
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 17 January 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1054488
This article is part of the Research Topic Addressing the Sexually Transmitted Infections Epidemic in the United States: A Sociomedical Perspective View all 14 articles
The emergence of the monkeypox virus (MPXV) outbreak in 2022 is a worldwide health issue. The rapid increase of monkeypox cases caused the WHO to designate the escalating global monkeypox outbreak a Public Health Emergency of International Concern on July 23, 2022. The WHO has called on the group currently most affected by the virus, men who have sex with men (MSM), to limit their sexual partners. The diminution in number of sexual partners not only decreases the proportion of infected MSM but could also increases the number of days needed to reach a given infection level among the general population. Several behavioral factors could be associated with high levels of different sexual partners, such as cannabis use and alcohol consumption. Firstly, this review focuses on the association between cannabis and alcohol consumption and the number of sexual partners, and their possible impact on the current MPXV outbreak by impairing the immune responses. Secondly, this review investigated in the UK Biobank cohort the relationship between alcohol and cannabis use and the number of sexual partners. Among the 115,604 participants, 1.8% declared to be MSM, 1.9% to be WSW (women having sex with women), 43.3% men heterosexuals and 53.0% women heterosexuals. MSM and WSW showed higher lifetime sexual partners (N = 17.4 (SD:17.52) and N = 13.65 (SD: 13.21), respectively) compared to heterosexual men (N = 6.89 (SD: 9.47) and women (N = 5.19 (SD:6.56), p < 0.001. After adjustment for age, body mass index, lifetime sexual activity, educational and income levels, tobacco and cardiovascular diseases, cannabis use and alcohol consumption remained significantly associated with increase in the number of different sexual partners in all four subgroups. Thus, cannabis use and alcohol consumption may have two detrimental effects on the MPXV outbreak: by participating in the increase of the number of sexual partners which are mainly responsible for the augmentation of the number of new MPXV infected cases and by impairing the immune response to a viral infection. Health and safety policies should address the factors and practices, including chemsex, leading to an increase in risk of sexual behaviors responsible for MPXV dissemination in the worldwide population.
The emergence of the monkeypox virus (MPXV) outbreak in 2022 is a worldwide health issue (1). To date, more than 58,000 laboratory-confirmed cases and 18 deaths have been observed by the World Health Organization (WHO) from 103 territories in all six WHO Regions (2). The rapid increase in monkeypox infected cases caused the WHO to designate the escalating global monkeypox outbreak a Public Health Emergency of International Concern on July 23, 2022. Like previous infectious diseases (3), Monkeypox is a contagious disease which requires physical or sexual contact with people infected with the virus (4). Although the risk to the overall population is considered to be rather low, the WHO has responded by making this pandemic as a high priority to avoid further outbreaks (5). In a recent report, the WHO has called on the group currently most affected by the virus, men who have sex with men (MSM), to limit their sexual partners. Recent analyses showed numerous risk determinants, such as being young men, having sex with other men (MSM), having risk behavioral attitudes and activities, including condomless sex, PrEP (pre-exposure prophylaxis) and HIV positivity (6, 7). Indeed, the transmission of MPXV has been correlated with close relationships, especially sexual contact, between men (4, 8) but also within heterosexual populations (9). Studies have suggested that gay, bisexual, and other MSM have taken steps to protect themselves and their sexual partners against MPXV, such as reducing their number of sexual relationships (10, 11). Thus, the decrease in the number of sexual partners not only decreases the percentage of infected MSM but could also increase the number of days needed to reach a given infection level among the overall population. This could allow more time for vaccination campaigns to reach targeted people (12). The decrease in sexual partners could significantly decrease the MPXV transmission rate (11) and slow down the trend toward a pandemic (13). Several behavioral comportments could be associated with high levels of different sexual partners, such as cannabis use (14) and alcohol consumption (15). Thus, this review focuses on the association between cannabis and alcohol consumption on the number of sexual partners, sexual behaviors, and their possible impact on the current MPXV outbreak.
Monkeypox (MPX) is a zoonotic viral disease which originates from the monkeypox virus (MPXV). This disease has been known for over 50 years but was limited to a restricted number of endemic territories localized in Central and West Africa. Nevertheless, since the years 2000, sporadic reports of imported cases have been observed in North America, Europe, and the Middle East. To date, a worldwide epidemic has shown major issues as the disease is quickly spreading, mainly in young MSM, showing a classic vesicular-pustular rash along with other clinical symptoms (16). More than 65,000 laboratory-confirmed cases and 26 deaths have been observed by the World Health Organization (WHO) from all the territories in the WHO Regions (Figure 1) (2)1. The skin is the major source of infection and contamination (17). Although respiratory droplets are thought to diffuse the infection from person to person, the US CDC (Centers for Disease Control and Prevention) declared that the transmission requires a long face-to-face relationship due to the inability of the droplets to cover a long distance. Whereas MPXV remains not only sexually transmitted by vaginal or sperm secretions, health authorities have declared that the current epidemic is due to human-to-human sexual intercourse (4, 18). MPXV is observed in seminal fluid, genital and rectal lesions, and feces and saliva from confirmed infected people in several countries (4, 19). Thus, human-to-human propagation needs contact with lesions, respiratory droplets, or bodily fluids. MPXV infection is characterized by first, a fever associated with headaches, body aches and asthenia. Two days after the fever, a blistering rash begins with formation of scab and scarring. The vesicles are mainly localized on the hands, the palms and the face, and the soles of the feet but can also be present around the mouth and the genital area. The incubation of the MPX ranges from 5 to 21 days. The MPX usually heals spontaneously after 2 to 3 weeks (20).
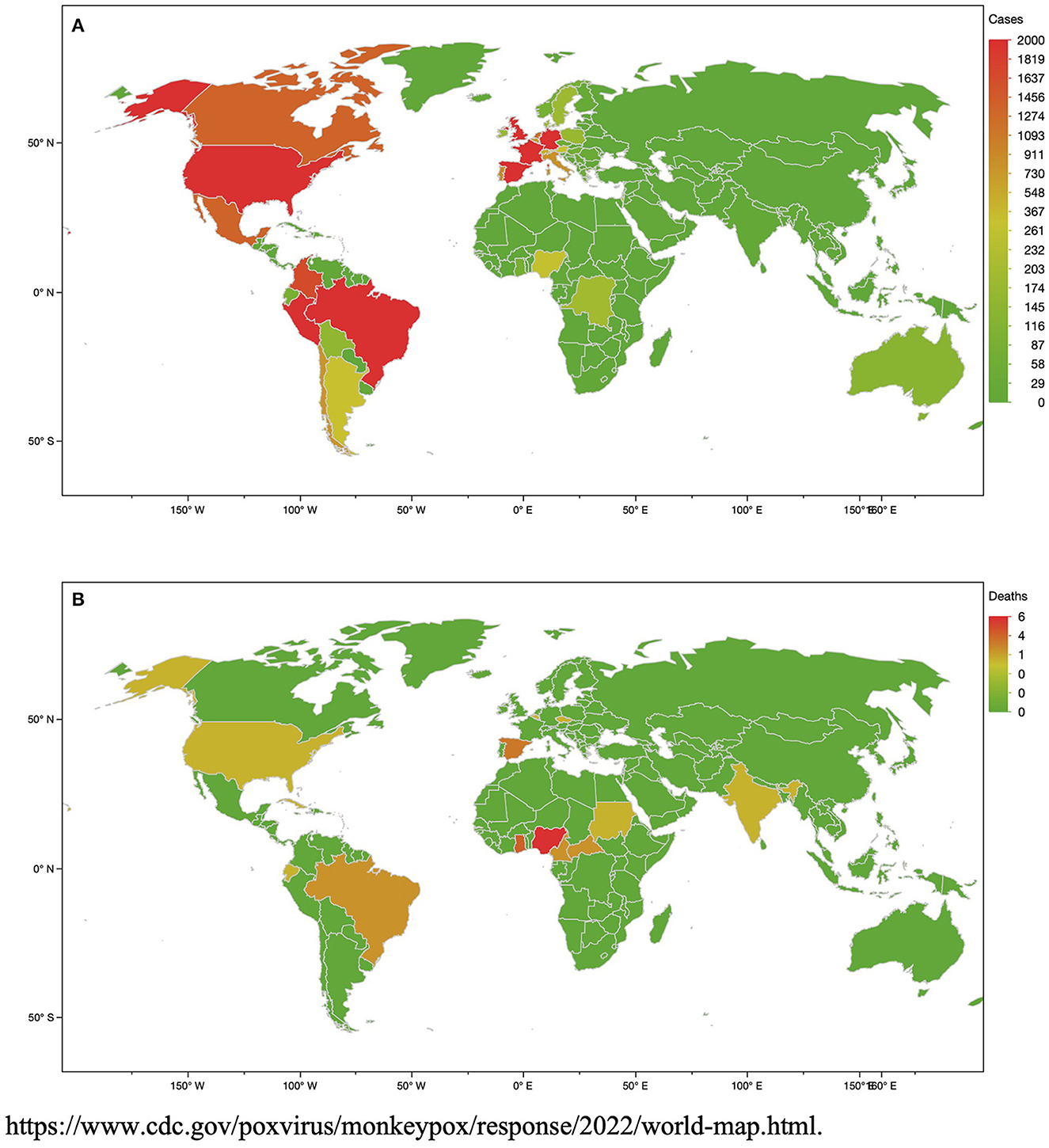
Figure 1. Number of monkeypox virus (MPXV) cases (A) and deaths (B) at the date of 23th September, 2022.
A recent French observational study found that complications affected more than a third of the patients (21). The main frequent complications were anal pain, and secondary bacterial skin infections, including cellulitis. The common neurologic symptoms were prodromal frontal headache occurring in the majority of patients (22) in association with asthenia and myalgias. Conjunctivitis can occur with corneal lesions and vision loss (23). Encephalitis has been observed in rare cases. Other complications can include pneumonitis, keratitis and secondary bacterial infections (23). Few patients were hospitalized but can represent up to 6% of them (21). From the beginning of the worldwide 2022 outbreak, the WHO reported 20 confirmed deaths for 64,290 cases in September, 2022 (24), and only six deaths have been reported in the United States for more than 25,000 cases (25). These data are consistent with the mortality rates of past MPXV outbreaks in Africa ranging from 1% in Nigeria to 10% in the Congo Basin (23). Most of the deaths have occurred in young people and people living with HIV (26).
Marijuana (cannabis) is the main worldwide consumed illicit drug, with over 188 million users, or around 2.5% of the population of 15–64 years (27). Cannabis use is correlated with poor economic, social, psychosocial and health levels. The psychosocial repercussions of cannabis use have been well reviewed (27), with drop out of school, antisocial behaviors and poor school performance. The health repercussions of cannabis use implicate several physiological and biochemical processes including immune, cardiovascular, hepatic, renal, endocrine and general health issues (28). Furthermore, around 38 million people are living with HIV, 170 million with hepatitis C virus, and 10 to 20 million with T-lymphotropic virus type 1. Most of them are cannabis users (29).
More than 100 million people worldwide are observed to have inappropriate alcohol consumption (30). Globally, 3 million deaths per years are the consequences of alcohol disorder. 5.3% of all causes of death are represented by alcohol disorder. Overall, 5.1% of the global burden of disease and injury is attributable to alcohol. Chronic alcohol consumption was associated with progression of community infections and complications in COVID-19 (31).
Several studies have shown that the relationship between alcohol and sexual behaviors increases the probability of HIV transmission and the absence of use of condoms in anal sexual practice (32–34). Among MSM, the consumption of alcohol is a major determinant of viral infection, such as HIV (33, 35). Nevertheless, the information regarding the effect of cannabis is limited (36). Around 40% of MSM reported to be heavy cannabis users, in comparison to seven percent of the overall population (37). Furthermore, in young MSM, the cannabis is more consumed than alcohol (daily 23 vs. 2%) (38). Recent investigations have suggested that MSM consume cannabis before having sex as frequently as alcohol consumption (63.5 vs. 61.5%) (39). Evidence also suggested that cannabis could increase condomless sex in heterosexual people (40–43).
The delta-9-tetrahydrocannabinol (THC) which composed cannabis could generate pharmacological actions on sexual-risk decision-making as could the consumption of alcohol (44). THC can lead to euphoric mood, impulsivity, risk-taking and aphrodisiac effects and can diminish the capacity of initiating responses (45, 46). Some studies have shown that, among MSM, cannabis and alcohol consumption may affect cognitive functions (47, 48). This risk is higher than the drug alone, with higher behavioral and social impacts and distress (34). Similar results have been observed among heterosexual young people, with higher risk of condomless sex (49, 50). In contrast, other studies have shown inconclusive results (36, 38, 51). The decision to use condoms or not may be impacted by other HIV prevention strategies, including pre-exposure prophylaxis (PrEP) and treatment-as-prevention (TasP), which could diminish the risk of sexually-based HIV infection, even in case of condomless sex (52, 53). The relationship between alcohol and sexual risk taking has been widely shown (54, 55). Alcohol consumption is correlated with disinhibition of behavioral sex comportments, leading to highly risk of exposure to sexually transmitted infections for individuals under its influence (56, 57). Alcohol consumption, especially in individuals who drink to intoxication levels, is correlated with higher risk of condomless sex (58, 59). Face to the MPXV outbreak, the WHO has called MSM to limit their sexual partners. In this review, we point the fact that several studies have shown that cannabis use was correlated with higher numbers of sexual partners for both genders (60, 61). Sexually transmitted diseases remain a major health issue, and new cases of infection are mainly attributable to the sexual relationships, among both MSM, women having sex with women (WSW) and heterosexuals (58). Thus, it is essential to investigate the role of both alcohol consumption and cannabis use with the number of sexual relationships in these different subgroups of populations.
The objective of this part of the work was to confirm the review information by original results of the association between cannabis use and alcohol consumption and the number of sexual partners among MSM, WSW and heterosexual men and women of the UK Biobank.
The UK Biobank is a prospective cohort for the investigation, prevention, diagnosis, and treatment of chronic diseases, such as CV diseases in adults. 502,478 Britons aged of 40–70 years across 22 UK cities from the UK National Health Service Register were included between 2006 and 2010. The cohort was phenotyped and genotyped, with participants who responded to a questionnaire; a computer-assisted interview; physical and functional measures; and blood, urine, and saliva samples (62–64). Data included socio-economic status, lifestyle behaviors, a mental health battery, clinical diagnoses and therapies, genetics, imaging and physiologic biomarkers from blood and urine samples. The cohort protocol can be found in the literature (65).
Sexual partners were reported by questionnaire. Participants were asked: “About how many sexual partners have you had in your lifetime?”. Lifetime sexual activity was defined as the difference between age at inclusion and the age of first sexual intercourse. Men having sex with men (MSM) and women having sex with women (WSW) were defined as participants who declared having same sex intercourse.
Cannabis use was reported by questionnaire. Participants were asked about their life-time cannabis use: “Have you taken cannabis (marijuana, grass, hash, ganja, blow, draw, skunk, weed, spliff, dope), even if it was a long time ago?”. Those who responded “no” were classified as controls (i.e., never users) and those endorsing “yes” options were classified as cannabis users. We separated these users into three groups: those reporting initial cannabis use (“yes, 1–2 times”, or “yes, 3–10 times”: low users) and continued cannabis use (“yes, 11–100 times”: moderate users; and “yes, more than 100 times”: high users).
Although the alcohol questionnaire has not been formally validated, several studies have shown expected associations with alcohol (66, 67). Alcohol level consumption was defined as reported in the questionnaire: high level (“daily or almost daily” or “three or four times a week”), moderate level (“once or twice a week,” or “one to three times a month”), and low level (“special occasions only” or “never”). Then participants self-reported the number of alcohol units (10 ml of pure ethanol) consumed, in “units per week” or “units per month” (for less frequent drinkers), across numerous beverage categories (red wine, white wine/champagne, beer/cider, spirits, fortified wine, and “other”). The UK Biobank assessment defined units of alcohol as: a pint or can of beer/lager/cider = two units; a 25 ml single shot of spirits = one unit; and a standard glass of wine (175 ml) = two units. The number of weekly units by summing the weekly units consumed in all categories was computed. When reported monthly, the intake was converted to units per week by dividing by 4.3. The number of weekly units was divided by 7 to determine units per day (68).
Current tobacco smokers were defined as participants who responded “yes, on most or all days” or “yes, only occasionally” to the question “do you smoke tobacco now.” Smoking pack-years are calculated as the average number of packs smoked per day multiplied by the total number of years smoking during lifetime. Body mass index was calculated as weight (in kg) divided by height2 (meters), and categorized as high (BMI > 30 kg/m2), moderate (BMI between 25 and 30 kg/m2) and low (< 25 kg/m2). Cardiovascular (CV) diseases were defined by heart attack, angina and stroke, as diagnosed by a doctor and reported in the questionnaires (69). Education level was defined in three categories, high (college or university degree), intermediate (A/AS levels or equivalent, O levels/GCSEs or equivalent), and low (none of the aforementioned) (70). Income level was defined as, high (“>£52,000 per year”), moderate (between £18,000 and £51,999 per year), and low (“ < £18,000 per year”) (71).
All participants provided electronic informed consent and UK Biobank received ethical approval from the North-West Multi-center Research Ethics Committee (MREC) covering the whole of the United Kingdom. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the North-West–Haydock Research Ethics Committee (protocol code: 21/NW/0157, date of approval: 21 June 2021). For details: https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
Inclusion criteria were participants who responded to the cannabis use questionnaire, to the questionnaire of number of sexual partners, and to alcohol consumption per day. Exclusion criteria were missing data for all covariates (like age, lifetime sexual activity, gender, income level, educational level, smoking pack years), 115,604 participants were included in the study.
Characteristics of the study population were described as the means with standard deviation (SD) for continuous variables. Categorical variables were described as numbers and proportions. To compare characteristics among the quartiles, we used the one-way ANOVA test for continuous variables and the chi-square test for categorical variables. Comparisons between two groups were performed using Student's test for continuous variables. Pearson's χ2 test was performed for categorical variables. Multivariate linear regression models were performed for the relationship between cannabis use and alcohol consumption, with adjustment for age, BMI, lifetime sexual activity, education, income, smoking pack years and CV diseases. Statistics were performed using SAS software (version 9.4; SAS Institute, Carry, NC). A p < 0.05 was considered statistically significant.
Among the 115,604 participants, 2,059 (1.78%) declared to be MSM, 2,243 (1.94%) to be WSW, 50,007 (43.26%) to be heterosexual men and 61,295 (53.02%) to be heterosexual women (Table 1). The gay population (both MSM and WSW) showed a significantly higher number of different sexual partners than the heterosexual population (15.4 vs. 6.0, p < 0.001). The gay population has a higher proportion of high cannabis users than the heterosexual population (10.58 vs. 2.356%, p < 0.001) and higher levels of alcohol consumption (27.96% vs 23.77%, p < 0.001). The same results were observed by stratifying by gender (Figure 2). The number of different sexual partners increases with cannabis use in all the groups, p < 0.001 (Figure 3). However, as the number of different sexual partners increases with alcohol consumption in heterosexual women (p < 0.001) and men (p < 0.001), and among WSW (p < 0.001), but this was not the case among MSM (p = 0.242) (Figure 4).
After adjustment for age, BMI, lifetime sexual activity, education, income, smoking pack years and CV diseases, the number of different sexual partners was significantly higher in high cannabis users compared to never users in all the groups (MSM, p = 0.031; WSW, p < 0.001; heterosexual women, p < 0.001 and heterosexual men, p < 0.001). The same results were observed for average daily alcohol consumption (MSM, p = 0.004; WSW, p < 0.001; heterosexual women, p = 0.002 and heterosexual men, p < 0.001) (Table 2). No interactions were observed between cannabis use and alcohol consumption in MSM (p = 0.674) and in WSW (p = 0.362) but significant interactions were observed in heterosexual women (p = 0.047) and in heterosexual men (p < 0.001).
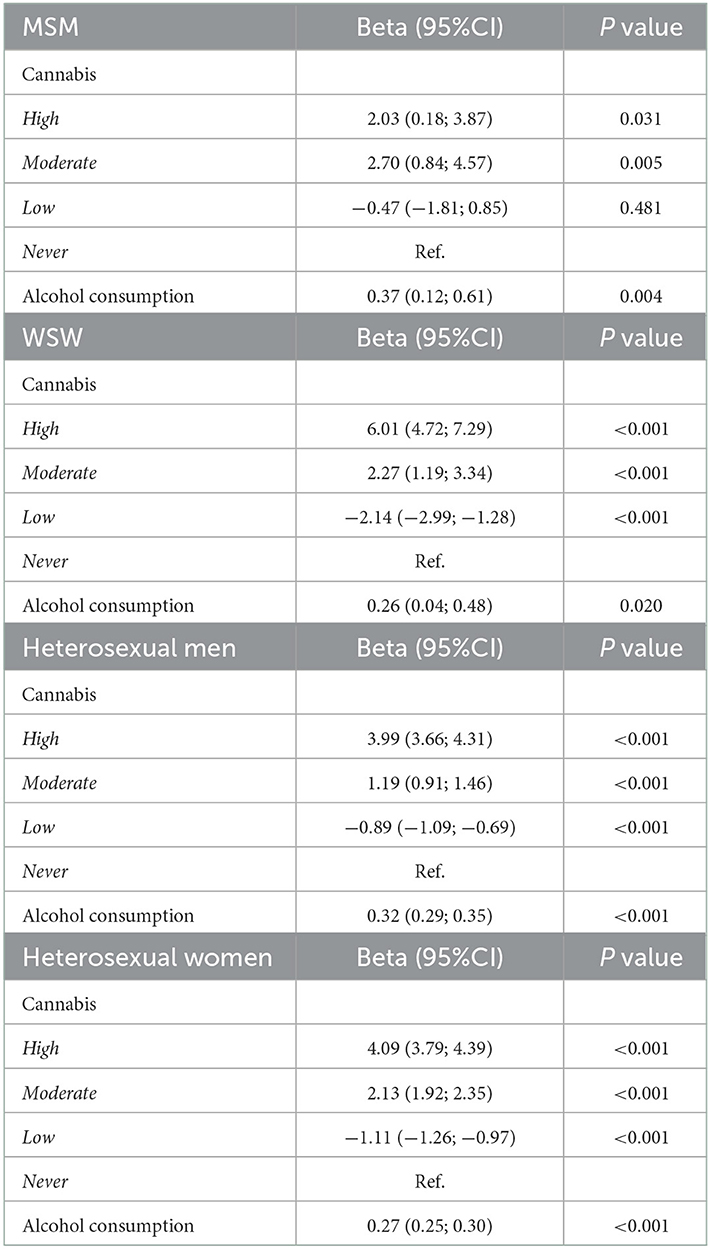
Table 2. Multivariate linear regression models for cannabis use and alcohol consumption with lifetime number of sexual partners, with adjustment for age, BMI, lifetime sexual activity, education, income, smoking pack years and CV diseases.
The cross-sectional observational design limits the relationship of causality. Reverse causation cannot be ruled out. The number of sexual partners was self-reported and could be considered as a major bias. Moreover, no historical indication of sexually transmitted infections has been reported in the UK Biobank cohort and could not allow us to investigate the relationship between number of sexual partners and sexually transmitted infection prevalence.
It is well-known that illicit or licit substances could affect several components of the immune system, by dysregulating the function of distinct immune response cells. Numerous studies have shown that some drugs could influence lymphocytes as well as dendritic cells and macrophages. Cannabis use, as well as alcohol consumption, has been reported to damage immune responsiveness. The recreational use of such drugs has been well described to affect resistance to microorganisms and alter susceptibility to infectious diseases (72). The consumption of cannabis and alcohol simultaneously is known to dysregulate the inflammatory responses through toll-like receptors (TLRs) (73).
Cannabidiol (CBD), one of the compound of cannabis, can damage the functional roles of the immune system (74). Cannabis acts as an immune modulator, damaging T-cells, B-cells, monocytes, and microglia, leading to reduction of the production of proinflammatory cytokines and an increase in the activity of anti-inflammatory cytokines. The role of cannabis immunity has been well discussed (75, 76). Cannabis use could predispose people to pulmonary infection, in patients showing a decrease in immune defenses by HIV infection chemotherapy (77). These people show that cannabis generates a concentration-dependent diminution in the proliferation of T cells and in the production of IFN-γ through CB2 receptor-dependent signaling. At the level of gene expression, cannabis stimulates Th1 cytokines (IFN-γ/IL-2) expression and decreasesTh2 cytokine (IL-4/IL-5) expression. Thus, both consumption of cannabis and alcohol significantly stimulates the production of IL-6 cytokine and toll-like receptors, TLR5, TLR7 and TLR9s. This suggests cannabis-related action on pulmonary innate immunity promoting airway inflammation (73). People living with viral infections can consume several illegal drugs, including cannabis. With regard to its negative actions, there are investigations which support the information that cannabis is detrimental for viral infections. Recent studies have shown that cannabis could control the immune system by modulating specific receptors on immune cells and decreasing immunity (78–83). Nevertheless, several investigations have shown that cannabis could have some positive actions (27). Thus, cannabis could affect the immune system (74, 75), could stimulate inflammatory cytokine production and CRP levels (84), and could decrease resistance to viral infections (85, 86). However, the literature remains inconsistent for the alteration of T cell lymphocytes, B cell lymphocytes, macrophages or immunoglobulin (27). Cannabis may reduce inflammation in HIV infection (87), and impairment of neurocognition of people living with HIV (88).
Similar results were observed for alcohol consumption. Alcohol may exert a dose-dependent interaction on the host response to viral infection (89). High alcohol consumption increases viral and bacterial infections (90), severity of infections (91), leads to viral infection expression, including HIV (92) and hepatitis C (93). However, moderate alcohol consumption was correlated with enhancement of immune response to infection and vaccination (94–96). The negative actions of high alcohol consumption on the immune system have been widely shown (97). Alcohol consumption can affect cell-mediated immunity and can be associated with high levels of post-operative infections (98). Ethanol diminishes cytokine production, affects macrophage responses to cytokines and LPS and stimulates the intracellular survival rate of Mycobacteria, Legionella, Salmonella or Listeria (99).
Few investigations have reported cannabis use and orthopoxvirus infection, none with the MPXV infection (100, 101). A team from Austria reported in 2016 that a young patient infected by an unusually severe cowpox virus (CPXV) with generalized rash and fever (102), showed abused substances associated with severe symptoms and diminution of the overall anti-CPXV antibody titer (100). They also showed that a single dose of cannabinoids increased the severity of CPXV infection in animals (101). Thus, a monodose of cannabis resin or THC before infection was associated with the reduction of the anti-CPXV anti-body formation in mice. The patient viewed by this team, developed a severe and generalized CPXV infection but without antibody response (100). The production of antibodies may have a major and protective role in CPXV infections (103). However, data are not consistent for the role of cannabis in antibody formation. A recent investigation showed a correlation between the diminution of immunoglobulin G and M rates and cannabis use (104) while other studies did not observe modifications in B-cell numbers rather than augmented IgE levels (105).
In a report of CPXV infection, the patient presented levels of lymphocytes within a normal range and serum analysis showed normal levels of immunoglobulin IgM but augmented serum IgG and IgE levels (100). These observations could be mainly attributed to a cannabinoid drug action showing an augmentation of the levels of IgE (99). Cannabis use can lead to immune-suppression leading to an enhancement of susceptibility to infection (100).
An association between cannabis use and herpes virus infections has been shown to reduce the phagocytic ability of alveolar macrophages, the NK-cell activity, interferon-gamma, and interleukin IL-12 levels (106). This observation of an increase of intracellular agent survival rate, and then of a decrease in survival rate of THC-treated mice with Legionella pneumophila infection (106).
Cannabis can influence the humoral immune response (107) and can control allergic immune responses (108) and neuro-inflammatory disorders (109). Even if no data clearly showed a negative role of cannabis in Orthopoxvirus, and its detrimental actions in viral infections, the significant reduction of Ig levels against vaccines and the reduction of complement protein in specific populations, like students, can highlight the dimension of the health problem (104).
No data linked alcohol consumption and Orthopoxvirus infection.
The incidence of STIs (sexually transmitted infections) has globally increased and continues to bear a disproportionate disease burden (110). Chemsex is sexualized drugs use and could be characterized as the intention to use illicit substances and/or drugs to enhance pleasure during sex. These substances include gamma-hydroxybutyrate (GHB), crystal meth, mephedrone, ecstasy, or cocaine (111). Chemsex is associated with a higher risk of reduction in the use of condoms (112, 113), increased risk of multiple sexual partners (114, 115) and higher risk of STI transmission, including HIV (116, 117). Face to the MPXV outbreak, chemsex practice could be a high-risk sexual practice for virus transmission. Furthermore, recent studies have observed that chemsex is often associated with cannabis use (118) and alcohol consumption (44).
Although investigations were mainly focused on LGBT (gay, lesbian, bisexual and transgender) populations, recent studies have shown that the practice of chemsex could be also frequently observed in both women and men, regardless of their sexual orientation (119, 120). Nevertheless, certain drug combinations should be considered according to the different populations. For example, alcohol and cannabis were mainly used in association with ecstasy among heterosexual sexual relationships (121, 122). To date, very few studies have investigated the role of chemsex in the MPXV outbreak. Wang et al. (123), showed that MSM using chemsex recently were less likely to perceive higher concern for MPXV infection (123). One of the explanations could be that MSM using chemsex tend to underestimate the STI risk in general (124, 125). However, Thornhill et al. (20), reported a prevalence of 20% of chemsex use among 528 infections diagnosed in 16 countries (20). Moreover, in a French cohort of patients infected by MPXV, 42% (90/216) reported having practiced chemsex in the last 3 months, and 40% (106/264) having condomless sex (21).
The emergence of the monkeypox virus (MPXV) outbreak in 2022 has become a worldwide health issue. Monkeypox is a contagious disease which requires physical or sexual contact with someone infected with the virus. In a recent report, the WHO has called on the group currently most affected by the virus, men who have sex with men (MSM), to limit their sexual partners. In this review, we observed that cannabis use and alcohol consumption are mainly correlated with a high number of sexual partners and at-risk sexual behaviors in both gay and heterosexual populations, which can lead to increase the dissemination of the MPXV and therefore lead to a sharp increase in this outbreak. Cannabis use and alcohol consumption may have two detrimental effects for the MPXV outbreak: by participating in the increase of the number of sexual partners which is mainly responsible for the augmentation of the number of new MPXV infected cases and by impairing immune responses to a viral infection. Health preventing policies should address the factors and practices leading to an increase in risk of sexual behaviors responsible for MPXV dissemination in the worldwide population (126). Health professionals should be aware of all risk behaviors concerning the MPXV outbreak to implement appropriate health policies without stigmatization to prevent discrimination and optimize compliance to these messages.
AV: conceptualization, methodology, formal analysis, and writing—original draft preparation.
The author thanks Polly Gobin for the English correction and proofreading.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mahase E. Seven monkeypox cases are confirmed in England. BMJ. (2022) 377:o1239. doi: 10.1136/bmj.o1239
2. WHO Director-General's Statement at the Press Conference Following IHR Emergency Committee Regarding the Multi-country Outbreak of Monkeypox. (2022). Available online at: https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi–country-outbreak-of-monkeypox (accessed September 17, 2022).
3. Farfour E, Lesprit P, Chan Hew Wai A, Mazaux L, Fourn E, Majerholc C, et al. Acute hepatitis A breakthrough in MSM in Paris area: implementation of targeted hepatitis a virus vaccine in a context of vaccine shortage. AIDS Lond Engl. (2018) 32:531–2. doi: 10.1097/QAD.0000000000001715
4. Vallée A, Farfour E, Zucman D. Monkeypox virus: a novel sexually transmitted disease? A case report from France. Travel Med Infect Dis. (2022) 49:102394. doi: 10.1016/j.tmaid.2022.102394
5. Multi-country Monkeypox Outbreak: Situation Update. Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed August 17, 2022).
6. Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. Monkeypox: an emerging global threat during the COVID-19 pandemic. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. (2022) 55:787–94. doi: 10.1016/j.jmii.2022.07.004
7. Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, Wu J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol. (2022) 95:e27931. doi: 10.1002/jmv.27931
8. Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases - United States, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1018–22. doi: 10.15585/mmwr.mm7132e3
9. Vallée A, Chatelain A, Carbonnel M, Racowsky C, Fourn E, Zucman D, et al. Monkeypox virus infection in 18-year-old woman after sexual intercourse, France, September 2022. Emerg Infect Dis. (2022) 29:219–22. doi: 10.3201/eid2901.221643
10. Spicknall IH. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men — United States, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1131–35. doi: 10.15585/mmwr.mm7135e2
11. Delaney KP, Sanchez T, Hannah M, Edwards OW, Carpino T, Agnew-Brune C, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission - United States, August 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1126–30. doi: 10.15585/mmwr.mm7135e1
12. Vallée A. Need for vaccination policies to face asymptomatic monkeypox virus infection. Vaccines. (2022) 10:2020. doi: 10.3390/vaccines10122020
13. Ajmera KM, Goyal L, Pandit T, Pandit R. Monkeypox - an emerging pandemic. IDCases. (2022) 29:e01587. doi: 10.1016/j.idcr.2022.e01587
14. Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Schootman M, Cottler LB, Bierut LJ. Number of sexual partners and associations with initiation and intensity of substance use. AIDS Behav. (2011) 15:869–74. doi: 10.1007/s10461-010-9669-0
15. Bazargan-Hejazi S, Gaines T, Bazargan M, Seddighzadeh B, Ahmadi A. Alcohol misuse and multiple sexual partners. West J Emerg Med. (2012) 13:151–9. doi: 10.5811/westjem.2011.6.6676
16. Farahat RA, Sah R, El-Sakka AA, Benmelouka AY, Kundu M, Labieb F, et al. Human monkeypox disease (MPX). Infez Med. (2022) 30:372–91. doi: 10.53854/liim-3003-6
17. Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. (2008) 225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x
18. Bragazzi NL, Kong JD, Wu J. Is monkeypox a new, emerging sexually transmitted disease? A rapid review of the literature. J Med Virol. (2022) 95:e28145. doi: 10.1002/jmv.28145
19. Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. (2022) 27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421
20. Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. (2022) 387:679–91. doi: 10.1056/NEJMoa2207323
21. Mailhe M, Beaumont AL, Thy M, Le Pluart D, Perrineau S, Houhou-Fidouh N, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2022) S1198-743X(22)00428-1. doi: 10.1016/j.cmi.2022.08.012
22. Billioux BJ, Mbaya OT, Sejvar J, Nath A. Neurologic complications of smallpox and monkeypox: a review. JAMA Neurol. (2022) 79:1180–6. doi: 10.1001/jamaneurol.2022.3491
23. Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. (2022) 22:1153–62. doi: 10.1016/S1473-3099(22)00228-6
24. Kumar R, Singh S, Singh SK. A Systematic Review of 5110 Cases of Monkeypox: What Has Changed Between 1970 and 2022? Cureus. (2022) 14:e30841. doi: 10.7759/cureus.30841
25. Kava CM, Rohraff DM, Wallace B, Mendoza-Alonzo JL, Currie DW, Munsey AE, et al. Epidemiologic features of the monkeypox outbreak and the public health response - United States, May 17-October 6, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1449–56. doi: 10.15585/mmwr.mm7145a4
26. Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. (2019) 13:e0007791. doi: 10.1371/journal.pntd.0007791
27. Maggirwar SB, Khalsa JH. The Link between cannabis use, immune system, and viral infections. Viruses. (2021) 13:1099. doi: 10.3390/v13061099
28. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. (2014) 370:2219–27. doi: 10.1056/NEJMra1402309
29. Pacek LR, Towe SL, Hobkirk AL, Nash D, Goodwin RD. Frequency of cannabis use and medical cannabis use among persons living with HIV in the united states. Findings from a nationally representative sample. AIDS Educ Prev Off Publ Int Soc AIDS Educ. (2018) 30:169–81. doi: 10.1521/aeap.2018.30.2.169
30. Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. (2018) 5:987–1012. doi: 10.1016/S2215-0366(18)30337-7
31. Lassen MCH, Skaarup KG, Sengeløv M, Iversen K, Ulrik CS, Jensen JUS, et al. Alcohol consumption and the risk of acute respiratory distress syndrome in COVID-19. Ann Am Thorac Soc. (2021) 18:1074–6. doi: 10.1513/AnnalsATS.202008-988RL
32. Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul Health Metr. (2017) 15:4. doi: 10.1186/s12963-017-0121-9
33. Boone MR, Cook SH, Wilson P. Substance use and sexual risk behavior in HIV-positive men who have sex with men: an episode-level analysis. AIDS Behav. (2013) 17:1883–7. doi: 10.1007/s10461-012-0167-4
34. Firkey M, Sheinfil A, Ramos J, Woolf-King SE. Cannabis and alcohol co-use and condomless anal sex among men who have sex with men living with HIV: an event-level analysis. AIDS Behav. (2021) 25:3770–81. doi: 10.1007/s10461-021-03228-6
35. Vosburgh HW, Mansergh G, Sullivan PS, Purcell DW. A review of the literature on event-level substance use and sexual risk behavior among men who have sex with men. AIDS Behav. (2012) 16:1394–410. doi: 10.1007/s10461-011-0131-8
36. Gorbach PM, Javanbakht M, Shover CL, Bolan RK, Ragsdale A, Shoptaw S. Associations between cannabis use, sexual behavior, and sexually transmitted infections/human immunodeficiency virus in a cohort of young men who have sex with men. Sex Transm Dis. (2019) 46:105–11. doi: 10.1097/OLQ.0000000000000919
37. Bruce D, Harper GW, Fernandez MI. The adolescent medicine trials network for HIV AIDS interventions (ATN). Heavy marijuana use among gay and bisexual male emerging adults living with HIV/AIDS. J HIVAIDS Soc Serv. (2013) 12:26–48. doi: 10.1080/15381501.2012.735171
38. Bruce D, Kahana S, Harper GW, Fernández MI. ATN Alcohol use predicts sexual risk behavior with HIV-negative or partners of unknown status among young HIV-positive men who have sex with men. AIDS Care. (2013) 25:559–65. doi: 10.1080/09540121.2012.720363
39. Torres TS, Bastos LS, Kamel L, Bezerra DRB, Fernandes NM, Moreira RI, et al. Do men who have sex with men who report alcohol and illicit drug use before/during sex (chemsex) present moderate/high risk for substance use disorders? Drug Alcohol Depend. (2020) 209:107908. doi: 10.1016/j.drugalcdep.2020.107908
40. Anderson BJ, Stein MD. A behavioral decision model testing the association of marijuana use and sexual risk in young adult women. AIDS Behav. (2011) 15:875–84. doi: 10.1007/s10461-010-9694-z
41. Brodbeck J, Matter M, Moggi F. Association between cannabis use and sexual risk behavior among young heterosexual adults. AIDS Behav. (2006) 10:599–605. doi: 10.1007/s10461-006-9103-9
42. Kerr DCR, Washburn IJ, Morris MK, Lewis KAG, Tiberio SS. Event-level associations of marijuana and heavy alcohol use with intercourse and condom use. J Stud Alcohol Drugs. (2015) 76:733–7. doi: 10.15288/jsad.2015.76.733
43. Zhang X, Wu LT. Marijuana use and sex with multiple partners among lesbian, gay and bisexual youth: results from a national sample. BMC Public Health. (2017) 17:19. doi: 10.1186/s12889-016-3905-0
44. Berry MS, Johnson MW. Does being drunk or high cause HIV sexual risk behavior? A systematic review of drug administration studies. Pharmacol Biochem Behav. (2018) 164:125–38. doi: 10.1016/j.pbb.2017.08.009
45. Lynn BK, López JD, Miller C, Thompson J, Campian EC. The relationship between marijuana use prior to sex and sexual function in women. Sex Med. (2019) 7:192–7. doi: 10.1016/j.esxm.2019.01.003
46. Skalski LM, Gunn RL, Caswell A, Maisto S, Metrik J. Sex-related marijuana expectancies as predictors of sexual risk behavior following smoked marijuana challenge. Exp Clin Psychopharmacol. (2017) 25:402–11. doi: 10.1037/pha0000138
47. Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry. (2004) 161:249–54. doi: 10.1176/appi.ajp.161.2.249
48. Skalski LM, Towe SL, Sikkema KJ, Meade CS. The impact of marijuana use on memory in HIV-infected patients: a comprehensive review of the HIV and marijuana literatures. Curr Drug Abuse Rev. (2016) 9:126–41. doi: 10.2174/1874473709666160502124503
49. Metrik J, Caswell AJ, Magill M, Monti PM, Kahler CW. Sexual risk behavior and heavy drinking among weekly marijuana users. J Stud Alcohol Drugs. (2016) 77:104–12. doi: 10.15288/jsad.2016.77.104
50. Parks KA, Collins RL, Derrick JL. The influence of marijuana and alcohol use on condom use behavior: findings from a sample of young adult female bar drinkers. Psychol Addict Behav. (2012) 26:888–94. doi: 10.1037/a0028166
51. Newcomb ME, Ryan DT, Greene R GJ, Garofalo, Mustanski B. Prevalence and patterns of smoking, alcohol use, and illicit drug use in young men who have sex with men. Drug Alcohol Depend. (2014) 141:65–71. doi: 10.1016/j.drugalcdep.2014.05.005
52. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. (2011) 365:493–505. doi: 10.1056/NEJMoa1105243
53. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. (2010) 363:2587–99. doi: 10.1056/NEJMoa1011205
54. Carey KB, Guthrie KM, Rich CM, Krieger NH, Norris AL, Kaplan C, et al. Alcohol use and sexual risk behavior in young women: a qualitative study. AIDS Behav. (2019) 23:1647–55. doi: 10.1007/s10461-018-2310-3
55. Scott-Sheldon LAJ, Carey KB, Cunningham K, Johnson BT, Carey MP. MASH research team. Alcohol use predicts sexual decision-making: a systematic review and meta-analysis of the experimental literature. AIDS Behav. (2016) 20 (Suppl 1):S19–39. doi: 10.1007/s10461-015-1108-9
56. Stall R, Paul JP, Greenwood G, Pollack LM, Bein E, Crosby GM, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men's Health Study. Addict Abingdon Engl. (2001) 96:1589–601. doi: 10.1046/j.1360-0443.2001.961115896.x
57. Woolf SE, Maisto SA. Alcohol use and risk of HIV infection among men who have sex with men. AIDS Behav. (2009) 13:757–82. doi: 10.1007/s10461-007-9354-0
58. George WH. Alcohol and Sexual Health Behavior: “What We Know and How We Know It.” J Sex Res. (2019) 56:409–24. doi: 10.1080/00224499.2019.1588213
59. Cooper ML. Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. J Stud Alcohol Suppl. (2002) 101−17. doi: 10.15288/jsas.2002.s14.101
60. Smith AMA, Ferris JA, Simpson JM, Shelley J, Pitts MK, Richters J. Cannabis use and sexual health. J Sex Med. (2010) 7:787–93. doi: 10.1111/j.1743-6109.2009.01453.x
61. N WC, A S. Associated risk factors of STIs and multiple sexual relationships among youths in Malawi. PLoS ONE. (2015) 10:e0134286. doi: 10.1371/journal.pone.0134286
62. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
63. Vallée A. Arterial stiffness determinants for primary cardiovascular prevention among healthy participants. J Clin Med. (2022) 11:2512. doi: 10.3390/jcm11092512
64. Vallée A. Association between lipids and arterial stiffness for primary cardiovascular prevention in a general middle-aged European population. Front Cardiovasc Med. (2022) 9:899841. doi: 10.3389/fcvm.2022.899841
65. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
66. Jani BD, McQueenie R, Nicholl BI, Field R, Hanlon P, Gallacher KI, et al. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: a prospective cohort study. BMC Med. (2021) 19:8. doi: 10.1186/s12916-020-01878-2
67. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet Lond Engl. (2018) 391:1513–23. doi: 10.1016/S0140-6736(18)30134-X
68. Daviet R, Aydogan G, Jagannathan K, Spilka N, Koellinger PD, Kranzler HR, et al. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. (2022) 13:1175. doi: 10.1038/s41467-022-28735-5
69. Vallée A. Association between serum uric acid and arterial stiffness in a large-aged 40-70 years old population. J Clin Hypertens. (2022) 24:885–97. doi: 10.1111/jch.14527
70. Chadeau-Hyam M, Bodinier B, Vermeulen R, Karimi M, Zuber V, Castagné R, et al. Education, biological ageing, all-cause and cause-specific mortality and morbidity: UK biobank cohort study. EClinicalMedicine. (2020) 29–30:100658. doi: 10.1016/j.eclinm.2020.100658
71. Tyrrell J, Jones SE, Beaumont R, Astley CM, Lovell R, Yaghootkar H, et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ. (2016) 352:i582. doi: 10.1136/bmj.i582
72. Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. (2006) 47:330–42. doi: 10.1111/j.1574-695X.2006.00097.x
73. Bailey KL, Wyatt TA, Katafiasz DM, Taylor KW, Heires AJ, Sisson JH, et al. Alcohol and cannabis use alter pulmonary innate immunity. Alcohol Fayettev N. (2019) 80:131–8. doi: 10.1016/j.alcohol.2018.11.002
74. Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. (2015) 10:193–203. doi: 10.1007/s11481-015-9615-z
75. Oláh A, Szekanecz Z, Bíró T. Targeting cannabinoid signaling in the immune system: “High”-ly exciting questions, possibilities, and challenges. Front Immunol. (2017) 8:1487. doi: 10.3389/fimmu.2017.01487
76. Almogi-Hazan O, Or R. Cannabis, the endocannabinoid system and immunity—the journey from the bedside to the bench and back. Int J Mol Sci. (2020) 21:4448. doi: 10.3390/ijms21124448
77. Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol. (2002) 42:71S−81S. doi: 10.1002/j.1552-4604.2002.tb06006.x
78. Rizzo MD, Henriquez JE, Blevins LK, Bach A, Crawford RB, Kaminski NE. Targeting cannabinoid receptor 2 on peripheral leukocytes to attenuate inflammatory mechanisms implicated in HIV-associated neurocognitive disorder. J Neuroimmune Pharmacol. (2020) 15:780–93. doi: 10.1007/s11481-020-09918-7
79. Rom S, Persidsky Y. Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol. (2013) 8:608–20. doi: 10.1007/s11481-013-9445-9
80. Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer's disease. Acta Biochim Biophys Sin. (2017) 49:853–66. doi: 10.1093/abbs/gmx073
81. Vallée A, Lecarpentier Y, Vallée JN. Cannabidiol and the canonical WNT/β-catenin pathway in glaucoma. Int J Mol Sci. (2021) 22:3798. doi: 10.3390/ijms22073798
82. Vallée A, Lecarpentier Y, Vallée JN. Possible actions of cannabidiol in obsessive-compulsive disorder by targeting the WNT/β-catenin pathway. Mol Psychiatry. (2021) 27:230–48. doi: 10.1038/s41380-021-01086-1
83. Vallée A, Vallée JN, Lecarpentier Y. Potential role of cannabidiol in Parkinson's disease by targeting the WNT/β-catenin pathway, oxidative stress and inflammation. Aging. (2021) 13:10796–813. doi: 10.18632/aging.202951
84. Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al. Low-dose cannabidiol is safe but not effective in the treatment for crohn's disease, a randomized controlled trial. Dig Dis Sci. (2017) 62:1615–20. doi: 10.1007/s10620-017-4540-z
85. Hernández-Cervantes R, Méndez-Díaz M, Prospéro-García Ó, Morales-Montor J. Immunoregulatory role of cannabinoids during infectious disease. Neuroimmunomodulation. (2017) 24:183–99. doi: 10.1159/000481824
86. Beji C, Loucif H, Telittchenko R, Olagnier D, Dagenais-Lussier X, van Grevenynghe J. Cannabinoid-induced immunomodulation during viral infections: a focus on mitochondria. Viruses. (2020) 12:E875. doi: 10.3390/v12080875
87. Coates RA, Farewell VT, Raboud J, Read SE, MacFadden DK, Calzavara LM, et al. Cofactors of progression to acquired immunodeficiency syndrome in a cohort of male sexual contacts of men with human immunodeficiency virus disease. Am J Epidemiol. (1990) 132:717–22. doi: 10.1093/oxfordjournals.aje.a115713
88. Watson CWM, Paolillo EW, Morgan EE, Umlauf A, Sundermann EE, Ellis RJ, et al. Cannabis exposure is associated with a lower likelihood of neurocognitive impairment in people living with HIV. J Acquir Immune Defic Syndr. (2020) 83:56–64. doi: 10.1097/QAI.0000000000002211
89. Barr T, Helms C, Grant K, Messaoudi I. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 65:242–51. doi: 10.1016/j.pnpbp.2015.09.001
90. Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. (1972) 33:171–85. doi: 10.15288/qjsa.1972.33.171
91. Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. (1997) 157:1446–52. doi: 10.1001/archinte.1997.00440340078008
92. Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. (2010) 26:511–8. doi: 10.1089/aid.2009.0211
93. Bhattacharya R, Shuhart MC. Hepatitis C and alcohol: interactions, outcomes, and implications. J Clin Gastroenterol. (2003) 36:242–52. doi: 10.1097/00004836-200303000-00012
94. Ouchi E, Niu K, Kobayashi Y, Guan L, Momma H, Guo H, et al. Frequent alcohol drinking is associated with lower prevalence of self-reported common cold: a retrospective study. BMC Public Health. (2012) 12:987. doi: 10.1186/1471-2458-12-987
95. Messaoudi I, Asquith M, Engelmann F, Park B, Brown M, Rau A, et al. Moderate alcohol consumption enhances vaccine-induced responses in rhesus macaques. Vaccine. (2013) 32:54–61. doi: 10.1016/j.vaccine.2013.10.076
96. Romeo J, Wärnberg J, Díaz LE, González-Gross M, Marcos A. Effects of moderate beer consumption on first-line immunity of healthy adults. J Physiol Biochem. (2007) 63:153–9. doi: 10.1007/BF03168226
97. Szabo G. Alcohol's contribution to compromised immunity. Alcohol Health Res World. (1997) 21:30–41.
98. Spies CD, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, et al. Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology. (2004) 100:1088–100. doi: 10.1097/00000542-200405000-00010
99. Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. (2003) 16:209–19. doi: 10.1128/CMR.16.2.209-219.2003
100. Huemer HP, Himmelreich A, Hönlinger B, Pavlic M, Eisendle K, Höpfl R, et al. “Recreational” drug abuse associated with failure to mount a proper antibody response after a generalised orthopoxvirus infection. Infection. (2007) 35:469–73. doi: 10.1007/s15010-007-6194-9
101. Huemer HP, Lassnig C, Bernhard D, Sturm S, Nowotny N, Kitchen M, et al. Cannabinoids lead to enhanced virulence of the smallpox vaccine (vaccinia) virus. Immunobiology. (2011) 216:670–7. doi: 10.1016/j.imbio.2010.11.001
102. Hönlinger B, Huemer HP, Romani N, Czerny CP, Eisendle K, Höpfl R. Generalized cowpox infection probably transmitted from a rat. Br J Dermatol. (2005) 153:451–3. doi: 10.1111/j.1365-2133.2005.06731.x
103. Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol Baltim Md. (2004) 172:6265–71. doi: 10.4049/jimmunol.172.10.6265
104. El-Gohary M, Eid MA. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum Exp Toxicol. (2004) 23:149–56. doi: 10.1191/0960327104ht426oa
105. Rachelefsky GS, Opelz G, Mickey MR, Lessin P, Kiuchi M, Silverstein MJ, et al. Intact humoral and cell-mediated immunity in chronic marijuana smoking. J Allergy Clin Immunol. (1976) 58:483–90. doi: 10.1016/0091-6749(76)90192-5
106. Roth MD, Whittaker K, Salehi K, Tashkin DP, Baldwin GC. Mechanisms for impaired effector function in alveolar macrophages from marijuana and cocaine smokers. J Neuroimmunol. (2004) 147:82–6. doi: 10.1016/j.jneuroim.2003.10.017
107. Kaminski NE, Koh WS, Yang KH, Lee M, Kessler FK. Suppression of the humoral immune response by cannabinoids is partially mediated through inhibition of adenylate cyclase by a pertussis toxin-sensitive G-protein coupled mechanism. Biochem Pharmacol. (1994) 48:1899–908. doi: 10.1016/0006-2952(94)90588-6
108. Jan TR, Farraj AK, Harkema JR, Kaminski NE. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol Appl Pharmacol. (2003) 188:24–35. doi: 10.1016/S0041-008X(03)00010-3
109. Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. (2004) 141:775–85. doi: 10.1038/sj.bjp.0705667
110. Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV. (2019) 6:e396–405. doi: 10.1016/S2352-3018(19)30043-8
111. Giorgetti R, Tagliabracci A, Schifano F, Zaami S, Marinelli E. Busardò FP. When “Chems” meet sex: a rising phenomenon called “ChemSex”. Curr Neuropharmacol. (2017) 15:762–70. doi: 10.2174/1570159X15666161117151148
112. Schumacher A, Marzell M, Toepp AJ, Schweizer ML. Association between marijuana use and condom use: a meta-analysis of between-subject event-based studies. J Stud Alcohol Drugs. (2018) 79:361–9. doi: 10.15288/jsad.2018.79.361
113. Simons JS, Simons RM, Maisto SA, Hahn AM, Walters KJ. Daily associations between alcohol and sexual behavior in young adults. Exp Clin Psychopharmacol. (2018) 26:36–48. doi: 10.1037/pha0000163
114. Glynn RW, Byrne N, O'Dea S, Shanley A, Codd M, Keenan E, et al. Chemsex, risk behaviours and sexually transmitted infections among men who have sex with men in Dublin, Ireland. Int J Drug Policy. (2018) 52:9–15. doi: 10.1016/j.drugpo.2017.10.008
115. Patel EU, White JL, Gaydos CA, Quinn TC, Mehta SH, Tobian AAR. Marijuana use, sexual behaviors, and prevalent sexually transmitted infections among sexually experienced males and females in the united states: findings from the national health and nutrition examination surveys. Sex Transm Dis. (2020) 47:672–8. doi: 10.1097/OLQ.0000000000001229
116. Flores Anato JL, Panagiotoglou D, Greenwald ZR, Blanchette M, Trottier C, Vaziri M, et al. Chemsex and incidence of sexually transmitted infections among Canadian pre-exposure prophylaxis (PrEP) users in the l'Actuel PrEP Cohort (2013-2020). Sex Transm Infect. (2022) 98:549–56. doi: 10.1136/sextrans-2021-055215
117. Curtis TJ, Rodger AJ, Burns F, Nardone A, Copas A, Wayal S. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: a comparison of cross-sectional studies conducted in 2013 and 2016. Sex Transm Infect. (2020) 96:197–203. doi: 10.1136/sextrans-2019-054139
118. Prestage G, Hammoud M, Jin F, Degenhardt L, Bourne A, Maher L. Mental health, drug use and sexual risk behavior among gay and bisexual men. Int J Drug Policy. (2018) 55:169–79. doi: 10.1016/j.drugpo.2018.01.020
119. Johnson MW, Herrmann ES, Sweeney MM, LeComte RS, Johnson PS. Cocaine administration dose-dependently increases sexual desire and decreases condom use likelihood: the role of delay and probability discounting in connecting cocaine with HIV. Psychopharmacology (Berl). (2017) 234:599–612. doi: 10.1007/s00213-016-4493-5
120. Xu Y, Towe SL, Causey ST, Meade CS. Using mobile health technologies to test the association of cocaine use with sexual desire and risky sexual behaviors among people with and without HIV who use illicit stimulants. Drug Alcohol Depend. (2021) 225:108744. doi: 10.1016/j.drugalcdep.2021.108744
121. Lawn W, Aldridge A, Xia R, Winstock AR. Substance-linked sex in heterosexual, homosexual, and bisexual men and women: an online, cross-sectional “global drug survey” report. J Sex Med. (2019) 16:721–32. doi: 10.1016/j.jsxm.2019.02.018
122. Íncera-Fernández D, Román FJ, Gámez-Guadix M. Risky sexual practices, sexually transmitted infections, motivations, and mental health among heterosexual women and men who practice sexualized drug use in Spain. Int J Environ Res Public Health. (2022) 19:6387. doi: 10.3390/ijerph19116387
123. Wang H, d'Abreu de Paulo KJI, Gültzow T, Zimmermann HML, Jonas KJ. Perceived monkeypox concern and risk among men who have sex with men: evidence and perspectives from The Netherlands. Trop Med Infect Dis. (2022) 7:293. doi: 10.3390/tropicalmed7100293
124. Zucman D, Fourn E, Touche P, Majerholc C, Vallée A. Monkeypox vaccine hesitancy in French men having sex with men with PrEP or living with HIV in France. Vaccines. (2022) 10:1629. doi: 10.3390/vaccines10101629
125. Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings ('chemsex') and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect. (2015) 91:564–8. doi: 10.1136/sextrans-2015-052052
Keywords: sexual behavior, cannabis, alcohol, monkeypox, MSM, outbreak, chemsex, epidemic
Citation: Vallée A (2023) Sexual behaviors, cannabis, alcohol and monkeypox infection. Front. Public Health 10:1054488. doi: 10.3389/fpubh.2022.1054488
Received: 26 September 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
Kimberly A. Koester, University of California, San Francisco, United StatesReviewed by:
Ramesh Pandit, Penn Medicine, United StatesCopyright © 2023 Vallée. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre Vallée,  YWwudmFsbGVlQGhvcGl0YWwtZm9jaC5jb20=
YWwudmFsbGVlQGhvcGl0YWwtZm9jaC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.