binding protein; EZH2, enhancer of zeste homolog 2; BRCA1, 2, breast cancer 1, 2; HNF- 6, hepatocyte nuclear factor 6; FAK and paxillin, two focal adhesion–associated proteins.
When red meat is cooked at high temperatures, carcinogen compounds such as heterocyclic amines, N-nitroso compounds, and polycyclic aromatic hydrocarbons are released, which potentially mediates the onset of BC (46–48). These compounds, especially heterocyclic amines, are involved in breast tissue-specific estrogen-based carcinogenic activity and support BC progression (49, 50). In other studies, researchers proposed that consuming processed red meat increases free radicals that are further involved in inducing lipid peroxidation (51, 52). Consumption of unprocessed red meat is thought to carry a 6% higher BC risk than not consuming such foods, whereas processed meat consumption is associated with a 9% higher BC risk (53, 54) due to higher levels of heme iron. The complete avoidance of meat is not usually advocated because it is a source of nutrients, such as proteins, iron, zinc, and vitamin B12. The evidence of its role in BC is inconclusive. However, suggestions to limit consumption of processed red meat are almost undoubtedly well-founded.
Menopausal status affects the influence of dietary fat consumption. Dietary fat poses a higher risk of BC in post-menopausal women, whereas for pre-menopausal women, dietary fats appear to have a protective effect (55). A recent study indicated that a high saturated-fat diet increases the risk of BC and, most conspicuously, of receptor-positive cancer, particularly ER+ (56). Cancer science suggests that dietary fats can influence the process of carcinogenesis by modulating intracellular signaling cascades (57). Although a high-fat diet, total cholesterol, and triglyceride levels have mostly been associated with increased risk, the evidence is limited.
Associations between BC and carbohydrates have been evaluated using the quality of the glycemic index (GI) and glycemic load (GL). The results are contradictory and inconclusive (58–61). A metadata study indicates that higher GI is associated with increased BC risk in post-menopausal women, but no effect was found in pre-menopausal women (62). Similarly, positive associations between GL and ER base and BC risk in post-menopausal women were explored in another study. The findings suggested that GL might increase the serum insulin that further mediates BC onset (63, 64). Whereas, the association between carbohydrate intake, GI, or GL and overall BC risk requires further research, glycemic control is advisable.
Red wine drinking is a prominent and modifiable lifestyle risk factor for BC. Some studies provide robust evidence that alcohol intake, regardless of the type of beverage (beer, wine, or spirits), and menopausal status are consistently associated with increased BC risk. A dose–response meta-analysis showed that 10 g of ethanol consumption in a single day contributed to a 5% increase in BC risk for pre-menopausal women and a 9% elevated risk for post-menopausal women (65). The findings reveal that ethanol's association with BC development is due to its ability to promote epithelial–mesenchymal transition, tumor growth, and metastasis formation (66, 67). Ethanol consumption causes an increase in estrogen concentration via different physiological mechanisms, for example, increased aromatase activity, prevention of estrogen breakdown, reduction of melatonin secretion, and increased hepatic oxidative stress that allows estrogen to exert its carcinogenic effect on breast tissue (68).
Soy is considered an optimal alternative to high-calorie protein sources such as meat. It contains soy isoflavones (known as phytoestrogen) and soy proteins (69). Research reveals that high levels of isolated soy proteins increase insulin-like growth factor 1, which may contribute to BC recurrence (70). The clinical findings indicate that soy protein isolate supplementation slightly stimulates epithelial BC in pre-menopausal women (69, 71). Prospective cohort studies that investigate the role of soy in BC risk reduction and its influence on BC recurrence and mortality rates yielded different results, often in support of soybean protein consumption (72–74). Thus, the relationship between soy consumption and BC remains unclear. Figure 2 provides a figurative description of dietary factors in BC incidence prevention and recurrence.
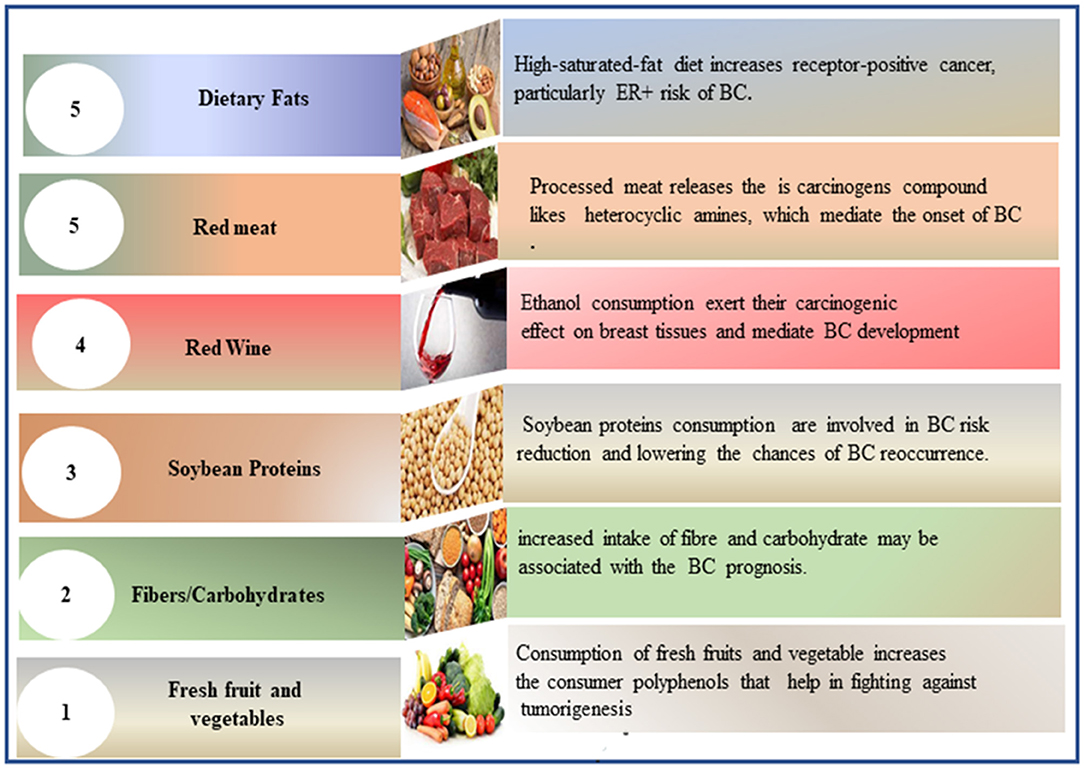
Figure 2. Figurative description of dietary factors involved in BC incidence prevention and recurrence. BC, breast cancer.
Physical activity has been considered a protective tool against BC since 1980, and the past two decades have witnessed many studies addressing the connection between physical activity and BC (75–77). Several studies have depicted the association between daily physical activity and better health outcomes which further reduce the risk of lifestyle-based risk factors for cancer (75, 78–81). The findings have revealed that physical exercise also lowers the chances of recurrence and is associated with a longer life expectancy in survivors (75, 82, 83). Although there is no direct link between physical activity and cancer risk reduction, however, the multiple interconnected physiological processes, which include sex hormones, insulin resistance and insulin levels, inflammation, oxidative stress, and adipokines, are believed to be involved in lowering the risk of tumorigenesis (84–86). Identifying the processes by which physical activity affects BC risk demonstrates biological plausibility for the observed link and provides evidence for the optimal exercise prescription for cancer risk reduction (15). Furthermore, understanding the exercise and BC link may provide fresh insights into cancer biology, which aids in developing additional preventive and treatment strategies for cancer (86). A more precise understanding of the nature of the association by type, dose, and timing of activity is needed to formulate public health recommendations regarding the association between physical activity and BC risk.
Researchers have well-explored the essential role of exercise in BC prevention and recurrence (87, 88). According to the World Cancer Research Fund/American Institute Cancer Research project findings, higher levels of physical exercise, with a dose–response connection, reduced the incidence of post-menopausal BC (89). In 2020, Physical Activity Guidelines for Americans from the American Cancer Society also advocated leads a physically active lifestyle to prevent and control BC (90). Physical activity influences cancer risk in several ways, which includes metabolic (calorie balance), hormonal, and immunological responses (91–93). Moore et al. analyzed data from the prospective US and European cohorts with self-reported physical activity measurements to evaluate the link between physical activity and the incidence of 26 different cancers. They concluded that physical activity levels are linked to a decreased risk of 13 malignancies, including BC (94). However, the current understanding of important levels of physical activity and their inverse relationships in patients with ER-negative BC needs further exploration. To address the challenges of women's lifestyle interventions and encourage them to be more physically active to lower their BC risk, many healthcare professionals employ technology-based interventions to raise daily physical activity levels (95–97).
Regardless of menopausal status, physical activity plays a significant role in reducing the onset of BC and supports survivors to overcome the disease. However, exercise has a more beneficial impact on pre-menopausal women (98, 99). Similar results were reported in a large-scale dose–response relationship study. Findings confirmed the association between physical activity and lowered risk of BC recurrence and increased survival rates. Furthermore, women with ER-positive tumors benefit the most from exercise (93, 100). However, the clinical oncologists can design the particular exercise program and prescription for women. There are three distinct study phases with different exercise prescriptions, which include treatment phase (three times per week for the length of chemotherapy, plus radiation, if received), post-treatment phase (two times per week for the following 10 week), and maintenance phase (one time per week for an additional 10 week) (101). Similarly, in a recent study, it has been found that a supervised therapeutic exercise program plus patient therapeutic education can significantly reduce the perceived fatigue and increase functional capacity in BC survivors suffering from cancer-related fatigue compared to an unsupervised physical exercise program based on individual preferences with patient therapeutic education (102). Taking all together, these findings suggest that physical exercise is a simple, cost-effective way of mitigating BC risk that could affect the outcome of millions of women with BC.
Based on the presence and absence of molecular markers on the plasma membrane of breast cells, BC is categorized into three major subtypes (i) estrogen and progesterone receptor-negative BC (HR-), (ii) human epidermal growth factor 2 (ERBB2) receptor BC, and (iii) both hormonal and growth factor negative (TNBC) (103). Totally, 90% of BC is not metastatic at diagnosis (104). All patients with non-metastatic BC are recommended to undergo local therapy that consists of surgical resection and consider postoperative radiation if lumpectomy is performed. However, patients are advised to undergo systemic therapy before surgery (105, 106). All metastatic BCs are treated according to their subtype with the ultimate goal of prolonging life and palliating the symptoms. Treatments of the 3 BC subtypes have distinct risk profile strategies. Optimal therapy for each patient depends on tumor subtype, anatomic cancer stage, and patient preferences (107). Figure 3 describes the different treatments according to the type of BC.
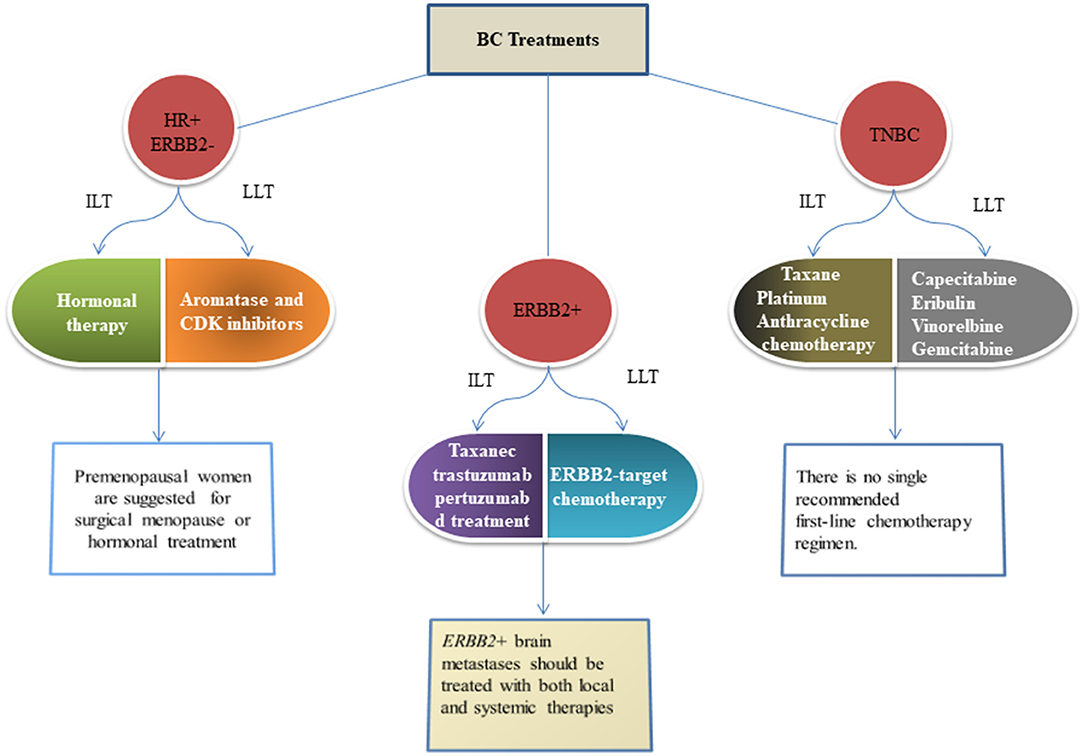
Figure 3. Figures give the recommended standard approaches to therapy of metastatic BC as per the guideline of the American Cancer Society. These recommendations are adopted in (108) and are given as per the guidelines of the National Comprehensives Cancer Network (109). HR, Hormonal receptor; ERBB2, Epidermal growth factor-2; TNBC, Triple negative breast cancer; ILT, Initial line of therapy; LLT, Later line of therapy.
Analysis of critical findings indicates that lifestyle factors, especially diet and physical activity, have the most robust effect on BC outcomes. A trial-based study showed that adapting routines to include 75 min of vigorous exercise per week and 2–3 weekly strength-training sessions can help to reduce the risk of BC recurrence and mortality (110, 111). To modify lifestyle-related health, these studies appeal to all healthcare professionals to promote and encourage exercise in BC survivors, as receiving advice from an oncologist to exercise will increase patients' physical activity levels (112). Considering the impact of primary care in BC risk reduction and prevention, oncologists should recommend and endorse specific exercise programs and non-pharmaceutical treatments.
Clear referral pathways need to be established, so that patients can be directly referred from a cancer care clinic to community-based exercise programs specialized for cancer survivors. Such community-based exercise programs can change individuals' daily routines and result in significant behavioral changes. These interventions can be supported using electronic gadgets or musical instruments for indoor activity, and self-motivation and enjoyment can also be increased through reminders or tracking activities on phones or email (Figure 4) (113). Women who participated in an aerobic exercise program while receiving adjuvant chemotherapy did not gain weight on average, whereas those who did not exercise gained 3.2 kg (114). In addition, exercise advantages include significant self-perceived improvements in attractiveness, cardiorespiratory fitness, lymphedema, and mental wellbeing (115).
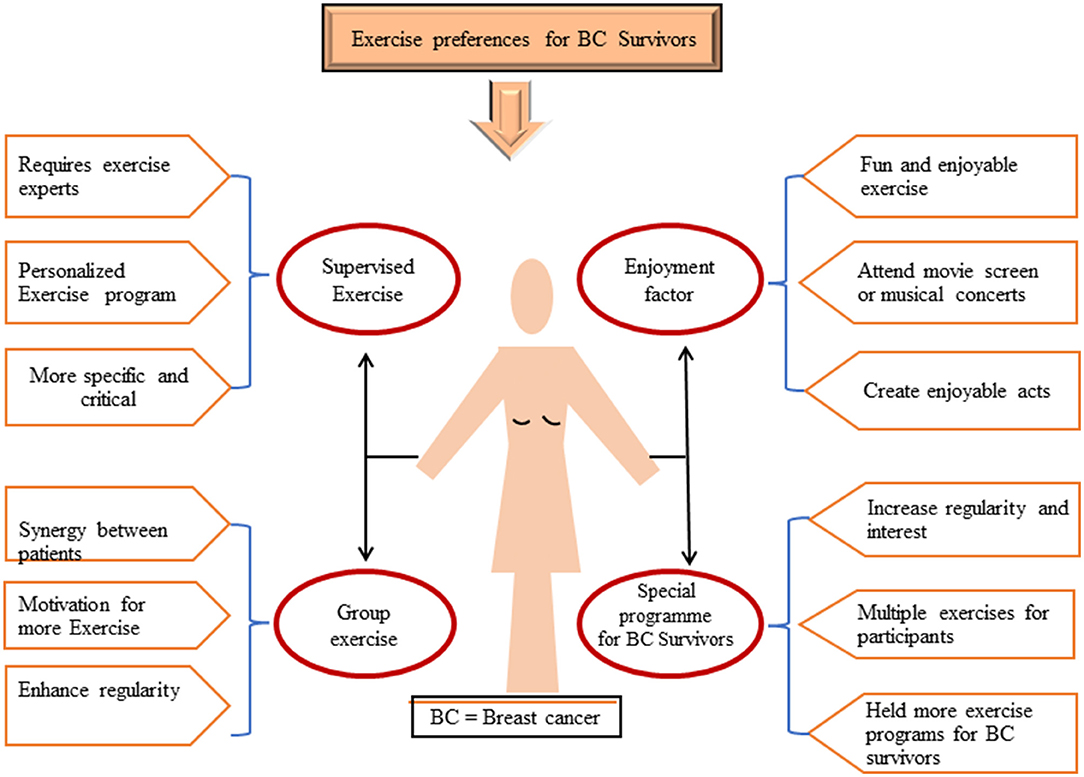
Figure 4. Available preferences of exercises for BC survivors in the form of (i) supervised exercise, (ii) group exercise, (iii) special program for BC survivors, (iv) enjoyment factors. BC, breast cancer.
While being overweight or obese at the time of diagnosis has a detrimental impact on BC prognosis, it is uncertain if losing weight improves the prognosis of overweight and obese women. To enhance overall survival—and perhaps BC-specific survival—patients should be strongly encouraged to quit smoking (116). Along with physical activity, there is a need to encourage an anticancer diet to help survivors reduce risks. Excessive consumption of western-style diets (high in processed grains, processed meats, and red meats) should be avoided as they are associated with increased rates of BC recurrence (117, 118). There is an association between BC and saturated fat, especially from high-fat dairy products, which may increase BC mortality (119–121). Soy products have not been found to increase BC recurrence and may even reduce it (122, 123).
Considering the clinical importance of developing fundamental guidelines for nutrition and physical activity toward health outcomes, especially BC onset, several international health organizations worldwide have developed the basic guidelines for both general and specific cancer prevention (Box 1) by adopting certain lifestyle interventions. The guidelines for risk reduction and survivorship by The National Comprehensive Cancer Network and The American Cancer Society are widely used by health institutes (124–127). However, incorporating these guidelines into survivorship care is challenging, and the transformation of scientific knowledge into practice in healthcare units is often problematic (128). There are several barriers to disseminate the oncological risk information and its essential association with nutrition and physical activity. These barriers include a lack of knowledge and interest from the general public, scarcity of public education seminars and conferences, uncertainty about when in the cancer continuum to introduce such information, a lack of support from hospital administration, and issues of time and patient flow (129, 130).

Box 1. For practices that do not have an in-house patient education department, according to the medical library association, there are several cancer patients' educational websites that provide in-house cancer guidelines for both patients and families.
Cancer education suggests that, at a minimum, oncology practice should provide evidence-based information on the association of BC and diet and physical activity in daily life (108). An oncology counselor's services should include answering questions from survivors about whether it is proper for them to be physically active and continue eating specific foods. An oncology counselor needs to address the lack of health education and manage the flow of misinformation from unqualified sources on the internet or within the community (109). They should provide credibly and trusted educational materials that help survivors make informed choices about physical activity and diet (131, 132). Provision of knowledge should be explicit, easily readable, attractive, and available in suitable languages. Survivors need basic information about health behavior recommendations, and patients with BC who cannot access these resources from care units should receive help from in-house patient education by following the advice of web resources mentioned in Box 1.
Risk assessment and counseling of patients on the prevention of BC is highly dependent on the oncology provider. Patients with cancer strongly prefer to receive information about physical activity, diet, and weight management from their oncology providers (133). Studies indicate that oncology providers play a pivotal role and their sessions with patients are very influential (50, 134, 135), which leads to increased physical activity among cancer survivors (136, 137). However, oncology providers may have limited time available for extensive counseling about physical activity, nutrition, and weight management—a further challenge that could be addressed if oncologists themselves advocate around the topic and show the importance of these discussions with their patients. Oncology providers should also become more involved in establishing an advanced infrastructure to reduce BC by emphasizing exercise and diet to cancer survivors and the general public (138). During oncology visits, many care providers demonstrate limited knowledge regarding exercise and diet despite established guidelines from health institutes (139). Considering physical activity and diet interventions as critical components of cancer care, many researchers believe non-pharmacological approaches that the oncology provider's role should include discussion of all and special attention to diet and exercise during patients' chemo-sessions to help support healthy lifestyle changes (140, 141).
To reassure patients who are uncertain about the safety or appropriateness of their diet and exercise regime or weight loss after treatment, comprehensive guidelines should be requested from providers. Furthermore, providers should indicate necessary precautions or additional testing that might be warranted (110). The oncology provider is the healthcare professional well-positioned to show the importance of advocating for cancer interventions, which include lifestyle-related secondary measurements, to cope with the burden of cancer, especially BC.
Increasing size of evidence has supported the interplay of BC development with diet and physical activity. Excessive consumption of saturated fats, red meat and red wine, and low intake of fresh fruits and vegetables leads to the accumulation of triglycerides, heterocyclic amines, and ethanol and a lack of polyphenols and fiber levels in the body which mediate the onset of BC and cause an increase in overall mortality in both pre- and post-menopausal women. Similarly, no or minimal physical activity leads to an alteration in sex and metabolic hormones, which raised oxidative stress and inflammation in the body, affecting the menopausal status of women and potentially facilitating the onset of BC. Considering the importance of these interventions, oncology education and the role of oncology providers can play a potential role in developing healthy interventions and reducing the BC burden effectively. Based on these findings, the current review emphasized the value of physical activity and diet management efforts in daily lifestyle to reduce BC risk and improve outcomes.
All authors made substantial contributions to conception and design, took part in drafting the article or revising it critically, agreed to be accountable for all aspects of the work, read and edited the submitted version of the manuscript, and approved to submit it to the current journal.
This study was supported by the National Natural Science Foundation of China (no. 81900375) and Henan Province Key R&D and Promotion Special (Scientific and Technological Research) Project (no. 212102310147).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge financial support from the National Natural Science Foundation of China and Henan Province Key R&D and Promotion Special (Scientific and Technological Research) Project. The authors would like to express their gratitude to Charlesworth Author Services for their language editing to improve this article.
1. Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. (2015) 5:8–17. doi: 10.1016/j.jcpo.2015.03.002
2. Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. (2013) 15:1–37. doi: 10.1186/bcr3493
3. Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European cancer observatory. Eur J Cancer. (2015) 51:1164–87. doi: 10.1016/j.ejca.2013.09.002
4. Khan NH, Duan S-F, Wu D-D, Ji X-Y. Better reporting and awareness campaigns needed for breast cancer in pakistani women. Cancer Manag Res. (2021) 13:2125. doi: 10.2147/CMAR.S270671
5. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. (2021) 149:778–89. doi: 10.1002/ijc.33588
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
7. Metcalfe K, Lubinski J, Lynch HT, Ghadirian P, Foulkes WD, Kim-Sing C, et al. Family history of cancer and cancer risks in women with brca1 or brca2 mutations. J Natl Cancer Inst. (2010) 102:1874–8. doi: 10.1093/jnci/djq443
8. Britt KL, Cuzick J, Phillips K-A. Key steps for effective breast cancer prevention. Nat Rev Cancer. (2020) 20:417–36. doi: 10.1038/s41568-020-0266-x
9. Cancer C. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet. (2019) 394:1159–68. doi: 10.1016/S0140-6736(19)31709-X
10. Wang K, Li F, Chen L, Lai Y-M, Zhang X, Li H-Y. Change in risk of breast cancer after receiving hormone replacement therapy by considering effect-modifiers: a systematic review and dose-response meta-analysis of prospective studies. Oncotarget. (2017) 8:81109. doi: 10.18632/oncotarget.20154
11. Bonfiglio R, Di Pietro M. The impact of oral contraceptive use on breast cancer risk: state of the art and future perspectives in the era of 4p medicine. Semin Cancer Biol. (2021) 72:11–18. doi: 10.1016/j.semcancer.2020.10.008
12. Johansson A, Christakou AE, Iftimi A, Eriksson M, Tapia J, Skoog L, et al. Characterization of benign breast diseases and association with age, hormonal factors, and family history of breast cancer among women in Sweden. JAMA Network Open. (2021) 4:e2114716. doi: 10.1001/jamanetworkopen.2021.14716
13. Inoue S, Kawaida H, Saito R, Nakayama Y, Ohmori M, Kimura A, et al. Clinical significance of past history of breast cancer screening for the prognosis of triple negative breast cancer. Anticancer Res. (2021) 41:1077–82. doi: 10.21873/anticanres.14865
14. American Cancer Society. Breast Cancer Facts and Figures 2017–2018. Atlanta, GA: American Cancer Society (2017).
15. Martel S, Lambertini M, Agbor-Tarh D, Ponde NF, Gombos A, Paterson V, et al. Body mass index and weight change in patients with Her2-positive early breast cancer: exploratory analysis of the altto big 2-06 trial. J Natl Compr Canc Netw. (2021) 19:181–9. doi: 10.6004/jnccn.2020.7606
16. Demark-Wahnefried W, Schmitz KH, Alfano CM, Bail JR, Goodwin PJ, Thomson CA, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. (2018) 68:64–89. doi: 10.3322/caac.21441
17. D'Souza V, Daudt H, Kazanjian A. Survivorship care plans for breast cancer patients: understanding the quality of the available evidence. Curr Oncol. (2017) 24:446–65. doi: 10.3747/co.24.3632
18. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. (2015) 13:1012–39. doi: 10.6004/jnccn.2015.0122
19. World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR (2007).
20. Cooper SL. Promoting physical activity for mental well-being. ACSM's Health Fit J. (2020) 24:12–6. doi: 10.1249/FIT.0000000000000569
21. Sfendla A, Hadrya F. Factors associated with psychological distress and physical activity during the Covid-19 pandemic. Health Secur. (2020) 18:444–53. doi: 10.1089/hs.2020.0062
22. Ruble K, Li HCW, Thornton CP, Hooke MC. Exercise and physical activity. In: Hinds PS, Linder L, editors. Pediatric Oncology Nursing: Defining Care Through Science. Cham: Springer (2020). p. 153–167. doi: 10.1007/978-3-030-25804-7_9
23. Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. (2018) 9:509. doi: 10.3389/fpsyg.2018.00509
24. Lipsett A, Barrett S, Haruna F, Mustian K, O'Donovan A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. The Breast. (2017) 32:144–55. doi: 10.1016/j.breast.2017.02.002
25. Carayol M, Ninot G, Senesse P, Bleuse J-P, Gourgou S, Sancho-Garnier H, et al. Short-and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the “apad1” randomized controlled trial. BMC Cancer. (2019) 19:1–20. doi: 10.1186/s12885-019-5896-6
26. Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr. (2010) 91:1294–302. doi: 10.3945/ajcn.2009.28796
27. Shapira N. The potential contribution of dietary factors to breast cancer prevention. Eur J Cancer Prev. (2017) 26:385. doi: 10.1097/CEJ.0000000000000406
28. De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. (2019) 11:1514. doi: 10.3390/nu11071514
29. Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, et al. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized mediterranean dietary intervention study. Eur J Nutr. (2018) 57:2133–45. doi: 10.1007/s00394-017-1489-9
30. International WCRF. Diet, Nutrition, Physical Activity Cancer: A Global Perspective: A Summary of the Third Expert Report. London: World Cancer Research Fund International (2018). Available online at: https://www.wcrf.org/diet-and-cancer/
31. Couto E, Sandin S, Löf M, Ursin G, Adami H-O, Weiderpass E. mediterranean dietary pattern and risk of breast cancer. PLoS ONE. (2013) 8:e55374. doi: 10.1371/journal.pone.0055374
32. Solans M, Castelló A, Benavente Y, Marcos-Gragera R, Amiano P, Gracia-Lavedan E, et al. Adherence to the western, prudent, and mediterranean dietary patterns and chronic lymphocytic leukemia in the Mcc-spain study. Haematologica. (2018) 103:1881. doi: 10.3324/haematol.2018.192526
33. Mourouti N, Papavagelis C, Plytzanopoulou P, Kontogianni M, Vassilakou T, Malamos N, et al. Dietary patterns and breast cancer: a case–control study in women. Eur J Nutr. (2015) 54:609–17. doi: 10.1007/s00394-014-0742-8
34. Kontou N. The mediterranean diet in cancer prevention. In: Victor R, Preedy RRW, editors. The Mediterranean Diet. Academic Press (2015). p. 393–406. doi: 10.1016/B978-0-12-407849-9.00036-1
35. Domínguez-López I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review. Nutrients. (2020) 12:2456. doi: 10.3390/nu12082456
36. Barrea L, Pugliese G, Laudisio D, Savastano S, Colao A, Muscogiuri G. Does mediterranean diet could have a role on age at menopause and in the management of vasomotor menopausal symptoms? The viewpoint of the endocrinological nutritionist. Curr Opin Food Sci. (2021) 39:171–81. doi: 10.1016/j.cofs.2021.02.018
37. Messeha SS, Zarmouh NO, Soliman KF. Polyphenols modulating effects of Pd-L1/Pd-1 checkpoint and Emt-mediated Pd-L1 overexpression in breast cancer. Nutrients. (2021) 13:1718. doi: 10.3390/nu13051718
38. Briguglio G, Costa C, Pollicino M, Giambò F, Catania S, Fenga C. Polyphenols in cancer prevention: new insights. Int J Funct Nutr. (2020) 1:9. doi: 10.3892/ijfn.2020.9
39. Thibane V, Ndhlala A, Finnie J, Van Staden J. Modulation of the enzyme activity of secretory phospholipase a2, lipoxygenase and cyclooxygenase involved in inflammation and disease by extracts from some medicinal plants used for skincare and beauty. S Afr J Bot. (2019) 120:198–203. doi: 10.1016/j.sajb.2018.06.001
40. Aichinger G, Beisl J, Marko D. The Hop polyphenols xanthohumol and 8-prenyl-naringenin antagonize the estrogenic effects of fusarium mycotoxins in human endometrial cancer cells. Front Nutr. (2018) 5:85. doi: 10.3389/fnut.2018.00085
41. Cipolletti M, Solar Fernandez V, Montalesi E, Marino M, Fiocchetti M. Beyond the Antioxidant activity of dietary polyphenols in cancer: the modulation of estrogen receptors (Ers) signaling. Int J Mol Sci. (2018) 19:2624. doi: 10.3390/ijms19092624
42. Nielsen AJ, McNulty J. Polyphenolic natural products and natural product–inspired steroidal mimics as aromatase inhibitors. Med Res Rev. (2019) 39:1274–93. doi: 10.1002/med.21536
43. Viggiani MT, Polimeno L, Di Leo A, Barone M. Phytoestrogens: dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients. (2019) 11:1709. doi: 10.3390/nu11081709
44. Zengul AG. Exploring the Link Between Dietary Fiber, the Gut Microbiota and Estrogen Metabolism Among Women With Breast Cancer. The University of Alabama at Birmingham, Ann Arbor, ProQuest Dissertations Publishing (2019).
45. Shannon OM, Stephan BC, Granic A, Lentjes M, Hayat S, Mulligan A, et al. Mediterranean diet adherence and cognitive function in older Uk Adults: the European prospective investigation into cancer and nutrition–norfolk (Epic-Norfolk) study. Am J Clin Nutr. (2019) 110:938–48. doi: 10.1093/ajcn/nqz114
46. Lu F, Kuhnle GK, Cheng Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control. (2018) 92:399–411. doi: 10.1016/j.foodcont.2018.05.018
47. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon: International Agency for Research on Cancer (2018). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK507971/
48. Ganesan K, Xu B. Deep frying cooking oils promote the high risk of metastases in the breast-a critical review. Food Chem Toxicol. (2020) 144:111648. doi: 10.1016/j.fct.2020.111648
49. Alsubait A, Aldossary W, Rashid M, Algamdi A, Alrfaei BM. Cyp1b1 gene: implications in glaucoma and cancer. J Cancer. (2020) 11:4652. doi: 10.7150/jca.42669
50. Phelps K, Drouillard JS, Jennings J, Depenbusch B, Vaughn M, Burnett DD, et al. Effect of the programmed nutrition beef program on moisture retention of cooked ground beef patties and enhanced strip loins. Meat Sci. (2015) 100:189–94. doi: 10.1016/j.meatsci.2014.10.021
51. Hur SJ, Yoon Y, Jo C, Jeong JY, Lee KT. Effect of dietary red meat on colorectal cancer risk—a review. Compr Rev Food Sci Food Saf. (2019) 18:1812–24. doi: 10.1111/1541-4337.12501
52. Czerwonka M, Tokarz A. Iron in red meat–friend or foe. Meat Sci. (2017) 123:157–65. doi: 10.1016/j.meatsci.2016.09.012
53. Gamage S, Dissabandara L, Lam AK-Y, Gopalan V. The role of heme iron molecules derived from red and processed meat in the pathogenesis of colorectal carcinoma. Crit Rev Oncol Hematol. (2018) 126:121–8. doi: 10.1016/j.critrevonc.2018.03.025
54. Demeyer D, Mertens B, De Smet S, Ulens M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: a review. Crit Rev Food Sci Nutr. (2016) 56:2747–66. doi: 10.1080/10408398.2013.873886
55. Chajès V, Assi N, Biessy C, Ferrari P, Rinaldi S, Slimani N, et al. A prospective evaluation of plasma phospholipid fatty acids and breast cancer risk in the epic study. Ann Oncology. (2017) 28:2836–42. doi: 10.1093/annonc/mdx482
56. Tan PY, Teng KT. Role of dietary fat on obesity-related postmenopausal breast cancer: insights from mouse models and methodological considerations. Breast Cancer. (2021) 28:556–71. doi: 10.1007/s12282-021-01233-0
57. Dydjow-Bendek D, Zagozdzon P. Total dietary fats, fatty acids, and omega-3/omega-6 ratio as risk factors of breast cancer in the polish population–a case-control study. In Vivo. (2020) 34:423–31. doi: 10.21873/invivo.11791
58. Farvid MS, Tamimi RM, Poole EM, Chen WY, Rosner BA, Willett WC, et al. Postdiagnostic dietary glycemic index, glycemic load, dietary insulin index, and insulin load and breast cancer survival. Cancer Epidemiol Biomarkers Prev. (2021) 30:335–43. doi: 10.1158/1055-9965.EPI-20-0764
59. Alboghobeish Z, Hekmatdoost A, Jalali S, Ahmadi M, Rashidkhani B. Carbohydrate intake, glycemic index, and glycemic load and the risk of breast cancer among Iranian women. Nutr Cancer. (2021) 73:785–93. doi: 10.1080/01635581.2020.1776886
60. Mullie P, Koechlin A, Boniol M, Autier P, Boyle P. Relation between breast cancer and high glycemic index or glycemic load: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. (2016) 56:152–9. doi: 10.1080/10408398.2012.718723
61. Turati F, Galeone C, Gandini S, Augustin LS, Jenkins DJ, Pelucchi C, et al. High glycemic index and glycemic load are associated with moderately increased cancer risk. Mol Nutr Food Res. (2015) 59:1384–94. doi: 10.1002/mnfr.201400594
62. Schlesinger S, Chan DS, Vingeliene S, Vieira AR, Abar L, Polemiti E, et al. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: a systematic review and dose–response meta-analysis of prospective studies. Nutr Rev. (2017) 75:420–41. doi: 10.1093/nutrit/nux010
63. Christopoulos PF, Corthay A, Koutsilieris M. Aiming for the insulin-like growth factor-1 system in breast cancer therapeutics. Cancer Treat Rev. (2018) 63:79–95. doi: 10.1016/j.ctrv.2017.11.010
64. Mourouti N, Kontogianni MD, Papavagelis C, Psaltopoulou T, Kapetanstrataki MG, Plytzanopoulou P, et al. Whole grain consumption and breast cancer: a case-control study in women. J Am Coll Nutr. (2016) 35:143–9. doi: 10.1080/07315724.2014.963899
65. Chen J-Y, Zhu H-C, Guo Q, Shu Z, Bao X-H, Sun F, et al. Dose-dependent associations between wine drinking and breast cancer risk-meta-analysis findings. Asian Pac J Cancer Prev. (2016) 17:1221–33. doi: 10.7314/APJCP.2016.17.3.1221
66. Xu M, Wang S, Ren Z, Frank JA, Yang XH, Zhang Z, et al. Chronic ethanol exposure enhances the aggressiveness of breast cancer: the role of P38γ. Oncotarget. (2016) 7:3489. doi: 10.18632/oncotarget.6508
67. Roswall N, Weiderpass E. Alcohol as a risk factor for cancer: existing evidence in a global perspective. J Prev Med Public Health. (2015) 48:1. doi: 10.3961/jpmph.14.052
68. Liu Y, Nguyen N, Colditz GA. Links between alcohol consumption and breast cancer: a look at the evidence. Women's Health. (2015) 11:65–77. doi: 10.2217/WHE.14.62
69. Kucuk O. Soy foods, isoflavones, and breast cancer. Cancer. (2017) 123:1901–3. doi: 10.1002/cncr.30614
70. Watson KL, Sauerzopf K, Moorehead RA. Isolated soy protein promotes mammary tumor development induced by the type I insulin-like growth factor receptor in transgenic mice. Nutr Cancer. (2021) 3:1340–9. doi: 10.1080/01635581.2020.1795210
71. Messina M. Impact of soy foods on the development of breast cancer and the prognosis of breast cancer patients. Complement Med Res. (2016) 23:75–80. doi: 10.1159/000444735
72. Park J, Kim HB. Suppression of metastasis-related Erbb2 and plau expressions in human breast cancerMcf 7 cells by fermented soybean extract. Korean J. Microbiol. (2018) 54:320–4. doi: 10.7845/kjm.2018.8073
73. Wang Y, Liu L, Ji F, Jiang J, Yu Y, Sheng S, et al. Soybean (Glycine Max) prevents the progression of breast cancer cells by downregulating the level of histone demethylase Jmjd5. J Cancer Res Ther. (2018) 14:609. doi: 10.4103/0973-1482.187292
74. Dukariya G, Shah S, Singh G, Kumar A. Soybean and its products: nutritional and health benefits. J Nutr Sci Health Diet. (2020) 1:22–9. Available online at: https://helicsgroup.net/assets/articles/1594789005.pdf
75. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. (2015) 54:635–54. doi: 10.3109/0284186X.2014.998275
76. McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. (2019) 51:1252. doi: 10.1249/MSS.0000000000001937
77. Hartman SJ, Nelson SH, Myers E, Natarajan L, Sears DD, Palmer BW, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. (2018) 124:192–202. doi: 10.1002/cncr.30987
78. Pizot C, Boniol M, Mullie P, Koechlin A, Boniol M, Boyle P, et al. Physical activity, hormone replacement therapy and breast cancer risk: a meta-analysis of prospective studies. Eur J Cancer. (2016) 52:138–54. doi: 10.1016/j.ejca.2015.10.063
79. Cannioto RA, Hutson A, Dighe S, McCann W, McCann SE, Zirpoli GR, et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: relationships with survival. J Natl Cancer Inst. (2021) 113:54–63. doi: 10.1093/jnci/djaa046
80. Bandera EV, Alfano CM, Qin B, Kang D-W, Friel CP, Dieli-Conwright CM. Harnessing nutrition and physical activity for breast cancer prevention and control to reduce racial/ethnic cancer health disparities. Am Soc Clin Oncol Educ Book. (2021) 41:e62–78. doi: 10.1200/EDBK_321315
81. Sturgeon K, Digiovanni L, Good J, Salvatore D, Fenderson D, Domchek S, et al. Exercise-induced dose-response alterations in adiponectin and leptin levels are dependent on body fat changes in women at risk for breast cancer. Cancer Epidemiol Biomarkers Prev. (2016) 25:1195–200. doi: 10.1158/1055-9965.EPI-15-1087
82. Schmidt ME, Chang-Claude J, Vrieling A, Seibold P, Heinz J, Obi N, et al. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. Int J Cancer. (2013) 133:1431–40. doi: 10.1002/ijc.28130
83. Lilleborge M, Falk RS, Sørlie T, Ursin G, Hofvind S. Can breast cancer be stopped? modifiable risk factors of breast cancer among women with a prior benign or premalignant lesion international. Int J Cancer. (2021) 149:1247–56. doi: 10.1002/ijc.33680
84. Dongre UJ. Adipokines in insulin resistance: current updates. Biosci Biotechnol Res. Asia. (2021) 18:357–66. doi: 10.13005/bbra/2922
85. Eraldemir FC, Korak T. Oxidative stress and cancer. In: Preedy VR, Patel VB, editors. Cancer: Oxidative Stress and Dietary Antioxidants. 2 ed. Academic Press (2021). p. 3–14. doi: 10.1016/B978-0-12-819547-5.00001-8
86. Hong BS, Lee KP. A systematic review of the biological mechanisms linking physical activity and breast cancer. Phys Act Nutr. (2020) 24:25. doi: 10.20463/pan.2020.0018
87. Ligibel JA, Basen-Engquist K, Bea JW. Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Educ Book. (2019) 39:e22–33. doi: 10.1200/EDBK_237423
88. Wirtz P, Baumann FT. Physical activity, exercise and breast cancer-what is the evidence for rehabilitation, aftercare, and survival a review. Breast Care. (2018) 13:92–100. doi: 10.1159/000488717
89. Chan DS, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, et al. World cancer research fund international: continuous update project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. (2019) 30:1183–200. doi: 10.1007/s10552-019-01223-w
90. Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American cancer society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. (2020) 70:245–71. doi: 10.3322/caac.21591
91. Schmidt T, Jonat W, Wesch D, Oberg H-H, Adam-Klages S, Keller L, et al. Influence of physical activity on the immune system in breast cancer patients during chemotherapy. J Cancer Res Clin Oncol. (2018) 144:579–86. doi: 10.1007/s00432-017-2573-5
92. Baumann FT, Bieck O, Oberste M, Kuhn R, Schmitt J, Wentrock S, et al. Sustainable impact of an individualized exercise program on physical activity level and fatigue syndrome on breast cancer patients in two german rehabilitation centers. Support Care Cancer. (2017) 25:1047–54. doi: 10.1007/s00520-016-3490-x
93. Cabelka CA, Baumann CW, Collins BC, Nash N, Le G, Lindsay A, et al. Effects of ovarian hormones and estrogen receptor α on physical activity and skeletal muscle fatigue in female mice. Exp Gerontol. (2019) 115:155–64. doi: 10.1016/j.exger.2018.11.003
94. Moore SC, Lee I-M, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM., et al. Association of leisure-time physical activity with risk of 26 types of cancer in 144 million adults. JAMA Intern Med. (2016) 176:816–25. doi: 10.1001/jamainternmed.2016.1548
95. Hartman SJ, Dunsiger SI, Marinac CR, Marcus BH, Rosen RK, Gans KM. Internet-based physical activity intervention for women with a family history of breast cancer. Health Psychol. (2015) 34:1296. doi: 10.1037/hea0000307
96. Lynch BM, Nguyen NH, Moore MM, Reeves MM, Rosenberg DE, Boyle T, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: the activate trial. Cancer. (2019) 125:2846–55. doi: 10.1002/cncr.32143
97. Phillips S, Solk P, Welch W, Auster-Gussman L, Lu M, Cullather E, et al. A technology-based physical activity intervention for patients with metastatic breast cancer (Fit2thrivemb): protocol for a randomized controlled trial. JMIR Res Protoc. (2021) 10:e24254. doi: 10.2196/24254
98. Spei M-E, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta-analysis on overall and breast cancer survival. The Breast. (2019) 44:144–52. doi: 10.1016/j.breast.2019.02.001
99. Falzone L, Grimaldi M, Celentano E, Augustin LS, Libra M. identification of modulated micrornas associated with breast cancer, diet, and physical activity. Cancers. (2020) 12:2555. doi: 10.3390/cancers12092555
100. Orlandella FM, De Stefano AE, Iervolino PLC, Buono P, Soricelli A, Salvatore G. Dissecting the molecular pathways involved in the effects of physical activity on breast cancers cells: a narrative review. Life Sci. (2020) 265:118790. doi: 10.1016/j.lfs.2020.118790
101. Kirkham AA, Bonsignore A, Bland KA, McKenzie DC, Gelmon KA, Van Patten CL, et al. Exercise prescription and adherence for breast cancer: one size does not fitt all. Med Sci Sports Exerc. (2018) 50:177–86. doi: 10.1249/MSS.0000000000001446
102. Prieto-Gómez V, Yuste-Sánchez MJ, Bailón-Cerezo J, Romay-Barrero H, de la Rosa-Díaz I, Lirio-Romero C, et al. Effectiveness of therapeutic exercise and patient education on cancer-related fatigue in breast cancer survivors: a randomised, single-blind, controlled trial with a 6-month follow-up. J Clin Med. (2022) 11:269. doi: 10.3390/jcm11010269
103. Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, et al. polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. (2019) 104:21–34. doi: 10.1016/j.ajhg.2018.11.002
104. Kniep HC, Madesta F, Schneider T, Hanning U, Schönfeld MH, Schön G, et al. Radiomics of brain mri: utility in prediction of metastatic tumor type. Radiology. (2019) 290:479–87. doi: 10.1148/radiol.2018180946
105. O'Neil DS, Nietz S, Buccimazza I, Singh U, Cačala S, Stopforth LW, et al. Neoadjuvant chemotherapy use for nonmetastatic breast cancer at five public south african hospitals and impact on time to initial cancer therapy. Oncologist. (2019) 24:933. doi: 10.1634/theoncologist.2018-0535
106. Khan MFA, Salman M, Khan NH, Masood T, Safdar M, Ansari MU, et al. Evaluation of errors in prescription writing: a cross-sectional study at community pharmacies and tertiary care hospitals of Lahore, Pakistan. Bangladesh J Med Sci. (2019) 18:260–6. doi: 10.3329/bjms.v18i2.40695
107. Caswell-Jin JL, Plevritis SK, Tian L, Cadham CJ, Xu C, Stout NK, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. (2018) 2:pky062. doi: 10.1093/jncics/pky062
108. Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. (2017) 67:194–232. doi: 10.3322/caac.21397
109. Hudson P, Zajo K, Gerhardt CA, Stanek J, Varga E. Defining the role of a genetic counselor within pediatric hematology and oncology comprehensive care teams: perspectives of the provider team and patients. J Genet Couns. (2019) 28:1139–47. doi: 10.1002/jgc4.1164
110. Ligibel JA, Jones LW, Brewster AM, Clinton SK, Korde LA, Oeffinger KC, et al. Oncologists' attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an asco survey of the oncology workforce. J Oncol Pract. (2019) 15:e520–e8. doi: 10.1200/JOP.19.00124
111. Balneaves LG, Truant TL, Van Patten C, Kirkham AA, Waters E, Campbell KL. Patient and medical oncologists' perspectives on prescribed lifestyle intervention—experiences of women with breast cancer and providers. J Clin Med. (2020) 9:2815. doi: 10.3390/jcm9092815
112. Kolak A, Kamińska M, Sygit K, Budny A, Surdyka D, Kukiełka-Budny B, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. (2017) 24:549–53. doi: 10.26444/aaem/75943
113. Anbari AB, Wanchai A, Graves R. Breast cancer survivorship in rural settings: a systematic review. Support Care Cancer. (2020) 28:3517–31. doi: 10.1007/s00520-020-05308-0
114. Ghose A, Kundu R, Toumeh A, Hornbeck C, Mohamed I. A review of obesity, insulin resistance, and the role of exercise in breast cancer patients. Nutr Cancer. (2015) 67:197–202. doi: 10.1080/01635581.2015.990569
115. Dieli-Conwright CM, Orozco BZ. Exercise after Breast Cancer Treatment: Current Perspectives. Breast Cancer. (2015) 7:353. doi: 10.2147/BCTT.S82039
116. Jizzini M, Raghavendra AS, Ibrahim NK, Kypriotakis G, Cinciripini PM, Seoudy K, et al. The impact of smoking cessation on breast cancer patients' survival. J Clin Oncol. (2019) 37:1542. doi: 10.1200/JCO.2019.37.15_suppl.1542
117. Wang F, Cai H, Gu K, Shi L, Yu D, Zhang M, et al. Adherence to dietary recommendations among long-term breast cancer survivors and cancer outcome associations. Cancer Epidemiol Biomarkers Prev. (2020) 29:386–95. doi: 10.1158/1055-9965.EPI-19-0872
118. Xiao Y, Xia J, Li L, Ke Y, Cheng J, Xie Y, et al. Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res. (2019) 21:1–22. doi: 10.1186/s13058-019-1096-1
119. Ligibel JA, Pierce LJ, Bender CM, Crane TE, Dieli-Conwright CM, Hopkins JO, et al. Attention to diet, exercise, and weight in the oncology clinic: Results of a national patient survey wolters kluwer health. J Clin Oncol. (2021). 39:10549.
120. Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ. (2017) 189:E268–74. doi: 10.1503/cmaj.160464
121. Makarem N, Chandran U, Bandera EV, Parekh N. Dietary fat in breast cancer survival. Annu Rev Nutr. (2013) 33:319–48. doi: 10.1146/annurev-nutr-112912-095300
122. Chi F, Wu R, Zeng Y-C, Xing R, Liu Y, Xu Z-G. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. (2013) 14:2407–12. doi: 10.7314/APJCP.2013.14.4.2407
123. Dong J-Y, Qin L-Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. (2011) 125:315–23. doi: 10.1007/s10549-010-1270-8
124. Lobascio F, Pedrazzoli P, Secondino S, Caccialanza R. Nutrition in survivorship care. In: Rauh S, editor. Survivorship Care for Cancer Patients. Cham: Springer (2021). doi: 10.1007/978-3-030-78648-9_18
125. Moraitis AM. Young Adult Cancer Survivors and Physical Activity: An Expert Consensus Study (Ph. D. Dissertion). University of Massachusetts, Amherst, United States (2021). doi: 10.7275/22158784.0
126. Sanft T, Denlinger CS, Armenian S, Baker KS, Broderick G, Demark-Wahnefried W, et al. Nccn guidelines insights: survivorship, version 2.2019: featured updates to the Nccn Guidelines. J Natl Compr Canc Netw. (2019) 17:784–94. doi: 10.6004/jnccn.2019.0034
127. Denlinger CS, Sanft T, Moslehi JJ, Overholser L, Armenian S, Baker KS, et al. Nccn guidelines insights: survivorship, version 2.2020: featured updates to the Nccn guidelines. J Natl Compr Canc Netw. (2020) 18:1016–23. doi: 10.6004/jnccn.2020.0037
128. Balas EA, Chapman WW. Road map for diffusion of innovation in health care. Health Aff. (2018) 37:198–204. doi: 10.1377/hlthaff.2017.1155
129. Fong AJ, Faulkner G, Jones JM, Sabiston CM. A qualitative analysis of oncology clinicians' perceptions and barriers for physical activity counseling in breast cancer survivors. Supportive Care Cancer. (2018) 26:3117–26. doi: 10.1007/s00520-018-4163-8
130. Cantwell M, Walsh D, Furlong B, Moyna N, McCaffrey N, Boran L, et al. Healthcare professionals' knowledge and practice of physical activity promotion in cancer care: challenges and solutions. Eur J Cancer Care. (2018) 27:e12795. doi: 10.1111/ecc.12795
131. Ahamad A, Wallner P, Salenius S, Ross R, Fernandez E. Information needs expressed during patient-oriented oncology consultations: quantity, variation, and barriers. J Cancer Educ. (2019) 34:488–97. doi: 10.1007/s13187-018-1329-5
132. Khan NH, Ullah F, Khan TA, Zafar U, Khan MFA, Mustaqeem M, et al. Personal-care cosmetic practices in Pakistan: current perspectives and management. Clin Cosmet Investig Dermatol. (2021) 14:9. doi: 10.2147/CCID.S270667
133. Keaver L, Callaghan H, Walsh L, Houlihan C. Nutrition guidance for cancer patients and survivors—a review of the websites of irish healthcare and charitable organisations and cancer centres. Eur J Cancer Care. (2020) 29:e13216. doi: 10.1111/ecc.13216
134. Clark LH, Ko EM, Kernodle A, Harris A, Moore DT, Gehrig PA, Bae-Jump V. Endometrial cancer survivors' perceptions of provider obesity counseling and attempted behavior change: Are we seizing the moment? Int J Gynecol Cancer. (2016) 26:318–24. doi: 10.1097/IGC.0000000000000596
135. Fisher A, Williams K, Beeken R, Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. (2015) 5:e006853. doi: 10.1136/bmjopen-2014-006853
136. Rogers LQ, Courneya KS, Oster RA, Anton PM, Robbs RS, Forero A, et al. Physical activity and sleep quality in breast cancer survivors: a randomized trial. Med Sci Sports Exerc. (2017) 49:2009. doi: 10.1249/MSS.0000000000001327
137. Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. (2013) 32:616. doi: 10.1037/a0029886
138. Nadler M, Bainbridge D, Tomasone J, Cheifetz O, Juergens RA, Sussman J. Oncology care provider perspectives on exercise promotion in people with cancer: an examination of knowledge, practices, barriers, and facilitators. Supportive Care Cancer. (2017) 25:2297–304. doi: 10.1007/s00520-017-3640-9
139. Nyrop KA, Callahan LF, Rini C, Altpeter M, Hackney B, DePue A, et al. Aromatase inhibitor associated arthralgia: the importance of oncology provider-patient communication about side effects and potential management through physical activity. Supportive Care Cancer. (2016) 24:2643–50. doi: 10.1007/s00520-015-3065-2
140. Treanor CJ, McMenamin UC, O'Neill RF, Cardwell CR, Clarke MJ, Cantwell M, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. (2016) 2016:CD011325. doi: 10.1002/14651858.CD011325.pub2
Keywords: breast cancer, diet, physical activity, oncology education, lifestyle
Citation: Jia T, Liu Y, Fan Y, Wang L and Jiang E (2022) Association of Healthy Diet and Physical Activity With Breast Cancer: Lifestyle Interventions and Oncology Education. Front. Public Health 10:797794. doi: 10.3389/fpubh.2022.797794
Received: 19 October 2021; Accepted: 22 February 2022;
Published: 23 March 2022.
Edited by:
Md Anwarul Azim Majumder, The University of the West Indies at Cave Hill, BarbadosReviewed by:
Tonia Vassilakou, University of West Attica, GreeceCopyright © 2022 Jia, Liu, Fan, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enshe Jiang, ZXNqaWFuZ0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.