- 1Department of Geriatrics Ward 2, The First Hospital of Lanzhou University, Lanzhou, China
- 2Evidence Based Social Science Research Center, School of Public Health, Lanzhou University, Lanzhou, China
- 3Health Technology Assessment Center of Lanzhou University, School of Public Health, Lanzhou University, Lanzhou, China
- 4Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 5Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou, China
- 6Department of Emergency Medicine, The First Hospital of Lanzhou University, Lanzhou, China
Background: Several studies have revealed a positive correlation between a Helicobacter pylori (HP) infection and the risk of non-alcoholic fatty liver disease (NAFLD). This meta-analysis was conducted to explore further the relationship between HP infection and NAFLD in the Asian and non-Asian populations.
Methods: Relevant studies published from inception to July 22, 2021, in the following databases: PubMed, EMBASE, the Cochrane library, and Web of Science were comprehensively searched. The odds ratio (OR) and hazard ratio (HR) with a 95% confidence interval (95%CI) were pooled by the random-effects model or fixed-effects model. Additionally, subgroup and sensitivity analyses were performed. The funnel plot and the Egger test were used to estimate publication bias.
Results: This meta-analysis included 25 studies involving 107,306 participants. Positive associations between HP infection and NAFLD were found both for the Asian (OR = 1.30, 95% CI: 1.13–1.49, P < 0.01; I2 = 94.30%, P < 0.01) and non-Asian populations (OR = 1.42, 95% CI: 1.04–1.94, P = 0.03; I2 = 44.90%, P = 0.09). Moreover, similar results were observed in the Asian female group (OR = 1.31, 95% CI: 1.17–1.46, P < 0.01; I2 = 46.30%, P = 0.07) but not for the Asian male group. Subgroup analyses for the Asian population showed that there were differences in the association among NAFLD diagnosis methods (P < 0.01) and the study design (P < 0.01). However, subgroup and sensitivity analyses results showed that the association for the non-Asian population was not stable enough.
Conclusions: The data obtained in this systematic review and meta-analysis suggested that an HP infection was associated with an increased risk of NAFLD for Asian and non-Asian populations. However, the association was not found for Asian males. Further studies are required to establish the causal association, especially for the non-Asian population.
Systematic review registration: Identifier: CRD42021266871.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease with a prevalence rate of ~25–30%, constantly increasing (1–3). Patients with NAFLD often progress to fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and ultimately death (4). In the coming decades, NAFLD could emerge as the leading cause of mortality due to end-stage liver disease (5). Therefore, NAFLD poses significant healthcare and economic burden to society (5–7).
Several factors are associated with NAFLD. A Helicobacter pylori (HP) infection, which is one of the most frequent gastrointestinal infections may be one of the factors. Approximately 50% of the global population suffers from HP infection (8, 9). In recent years, several studies have tried to elucidate the association between HP and intestinal dysregulation disease (10, 11). Moreover, several epidemiological studies and experimental trials have identified an increased risk of NAFLD among patients infected with HP (3, 12, 13).
Considering the need of strong evidence for risk of NAFLD with HP, meta-analysis and systematic review could be helpful. Meta-analysis is commonly used for achieving high-quality evidence (14–16) and is widely used in natural science and social science research (17, 18). In five recently published meta-analyses, the potential association between HP and NAFLD was explored (19–23). All five meta-analyses showed a positive association between the HP infection and NAFLD risk, but most of the included populations were Asian. Non-Asian populations usually have lower rates of a HP infection, which may prove consistency of this association (24). Subgroup analyses by region for four of these five meta-analyses (19–22) further indicated the positive relationship between HP infection and NAFLD for the Asian population. However, in one of the four meta-analyses, it was found that the relationship was no longer significant in the non-Asian population, and the other three meta-analyses had different results. Several new studies on this topic in the Western population were published (3, 25–28). The results showed that, different from the Asian population, no overall associations were observed between HP and NAFLD (24, 29).
Therefore, this systematic review and meta-analysis were conducted to explore further the relationship between HP infection and NAFLD in the Asian and non-Asian populations to update the evidence and fill in the gaps mentioned above. Besides, we did subgroup analysis based on the different diagnosis methods of NAFLD and HP, the degree of covariate adjustment, the study design, and gender.
Methods
This systematic review and meta-analysis complied with the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (30). This study has been registered in PROSPERO (Registration ID: CRD42021266871).
Search strategy
The PubMed (1951), EMBASE (1966), the Cochrane Library (2000), and Web of Science (1900) were systematically searched to July 22, 2021 using the medical subject headings (MeSH) and related text words. Supplementary Table 1 presents the detailed search strategy. The reference lists of the original studies included in our analysis were also searched as well as those listed in the published review and meta-analyses (19–23).
Eligibility criteria and study selection
This study included original publications to evaluate the association between HP infection and the risk of NAFLD. We excluded the followings studies: (1) no information of the country for included population; (2) animal experiments, case reports, case series, reviews, practice guidelines, commentaries, and editorials; (3) unavailable data; (4) non-English or non-Chinese language publications. If more than one publication on the same study population was available, only the most recent publication was included. Two reviewers (Xiajing Chu, Zhiyuan Ma) independently screened the titles and abstracts, selected relevant full texts, and assessed them for eligibility. Non-conformity was resolved by discussion.
Data extraction
Using a predefined data collection form, two reviewers (Xiajing Chu, Zhiyuan Ma) independently extracted the following data: the first author, year of publication, country of participants, study design, publication type, sample size, participants setting, time of the study, age of participants, methods used for identification, diagnosis of the HP infection and NAFLD, adjusted in the multivariate analysis or non-adjusted effect estimates with the 95% CI. If effect estimates were not provided, the odds ratio (OR) and 95% CI were calculated. Any discrepancies between the reviewers were resolved by discussion, or by resort to a third reviewer (Xiang Yan) if consensus could not be reached.
Risk of bias assessment
Two independent reviewers (Zhiyuan Ma, Xiang Yan) assessed the risk of bias based on the Newcastle-Ottawa Scale (NOS) (31) for the cohort study and case-control study and the Agency for Healthcare Research and Quality (AHRQ) for the cross-sectional study (32). In the case of disagreement, a third investigator (Xiajing Chu) was consulted. The included cohort studies and case-control studies were rated as “low quality” (0–3 points), “moderate quality” (4–6 points) or “high quality” (7–9 points) based on their overall score on the NOS. The included cross-sectional studies were regarded as high quality (8–11 points), moderate quality (4–6 points), and low quality (< 4 points) based on their overall score on the AHRQ.
Statistical analyses
This meta-analysis was performed using R software v3.6.1. The odds ratio (OR), hazard ratio (HR) and 95% CI were used to explore the relationship between HP infection and NAFLD. The extent of heterogeneity was interpreted based on the total percentage of variation between the relevant studies, as measured by the I2 statistical parameter. The heterogeneity was categorized as low if I2 was 0–25%, moderate if I2 was 25–50%, and high if I2 was more than 50% (33). Additionally, Cochrane's Q-test was used to assess the presence of heterogeneity. The P-value by the Cochrane's Q-test > 0.05 indicated no significant heterogeneity among the included studies (22). When the heterogeneity was low, a fixed-effects model was used. Otherwise, a random-effects model was used (19). If necessary, multiple reported analyses per outcome were combined using fixed-effects models, so that each study contributed at most one effect size for each outcome.
Previous studies found a difference in the association of the HP infection with the risk of NAFLD between the Asian and non-Asian populations. Therefore, we analyzed Asian and non-Asian populations, respectively. The Asian population was defined as the population from Asian countries, such as China, India, Iran, Japan, and South Korea. The Non-Asian population was defined as the population from a non-Asian country.
Subgroup and sensitivity analyses were performed to explore the sources of heterogeneity. Subgroup analysis was conducted based on the different diagnosis methods of NAFLD and HP. The common NAFLD methods included liver biopsy, ultrasound, hepatic steatosis index (HIS), NAFLD liver fat score (NAFLD-LFS), and fatty liver index (FLI). HP methods included invasive tests, serology, breath test (UBT), and Stool antigen tests. Furthermore, subgroup analysis was also based on the degree of covariate adjustment, the study design, and gender. Because these factors may have an influence on the overall association between NAFLD and HP based on previous studies (19–23).
Additionally, sensitivity analyses were performed to determine the influence of individual studies on the overall estimates by serially excluding each study.
Funnel plots and the Egger test were used to assess potential publication bias. If the number of included studies were < 10, testing for publication bias was not performed (34).
Results
Study selection
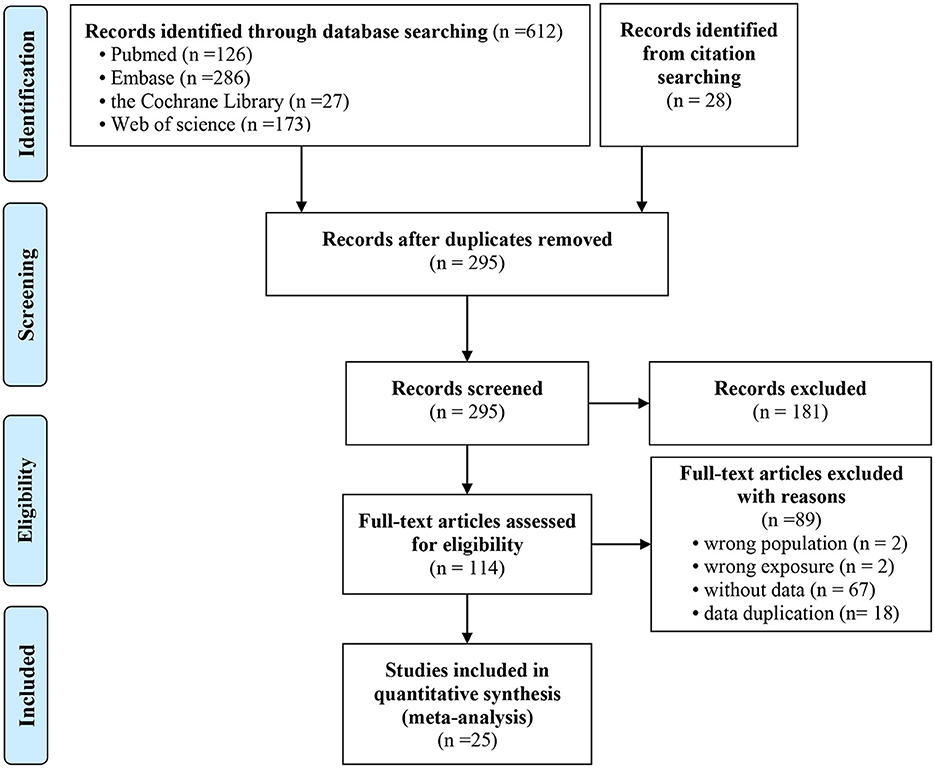
For this study, 612 potentially relevant studies were retrieved using the predefined search strategy, and another 28 studies were retrieved through other sources. Among these articles, 345 were duplicate publications. A total of 181 studies were excluded by screening the titles and abstracts. Of the remaining 114 potential eligible articles, 89 studies were excluded by carefully examining the abstracts or full texts. Finally, 25 studies met the inclusion criteria and were included in our meta-analysis. Two studies reported the HR (35, 36), and 23 studies reported the OR (3, 25–29, 37–53). In these 23 studies, one study reported the OR values based on different levels of NAFLD (mild, moderate and severe) (50); another study reported the OR values based on the different white blood cell (WBC) count (52). Therefore, 25 studies were included in this meta-analysis. Figure 1 shows the detailed flowchart of the selection process of eligible studies.
Study characteristics
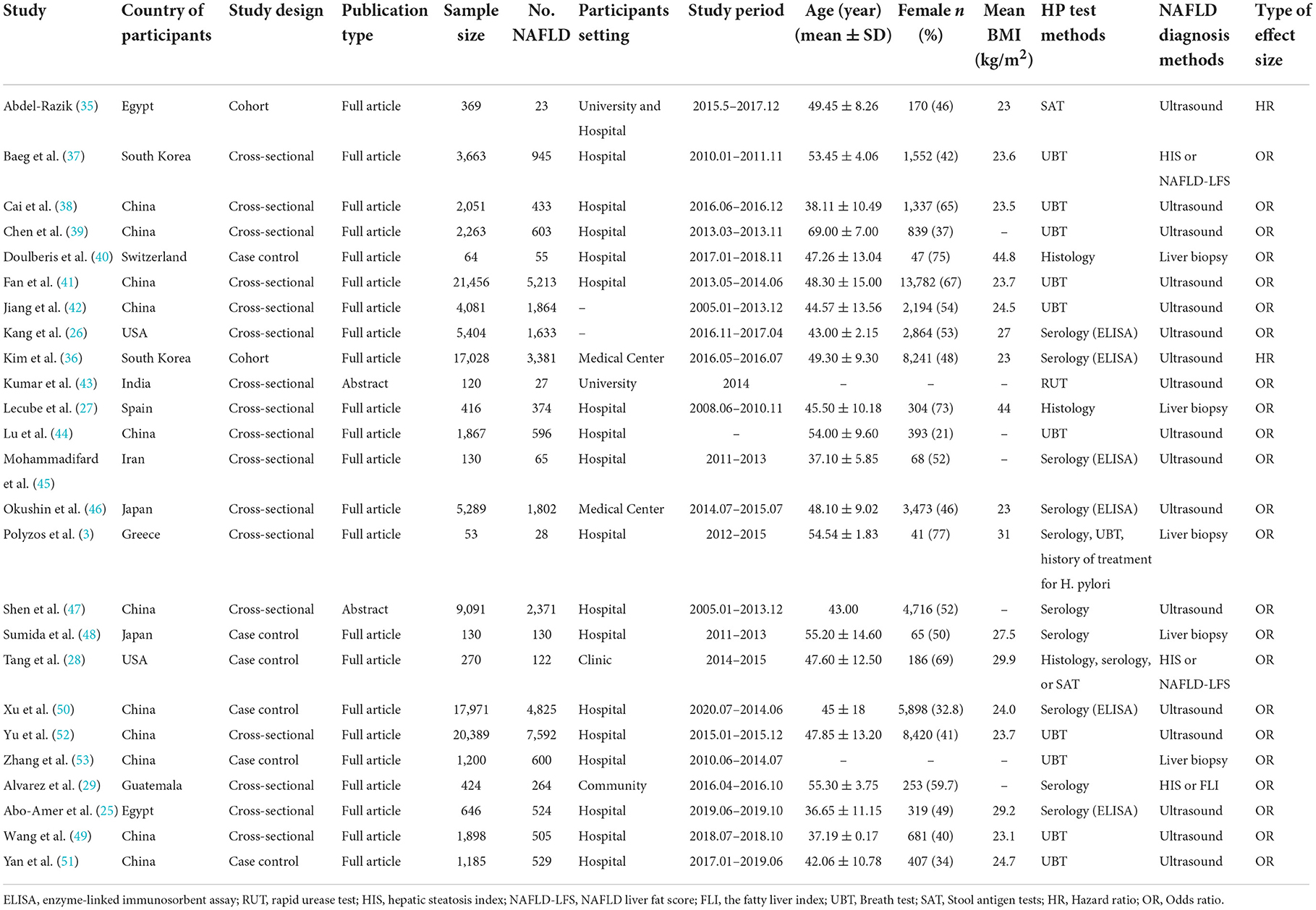
Twenty-five studies involving 117,458 participants were included (Table 1). This review contained two cohort studies (35, 36), 17 cross-sectional studies (3, 25–27, 29, 37–39, 41–47, 49, 52), and six case-control studies (28, 40, 48, 50, 51, 53). These studies were published between 2013 and 2021, three of which were conference abstracts (43, 47, 54). The participants from 17 studies were from Asia, and eight studies involved participants who were not from Asia. Regarding the HP test method, two studies used multiple methods. Of the remaining studies, three used only invasive tests, 10 used the breath test, nine used the serology, and one used stool Ag tests. Additionally, most studies (17 studies) used ultrasound to diagnose NAFLD.
Quality assessment
In this study, quality assessments were conducted on full texts. Supplementary Table 2 presents the quality assessment results according to NOS for the cohort study and the case-control study. The scores of included studies ranged from six to nine (mean 7.75), and the comparability had a low score for most included studies. Supplementary Table 3 presents the results of AHRQ for the cross-sectional study. The scores of included studies ranged from one to 15 (mean 6.53). In only one study, the handling of missing data in the analysis was explained (49), and in only one study, the patient response rates and completeness of the data collector were summarized (41).
HP infection and NAFLD for Asian and non-Asian
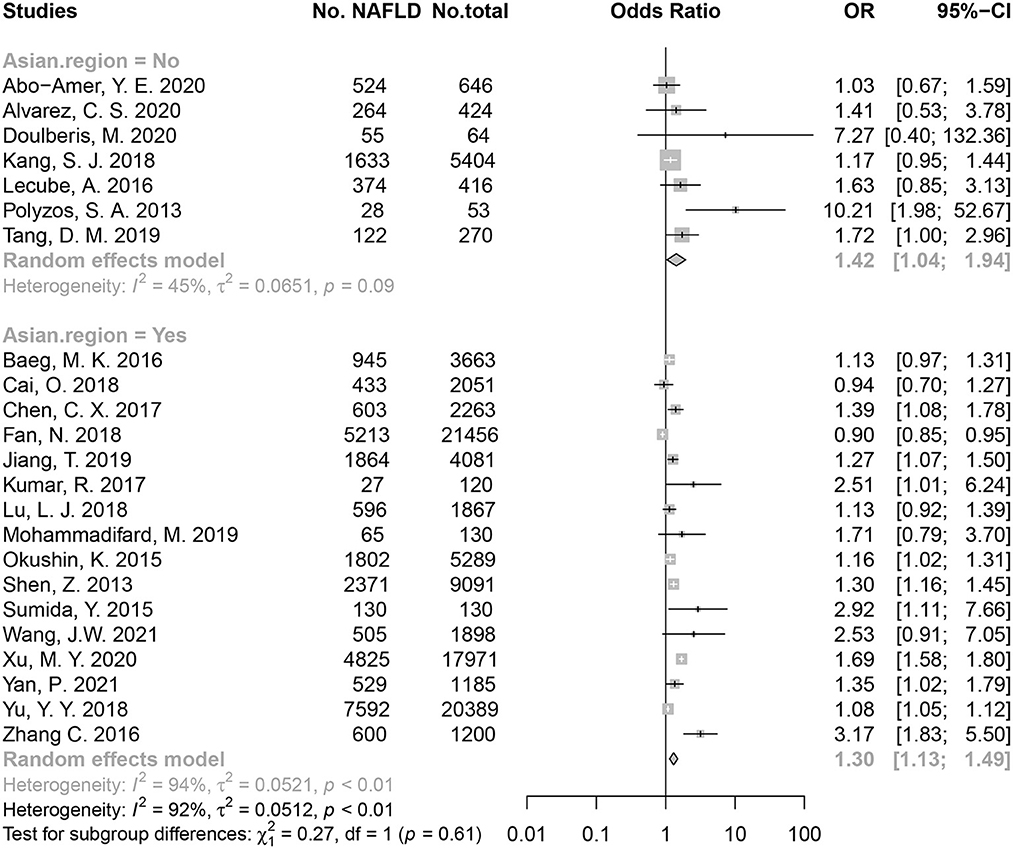
In an overall pooled analysis, HP-positive populations had a higher risk of NAFLD than HP-negative populations (OR = 1.30) both in Asian and non-Asian countries. Regarding The Asian population, the meta-analysis using random-effects model showed HP infection was associated with a risk of 1.30 of developing NAFLD (OR = 1.30, 95% CI: 1.13–1.49, P < 0.01; I2 = 94.30%, P < 0.01; Figure 2). The HP infection was also associated with an increase risk of NAFLD in non-Asian population (OR = 1.42, 95% CI: 1.04–1.94, P = 0.03; I2 = 44.90%, P = 0.09; Figure 2). No significant difference in the association was found between non-Asia and Asia populations (P = 0.61). Besides, two studies reported the effect size of HR (35, 36), and the pooled HR supported the association between HP infection and risk of NAFLD (HR = 1.13, 95% CI: 1.04–1.23) (Supplementary Figure 1).
Subgroup analysis for the Asian population
Country of population
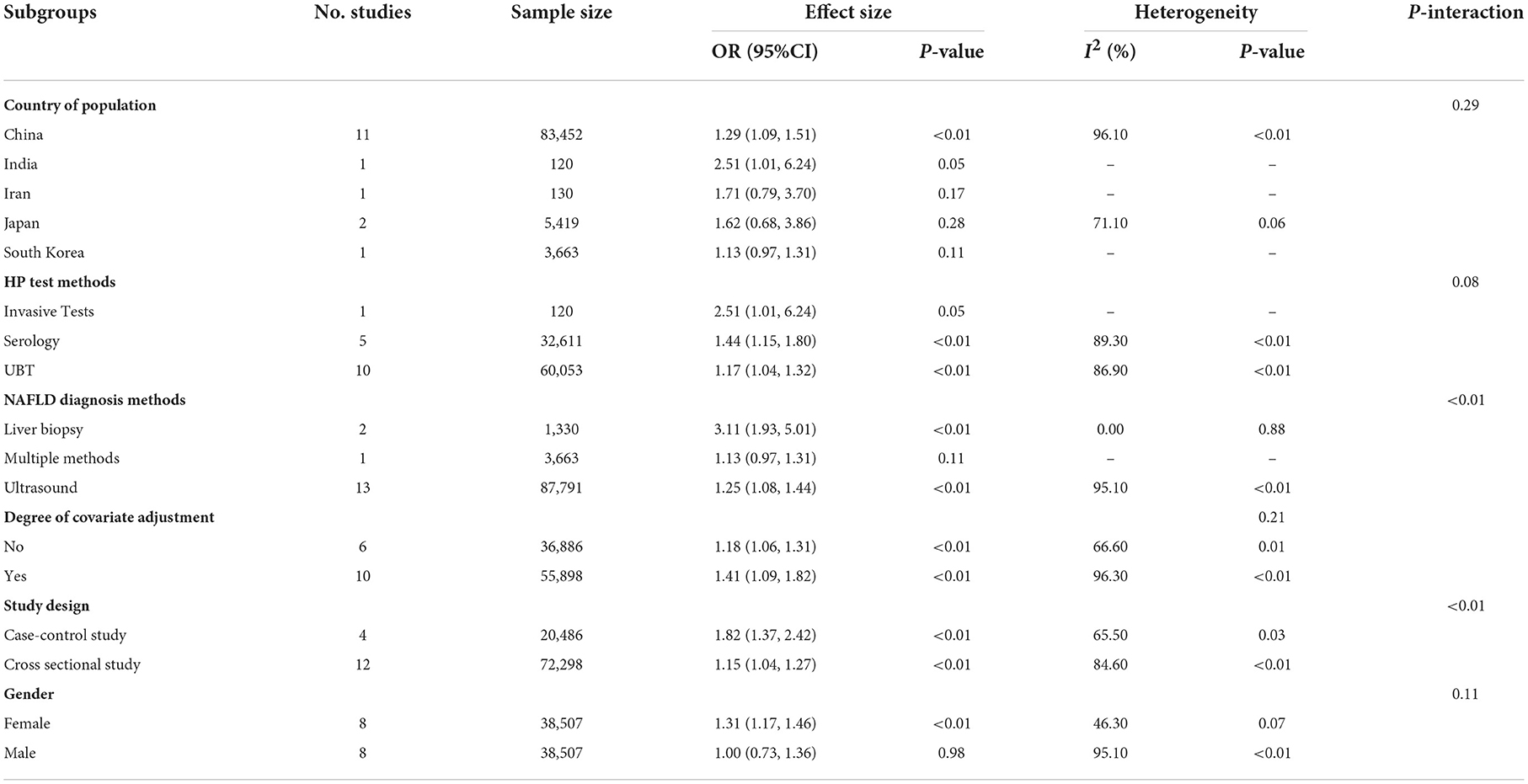
Table 2 shows the subgroup analyses of the Asian population for 16 studies that used OR as an effect size. The HP infection was associated with the risk of NAFLD (OR = 1.29, 95% CI: 1.09–1.51, P < 0.01; I2 = 96.10%, P < 0.01) for Chinese (11 studies, 36,942 participants). Subgroup analysis showed no significant difference among countries (P = 0.29).
HP test methods
For the three main HP test methods, the positive association between HP infection and the risk of NAFLD were observed in one study that used invasive tests (OR = 2.51, 95% CI 1.01–6.24, P = 0.05; Table 2), in five studies that used serology (OR = 1.44, 95% CI 1.15–1.80, P < 0.01; I2 = 89.30%, P < 0.01; Table 2), and in ten studies that used UBT (OR = 1.17, 95% CI 1.04–1.32, P < 0.01; I2 = 86.90%, P < 0.01; Table 2). Subgroup analysis showed significant differences among invasive tests, serology, and UBT groups (P = 0.08; Table 2).
NAFLD diagnosis methods
For the three main NAFLD diagnosis methods, the positive association between HP infection and the risk of NAFLD were observed in two studies that used liver biopsy (OR = 3.11, 95% CI 1.93–5.01, P < 0.01; I2 = 0.00%, P = 0.88; Table 2), and in 13 studies that used ultrasound (OR = 1.25, 95% CI 1.08–1.44, P < 0.01; I2 = 95.10%, P < 0.01; Table 2). Subgroup analysis showed a significant difference among liver biopsy, multiple methods, and ultrasound groups (P < 0.01; Table 2).
Degree of covariate adjustment
The meta-analysis on six studies with 33,770 participants without covariate adjustment showed the positive association between HP infection and the risk of NAFLD (OR = 1.18, 95% CI 1.06–1.31, P < 0.01; I2 = 66.60%, P = 0.01; Table 2). The positive association between HP infection and the risk of NAFLD was confirmed by 10 studies with 34,213 participants with covariate adjustment (OR = 1.41, 95% CI 1.09–1.82, P < 0.01; I2 = 96.30%, P < 0.01; Table 2), the adjusted covariates included sex, age, education level, medical history, lifestyles, and biomarks (see Supplementary Table 4). Subgroup analysis showed no significant differences between studies without covariate adjustment and with covariate adjustment groups (P = 0.21; Table 2).
Study design
Subgroup analysis showed a significant difference between case-control studies and cross-sectional studies (P < 0.01; Table 2), although neither group found the positive association between HP infection and the risk of NAFLD. The pooled OR of four case-control studies with 7,480 participants was 1.82 (OR = 1.82, 95% CI 1.37–2.42, P < 0.01; I2 = 65.50%, P = 0.03; Table 2). The pooled OR from 12 studies with 60,503 participants (cross-sectional studies) was 1.15 (OR = 1.15, 95% CI 1.04–1.27, P < 0.01; I2 = 84.60%, P < 0.01; Table 2).
Gender
Subgroup analysis based on gender showed the HP infection had positive association with risk of NAFLD in female group (OR = 1.31, 95% CI: 1.17–1.46, P < 0.01; I2 = 46.30%, P = 0.07) but not in male group (P = 0.98). Subgroup analysis showed no significant differences between males and females (P = 0.11; Table 2).
Subgroup analysis in non-Asian countries
Country of population
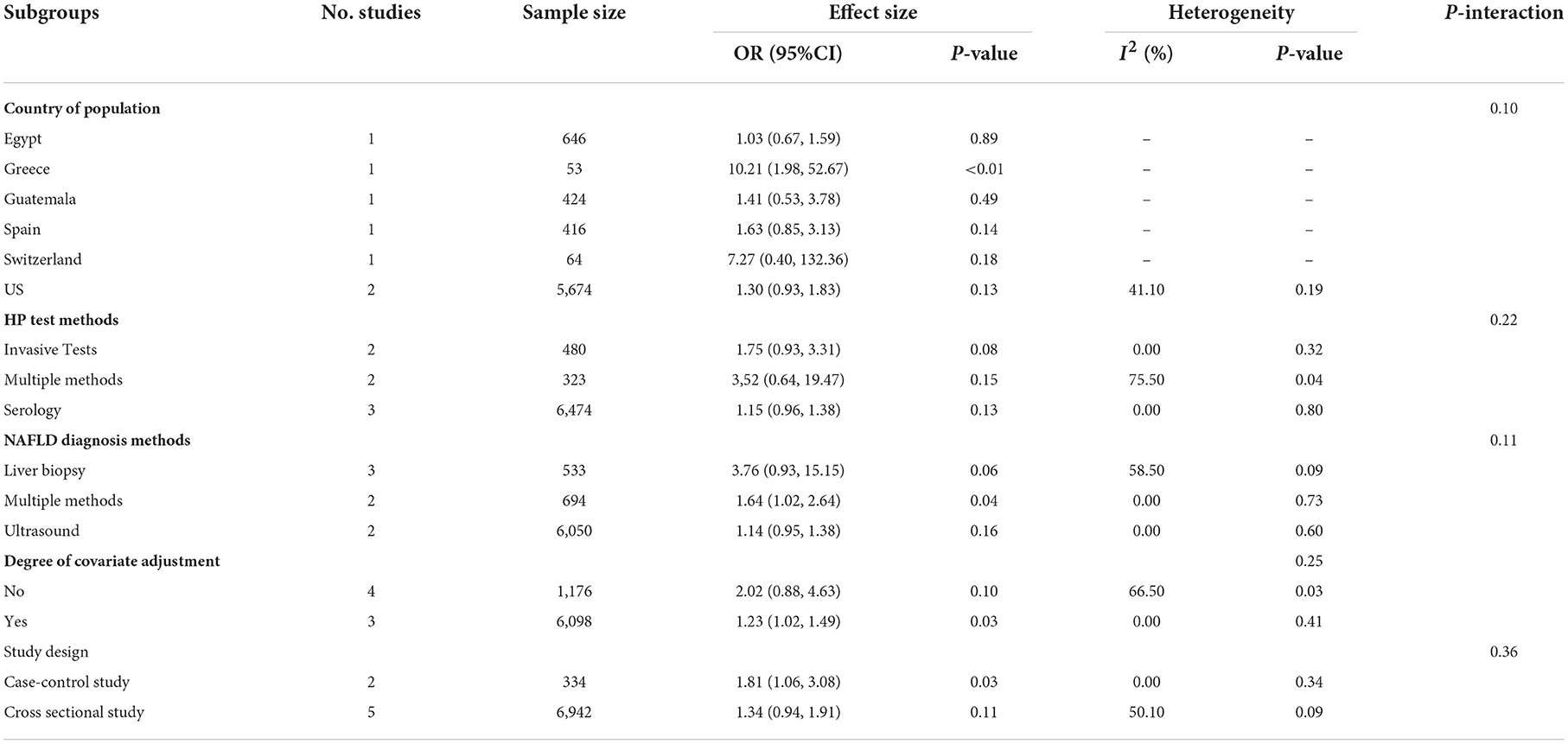
Table 3 shows the subgroup analyses of the non-Asian population for seven studies that used OR as an effect size. Subgroup analysis showed no significant differences among countries (P = 0.10; Table 3). No association between the HP infection and NAFLD risk was found except for Greece (Table 3).
HP test methods
For the three main HP test methods, no association between HP infection and the risk of NAFLD were observed in two studies that used invasive tests (OR = 1.75, 95% CI 0.93–3.31, P = 0.08; I2 = 0.00%, P = 0.32; Table 3), in three studies that used serology (OR = 3.52, 95% CI 0.64–19.47, P = 0.15; I2 = 75.50%, P = 0.04; Table 3), or in two studies that used multiple methods (OR = 1.15, 95% CI 0.96–1.38, P = 0.13; I2 = 0.00%, P = 0.80; Table 3). Subgroup analysis showed no significant differences among invasive tests, serology and multiple methods groups (P = 0.22; Table 3).
NAFLD diagnosis methods
For the three main NAFLD diagnosis methods, the positive association between HP infection and the risk of NAFLD were observed in two studies that used multiple methods (OR = 1.64, 95% CI 1.02–2.64, P = 0.04; I2 = 58.50%, P = 0.09; Table 3) but not for the studies using serology (P = 0.13) and multiple methods (P = 0.15). Subgroup analysis showed no significant differences among liver biopsy, multiple methods and ultrasound groups (P = 0.11; Table 3).
Degree of covariate adjustment
The meta-analysis on four studies with 33,770 participants without covariate adjustment showed no association between the HP infection and the risk of NAFLD (OR = 2.02, 95% CI 0.88–4.63, P = 0.10; I2 = 66.50%, P = 0.03; Table 3). But positive association between HP infection and the risk of NAFLD was observed for three studies with 34,213 participants with covariate adjustment (OR = 1.23, 95% CI 1.02–1.49, P = 0.03; I2 = 0.00%, P = 0.41; Table 3), the adjusted covariates included sex, age, education level, medical history, lifestyles, and biomarks (see Supplementary Table 4). The subgroup analysis showed no significant difference was observed among studies without covariate adjustment and with covariate adjustment groups (P = 0.25; Table 3).
Study design
Subgroup analysis showed no significant differences among case-control studies and cross-sectional studies groups (P = 0.36; Table 3). The positive association between HP infection and the risk of NAFLD was observed for two case-control studies with 31,834 participants (OR = 1.81, 95% CI 1.06–3.08, P = 0.03; I2 = 0.00%, P = 0.34; Table 3). But no association was observed for five cross-sectional studies with 5,448 participants (OR = 1.34, 95% CI 0.94–1.91, P = 0.11; I2 = 50.10%, P = 0.09; Table 3).
Sensitivity analysis and publication bias
Sensitivity analysis was performed by removing one study at a time, and the P-value confirmed the stability of the results for the Asian population. However, the results of the non-Asian population were not stable enough (Supplementary Figures 1–3). Analysis of the funnel plot of the OR for publication bias suggested the absence of bias because of plot symmetry (Supplementary Figure 4). Furthermore, the Egger test showed no publication bias (P = 0.17).
Discussion
Main findings
This meta-analysis included 25 studies (two control studies, six case-control studies, and 17 cross-sectional studies) involving 107,306 participants from 11 countries. The data studies showed a positive association between HP and the risk of development of NAFLD both for Asian and non-Asian populations. Similar results were observed in most subgroups of the Asian populations, except for the male group. However, the association for the non-Asian population was not stable enough.
Potential explanations and implications
In several studies, the underlying mechanism involved in HP infection and the risk of NAFLD development were explained. HP infection is known to cause chronic low-grade systemic inflammation by increasing the levels of proinflammatory cytokines (55). Additionally, HP influences the development of NAFLD through hormonal effects (13). Moreover, HP-induced bacterial translocation in chronic liver disease can be detected by human β-defensin-1(40).
A prospective multicenter pilot cohort study (35) showed that after eradication therapy of HP infection, there was a significant reduction in levels of C-reactive protein, leptin, insulin resistance, NAFLD-LFS, TNF-α, and IL-6. After a 24-month follow-up, the incidence rate of NAFLD in patients with eradication therapy was five times lower compared to that in untreated patients. Additionally, a randomized controlled trial (56) reported a significant improvement in insulin resistance after 24 weeks of successful eradication of HP. Thus, the eradication of HP had an advantageous effect on metabolic diseases, such as NAFLD. These findings were consistent with our results. Although the forest plot of the OR both for Asian and non-Asian populations showed a moderate or high heterogeneity, the sensitivity analysis and publication bias substantiated the robustness of our results for the Asian population. NAFLD diagnosis methods and study design contributed to the heterogeneity of the association for the Asian population.
Subgroup analysis based on different country populations showed positive associations between HP infection and the risk of NAFLD in China, India, and Greece. Considering the small size effect, we thought that the positive association in China was more robust. This observation was consistent with the data presented in previous studies (24, 29). The pathogenesis of NAFLD is known to include adipose tissue-derived hormones, nutritional factors, genetic and epigenetic factors, gut microbiota, and insulin resistance (57, 58). Because some information on these pathogenesis factors and metabolic risk factors (e.g., body weight, hypertension, hyperglycemia, and dyslipidemia) was lacking, subgroup analysis based on these factors was not conducted. Further studies are required to verify these results by well-designed human studies, considering the complex interactions with confounding factors, such as environmental and genetic susceptibility factors (19). Additionally, we found that females with HP infection had a higher risk of NAFLD in the Asian population. Although the underlying mechanism of action is unclear, many studies have shown that gender is a factor influencing the risk of an infection (36, 41, 48). Besides, the observed differences in associations are most likely due to social determinants of health, but not decided by biological or genetic factor only. The difference on associations between Asian and non-Asian population were possibly caused by adoption of dietary and lifestyle habits, crowded living conditions, poor sanitation, and lack of access to care (24, 59). However, the small sample size in our included populations might be a confounding variable.
Comparison with previous work
Five studies (19–23) assessed the association between HP infection and NAFLD, and all studies concluded that the presence of a significant relationship between HP and NAFLD, and one study noted a 36% increased risk of NAFLD in patients with HP infection (20). HP infection indeed showed a positive association with NAFLD for the Asian population. These findings were similar to the findings of our study. Compared with previous meta-analyses, this study used a larger sample size to explore the association between HP infection and NAFLD in the non-Asian population than in a previous Meta-analysis. In our review, we included additional studies, including cross-sectional, case-control, and cohort studies. The comprehensive subgroup analysis, which was based on the region of the population, the different diagnosis methods of NAFLD and HP, the degree of covariate adjustment, and the study design, confirmed the robustness of our results.
Strengths and limitations
A specific subgroup analysis was performed based on the country of the population, the different diagnosis methods used for NAFLD and HP, the degree of covariate adjustment, and the study design. These methods supported our conclusions. Our study had several limitations. First, the included studies had a small sample size of fewer than 500 participants, which might affect the quality of evidence. Second, the bias of the included retrospective studies might affect the quality of evidence. Additionally, the Asian and Non-Asian populations were defined as a population from the Asian or Non-Asian countries, respectively. The designation may not be reliable enough, but there is no more precise approach due to lack of data. Finally, subgroup analyses were performed based on potential confounding variables, but subgroup analyses by several important pathogenesis factors, HP test ranges, and metabolic risk factors (e.g., body weight, hypertension, hyperglycemia, and dyslipidemia) could not be conducted because of lacking information. Further prospective studies are required to perform an in-depth analysis of the heterogeneity.
Conclusions
This systematic review and meta-analysis suggested that HP infection was associated with an increased risk of NAFLD in Asian and non-Asian populations. However, the association was not found for Asian males; the association for the non-Asian population was not stable enough. Further studies are required to establish a causal association between HP infection and NAFLD. Thus, eradicating HP infection might be a new approach to the clinical prevention and treatment of NAFLD.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to d3dqb3VAMTI2LmNvbQ==.
Author contributions
ZM and XC conceptualized and designed the protocol, drafted the initial manuscript, and reviewed the manuscript. XY and ZM defined the concepts and search items and data extraction process as well as methodological appraisal of the studies. XC, XY, and WW planned the data extraction and statistical analysis. XY and WW provided critical insights. All authors have approved and contributed to the final written manuscript.
Acknowledgments
We would like to thank the authors of the included primary studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1062942/full#supplementary-material
References
1. Maher JJ, Schattenberg JM. Non-alcoholic fatty liver disease in 2020. Gastroenterology. (2020) 158:1849–50. doi: 10.1053/j.gastro.2020.04.013
2. Iqbal U, Perumpail B, Akhtar D, Kim D, Ahmed A. The epidemiology, risk profiling and diagnostic challenges of non-alcoholic fatty liver disease The epidemiology, risk profiling and diagnostic challenges of non-alcoholic fatty liver disease. Medicines. (2019) 6:41. doi: 10.3390/medicines6010041
3. Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E, Zafeiriadou E, et al. Helicobacter pylori infection in patients with non-alcoholic fatty liver disease. Metabolism. (2013) 62:121–6. doi: 10.1016/j.metabol.2012.06.007
4. Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol. (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
5. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
6. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. (2018) 69:896–904. doi: 10.1016/j.jhep.2018.05.036
7. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology. (2019) 69:2672–82. doi: 10.1002/hep.30251
8. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
9. McColl KE. Clinical practice. Helicobacter pylori infection. New England J Med. (2010) 362:1597–604. doi: 10.1056/NEJMcp1001110
10. de Korwin JD, Ianiro G, Gibiino G, Gasbarrini A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter. (2017) 22:e12411. doi: 10.1111/hel.12411
11. Cheng DD, He C, Ai HH, Huang Y, Lu NH. The possible role of helicobacter pylori infection in non-alcoholic fatty liver disease. Front Microbiol. (2017) 8:743. doi: 10.3389/fmicb.2017.00743
12. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. (2018) 15:397–411. doi: 10.1038/s41575-018-0011-z
13. Castaño-Rodríguez N, Mitchell HM, Kaakoush NO. NAFLD helicobacter species and the intestinal microbiome. Best practice & research. Clin Gastroenterol. (2017) 31:657–68. doi: 10.1016/j.bpg.2017.09.008
14. Tian J, Zhang J, Ge L, Yang K, Song F. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. (2017) 85:50–8. doi: 10.1016/j.jclinepi.2016.12.004
15. Xiu-xia L, Ya Z, Yao-long C, Ke-hu Y, Zong-jiu Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. (2015) 119:503–10. doi: 10.1016/j.healthpol.2014.09.002
16. Yao L, Sun R, Chen Y-L, Wang Q, Wei D, Wang X, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol. (2016) 74:73–9. doi: 10.1016/j.jclinepi.2016.01.003
17. Yang K. Evidence-based social science: the origin, development and prospects. J Librarianship Inf Sci. (2018) 3:1e10. doi: 10.11968/tsyqb.1003-6938.2018038
18. Yang K, Li X, Bai Z. Evidence-Based Social Science Research Methods: Systematic Review and Meta-Analysis. Lanzhou: Lanzhou University Press (2018).
19. Zhou BG, Yang HJ, Xu W, Wang K, Guo P, Ai YW. Association between Helicobacter pylori infection and non-alcoholic fatty liver disease: a systematic review and meta-analysis of observational studies. Helicobacter. (2019) 24:e12576. doi: 10.1111/hel.12576
20. Ning L, Liu R, Lou X, Du H, Chen W, Zhang F, et al. Association between Helicobacter pylori infection and non-alcoholic fatty liver disease: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. (2019) 31:735–42. doi: 10.1097/MEG.0000000000001398
21. Mantovani A, Turino T, Altomari A, Lonardo A, Zoppini G, Valenti L, et al. Association between Helicobacter pylori infection and risk of non-alcoholic fatty liver disease: an updated meta-analysis. Metabolism. (2019) 96:56–65. doi: 10.1016/j.metabol.2019.04.012
22. Liu R, Liu Q, He Y, Shi W, Xu Q, Yuan Q, et al. Association between helicobacter pylori infection and non-alcoholic fatty liver: a meta-analysis. Medicine. (2019) 98:e17781. doi: 10.1097/MD.0000000000017781
23. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Manatsathit W, Jaruvongvanich V, Ungprasert P. Helicobacter pylori and risk of non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol. (2018) 52:386–91. doi: 10.1097/MCG.0000000000000784
24. Polyzos SA, Kountouras J, Mantzoros CS. Helicobacter pylori infection and non-alcoholic fatty liver disease: are the four meta-analyses favoring an intriguing association pointing to the right direction? Metabol. Clin. Exp. (2019) 96:iii–v. doi: 10.1016/j.metabol.2019.05.006
25. Abo-Amer YE, Sabal A, Ahmed R, Hasan NFE, Refaie R, Mostafa SM, et al. Relationship between helicobacter pylori infection and non-alcoholic fatty liver disease (NAFLD) in a developing country: a cross-sectional study. Diabetes Metabol Syndrome Obesity Targets Therapy. (2020) 13:619–25. doi: 10.2147/DMSO.S237866
26. Kang SJ, Kim HJ, Kim D, Ahmed A. Association between cagA negative helicobacter pylori status and non-alcoholic fatty liver disease among adults in the United States. PLoS ONE. (2018) 13:e0202325. doi: 10.1371/journal.pone.0202325
27. Lecube A, Valladares S, Lopez-Cano C, Gutierrez L, Ciudin A, Fort JM, et al. The role of morbid obesity in the promotion of metabolic disruptions and non-alcoholic steatohepatitis by helicobacter pylori. PLoS ONE. (2016) 11:9. doi: 10.1371/journal.pone.0166741
28. Tang DM, Chascsa DM, Chou JY, Ho N, Auh S, Wank SA, et al. Helicobacter pylori infection is strongly associated with metabolic syndrome, and weakly associated with non-alcoholic fatty liver disease, in a US Hispanic population. GastroHep. (2019) 1:325–31. doi: 10.1002/ygh2.375
29. Alvarez CS, Florio AA, Butt J, Rivera-Andrade A, Kroker-Lobos MF, Waterboer T, et al. Associations between Helicobacter pylori with non-alcoholic fatty liver disease and other metabolic conditions in Guatemala. Helicobacter. (2020). doi: 10.1111/hel.12756
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
31. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
32. Rostom A, Dube C, Cranney A, Saloojee N, Richmond Sy MD. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US). (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK35156/.
33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
34. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
35. Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elhelaly R, Elzehery R, et al. Helicobacter pylori and non-alcoholic fatty liver disease: a new enigma? Helicobacter. (2018) 23:e12537. doi: 10.1111/hel.12537
36. Kim TJ, Sinn DH, Min YW, Son HJ, Kim JJ, Chang Y, et al. A cohort study on Helicobacter pylori infection associated with non-alcoholic fatty liver disease. J Gastroenterol. (2017) 52:1201–10. doi: 10.1007/s00535-017-1337-y
37. Baeg MK, Yoon SK, Ko SH, Noh YS, Lee IS, Choi MG. Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease. World J Gastroenterol. (2016) 22:2592–600. doi: 10.3748/wjg.v22.i8.2592
38. Cai O, Huang Z, Li M, Zhang C, Xi F, Tan S. Association between helicobacter pylori infection and non-alcoholic fatty liver disease: a single-center clinical study. Gastroenterol Res Pract. (2018) 2018:8040262. doi: 10.1155/2018/8040262
39. Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and non-alcoholic fatty liver disease. Appl Physiol Nutr Metab. (2017) 42:295–301. doi: 10.1139/apnm-2016-0499
40. Doulberis M, Srivastava S, Polyzos SA, Kountouras J, Papaefthymiou A, Klukowska-Rötzler J, et al. Active helicobacter pylori infection is independently associated with non-alcoholic steatohepatitis in morbidly obese patients. J Clin Med. (2020) 9:933. doi: 10.3390/jcm9040933
41. Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease: a cross-sectional study in China. Front Microbiol. (2018) 9:73. doi: 10.3389/fmicb.2018.00073
42. Jiang T, Chen X, Xia C, Liu H, Yan H, Wang G, et al. Association between helicobacter pylori infection and non-alcoholic fatty liver disease in North Chinese: a cross-sectional study. Sci Rep. (2019) 9:4874. doi: 10.1038/s41598-019-41371-2
43. Kumar R, Dixit VK, Shukla SK, Sachan S, Agarwal S. The association of Helicobacter pylori infection in non-alcoholic fatty liver disease patients. Indian J Gastroenterol. (2017) 36:A33. doi: 10.1007/s12664-017-0798-5
44. Lu LJ, Hao NB, Liu JJ Li X, Wang RL. Correlation between Helicobacter pylori Infection and Metabolic Abnormality in General Population: A Cross-Sectional Study. Gastroenterol Res Pract. (2018) 2018:7410801. doi: 10.1155/2018/7410801
45. Mohammadifard M, Saremi Z, Rastgoo M, Akbari E. Relevance between Helicobacter pylori Infection and Non-Alcoholic Fatty Liver Disease in Birjand, Iran. J Med Life. (2019) 12:168–72. doi: 10.25122/jml-2019-0012
46. Okushin K, Takahashi Y, Yamamichi N, Shimamoto T, Enooku K, Fujinaga H, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. (2015) 15:25. doi: 10.1186/s12876-015-0247-9
47. Shen Z, Qin Y, Lu Y, Yu C, Li Y. Association between helicobacter pylori infection diagnosed by serological status and non-alcoholic fatty liver disease: A cross-sectional study. United Eur Gastroenterol J. (2013) 1:A99. doi: 10.1177/2050640613502899
48. Sumida Y, Kanemasa K, Imai S, Mori K, Tanaka S, Shimokobe H, et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in non-alcoholic fatty liver disease. J Gastroenterol. (2015) 50:996–1004. doi: 10.1007/s00535-015-1039-2
49. Wang J, Dong F, Su H, Zhu L, Shao S, Wu J, et al. Pylori is related to nafld but only in female: a cross-sectional study. Int J Med Sci. (2021) 18:2303–11. doi: 10.7150/ijms.50748
50. Xu MY, Ma JH, Du J, Yin J, Liu L, Cui F, et al. Non-alcoholic Fatty Liver Disease Is Associated with Helicobacter pylori Infection in North Urban Chinese: A Retrospective Study. Gastroenterol Res Pract. (2020) 2020:9797841. doi: 10.1155/2020/9797841
51. Yan P, Yu B, Li M, Zhao W. Association between non-alcoholic fatty liver disease and infection in Dali City, China. Saudi Med J. (2021) 42:735–41. doi: 10.15537/smj.2021.42.7.20210040
52. Yu YY, Cai JT, Song ZY, Tong YL, Wang JH. The associations among Helicobacter pylori infection, white blood cell count and non-alcoholic fatty liver disease in a large Chinese population. Medicine. (2018) 97:e13271. doi: 10.1097/MD.0000000000013271
53. Zhang C, Guo L, Qin Y, Li G. Correlation between Helicobacter pylori infection and polymorphism of adiponectin gene promoter-11391G/A, superoxide dismutase gene in non-alcoholic fatty liver disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2016) 41:359–66. doi: 10.11817/j.issn.1672-7347.2016.04.004
54. Roberts J, Le Blanc W, Bambha K. Associations between helicobacter pylori, leptin, and non-alcoholic fatty liver disease (NAFLD). Hepatology. (2014) 61:8A. doi: 10.1002/hep.27514
55. Waluga M, Kukla M, Zorniak M, Bacik A, Kotulski R. From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol. (2015) 7:2136–46. doi: 10.4254/wjh.v7.i18.2136
56. Maharshi V, Gupta P, Kumar VL, Upadhyay AD, Das P, Yadav R, et al. Helicobacter pyloriEffect of -eradication therapy on hepatic steatosis in patients with non-alcoholic fatty liver disease: a randomized-controlled pilot study. Gastroenterology report. (2020). doi: 10.1093/gastro/goz058
57. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
58. Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. (2013) 14:20704–28. doi: 10.3390/ijms141020704
Keywords: Helicobacter pylori infection, non-alcoholic fatty liver disease, risk factors, systematic review, meta-analysis
Citation: Ma Z, Chu X, Yan X and Wang W (2022) Association between Helicobacter pylori infection and non-alcoholic fatty liver disease for Asian and non-Asian population: A systematic review and meta-analysis. Front. Public Health 10:1062942. doi: 10.3389/fpubh.2022.1062942
Received: 06 October 2022; Accepted: 22 November 2022;
Published: 08 December 2022.
Edited by:
Sonia Michael Najjar, Ohio University, United StatesReviewed by:
Laura Jensen, United States Department of Veterans Affairs, United StatesElizabeth Ann Beverly, Ohio University, United States
Cosmin Mihai Vesa, University of Oradea, Romania
Copyright © 2022 Ma, Chu, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjin Wang, d3dqb3VAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Zhiyuan Ma1†
Zhiyuan Ma1† Wenjin Wang
Wenjin Wang