
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 10 November 2022
Sec. Clinical Diabetes
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1052485
 Bao Sun1,2,3
Bao Sun1,2,3 Yongchao Gao1,4,5,6
Yongchao Gao1,4,5,6 Fazhong He7
Fazhong He7 Zhaoqian Liu1,4,5,6
Zhaoqian Liu1,4,5,6 Jiecan Zhou8,9*
Jiecan Zhou8,9* Xingyu Wang10*
Xingyu Wang10* Wei Zhang1,4,5,6*
Wei Zhang1,4,5,6*Background: Although a growing attention has been recently paid to the role of HbA1c variability in the risk of diabetic complications, the impact of HbA1c variability on cardiovascular diseases (CVD) in type 2 diabetes is still debated. The aim of the study is to investigate the association of HbA1c variability with CVD in individuals within or outside the target range of HbA1c.
Methods: Using data from Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE), we enrolled 855 patients with type 2 diabetes in China. The primary outcomes included major macrovascular events and major microvascular events. Visit-to-visit HbA1c variability was expressed as the coefficient of variation (CV) of five measurements of HbA1c taken 3–24 months after treatment. Cox proportional hazard models were used to estimate adjusted hazard ratios (aHR).
Results: Among 855 patients in the intensive glucose treatment group, 563 and 292 patients were assigned to the group of “within the target range of HbA1c” (WTH) (updated mean HbA1c ≤ 7.0%) and “outside the target range of HbA1c” (OTH) (updated mean HbA1c > 7.0%), respectively. HbA1c variability was positively associated with the risk of major microvascular events in all patients and both the subgroups during a median follow-up period of 4.8 years. Particularly, the risk related to HbA1c variability was higher in patients in WTH group for the new or worsening nephropathy [aHR: 3.35; 95% confidence interval (CI): 1.05–10.74; P = 0.042].
Conclusions: This retrospective cohort study confirmed the positive correlation between HbA1c variability and major microvascular events, especially in subjects in WTH or OTH.
Diabetes is a major driver of cardiovascular diseases (CVD) and mortality worldwide (1), and associations between glycemic control and CVD are still debated (2). Although HbA1c, an integral marker of glycemic exposure over the past 2–3 months, has become the gold standard to assess glycemic control, it cannot describe interday or intraday glucose fluctuations. In recent years, a growing attention has been paid to the important role of glycemic variability (GV) in the development of CVD (3–5). GV refers to oscillations in blood glucose levels over a certain interval of time. The widespread use of continuous glucose monitoring (CGM) and self-monitored blood glucose (SMBG) technology has provided the opportunity to assess short-term GV (both within-day and between-day GV) and long-term GV (based on serial determinations in blood glucose over a longer period of time) (6). A meta-analysis indicated that HbA1c variability was superior at predicting diabetic complications than mean HbA1c (7). Characterized by its simplicity for glucose concentration, coefficient of variation (%CV) is considered as the most appropriate indicator for assessing GV because it is easy to calculate and is independent of the mean glucose level (8).
Many observational studies and randomized controlled trials suggested that GV was closely associated with CVD in patients with type 1 or type 2 diabetes mellitus (T2DM) (7, 9–17). However, there were several heterogeneous results regarding the association between GV and diabetic complications (6, 18–20). A recent study revealed that HbA1c variability seemed to play a greater role in microvascular complications among patients with relatively optimal baseline glycemic control (21). Nevertheless, there was not yet complete understanding of the possible impact of HbA1c variability on CVD in patients within or outside the recommended HbA1c target (22). Given that the current guidelines from the Chinese Diabetes Society and American Diabetes Association recommend an HbA1c level of <7.0% as the treatment goal (23, 24), we explore the impact of HbA1c variability on the development of CVD among patients within or outside the recommended HbA1c target.
This study was part of the ADVANCE, a factorial randomized and controlled trial of intensive blood glucose and blood pressure lowering treatment in patients with type 2 diabetes (25). The patients were enrolled from 61 centers in China, and the details of these patients had been described in our previous study (26). The study was approved by each center's institutional review board, and all participants provided written informed consent.
Patients were randomly assigned (1:1) to receive modified release gliclazide-based intensive or standard therapy for glycemic control, and perindopril-indapamide or matching placebo for blood pressure control. The intensive glucose control received gliclazide-modified release-based strategy (target HbA1c ≤ 6.5%).
Fasting HbA1c samples were collected at baseline, at 3, 6, 12, 18, 24 months, and every 6 months thereafter in the intensive glucose treatment group. Standard glucose treatment group was not included in our study, due to insufficient measurements taken (fasting HbA1c was only measured at 6, 12, and 24 months during the follow up of first 2 years). To eliminate the effect of multiple HbA1c measures in a short space of time, we used the mean value of serially measured HbA1c in each participant. The %CV of HbA1c was calculated as the standard deviations (SD) divided by the updated mean value of HbA1c [%CV = (SD/mean HbA1c) × 100], which was independent of the mean glucose level. Based on the previous studies (15, 27), participants were divided into high HbA1c variability and low HbA1c variability for further analyses.
The primary outcomes included major macrovascular events and major microvascular events. The major microvascular events comprised of new or worsening nephropathy or retinopathy, which was considered as the secondary outcomes. A previous study clearly described the primary and secondary outcomes (28). The duration of follow-up for each participant ranged from their 24-month visit until they experienced events, deaths, or completed the final visit at the end of the study.
Data are summarized as means ± SD for continuous variables and as percentages for categorical variables. Baseline clinical characteristics were compared with the use of Student's t-test, Wilcoxon rank sum test or χ2 tests. Multivariable Cox regression analyses was used to explore the association between HbA1c variability and the risk of CVD. A backward stepwise was utilized for baseline covariates in the multivariable model including age, duration of diabetes, gender, body mass index (BMI), current smoking status, systolic and diastolic blood pressure, total cholesterol, triglycerides, high- and low-density lipoprotein cholesterol, history of major macrovascular diseases and microvascular diseases, baseline use of insulin and mean HbA1c during the first 24 months. In addition, stratified analyses were performed for patients with average HbA1c levels ≤ 7.0% or > 7.0% during the follow-up. High HbA1c variability and low HbA1c variability was estimated separately for these two subgroups. Kaplan–Meier estimates were employed to compare the freedom from CVD within groups defined by HbA1c variability. The SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) was performed for all statistical analyses, and a two-sided P value < 0.05 was considered statistically significant.
A total of 855 patients with type 2 diabetes in the intensive glycemic control were included for the final analysis (excluding intra-individual missing values of HbA1c) (Figure 1), including 424 males and 431 females. The detailed clinical characteristics of patients at baseline are described in Table 1. Based on the updated mean of intra-individual HbA1c, 563 (65.8%) and 292 (34.2%) patients were assigned to the group of “within the target range of HbA1c” (WTH) and “outside the target range of HbA1c” (OTH), respectively.
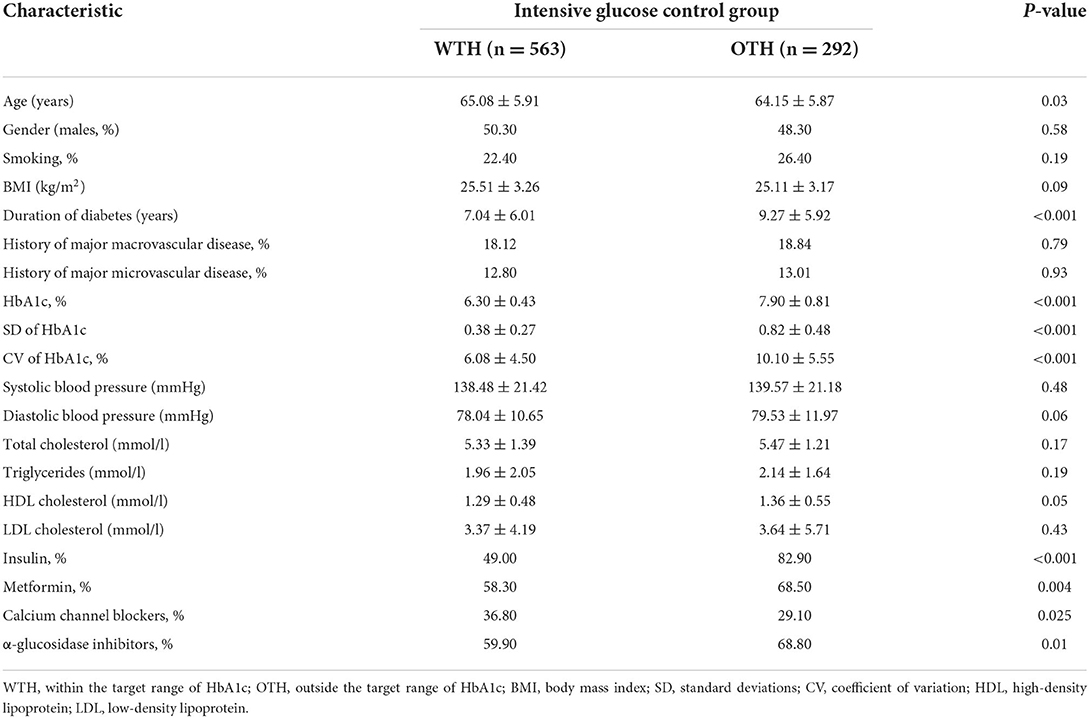
Table 1. Characteristics of the study population by the target range of HbA1c in the intensive glucose control.
The %CV of HbA1c was significantly lower in group of WTH (6.08 ± 4.50) than those in OTH (10.10 ± 5.55) during a median follow-up period of 4.8 years. The median value of %CV of HbA1c was used to define high and low HbA1c variability.
Compared with patients with the low HbA1c variability, the risk of major microvascular events was significantly increased in patients with the high HbA1c variability (aHR = 1.97, 95% CI 1.18–3.30, P = 0.010) after adjusting the potential confounding factors (Table 2). However, there was no association between high HbA1c variability and major macrovascular events. The Kaplan–Meier plot of freedom from major microvascular events between low and high HbA1c variability was presented in Figure 2. In addition, we further performed the multiple regression analyses for secondary outcomes and found that aHRs for new or worsening nephropathy and retinopathy were 3.12 (95% CI 1.32–7.35, P = 0.0090) and 1.75 (95% CI 0.96–3.17, P = 0.066), respectively, in patients with high HbA1c variability (Table 2).

Table 2. The risk of cardiovascular events according to visit-to-visit HbA1c variability in all patients.
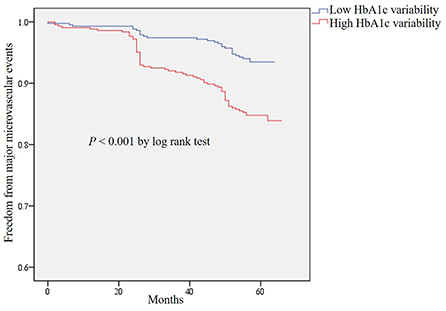
Figure 2. Kaplan–Meier curves of freedom from major microvascular events for HbA1c variability in total patients.
Subgroup analyses were performed using multivariable Cox regression analyses in subjects of WTH and OTH group (Tables 3, 4). In general, the associations of HbA1c variability with the risk of major microvascular events were of statistical significance in both patients of WTH and OTH group. In WTH group, high HbA1c variability increased the risk of developing major microvascular events by 2.20 folds (Table 3), and high HbA1c variability increased such risk by 2.26 folds in OTH group (Table 4). The Kaplan–Meier plot of freedom from major microvascular events between low and high HbA1c variability in subgroup analyses was presented in Supplementary Figure 1. Of note, this consistent trend was also found for new or worsening nephropathy in patients of WTH group.

Table 3. The risk of cardiovascular events according to visit-to-visit HbA1c variability in patients of WTH group.
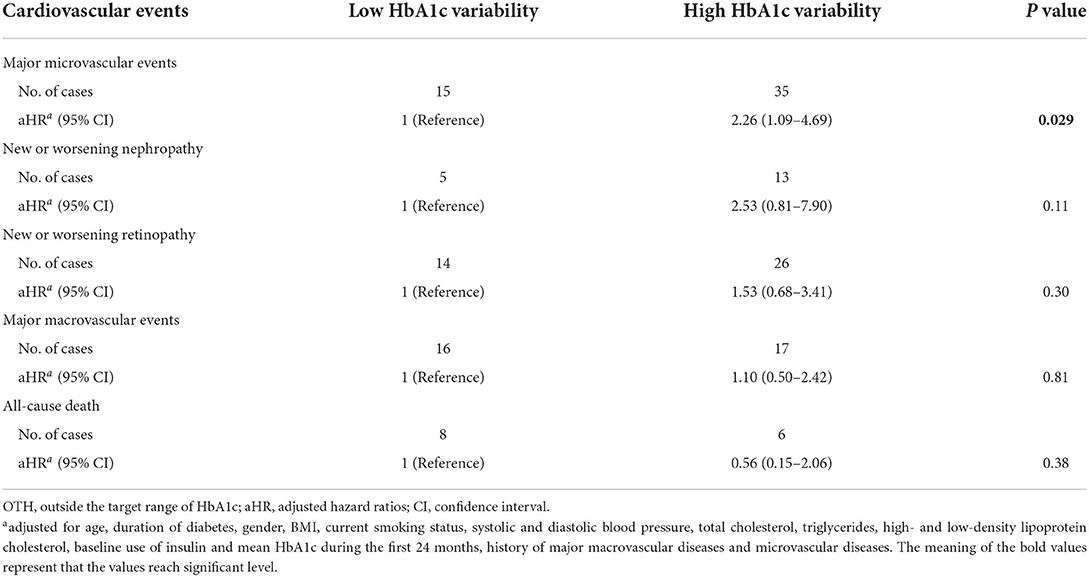
Table 4. The risk of cardiovascular events according to visit-to-visit HbA1c variability in patients of OTH group.
In the present study, we found that high HbA1c variability was associated with risk of major microvascular events in patients with T2DM after long-term follow-up. Particularly, our result highlighted the importance of HbA1c variability even in patients within or outside the target range of HbA1c.
The possible role of HbA1c variability in the development of CVD is still a remaining unanswered question in T2DM. Several studies suggested an association between HbA1c variability and CVD (14, 16, 17). In contrast, others showed no association of HbA1c variability with cardiovascular outcomes (18–20). Interestingly, a previous study also indicated that HbA1c variability (SD of HbA1c) was correlated with combined macro/microvascular events and macrovascular events, but not with microvascular events (10). In this study, we revealed an association of HbA1c variability (%CV of HbA1c) with major microvascular events in patients regardless of being within the target range of HbA1c, but not with major macrovascular events. One of the possible explanations for such disparity may be due to the absence of standardized definitions for HbA1c variability. For instance, EI Malahi et al. (29) demonstrated that GV [assessed by time in range (TIR)] was independently associated with the presence of composite microvascular complications, while it (assessed by TIR, SD, and CV) did not show a link with macrovascular complications. Thus, further research defining the standardized GV is required to elucidate these controversial results.
On the other hand, several related confounding factors might be involved in CVD. Drug therapy including insulin, metformin, α-glucosidase inhibitors had direct or indirect impacts on CVD. Pieber et al. (30) found that severe hypoglycemia induced by insulin was significantly associated with the risk of CVD. A recent study provided that metformin could ameliorate the prognosis of heart failure by the modulation of glucose and lipid metabolism, the attenuation of oxidative stress and inflammation, and the inhibition of myocardial cell apoptosis (31). Another study considered that α-glucosidase inhibitors contributed to the significant beneficial CVD outcome via affecting endothelial dysfunction and carotid intima media thickening (32). In the present study, there were significant differences in the use of anti-diabetic drugs, which might affect the HbA1c variability and confound the ultimate results. Now-a-days, cholesterol-lowering drugs should also be taken into account in diabetic patients with lipid abnormalities. Gentile et al. found that statins not only reduced low density lipoprotein (LDL)-cholesterol levels, but also resulted in the reduction of inflammation and CVD mortality (33). In this study, we did not provide the information about the statins use, but the LDL-cholesterol levels were not significant differences between WTH and OTH group. In addition, a previous study observed that people with young-onset T2DM had a higher prevalence of diabetic complications than those with late-onset T2DM because of the longer duration of diabetes (34). Similarly, duration of diabetes in OTH group was longer and more significant than that in WTH group, which might also affect the risk of major macrovascular events in our study. Nevertheless, the results remained present after adjusting for these confounding factors in the present study. Alternatively, another likely explanation is that the effect of HbA1c variability may be diluted in the general diabetes patients due to the different related confounding factors.
Several pathways and mechanisms linking HbA1c variability to CVD have been proposed. Endothelial dysfunction, inflammatory cytokines and oxidative stress were proposed as mediators of the HbA1c variability involved in CVD (35, 36). A recent study provided the evidence that oxidative damage was even more serious in glucose variability model than that in prolonged hyperglycemia model (37). Therefore, basic research regarding the elaborated mechanisms of HbA1c variability in the development of CVD is still needed.
The strengths of our study include the use of a database with a long-term follow-up and a large number of HbA1c measurements. Moreover, in addition to the major microvascular events, we further analyze the association of HbA1c variability with new or worsening nephropathy and retinopathy. Inevitably, several limitations to our study should be noted. Since this is a retrospective cohort study, there may be uncorrected confounding factors such as drug therapy and duration of diabetes. To ensure the robustness of the findings, we adjusted the confounding factors in the analyses and performed subgroup analyses. Despite the adjustments for a broad set of confounding factors, we could not exclude the possibility of residual or unmeasured confounding factors. Selection bias may be another limitation due to the exclusion of patients with intra-individual missing values of HbA1c. In addition, the included subjects are from centers in China, which may not be generalizable to other ethnic lines. Also, the sample size is small in our study, especially in the subgroup of OTH, which needs to be verified by large samples in future studies. Finally, we do not investigate the elaborated mechanisms linking HbA1c variability and the risk of major microvascular events, which deserves future experiments to figure them out.
In conclusion, our study showed that HbA1c variability was an independent risk factor for major microvascular events in T2DM patients within or outside the target range of HbA1c.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
BS contributed to the study design and data analysis and edited the manuscript. YG and FH collected the data and performed the statistical analysis. ZL contributed to revising the manuscript. JZ, XW, and WZ are the guarantor of this work and takes responsibility for the accuracy of the data analysis. All authors reviewed and approved the final submitted report.
This study was supported by National Scientific Foundation of China (Nos. 82104307, 81573511, 81874329, 82003872, and 81522048), National Key Research and Development Program (Nos. 2016YFC0905000 and 2016YFC0905001), Natural Science Foundation of Hunan Province (Nos. 2021JJ40865 and 2020JJ5513), Scientific Research Fund Project of Hunan Provincial Health Commission (Nos. 20201973 and B202313016776), Scientific Research Fund of Hunan Provincial Education Department (No. 19A418), Central Government Funds for Guiding Local Scientific and Technological Development (2021QZY016), Hunan Province Clinical Medical Technology Innovation Guidance Project (2020SK51823), and Scientific Research Launch Project of the Second Xiangya Hospital of Central South University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1052485/full#supplementary-material
Supplementary Figure 1. Kaplan–Meier curves of freedom from major microvascular events for HbA1c variability in subgroup analyses. (A) Kaplan–Meier curves of freedom from major microvascular events for HbA1c variability in WTH group. (B) Kaplan–Meier curves of freedom from major microvascular events for HbA1c variability in OTH group.
CVD, cardiovascular diseases; ADVANCE, Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation; CV, coefficient of variation; aHR, adjusted hazard ratios; WTH, within the target range of HbA1c; OTH, outside the target range of HbA1c; CI, confidence interval; GV, glycemic variability; CGM, continuous glucose monitoring; SMBG, self-monitored blood glucose; T2DM, type 2 diabetes mellitus; SD, standard deviations; BMI, body mass index; TIR, time in range; LDL, low density lipoprotein.
2. Prattichizzo F, de Candia P, De Nigris V, Nicolucci A, Ceriello A. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. (2020) 110:154308. doi: 10.1016/j.metabol.2020.154308
3. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. (2019) 7:221–30. doi: 10.1016/S2213-8587(18)30136-0
4. Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. (2020) 19:102. doi: 10.1186/s12933-020-01085-6
5. Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. (2021) 20:9. doi: 10.1186/s12933-020-01200-7
6. Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. (2022) 10:75–84. doi: 10.1016/S2213-8587(21)00245-X
7. Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. (2015) 38:2354–69. doi: 10.2337/dc15-1188
8. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. (2017) 40:1631–40. doi: 10.2337/dc17-1600
9. Marcovecchio ML, Dalton RN, Chiarelli F, Dunger DB. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care. (2011) 34:1011–3. doi: 10.2337/dc10-2028
10. Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. (2014) 37:2359–65. doi: 10.2337/dc14-0199
11. Laiteerapong N, Karter AJ, Moffet HH, Cooper JM, Gibbons RD, Liu JY, et al. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: the diabetes and aging study. J Diabetes Complications. (2017) 31:94–100. doi: 10.1016/j.jdiacomp.2016.07.023
12. Takao T, Suka M, Yanagisawa H, Matsuyama Y, Iwamoto Y. Predictive ability of visit-to-visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract. (2017) 128:15–23. doi: 10.1016/j.diabres.2017.03.027
13. Mo Y, Zhou J, Ma X, Zhu W, Zhang L, Li J, et al. Haemoglobin A1c variability as an independent correlate of atherosclerosis and cardiovascular disease in Chinese type 2 diabetes. Diab Vasc Dis Res. (2018) 15:402–8. doi: 10.1177/1479164118778850
14. Su JB, Zhao LH, Zhang XL, Cai HL, Huang HY, Xu F, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. (2018) 17:47. doi: 10.1186/s12933-018-0693-0
15. Sun B, He F, Gao Y, Zhou J, Sun L, Liu R, et al. Prognostic impact of visit-to-visit glycemic variability on the risks of major adverse cardiovascular outcomes and hypoglycemia in patients with different glycemic control and type 2 diabetes. Endocrine. (2019) 64:536–43. doi: 10.1007/s12020-019-01893-1
16. Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-to-visit HbA(1c) variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care. (2020) 43:426–32. doi: 10.2337/dc19-0823
17. Wan EYF, Yu EYT, Chin WY, Ng FTY, Chia SMC, Wong ICK, et al. Age-specific associations of glycated haemoglobin variability with cardiovascular disease and mortality in patients with type 2 diabetes mellitus: a 10- year cohort study. Diabetes Obes Metab. (2020) 22:1316–27. doi: 10.1111/dom.14034
18. Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care. (2017) 40:777–83. doi: 10.2337/dc16-2426
19. Zhou JJ, Schwenke DC, Bahn G, Reaven P. Glycemic variation and cardiovascular risk in the veterans affairs diabetes trial. Diabetes Care. (2018) 41:2187–94. doi: 10.2337/dc18-0548
20. Martinez M, Santamarina J, Pavesi A, Musso C, Umpierrez GE. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. (2021) 9:e002032. doi: 10.1136/bmjdrc-2020-002032
21. Yang CY, Su PF, Hung JY, Ou HT, Kuo S. Comparative predictive ability of visit-to-visit HbA1c variability measures for microvascular disease risk in type 2 diabetes. Cardiovasc Diabetol. (2020) 19:105. doi: 10.1186/s12933-020-01082-9
22. Ceriello A, Lucisano G, Prattichizzo F, La Grotta R, Franzén S, Svensson AM, et al. HbA1c variability predicts cardiovascular complications in type 2 diabetes regardless of being at glycemic target. Cardiovasc Diabetol. (2022) 21:13. doi: 10.1186/s12933-022-01445-4
23. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41:S55–64. doi: 10.2337/dc18-S006
24. Chinese Diabetes Society. China guideline for type 2 diabetes. China J Diabetes Mellitus. (2021) 13:315–409.
25. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. doi: 10.1056/NEJMoa0802987
26. He F, Liu M, Chen Z, Liu G, Wang Z, Liu R, et al. Assessment of human tribbles homolog 3 genetic variation (rs2295490) effects on type 2 diabetes patients with glucose control and blood pressure lowering treatment. EBioMedicine. (2016) 13:181–9. doi: 10.1016/j.ebiom.2016.10.025
27. Gu J, Fan YQ, Zhang JF, Wang CQ. Association of hemoglobin A1c variability and the incidence of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and arterial hypertension. Hellenic J Cardiol. (2018) 59:91–7. doi: 10.1016/j.hjc.2017.08.001
28. Action in Diabetes and Vascular Disease: PreterAx and Diamicron Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens Suppl. (2001) 19:S21–8.
29. El Malahi A, Van Elsen M, Charleer S, Dirinck E, Ledeganck K, Keymeulen B, et al. Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes mellitus. J Clin Endocrinol Metab. (2022) 107:e570–81. doi: 10.1210/clinem/dgab688
30. Pieber TR, Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. (2018) 61:58–65. doi: 10.1007/s00125-017-4422-0
31. Salvatore T, Galiero R, Caturano A, Vetrano E, Rinaldi L, Coviello F, et al. (2021). Effects of metformin in heart failure: from pathophysiological rationale to clinical evidence. Biomolecules. (2021) 11:834. doi: 10.3390/biom11121834
32. Standl E, Schnell O. Alpha-glucosidase inhibitors 2012 - cardiovascular considerations and trial evaluation. Diab Vasc Dis Res. (2012) 9:163–9. doi: 10.1177/1479164112441524
33. Gentile S, Turco S, Guarino G, Sasso CF, Amodio M, Magliano P, et al. Comparative efficacy study of atorvastatin vs simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes Obes Metab. (2000) 2:355–62. doi: 10.1046/j.1463-1326.2000.00106.x
34. Cho Y, Park H, Huh BW, SeoHS, Seo DH, Ahn SH, et al. Prevalence and risk of diabetic complications in young-onset versus late-onset type 2 diabetes mellitus. Diabetes Metab. (2022) 48:101389. doi: 10.1016/j.diabet.2022.101389
35. Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA(1c) Levels. Diabetes. (2017) 66:2472–82. doi: 10.2337/db17-0294
36. Ohara M, Kohata Y, Nagaike H, Koshibu M, Gima H, Hiromura M, et al. Association of glucose and blood pressure variability on oxidative stress in patients with type 2 diabetes mellitus and hypertension: a cross-sectional study. Diabetol Metab Syndr. (2019) 11:29. doi: 10.1186/s13098-019-0425-y
Keywords: HbA1c variability, type 2 diabetes, major microvascular events, within the target range of HbA1c, outside the target range of HbA1c
Citation: Sun B, Gao Y, He F, Liu Z, Zhou J, Wang X and Zhang W (2022) Association of visit-to-visit HbA1c variability with cardiovascular diseases in type 2 diabetes within or outside the target range of HbA1c. Front. Public Health 10:1052485. doi: 10.3389/fpubh.2022.1052485
Received: 24 September 2022; Accepted: 27 October 2022;
Published: 10 November 2022.
Edited by:
Ferdinando Carlo Sasso, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Alfredo Caturano, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Sun, Gao, He, Liu, Zhou, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, Y3N1emhhbmd3ZWlAY3N1LmVkdS5jbg==; Jiecan Zhou, emhvdWppZWNhbkBmc3l5LnVzYy5lZHUuY24=; Xingyu Wang, eGluZ3l1d0B5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.