
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 13 January 2023
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1069162
Background: The existing literature has repeatedly assessed the association between sugar-sweetened beverages and depressive symptoms, but studies of the association of total dietary sugar with depressive symptoms and of this association in obese adults are scarce. Thus, the purpose of this cross-sectional study was to assess the association between total sugar consumption and depressive symptoms in the study population and then in the population stratified by body mass index.
Methods: This study was conducted in a nationally representative sample of 16,009 adults from the 2011–2018 National Health and Nutrition Examination Survey in the US. Total sugar intake was assessed by 24 h dietary recalls, and depressive symptoms were assessed by the nine-item Patient Health Questionnaire. Logistic regression models were used to evaluate the association between total sugar consumption and depressive symptoms.
Results: Total sugar intake was positively associated with higher prevalence of depressive symptoms, and the adjusted odds ratio (95% confidence interval) of depressive symptoms for the highest vs. lowest quintile of total sugar intake was 1.56 (1.18, 2.05). In stratified analysis, we found a positive association between total sugar intake and depressive symptoms in adults with body mass index ≥30 kg/m2 (P for trend = 0.013), whereas no association was found in normal weight or overweight adults.
Conclusions: A higher intake of total sugar was associated with increased odds of clinically relevant depressive symptoms among obese adults. Further studies are necessary to confirm the role of total sugar in depressive symptoms among obese adults.
Depression is a primary cause of disability and a significant contributor to the global burden of disease (1). According to the World Health Organization, depression affects more than 350 million people worldwide (2), and this disorder disproportionately affects individuals with obesity. The prevalence of depressive symptoms in obese people is twice as high as that in individuals of normal weight (3). According to the Global Burden of Disease Study 2019, obesity is also an important contributor to the global burden disease (4). Obesity as a major risk factor for non-communicable diseases is associated with reduced quality of life and life expectancy (5). The underlying cause of obesity is an energy imbalance between calories consumed and calories expended (5). There is evidence that diets high in sugar promote the development of obesity (6). Sugar consumption is strongly associated with the rise of obesity. In addition, obesity and depressive symptoms have a bidirectional relationship (2, 3, 7), as risk factors for depressive symptoms are also linked to the development of obesity (7). A meta-analysis concluded that obese individuals had a 55% increased risk of developing depressive symptoms, while depressed individuals had a 58% increased risk of becoming obese (7).
Although the pathogenesis of depressive symptoms is not well understood, there is evidence that nutrition and diet play important roles in the development of depressive symptoms. In recent years, a growing number of studies have explored the relationship between sugary foods/beverages and depressive symptoms (8–11). However, most studies focused on sugar-sweetened beverages rather than dietary total sugar intake, and the results were inconsistent. A meta-analysis of 10 observational studies indicated that consumption of sugar-sweetened beverages was associated with a higher prevalence of depressive symptoms (8). In contrast, the Seguimiento Universidad de Navarra project, which was a 10-year follow-up study involving 15,546 participants, found no significant association between the consumption of sugar-sweetened beverages and depressive symptoms (9). The association between total sugar intake and depressive symptoms has rarely been studied, with only two reports on this topic available to date. The Whitehall II cohort study found that high sugar intake was positively associated with depressive symptoms (10). However, the analysis was conducted in a sample of non-industrial civil servants aged 39–83 years, which limits the generalization of their findings. A similar positive association was found in a cross-national study involving six countries (11), but it did not take into account any confounding factors such as gender, physical activity, marital status, education level, family income, body mass index (BMI), etc. There is evidence that depressive disorders are twice as common in women as in men (12). Sociocultural roles, vulnerability to life events, and coping skills are reasons why women are more likely to suffer from depression. Besides, the evidence suggests that physical activity has a beneficial effect on depressive symptoms (13). Living with a spouse, higher levels of education and higher economic income are also associated with a lower prevalence of depressive symptoms too (12, 14, 15). Moreover, none of these studies focused on obese adults or conducted stratified analysis by BMI.
Therefore, the goal of the current study was to investigate the association between total sugar consumption and depressive symptoms in a cross-sectional study of US adults, adjusting for numerous confounding factors (including gender, physical activity, marital status, education level, family income, etc.). The data were further stratified by BMI to assess the patterns in obese adults.
The data analyzed in this study came from the National Health and Nutrition Examination Survey (NHANES), which is a nationally representative survey conducted by the Centers for Disease Control and Prevention of the United States. The Research Ethics Review Board of the National Center for Health Statistics approved the NHANES study protocol, with all participants providing signed informed consent. From 2011 to 2018, a total of 39,156 individuals participated in the NHANES, and our analyses were confined to 23,826 individuals over the age of 18 years. Pregnant females (n = 247) and those who did not complete the depression scale (n = 3,289) were omitted from the study. Moreover, individuals with incomplete 24 h dietary recall data (n = 3,355) or with implausible energy intake (<500 or >5,000 kcal/day; n = 926) (16, 17) were further excluded. Finally, 16,009 participants were included in the analyses.
The nine-item Patient Health Questionnaire (PHQ-9), a valid criteria instrument based on DSM-V, was used to measure depressive symptoms (18, 19). The PHQ-9 consists of nine items. The PHQ score of each participant is the sum of all answers to the PHQ question. Because a PHQ-9 score ≥10 points has 88% sensitivity and 88% specificity in diagnosing significant depression symptoms (18), it was chosen as the binary threshold to determine the existence of depressive symptoms in this study.
Two 24 h dietary recall interviews were used to examine dietary data. The first interview was conducted in-person at the Mobile Examination Center, and the second interview was conducted over the phone 3–10 days later. The interviewer inquired about all foods and beverages ingested in the previous 24 h. To assist respondents in reporting food amounts during the interview, participants were given a set of measurement instructions and a food model booklet. The Food and Nutrient Database for Dietary Studies was used to calculate dietary intakes. Data from the two 24 h recalls were utilized to calculate the mean of dietary intake.
The following covariates were included in this study: age, gender, BMI, energy intake, physical activity, marital status, race, education level, family income, smoking/drinking history, hypertension, and diabetes. Physical activity was assessed using a physical activity questionnaire based on the Global Physical Activity Questionnaire, which provided respondent-level interview data on physical activity. Total physical activity was calculated by weighting the metabolic equivalent of task (MET) minutes of each activity and adding them together. The MET score for each activity was suggested by the NHANES database. Marital status was categorized into three groups: married/living with partner, widowed/divorced/separated/never-married, and other. Race was categorized as Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other. Education was divided into three levels: pre-high school, high school, and post-high school. Family income was categorized as <$20,000, $20,000–$74,999, and ≥$75,000. Alcohol status was evaluated according to the survey question “Had at least 12 alcohol drinks a year?”. Lifetime smoking of at least 100 cigarettes was considered as having a smoking history. Hypertension or diabetes was determined by a doctor's diagnosis.
Categorical variables were expressed by proportions (%) while continuous variables were expressed by the mean ± standard deviation or median (interquartile range). One-way analysis of variance, the Kruskal-Wallis H-test, or the chi-square test was used to analyze the distribution among the quintiles of dietary sugar intake according to the characteristics of the variables. Odds ratio (OR) and 95% confidence interval (CI) were calculated to assess the association between total sugar intake and depressive symptoms using logistic regression models. Model 1 was adjusted for age, gender, and BMI. Model 2 was fully adjusted, including age, gender, BMI, energy intake, physical activity, marital status, race, education level, family income, smoking/drinking history, hypertension, and diabetes. Restricted cubic spline (RCS) models were applied to assess the non-linear association between total sugar intake and depressive symptoms after adjusting for variables in Model 2. We further stratified the analyses by BMI (<25, 25–30, or ≥30 kg/m2) and gender (male or female). All analyses conducted in this study were weighted. All statistical analyses were carried out using R (http://www.R-project.org, The R Foundation), Free Statistics software, and EmpowerStats (20). A two-sided P < 0.05 was considered to be statistically significant.
Among the 16,009 participants, 7,723 males and 8,286 females were eligible for the final analyses, and 1,365 (8.53%) reported depressive symptoms. Table 1 summarizes the characteristics of the study participants by categories of total sugar intake. Adults with higher intake of sugar tended to be younger and male, and they had higher levels of energy intake and physical activity. Statistically significant differences were also observed for marital status, race, educational level, family income, smoking and drinking status, and history of diabetes and hypertension (all P < 0.05). No significant difference was observed for BMI (P > 0.05).
Table 2 presents the association between total sugar intake and depressive symptoms. In the crude model, total sugar intake was positively associated with depressive symptoms (P for trend = 0.047). After multivariable adjustments, the positive association remained significant (P for trend = 0.001). Compared with the participants in the lowest quintile, the ORs (95% CIs) across increasing intake of total sugar were 0.81 (0.61, 1.06), 1.00 (0.76, 1.32), 1.25 (0.94, 1.66), and 1.56 (1.18, 2.05). To assess the dose-response association between sugar intake and depressive symptoms, RCS analysis was conducted. Figure 1 shows a linear association between sugar intake and depressive symptoms in all participants (P for non-linearity = 0.128).
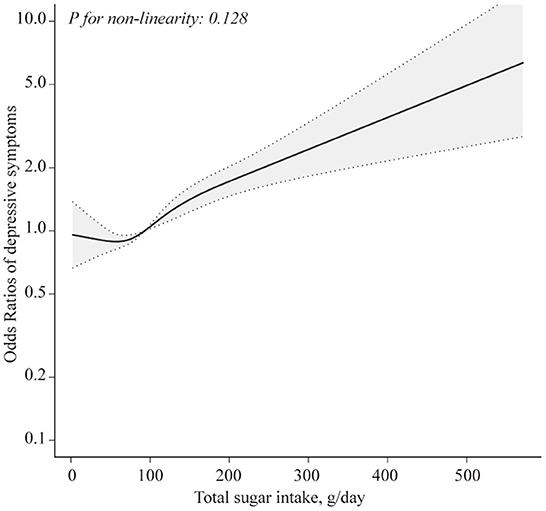
Figure 1. Restricted cubic spline model of the odds ratios of total sugar intake with depressive symptoms. Adjusted for age, gender, BMI, total energy intake, physical activity, marital status, race, education level, household income, smoking status, drinking history, hypertension, and diabetes. The dashed lines represent the 95% confidence intervals.
Table 3 shows the result of stratified analysis by BMI. In obese adults (BMI > 30 kg/m2), after multivariable adjustments, total sugar intake was positively associated with depressive symptoms (P for trend = 0.013). The corresponding ORs (95% CIs) were 1 (reference), 0.87 (0.61, 1.25), 1.04 (0.70, 1.55), 1.27 (0.85, 1.89), and 1.66 (1.13, 2.46). However, among adults with BMI <30 g/m2, no significant association between total sugar intake and depressive symptoms was found in any of the models. When RCS analyses were conducted in normal weight, overweight, and obese adults, respectively, the results did not indicate evidence against linearity (all P for non-linearity > 0.05) (Supplementary Figure 1).
Table 4 shows the associations between total sugar intake and depressive symptoms by gender in normal weight, overweight, and obese adults. In obese adults (BMI ≥30 kg/m2), after full adjustments, total sugar intake was positively associated with depressive symptoms in both females (P for trend = 0.034) and males (P for trend = 0.015). For those with BMI <30 g/m2, there was no association between total sugar consumption and depressive symptoms in either females or males.
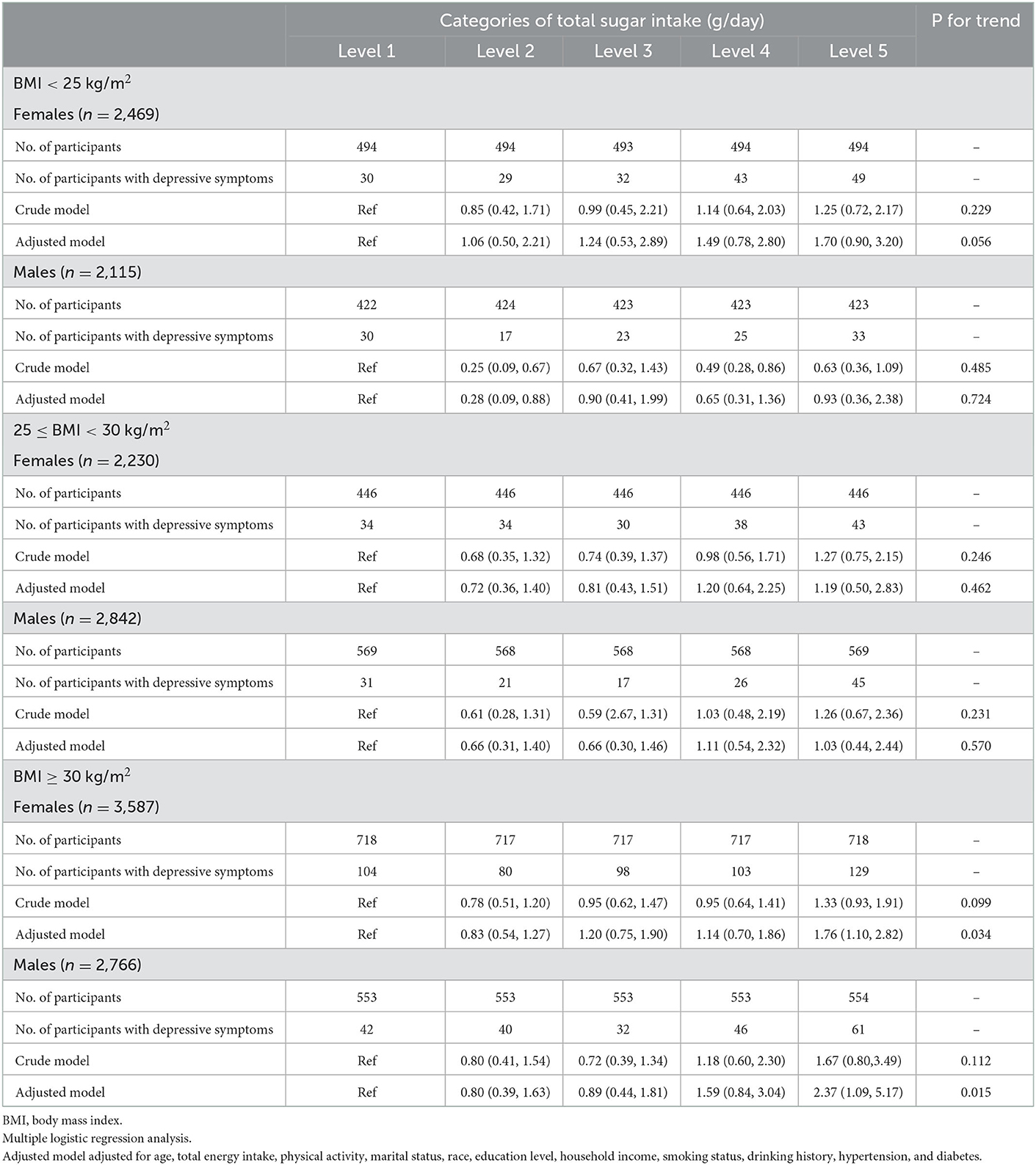
Table 4. Associations between total sugar intake and depressive symptoms by gender in normal weight, overweight and obese adults.
This nationally representative cross-sectional study demonstrated a positive association between total sugar intake and depressive symptoms in adults when adjusted for potentially important confounders. A similar significant association was observed in obese females and males, but not in normal weight or overweight adults. To the best of our knowledge, this is the first study to explore the association between total sugar intake and depressive symptoms in a stratified analysis based on BMI in the US adult population.
In previous studies, researchers evaluated the association between sugary foods/beverages and depressive symptoms (8, 21, 22). A meta-analysis of 10 observational studies including 365,289 participants indicated that drinking sugar-sweetened beverages was linked to a higher frequency of depression symptoms (8). Another two cross-sectional studies conducted in Brazilian adults and Chinese adolescents consistently showed a positive association between the consumption of sugar-sweetened beverage and depressive symptoms (21, 22). In other cases, the results were based on analyses of dietary patterns. The Whitehall II prospective cohort indicated that a diet rich in processed foods, such as chocolate, sweet desserts, processed meat, and fried food, was deleterious for depressive symptoms (OR = 1.58; 95% CI 1.11, 2.23) (23). In a case-control study, Khosravi et al. (24) showed that adherence to unhealthy dietary patterns, such as those defined by a high intake of sweets and commercial fruit drinks, increased the OR of depressive symptoms. In contrast to numerous studies of the effects of sugary foods/beverages on depressive symptoms, there are only two reports about the association between total sugar intake and depressive symptoms. The results of the Whitehall II study provided evidence that total sugar intake increased the risk of depressive symptoms in males (10). In addition, a study based on data from six countries reported a relationship between total sugar intake and the prevalence of major depression (11).
Although little is known about the underlying mechanisms of the positive association between sugar intake and depressive symptoms, various possibilities have been proposed. The pathophysiology of depressive symptoms includes dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in reduced volumes in the hippocampus, prefrontal cortex, and striatum (25), and the activity of the HPA axis can be inhibited via the consumption of dietary sugar (26). A recent review showed that the neuroadaptations that occurred in the hippocampus, prefrontal cortex, and amygdala after consuming sugar increased the likelihood of developing depressive symptoms (27). Inflammation is another potential biological explanation for the link between sugar consumption and depressive symptoms. In both humans (28) and rats (29), dietary sugar has been shown to induce proinflammatory states that are characterized by increased expression of inflammatory genes and raised levels of inflammatory factors. Inflammation is recognized as a potent physiological trigger of depressive symptoms, especially fatigue, lack of energy, sleep problems, and changes in appetite (30, 31). Anti-inflammatory drugs have antidepressant therapeutic characteristics, while pro-inflammatory drugs may induce depressive symptoms and raise the likelihood of developing full-blown depressive symptoms (32). Excessive sugar consumption can also drive insulin resistance and impair glucose homoeostasis. Previous studies demonstrated that insulin resistance increased the risk for the development of future depressive symptoms, and higher insulin resistance biomarkers were more prevalent in those who reported having depressive symptoms (33, 34). Evidence also revealed that insulin resistance in the brain and the consequent disruption in energy use might be a direct cause of depressive symptoms (32).
Given that the relationships among sugar intake, depressive symptoms, and obesity are of particular interest, data were further stratified by BMI. Total sugar intake was shown to be associated with higher prevalence of depressive symptoms in obese adults. Several previous studies suggested a bidirectional association between depressive symptoms and obesity (2, 7, 35). A meta-analysis of eight longitudinal studies concluded that obese individuals had a 55% increased risk of developing depressive symptoms, whereas depressed people had a 58% increased risk of becoming obese (7). Furthermore, studies of both humans and animals have demonstrated a connection between depressive symptoms and sweet desires (36). Sweet foods can enhance mood and relieve negative emotional states (27), but excessive sugar intake is one of the leading contributors to weight gain (37), and obesity has been found to increase risk of depressive symptoms. These results suggest a tight link among dietary sugar, depressive symptoms, and obesity. The association between sugar consumption and depressive symptoms may be mediated by obesity, as obesity can activate inflammatory pathways, increase risk of insulin resistance, and contribute to HPA-axis dysregulation (7, 38, 39), all of which may be involved in depressive symptoms.
The co-existence of depressive symptoms with obesity has raised as an important public health issue. When obese adults with depressive symptoms consult a dietitian/doctor for weight loss, it is crucial that they receive scientific dietary advice. Losing weight and easing depressed mood at the same time by adjusting diet is a win-win approach. However, research in this field is scarce. Studies of the association between sugar consumption and depression symptoms have mostly focused on sugar-sweetened drinks, with little attention paid to total sugar intake, and studies of the association between total sugar intake and depressive symptoms in obese adults are extremely rare. The findings of our study can be utilized to provide a scientific basis for gradually reducing sugar intake in obese adults with depressive symptoms. For these adults, good diet and lifestyle habits can help control weight while alleviating depressed mood. We should do more to reduce total sugar consumption through public policy. Besides energy intake control, energy expenditure constitutes the other important factor in the energy balance formula. Physical activity can help with energy expenditure. However, strong evidence reveals that insufficient physical activity presents a major global public health issue. In 2019, low physical activity potentially contributed to 0.83 million deaths and 15.75 million disability-adjusted life years globally (40). Therefore, the dissemination of appropriate diet and physical activity recommendations is crucial today.
The current study has several strengths. First, we used a large nationally representative sample, which provided sufficient statistical power. We also included and adjusted for numerous confounding factors, including sociodemographic, behavioral, anthropometric, and clinical factors. To the best of our knowledge, this study is the first to investigate the association between total sugar consumption and depressive symptoms in a stratified analysis based on BMI. Possible limitations of this study are as follows: (1) due to the cross-sectional design, causal association between dietary sugar and depressive symptoms cannot be confirmed; (2) although the study used two 24 h dietary recalls to assess total food intake in a detailed way, recall bias and day-to-day variation are unavoidable; (3) the PHQ-9 scores do not correspond with a clinical diagnosis of depression but rather indicate the level of depressive symptoms that may be of clinical relevance; and (4) even though a variety of confounders were considered, we cannot rule out the possibility that some unknown or unmeasured factors might partly explain the results. For example, the NHANES database did not assign METs by age groups, which might lead to bias.
In summary, we identified a positive association between total sugar intake and prevalence of depressive symptoms in obese adults. Further cohort studies and randomized controlled trials are needed to provide more powerful evidence to elucidate the association.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Research Ethics Review Board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
The study was conceived and designed by PL and JZ. The data was examined by PL, YZ, RZ, and QW. The manuscript was drafted and revised by PL, FY, YL, JW, WL, and QW. FY, QW, and JZ supervised the analyses and suggested revisions of the paper. The final paper has been approved by all the authors.
We thank the National Center for Health Statistics team at the CDC for designing, gathering, managing, and releasing the NHANES data available for public use. We appreciate the cooperation of all the participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1069162/full#supplementary-material
1. Wang J, Wu X, Lai W, Long E, Zhang X, Li W, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ Open. (2017) 7:e017173. doi: 10.1136/bmjopen-2017-017173
2. Blasco BV, García-Jiménez J, Bodoano I, Gutiérrez-Rojas L. Obesity and depression: its prevalence and influence as a prognostic factor: a systematic review. Psychiatry Investig. (2020) 17:715–24. doi: 10.30773/pi.2020.0099
3. Fuller NR, Burns J, Sainsbury A, Horsfield S, da Luz F, Zhang S, et al. Examining the association between depression and obesity during a weight management programme. Clin Obes. (2017) 7:354–9. doi: 10.1111/cob.12208
4. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
5. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
6. Faruque S, Tong J, Lacmanovic V, Agbonghae C, Minaya DM, Czaja K. The dose makes the poison: sugar and obesity in the United States - a review. Pol J Food Nutr Sci. (2019) 69:219–33. doi: 10.31883/pjfns/110735
7. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. (2010) 67:220–9. doi: 10.1001/archgenpsychiatry.2010.2
8. Hu D, Cheng L, Jiang W. Sugar-sweetened beverages consumption and the risk of depression: a meta-analysis of observational studies. J Affect Disord. (2019) 245:348–55. doi: 10.1016/j.jad.2018.11.015
9. Sanchez-Villegas A, Zazpe I, Santiago S, Perez-Cornago A, Martinez-Gonzalez MA, Lahortiga-Ramos F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. Br J Nutr. (2018) 119:211–21. doi: 10.1017/S0007114517003361
10. Knüppel A, Shipley MJ, Llewellyn CH, Brunner EJ. Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Sci Rep. (2017) 7:6287. doi: 10.1038/s41598-017-05649-7
11. Westover AN, Marangell LB. A cross-national relationship between sugar consumption and major depression? Depress Anxiety. (2002) 16:118–20. doi: 10.1002/da.10054
12. Oh DH, Kim SA, Lee HY, Seo JY, Choi BY, Nam JH. Prevalence and correlates of depressive symptoms in Korean adults: results of a 2009 Korean community health survey. J Korean Med Sci. (2013) 28:128–35. doi: 10.3346/jkms.2013.28.1.128
13. Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Ir J Med Sci. (2011) 180:319–25. doi: 10.1007/s11845-010-0633-9
15. Ross CE, Mirowsky J. Sex differences in the effect of education on depression: resource multiplication or resource substitution? Soc Sci Med. (2006) 63:1400–13. doi: 10.1016/j.socscimed.2006.03.013
16. Kim SA, Ha J, Lim B, Kim JM, Shin S. The association between major dietary pattern and low muscle mass in korean middle-aged and elderly populations: based on the Korea National Health and Nutrition Examination Survey. Nutrients. (2020) 12:3543. doi: 10.3390/nu12113543
17. Li Y, Zhang C, Li S, Zhang D. Association between dietary protein intake and the risk of depressive symptoms in adults. Br J Nutr. (2020) 123:1290–301. doi: 10.1017/S0007114520000562
18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
19. Lamers F, Jonkers CC, Bosma H, Penninx BW, Knottnerus JA, van Eijk JT. Summed score of the Patient Health Questionnaire-9 was a reliable and valid method for depression screening in chronically ill elderly patients. J Clin Epidemiol. (2008) 61:679–87. doi: 10.1016/j.jclinepi.2007.07.018
20. Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. (2018) 17:130. doi: 10.1186/s12944-018-0776-7
21. Werneck AO, Schuch FB, Stubbs B, Oyeyemi AL, Szwarcwald CL, Vancampfort D, et al. Independent and combined associations of sugar-sweetened beverage consumption, TV viewing, and physical activity with severe depressive symptoms among 59,402 adults. Braz J Psychiatry. (2021) 43:574–83. doi: 10.1590/1516-4446-2020-1073
22. Xu H, Guo J, Wan Y, Zhang S, Yang R, Xu H, et al. Association between screen time, fast foods, sugar-sweetened beverages and depressive symptoms in Chinese adolescents. Front Psychiatry. (2020) 11:458. doi: 10.3389/fpsyt.2020.00458
23. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. (2009) 195:408–13. doi: 10.1192/bjp.bp.108.058925
24. Khosravi M, Sotoudeh G, Majdzadeh R, Nejati S, Darabi S, Raisi F, et al. Healthy and unhealthy dietary patterns are related to depression: a case-control study. Psychiatry Investig. (2015) 12:434–42. doi: 10.4306/pi.2015.12.4.434
25. Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. (2009) 30:3719–35. doi: 10.1002/hbm.20801
26. Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. (2011) 103:104–10. doi: 10.1016/j.physbeh.2010.12.011
27. Jacques A, Chaaya N, Beecher K, Ali SA, Belmer A, Bartlett S. The impact of sugar consumption on stress driven, emotional and addictive behaviors. Neurosci Biobehav Rev. (2019) 103:178–99. doi: 10.1016/j.neubiorev.2019.05.021
28. Jameel F, Phang M, Wood LG, Garg ML. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. (2014) 13:195. doi: 10.1186/1476-511X-13-195
29. Kovačević S, Nestorov J, Matić G, Elaković I. Fructose-enriched diet induces inflammation and reduces antioxidative defense in visceral adipose tissue of young female rats. Eur J Nutr. (2017) 56:151–60. doi: 10.1007/s00394-015-1065-0
30. Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry. (2016) 73:87–8. doi: 10.1001/jamapsychiatry.2015.1977
31. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. (2014) 140:774–815. doi: 10.1037/a0035302
32. Reis DJ, Ilardi SS, Namekata MS, Wing EK, Fowler CH. The depressogenic potential of added dietary sugars. Med Hypotheses. (2020) 134:109421. doi: 10.1016/j.mehy.2019.109421
33. Ford AH, Flicker L, Hankey GJ, Yeap BB, Chubb SA, Golledge J, et al. Insulin resistance and depressive symptoms in older men: the health in men study. Am J Geriatr Psychiatry. (2015) 23:872–80. doi: 10.1016/j.jagp.2014.10.010
34. Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. (2013) 36:480–9. doi: 10.2337/dc12-1442
35. Jeffery RW, Linde JA, Simon GE, Ludman EJ, Rohde P, Ichikawa LE, et al. Reported food choices in older women in relation to body mass index and depressive symptoms. Appetite. (2009) 52:238–40. doi: 10.1016/j.appet.2008.08.008
36. Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, et al. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl). (1998) 136:272–83. doi: 10.1007/s002130050566
37. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. (2013) 98:1084–102. doi: 10.3945/ajcn.113.058362
38. Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord. (2000) 24 Suppl 2:S47–9. doi: 10.1038/sj.ijo.0801277
39. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. (2007) 132:2169–80. doi: 10.1053/j.gastro.2007.03.059
40. Ammar A, Trabelsi K, Hermassi S, Kolahi A, Mansournia MA, Jahrami H, et al. Global disease burden attributed to low physical activity in 204 countries and territories from 1990 to 2019: insights from the Global Burden of Disease 2019 Study. Biology of Sport. (2023) 40:835–55. doi: 10.5114/biolsport.2023.121322
Keywords: depressive symptoms, sugar intake, obesity, cross-sectional study, NHANES
Citation: Li P, Yin F, Zhao Y, Liu Y, Zhang R, Wang J, Lu W, Wang Q and Zhang J (2023) Total sugar intake is associated with higher prevalence of depressive symptoms in obese adults. Front. Public Health 10:1069162. doi: 10.3389/fpubh.2022.1069162
Received: 31 October 2022; Accepted: 28 December 2022;
Published: 13 January 2023.
Edited by:
Achraf Ammar, Johannes Gutenberg University Mainz, GermanyReviewed by:
Asma Salari-Moghaddam, Tehran University of Medical Sciences, IranCopyright © 2023 Li, Yin, Zhao, Liu, Zhang, Wang, Lu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang,  amluZ3hpbjMxNUAxMjYuY29t
amluZ3hpbjMxNUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.