
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 14 October 2022
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1041136
Background: Anemia in pregnancy is a serious threat to maternal and child health and is a major public health problem. However, the risk factors associated with its incidence are unclear and controversial.
Methods: PubMed, Ovid Embase, Web of Science, and Cochrane databases were systematically searched (inception to June 27, 2022). The screening of search results, extraction of relevant data, and evaluation of study quality were performed independently by two reviewers.
Results: A total of 51 studies of high quality (NOS score ≥ 7) were included, including 42 cross-sectional studies, six case-control studies, and three cohort studies. Meta-analysis showed that infected parasite, history of malarial attack, tea/coffee after meals, meal frequency ≤ 2 times per day, frequency of eating meat ≤ 1 time per week, frequency of eating vegetables ≤ 3 times per week, multiple pregnancies, multiparous, low household income, no antenatal care, rural residence, diet diversity score ≤ 3, have more than 3 children, history of menorrhagia, underweight, family size ≥ 5, middle upper arm circumference < 23, second trimester, third trimester, birth interval ≤ 2 year were all risk factors for anemia in pregnancy.
Conclusions: Prevention of anemia in pregnancy is essential to promote maternal and child health. Sufficient attention should be paid to the above risk factors from the social level and pregnant women's own aspects to reduce the occurrence of anemia in pregnancy.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022344937.
As a global public health problem, anemia in pregnancy has been shown to be an independent risk factor for adverse maternal and infant outcomes such as blood transfusion, postpartum hemorrhage, cesarean section, hysterectomy, preterm birth, and infectious diseases (1). It directly threatens the health of about 32 million pregnant women around the world. Especially in developing countries, 56% of pregnant women are affected by it (2, 3). Anemia in pregnancy is a global concern as it impairs physical health, cognitive development, productivity, and reflects lagging economic status (2, 4). Improving anemia in pregnancy is essential to reduce maternal and infant mortality and serious complications. Unfortunately, even though extensive studies have been conducted over the past 20 years and various national nutrition programs have been implemented to reduce anemia in pregnancy, there has not been much success in eliminating anemia in pregnancy, and it remains a major public health problem (4, 5).
It is critical to explore the risk factors that may cause anemia in pregnancy and take preventive strategies as soon as possible. However, the risk factors for anemia in pregnancy are controversial. For example, the findings of Kedir et al. suggest that parasite infection is not a risk factor for anemia in pregnancy (6). However, other studies in the same area identified parasitic infection as a risk factor for anemia in pregnancy (7, 8). It has also been shown that tea/coffee after meals is not a risk factor for anemia in pregnancy (AOR = 1.03, 95% CI: 0.88–2.06) (9), but the results of Teshome et al. showed a very significant association between tea/coffee after meals and anemia in pregnancy (AOR = 18.49, 95% CI: 6.89–40) (10). In addition, iron deficiency is considered to be the most common cause of anemia in pregnancy, therefore, most studies recommend that pregnant women should take adequate iron supplements to prevent anemia in pregnancy (11, 12). On the contrary, some studies have shown that iron supplementation did not reduce the incidence of anemia in pregnancy (13, 14). Some studies have even concluded that even without iron supplementation during pregnancy, the incidence of anemia in pregnant women is not significantly higher (15). In conclusion, disparate findings on the same exposure factors pose an obstacle to the prevention of anemia in pregnancy and further public health decisions.
The current field lacks definitive evidence on the risk factors for anemia in pregnancy. Therefore, as the first study to systematically summarize the risk factors of anemia in pregnancy, the results of this study can provide a reference for the prevention and treatment of anemia in pregnancy in the future.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). The review protocol has been registered with PROSPERO, number CRD42022344937 (https://www.crd.york.ac.uk/prospero/).
Pregnant women.
Report at least one exposure factor associated with anemia in pregnancy.
Studies where adjusted odds ratio (AOR) for exposure factors were available or calculated.
Anemia in pregnancy occurs. The diagnostic criteria are hemoglobin ≤ 11 g/dL.
Cross-sectional studies, case-control studies, and cohort studies.
Animal studies and cell experiments were excluded. Reviews, case reports, opinion articles, conference abstracts, and non-published data were also excluded.
Candidate studies were identified through searches of the PubMed, Web of Science, Cochrane, and Ovid Embase databases from inception until June 27, 2022. Also, the reference lists of the included studies were searched. The retrieval approach of the combination of free words and subject words was adopted. The following terms were combined to generate search keywords: [gestational anemia OR anemia in pregnancy OR (pregnancy OR pregnant OR gestation) AND anemia] AND (hazard OR risk factors OR risk factor OR related factors OR factors OR influence factors OR influencing factors). Further details of the search strategy are shown in Supplementary Table 1.
Literature screening and data extraction were performed by 2 trained researchers according to the inclusion and exclusion criteria as indicated previously. Extracted content includes: (1) Basic information of included studies: authors, year, country, type of study, sample size, age, method of obtaining information, diagnostic criteria for anemia, and data analysis methods. (2) Exposure factors: Risk factors related to dietary habits, self-condition, and disease history of pregnant women. (3) Key elements of risk of bias assessment.
Based on the Newcastle-Ottawa Scale (NOS), two qualified researchers independently evaluated the inherent risk of bias of included studies from three aspects, including the selection of participants, confounding variables, and measurement of exposure (17). The evaluation results were scored as low, medium, and high quality, respectively, with scores of 0–3, 4–6, and 7–9.
Statistical analysis was performed using STATA 16 software. Results were calculated using adjusted odds ratio (AOR) and 95% confidence interval (95% CI). The χ2 test was used to evaluate the heterogeneity of the included studies (the test level was α = 0.05), and the size of the heterogeneity was judged according to the I2 value. When P > 0.05 and I2 ≤ 50%, it indicated that the heterogeneity of the results of each study was not statistically significant, and a fixed-effects model was used for meta-analysis; otherwise, after further analysis of the source of heterogeneity, a random-effects model was used.
A total of 4,638 relevant records were obtained from the initial inspection, which were excluded from repeated studies, non-risk factor studies (prevalence, diagnosis, and treatment of anemia in pregnancy), pregnant women without anemia (postpartum anemia, women of childbearing age) and study types that are not consistent (review, conference summary, case report, etc.), 51 studies were finally included. The article screening process is shown in Figure 1.
A total of 51 studies were included (6–10, 13–15, 18–60), including 42 cross-sectional studies (6–9, 13–15, 18–29, 31–37, 39–43, 45, 47–49, 51, 53, 54, 56–58, 60), six case-control studies (10, 30, 38, 50, 52, 55), and three cohort studies (44, 46, 59). The entire population was from developing countries, and 36 study sites were in Ethiopia (6–10, 13–15, 18–20, 23, 26–31, 34–43, 50, 52, 55–60). The total number of patients was 73,919 and in individual study ranged from 163 (49)−12,403 (53). The patients were aged between 15 and 49 years (40). Information was obtained through structured questionnaires (6–10, 13–15, 18–39, 41–45, 47–50, 52–56, 59, 60), outpatient medical record data (40, 46, 51, 58), and databases (57). The diagnostic criteria for anemia were hemoglobin < 11 g/dL, and the statistical analysis methods were multivariable logistic regression. See Table 1 for details.
The NOS scores of the 51 included studies were all ≥ 7 points, of which 38 studies had a NOS score of 8 points, and 13 studies had 7 points, indicating that the included studies had high research quality. See Supplementary Table 2 for details.
A total of four exposure factors associated with medical history may contribute to anemia in pregnancy. Since I2 = 0, P > 0.05, indicating that there is little possibility of heterogeneity among the studies, a fixed-effect model was used for combined analysis. Meta-analysis showed that parasitic infection (AOR = 2.20, 95% CI: 1.63–2.76) and history of malarial attack (AOR = 2.86, 95% CI: 1.98–3.73) were risk factors for anemia in pregnancy, while HIV status (AOR = 1.36, 95% CI: 0.97–1.75) and abortion history (AOR = 1.05, 95% CI: 0.47–1.63) were not associated with anemia in pregnancy (Figure 2).
A total of eight exposure factors related to dietary habits may contribute to anemia in pregnancy. The fixed effect model was used to analyze the exposure factors of I2 < 50%. The results showed that tea/coffee after meals, meal frequency ≤ 2 times per day, frequency of eating meat ≤ 1 time per week, diet diversity score ≤ 3 were risk factors for anemia in pregnancy. Iron supplementation was a protective factor for anemia in pregnancy (Table 2). Meta-analysis of the potential risk factors for I2 > 50 % using a random effect model showed that frequency of eating vegetables ≤ 3 times per week was a risk factor for anemia in pregnancy, while no iron supplementation and drinking were not associated with anemia in pregnancy (Table 2).
A total of 20 exposure factors associated with maternal conditions may contribute to anemia in pregnancy. Multiple pregnancies, multiparous, low household income, no antenatal care, rural residence, have more than 3 children, history of menorrhagia, underweight, family size ≥ 5, middle upper arm circumference < 23, second trimester, third trimester, and birth interval ≤ 2 year were all risk factors for anemia in pregnancy. Overweight was a protective factor, and the remaining exposure factors were not associated with anemia in pregnancy (Table 3).
Funnel plots were drawn for exposure factors with more than 10 studies to detect publication bias. The results showed that the funnel plots were basically symmetrical, suggesting a small possibility of publication bias (Figure 3).
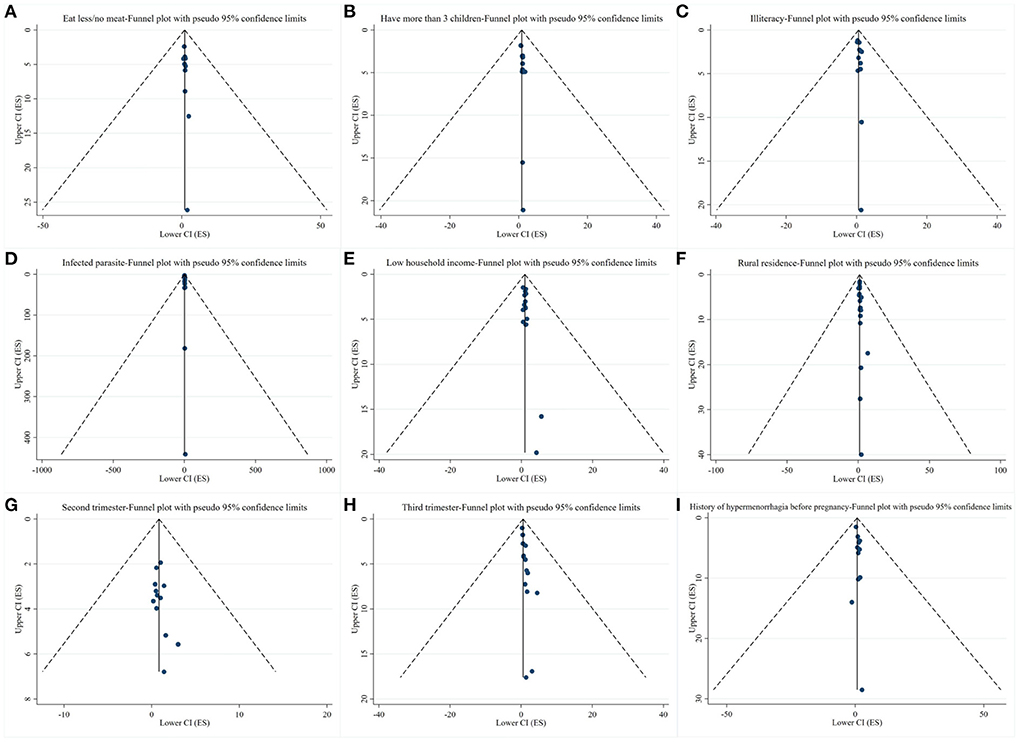
Figure 3. Results of publication bias. (A–I) Funnel plots of exposure factors with more than 10 studies.
Due to poverty, lack of safe drinking water, poor hygiene, and malnutrition, combined with the immunomodulatory and physiological changes that occur during pregnancy, pregnant women are often more vulnerable than non-pregnant women to intestinal parasite invasion, especially in developing countries (61). More than 7 million pregnant women worldwide are infected with hookworm, and 10 million pregnant women in Africa are infected with schistosomiasis (62, 63). Parasites entering the gut can attach to the mucosa and submucosa of the small intestine, destroy capillaries and arterioles and feed on the exuding blood (64). Our findings suggest that parasitic infection is one of the risk factors for anemia in pregnancy. This finding is consistent with the study conducted by Alem et al. (65). In developing countries, infection of young women, pregnant women, and their infants with intestinal parasites, especially hookworms, can lead to deficiencies in iron, total energy, protein, and folic acid and zinc, leading to low birth weight, intrauterine growth retardation, and higher morbidity and mortality of anemia in pregnancy (58). Moreover, as another common risk factor, infection with malaria is also susceptible to anemia in pregnancy. Notably, studies on malaria have come from both Ethiopia and Ghana. Their geographical location in the tropics has an important impact on the distribution of malaria. Sequestration of plasmodium in the placenta avoids spleen clearance, thereby predisposing pregnant women to malaria. Malaria causes anemia in a variety of ways, including excessive depletion of non-parasitic red blood cells, immune destruction of parasitic red blood cells, and impaired erythropoiesis due to bone marrow dysfunction (66, 67). Other studies have shown that pregnant women infected with HIV are more likely to develop anemia than those who are not infected with HIV (41). This may be due to the properties of the virus that lead to increased metabolic and nutritional requirements and directly inhibit the production of red blood cells in the body (68). Although our meta-analysis showed no significant association between HIV infection and anemia in pregnancy. However, the lower limit of confidence interval of our results is close to 1, suggesting to some extent the correlation between HIV infection and anemia in pregnancy.
Previous study has shown that the risk of anemia in pregnancy increases with the number of births. The risk of developing anemia in pregnancy was nearly 3 times higher for women with 2–3 children and 4 times higher for women with 4 or more children compared to only one child (69, 70). This is because pregnant women do not have enough time to recover from the nutritional burden of their previous pregnancy, especially folic acid, and iron deficiency. Maternal serum and erythrocyte folate concentrations also decline from the fifth month of pregnancy and remain low for a considerable time after delivery (6). The same is true for our combined analysis of 12 studies, finding that women with more than 3 children were more likely to develop anemia in pregnancy. In addition, multiple pregnancies, multiparous, and birth interval ≤ 2 years are also risk factors for anemia in pregnancy. Like the reasons for having more children, these factors lead to impaired iron stores in pregnant women, and to a certain extent, they impair the normal physiological functions and anatomical structures of pregnant women. Studies have shown that during pregnancy, the incidence of anemia increases more than 4 times from the first trimester to the third trimester, and the prevalence in the third trimester is as high as 30–45% (71). This is consistent with our findings that early pregnancy is less prone to anemia, whereas second and third trimesters are significantly associated with anemia. It may be related to the rapid growth of the fetus in the second and third trimesters and the significant increase in the demand for nutrients such as iron (72).
According to the World Health Organization, anemia in pregnancy is more prevalent in developing countries, such as Africa and Southeast Asia, where dietary diversity, living standards, and education levels are all poorer (73). In addition, lack of knowledge about anemia, infrequent antenatal check-ups, and unplanned pregnancies naturally lead to more threats of anemia in local pregnant women (74). As our study shows, 51 studies are from developing countries, especially Ethiopia, Ghana, and other countries. Also, low household income, no antenatal care, rural residence, underweight, middle upper arm circumference < 23, and illiteracy are all risk factors for anemia in pregnancy. Although unplanned pregnancy and lack of understanding of anemia were not statistically associated with anemia in pregnancy. However, according to the OR value of more than 1, and the lower limit of the 95% confidence interval close to 1, it is suggested that these two exposure factors are related to anemia in pregnancy to a certain extent.
In fact, the most common cause of anemia in pregnancy is iron deficiency, while other causes are rare (2). Although our study shows that lack of iron supplementation during pregnancy is not associated with anemia, the likely reason is that pregnant women obtain adequate iron intake through other means such as diet. However, our study also shows that iron supplementation is a protective factor in reducing the occurrence of anemia. This fully illustrates the importance of ingesting or supplementing adequate iron during pregnancy. Adequate intake of macro- and micronutrients, quantity and variety of diets is a challenge in many countries, especially developing ones (75, 76). After combined analysis of exposure factors related to dietary habits of pregnant women, we found that meal frequency ≤ 2 times per day, frequency of eating meat ≤ 1 time per week, tea/coffee after meals, diet diversity score ≤ 3, frequency of eating vegetables ≤ 3 times per week were all risk factor for anemia in pregnancy. This is consistent with the findings of Roess et al. Tea and coffee contain compounds that affect iron absorption such as tannins and polyphenol, meat is a good source of heme iron and protein of high biological value, and fruits rich in ascorbic acid can enhance iron absorption (77). Therefore, eating less or not eating meat and fruits will also lead to insufficient iron intake, which will eventually lead to the occurrence of anemia (78, 79). In addition, vegetables are a food source of folic acid, and folic acid deficiency is associated with anemia in pregnancy (80).
Although anemia in pregnancy is a global public health problem, we must acknowledge that anemia in pregnancy often differs between developed and developing countries and is one of the distinct health disparities between developed and developing countries (81). The prevalence of anemia in pregnancy in developing countries ranged between 53.8 and 90.2%, compared with 8.3% in developed countries (82). There are many factors that contribute to this difference. Compared with developed countries, medical resources are scarce in developing countries, pregnant women are less likely to receive adequate or quality health care, and they are at higher risk of exposure to diseases such as malaria and parasitic infections that cause anemia in pregnancy (83). Although we conducted a comprehensive search of current mainstream databases, the studies we included were all from developing countries, and evidence from developed countries was lacking. Therefore, our findings are only applicable to developing countries. More findings from developed countries are needed in the future to provide a global picture of risk factors for anemia in pregnancy.
Strengths: (1) As the first study in the current field to systematically summarize the risk factors of anemia in pregnancy, this study may serve as the best evidence for the prevention of anemia in pregnancy in the future. (2) This study was based on AOR rather than OR analysis, avoiding the interaction between multiple exposure factors, and the results were more in line with the actual situation. (3) According to the NOS scoring results, we found that the quality of evidence of the 51 included studies was high, which ensured the reliability of the meta-analysis results.
Limitations: (1) The included studies were all from developing countries, especially Ethiopia, therefore, our findings are only applicable to some countries, not all countries. (2) Include only English literature, which may lead to language bias. (3) The gray literature and conference abstracts were not searched, which may lead to publication bias.
The high incidence and serious harm of anemia in pregnancy make it urgent to systematically summarize its risk factors. Evidence from 51 high-quality studies showing infected parasite, history of malarial attack, tea/coffee after meals, meal frequency ≤ 2 times per day, frequency of eating meat ≤ 1 time per week, frequency of eating vegetables ≤ 3 times per week, multiple pregnancies, multiparous, low household income, no antenatal care, rural residence, diet diversity score ≤ 3, have more than 3 children, history of menorrhagia, underweight, family size ≥ 5, middle upper arm circumference < 23, second trimester, third trimester, birth interval ≤ 2 year, these 20 exposure factors were all risk factors for Anemia in Pregnancy. Therefore, health institutions and pregnant women themselves should focus on the above risk factors for better prevention and early detection of anemia in pregnancy.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
YSu proposed ideas and designed protocol. JZ and QL were responsible for data analysis and writing of the paper. YSo, LF, and LH were responsible for literature screening, data extraction, and quality evaluation. All authors contributed to the article and approved the submitted version.
We acknowledge the financial support from Kunming Health Science and Technology Personnel Training Project (No. 2022-SW-93).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1041136/full#supplementary-material
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; AOR, adjusted odds ratio; NOS, Newcastle-Ottawa Scale.
1. Harrison RK, Lauhon SR, Colvin ZA, McIntosh JJ. Maternal anemia and severe maternal morbidity in a US cohort. Am J Obstet Gynecol MFM. (2021) 3:100395. doi: 10.1016/j.ajogmf.2021.100395
2. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. (2013) 1:e16–25. doi: 10.1016/S2214-109X(13)70001-9
3. Kumera G, Haile K, Abebe N, Marie T, Eshete T. Anemia and its association with coffee consumption and hookworm infection among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia. PLoS One. (2018) 13:e0206880. doi: 10.1371/journal.pone.0206880
4. Liyew AM, Tesema GA, Alamneh TS, Worku MG, Teshale AB, Alem AZ, et al. Prevalence and determinants of anemia among pregnant women in East Africa; a multi-level analysis of recent Demographic and Health Surveys. PLoS ONE. (2021) 16:e0250560. doi: 10.1371/journal.pone.0250560
5. Lone FW, Qureshi RN, Emanuel F. Maternal anaemia and its impact on perinatal outcome. Trop Med Int Health. (2004) 9:486–90. doi: 10.1111/j.1365-3156.2004.01222.x
6. Kedir RD, Halil HM, Reta AE, Helill SE, Abdo RA. Prevalence and factors associated with anaemia among pregnant women in Hossana Town, Southern Ethiopia: a cross-sectional study. J Nepal Paediatr Soc. (2021) 41:218–25. doi: 10.3126/jnps.v41i2.32436
7. Kefiyalew F, Zemene E, Asres Y, Gedefaw L. Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors. BMC Res Notes. (2014) 7:771. doi: 10.1186/1756-0500-7-771
8. Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A. Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasit Vectors. (2012) 5:296. doi: 10.1186/1756-3305-5-296
9. Balis B, Dessie Y, Debella A, Alemu A, Tamiru D, Negash B, et al. Magnitude of anemia and its associated factors among pregnant women attending antenatal care in hiwot fana specialized University Hospital in Eastern Ethiopia. Front. Public Health. (2022) 10:867888. doi: 10.3389/fpubh.2022.867888
10. Teshome MS, Meskel DH, Wondafrash B. Determinants of anemia among pregnant women attending antenatal care clinic at public health facilities in Kacha Birra District, Southern Ethiopia. J Multidiscip Healthc. (2020) 13:1007–15. doi: 10.2147/JMDH.S259882
11. James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. (2021) 138:663–74. doi: 10.1097/AOG.0000000000004559
12. Mishra A, Marwah S, Divedi P, Dewan R, Ahluwalia H. A cross-sectional study of barriers in prevention of anemia in pregnancy. Cureus. (2021) 13:e12802. doi: 10.7759/cureus.12802
13. Debella A, Dheresa M, Geda B, Tiruye G, Fage SG. A third of pregnant women are affected by anemia in Eastern Ethiopia: a facility-based study. J Blood Med. (2021) 12:299–306. doi: 10.2147/JBM.S305567
14. Abdella B, Ibrahim M, Tadesse I, Hassen K, Tesfa M. Association between Helicobacter pylori infection and occurrence of anemia among pregnant women attending antenatal care in Kulito Health Center, Halaba Zone, South Ethiopia, 2018. Anemia. (2020) 2020:6574358. doi: 10.1155/2020/6574358
15. Kejela G, Wakgari A, Tesfaye T, Turi E, Adugna M, Alemu N, et al. Prevalence of anemia and its associated factors among pregnant women attending antenatal care follow up at Wollega University referral hospital, Western Ethiopia. Contracept Reprod Med. (2020) 5:26. doi: 10.1186/s40834-020-00130-9
16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Abdu S, Ali T, Debella A, Assefa N, Teji Roba K. Magnitude and factors associated with anemia among pregnant women admitted to labor ward of Hiwot Fana Specialized University Hospital, Eastern Ethiopia. SAGE Open Med. (2021) 9:20503121211047389. doi: 10.1177/20503121211047389
19. Abriha A, Yesuf ME, Wassie MM. Prevalence and associated factors of anemia among pregnant women of Mekelle town: a cross sectional study. BMC Res Notes. (2014) 7:888. doi: 10.1186/1756-0500-7-888
20. Addis Alene K, Mohamed Dohe A. Prevalence of anemia and associated factors among pregnant women in an urban area of Eastern Ethiopia. Anemia. (2014) 2014:e0188783. doi: 10.1155/2014/561567
21. Alreshidi MA, Haridi HK. Prevalence of anemia and associated risk factors among pregnant women in an urban community at the North of Saudi Arabia. J Prev Med Hyg. (2021) 62:E653. doi: 10.15167/2421-4248/jpmh2021.62.3.1880
22. Anwary Z, Stanikzai MH, Wyar WM, Wasiq AW, Farooqi K. Anemia among women who visit Bost Hospital for delivery in Helmand Province, Afghanistan. Anemia. (2021) 2021:9358464. doi: 10.1155/2021/9358464
23. Asri F. Prevalence of anemia and its associated factors among pregnant women receiving antenatal care at Aymiba Health Center, northwest Ethiopia. J Blood Med. (2017) 8:35–40. doi: 10.2147/JBM.S134932
24. Nonterah EA, Adomolga E, Yidana A, Kagura J, Agorinya I, Ayamba EY, et al. Descriptive epidemiology of anaemia among pregnant women initiating antenatal care in rural Northern Ghana. Afr J Primary Health Care Fam Med. (2019) 11:1–7. doi: 10.4102/phcfm.v11i1.1892
25. Azhar BS, Islam MS, Karim MR. Prevalence of anemia and associated risk factors among pregnant women attending antenatal care in Bangladesh: a cross-sectional study. Prim Health Care Res Dev. (2021) 22:e61. doi: 10.1017/S146342362100061X
26. Bekele A, Tilahun M, Mekuria A. Prevalence of anemia and its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch Town, Gamo Gofa Zone, Ethiopia: a cross-sectional study. Anemia. (2016) 2016:1073192. doi: 10.1155/2016/1073192
27. Berhe B, Mardu F, Legese H, Gebrewahd A, Gebremariam G, Tesfay K, et al. Prevalence of anemia and associated factors among pregnant women in Adigrat General Hospital, Tigrai, northern Ethiopia, 2018. BMC Res Notes. (2019) 12:1–6. doi: 10.1186/s13104-019-4347-4
28. Berhe K, Fseha B, Gebremariam G, Teame H, Etsay N, Welu G, et al. Risk factors of anemia among pregnant women attending antenatal care in health facilities of Eastern Zone of Tigray, Ethiopia, case-control study, 2017/18. Pan Afr Med J. (2019) 34:121. doi: 10.11604/pamj.2019.34.121.15999
29. Beyene T. Prevalence and factors associated with anemia among pregnant women attending antenatal care in Shalla Woreda, W/Arsi Zone, Oromia region. Int J Green Pharm. (2018) 12:165–73. doi: 10.22377/ijgp.v12i01.1629
30. Deriba BS, Bulto GA, Bala ET. Nutritional-related predictors of anemia among pregnant women attending antenatal care in central Ethiopia: an unmatched case-control study. Biomed Res Int. (2020) 2020:8824291. doi: 10.1155/2020/8824291
31. Derso T, Abera Z, Tariku A. Magnitude and associated factors of anemia among pregnant women in Dera District: a cross-sectional study in northwest Ethiopia. BMC Res Notes. (2017) 10:1–8. doi: 10.1186/s13104-017-2690-x
32. Engmann C, Adanu R, Lu T-S, Bose C, Lozoff B. Anemia and iron deficiency in pregnant Ghanaian women from urban areas. Int J Gynecol Obstetr. (2008) 101:62–6. doi: 10.1016/j.ijgo.2007.09.032
33. Fondjo LA, Addai-Mensah O, Annani-Akollor ME, Quarshie JT, Boateng AA, Assafuah SE, et al. A multicenter study of the prevalence and risk factors of malaria and anemia among pregnant women at first antenatal care visit in Ghana. PLoS ONE. (2020) 15:e0238077. doi: 10.1371/journal.pone.0238077
34. Gari W, Tsegaye A, Ketema T. Magnitude of anemia and its associated factors among pregnant women attending antenatal care at Najo General Hospital, Northwest Ethiopia. Anemia. (2020) 2020:8851997. doi: 10.1155/2020/8851997
35. Gebre A, Mulugeta A. Prevalence of anemia and associated factors among pregnant women in North Western zone of Tigray, Northern Ethiopia: a cross-sectional study. J Nutr Metab. (2015) 2015:165430. doi: 10.1155/2015/165430
36. Grum T, Brhane E, Hintsa S, Kahsay G. Magnitude and factors associated with anemia among pregnant women attending antenatal care in public health centers in central zone of Tigray region, northern Ethiopia: a cross sectional study. BMC Pregnancy Childbirth. (2018) 18:433. doi: 10.1186/s12884-018-2063-z
37. Gudeta TA, Regassa TM, Belay AS. Magnitude and factors associated with anemia among pregnant women attending antenatal care in Bench Maji, Keffa and Sheka zones of public hospitals, Southwest, Ethiopia, 2018: A cross-sectional study. PLoS ONE. (2019) 14:e0225148. doi: 10.1371/journal.pone.0225148
38. Hailu T, Kassa S, Abera B, Mulu W, Genanew A. Determinant factors of anaemia among pregnant women attending antenatal care clinic in Northwest Ethiopia. Tropical Dis Travel Med Vaccines. (2019) 5:1–7. doi: 10.1186/s40794-019-0088-6
39. Helion Belay AM, Tariku A, Woreta SA, Demissie GD, Asrade G. Anemia and associated factors among pregnant women attending prenatal care in rural Dembia district, North West Ethiopia: a cross-sectional study. Ecol Food Nutr. (2020) 59:154–74. doi: 10.1080/03670244.2019.1680551
40. Kare AP, Gujo AB. Anemia among pregnant women attending ante natal care clinic in Adare General Hospital, southern Ethiopia: prevalence and associated factors. Health Serv Insights. (2021) 14:11786329211036303. doi: 10.1177/11786329211036303
41. Kebede A, Gerensea H, Amare F, Tesfay Y, Teklay G. The magnitude of anemia and associated factors among pregnant women attending public institutions of Shire Town, Shire, Tigray, Northern Ethiopia, 2018. BMC Res Notes. (2018) 11:595. doi: 10.1186/s13104-018-3706-x
42. Kenea A, Negash E, Bacha L, Wakgari N. Magnitude of anemia and associated factors among pregnant women attending antenatal care in public hospitals of ilu Abba Bora zone, south west Ethiopia: a cross-sectional study. Anemia. (2018) 2018:9201383. doi: 10.1155/2018/9201383
43. Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: a community based cross-sectional study. PLoS ONE. (2017) 12:e0188783. doi: 10.1371/journal.pone.0188783
44. Lin L, Wei Y, Zhu W, Wang C, Su R, Feng H, et al. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: a multicentre retrospective study. BMC Pregnancy Childbirth. (2018) 18:1–8. doi: 10.1186/s12884-018-1739-8
45. Ngimbudzi EB, Massawe SN, Sunguya BF. The burden of anemia in pregnancy among women attending the antenatal clinics in Mkuranga District, Tanzania. Front Public Health. (2021) 9:724562. doi: 10.3389/fpubh.2021.724562
46. Noronha JA, Bhaduri A, Bhat HV, Kamath A. Maternal risk factors and anaemia in pregnancy: a prospective retrospective cohort study. J Obstet Gynaecol. (2010) 30:132–6. doi: 10.3109/01443610903267457
47. Obai G, Odongo P, Wanyama R. Prevalence of anaemia and associated risk factors among pregnant women attending antenatal care in Gulu and Hoima Regional Hospitals in Uganda: A cross sectional study. BMC Pregnancy Childbirth. (2016) 16:76. doi: 10.1186/s12884-016-0865-4
48. Oboro V, Tabowei T, Jemikalajah J. Prevalence and risk factors for anaemia in pregnancy in South Southern Nigeria. J Obstet Gynaecol. (2002) 22:610–613. doi: 10.1080/0144361021000020367
49. Okia CC, Aine B, Kiiza R, Omuba P, Wagubi R, Muwanguzi E, et al. Prevalence, morphological classification, and factors associated with anemia among pregnant women accessing antenatal clinic at Itojo Hospital, south western Uganda. J Blood Med. (2019) 10:351. doi: 10.2147/JBM.S216613
50. Osman MO, Nour TY, Bashir HM, Roble AK, Nur AM, Abdilahi AO. Risk factors for anemia among pregnant women attending the antenatal care unit in selected Jigjiga public health facilities, Somali region, East Ethiopia 2019: unmatched case–control study. J Multidiscip Healthc. (2020) 13:769. doi: 10.2147/JMDH.S260398
51. Ribot B, Díez FR, Abajo S, March G, Fargas F, Arija V. Prevalence of anaemia, risk of haemoconcentration and risk factors during the three trimesters of pregnancy. Nutrición Hospitalaria. (2018) 35:123–30. doi: 10.20960/nh.1045
52. Tadesse SE, Seid O, G/Mariam Y, Fekadu A, Wasihun Y, Endris K, et al. Determinants of anemia among pregnant mothers attending antenatal care in Dessie town health facilities, northern central Ethiopia, unmatched case-control study. PLoS ONE. (2017) 12:e0173173. doi: 10.1371/journal.pone.0173173
53. Tan J, He G, Qi Y, Yang H, Xiong Y, Liu C, et al. Prevalence of anemia and iron deficiency anemia in Chinese pregnant women (IRON WOMEN): a national cross-sectional survey. BMC Pregnancy Childbirth. (2020) 20:1–12. doi: 10.1186/s12884-020-03359-z
54. Tan J, Qi Y-N, He G-L, Yang H-M, Zhang G-T, Zou K, et al. Association between maternal weight indicators and iron deficiency anemia during pregnancy: a cohort study. Chinese Med J. (2018) 131:2566–74. doi: 10.4103/0366-6999.244109
55. Tulu BD, Atomssa EM, Mengist HM. Determinants of anemia among pregnant women attending antenatal care in Horo Guduru Wollega Zone, West Ethiopia: Unmatched case-control study. PLoS ONE. (2019) 14:e0224514. doi: 10.1371/journal.pone.0224514
56. Weldekidan F, Kote M, Girma M, Boti N, Gultie T. Determinants of anemia among pregnant women attending antenatal clinic in public health facilities at Durame Town: unmatched case control study. Anemia. (2018) 2018:8938307. doi: 10.1155/2018/8938307
57. Woldegebriel AG, Gebregziabiher Gebrehiwot G, Aregay Desta A, Fenta Ajemu K, Berhe AA, Woldearegay TW, et al. Determinants of anemia in pregnancy: findings from the Ethiopian health and demographic survey. Anemia. (2020) 2020:2902498. doi: 10.1155/2020/2902498
58. Yesuf NN, Agegniche Z. Prevalence and associated factors of anemia among pregnant women attending antenatal care at Felegehiwot Referral Hospital, Bahirdar City: institutional based cross-sectional study. Int J Africa Nurs Sci. (2021) 15:100345. doi: 10.1016/j.ijans.2021.100345
59. Zerfu TA, Baye K, Faber M. Dietary diversity cutoff values predicting anemia varied between mid and term of pregnancy: a prospective cohort study. J Health Populat Nutr. (2019) 38:44. doi: 10.1186/s41043-019-0196-y
60. Zillmer K, Pokharel A, Spielman K, Kershaw M, Ayele K, Kidane Y, et al. Predictors of anemia in pregnant women residing in rural areas of the Oromiya region of Ethiopia. BMC Nutr. (2017) 3:65. doi: 10.1186/s40795-017-0166-y
61. Derso A, Nibret E, Munshea A. Prevalence of intestinal parasitic infections and associated risk factors among pregnant women attending antenatal care center at Felege Hiwot Referral Hospital, northwest Ethiopia. BMC Infect Dis. (2016) 16:530. doi: 10.1186/s12879-016-1859-6
62. Hotez P, Whitham M. Helminth infections: a new global women's health agenda. Obstet Gynecol. (2014) 123:155–60. doi: 10.1097/AOG.0000000000000025
63. Verjee MA. Schistosomiasis: still a cause of significant morbidity and mortality. Res Rep Trop Med. (2019) 10:153–63. doi: 10.2147/RRTM.S204345
64. Loukas A, Gaze S, Mulvenna JP, Gasser RB, Brindley PJ, Doolan DL, et al. Vaccinomics for the major blood feeding helminths of humans. OMICS. (2011) 15:567–77. doi: 10.1089/omi.2010.0150
65. Alem M, Enawgaw B, Gelaw A, Kena T, Seid M, Olkeba Y. Prevalence of anemia and associated risk factors among pregnant women attending antenatal care in Azezo Health Center Gondar town, Northwest Ethiopia. J Interdiscip Histopathol. (2013) 1:137–44. doi: 10.5455/jihp.20130122042052
66. Bauserman M, Conroy AL, North K, Patterson J, Bose C, Meshnick S. An overview of malaria in pregnancy. Semin Perinatol. (2019) 43:282–90. doi: 10.1053/j.semperi.2019.03.018
67. Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. (2004) 17:760–9. doi: 10.1128/CMR.17.4.760-769.2004
68. Jufar AH, Zewde T. Prevalence of anemia among pregnant women attending antenatal care at tikur anbessa specialized hospital, Addis Ababa Ethiopia. J Hematol Thromb Dis. (2014) 2:2. doi: 10.4172/2329-8790.1000125
69. Karaoglu L, Pehlivan E, Egri M, Deprem C, Gunes G, Genc MF, et al. The prevalence of nutritional anemia in pregnancy in an east Anatolian province, Turkey. BMC Public Health. (2010) 10:329. doi: 10.1186/1471-2458-10-329
70. Uche-Nwachi EO, Odekunle A, Jacinto S, Burnett M, Clapperton M, David Y, et al. Anaemia in pregnancy: associations with parity, abortions and child spacing in primary healthcare clinic attendees in Trinidad and Tobago. Afr Health Sci. (2010) 10:66–70.
71. Al-Mehaisen L, Khader Y, Al-Kuran O, Abu Issa F, Amarin Z. Maternal anemia in rural jordan: room for improvement. Anemia. (2011) 2011:381812. doi: 10.1155/2011/381812
72. Srinivasa Rao P, Srikanth S. Prevalence of anemia in the first trimester of pregnancy in rural population of Krishna District in Andhra Pradesh. Sch J App Med Sci. (2013) 1:570–4.
73. Sharma S, Kaur SP, Lata G. Anemia in pregnancy is still a public health problem: a single center study with review of literature. Indian J Hematol Blood Transfus. (2020) 36:129–34. doi: 10.1007/s12288-019-01187-6
74. Sultana F, Ara G, Akbar T, Sultana R. Knowledge about Anemia among pregnant women in tertiary hospital. Med Today. (2019) 31:105–10. doi: 10.3329/medtoday.v31i2.41962
75. Asayehu TT, Lachat C, Henauw S, Gebreyesus SH. Dietary behaviour, food and nutrient intake of women do not change during pregnancy in Southern Ethiopia. Matern Child Nutr. (2017) 13:e12343. doi: 10.1111/mcn.12343
76. Bailey RL, Pac SG, Fulgoni VL, 3rd, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open. (2019) 2:e195967. doi: 10.1001/jamanetworkopen.2019.5967
77. Dattijo L, Daru P, Umar N. Anaemia in pregnancy: prevalence and associated factors in Azare, North-East Nigeria. Int J Trop Dis Health. (2016) 11:1–9. doi: 10.9734/IJTDH/2016/20791
78. Roess AA, Jacquier EF, Catellier DJ, Carvalho R, Lutes AC, Anater AS, et al. Food consumption patterns of infants and toddlers: findings from the feeding infants and toddlers study (FITS) 2016. J Nutr. (2018) 148:1525S−35S. doi: 10.1093/jn/nxy171
79. Milman NT. A review of nutrients and compounds, which promote or inhibit intestinal iron absorption: making a platform for dietary measures that can reduce iron uptake in patients with genetic haemochromatosis. J Nutr Metab. (2020) 2020:7373498. doi: 10.1155/2020/7373498
80. Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Med Clin North Am. (2016) 100:1199–215. doi: 10.1016/j.mcna.2016.06.004
81. McMahon LP. Iron deficiency in pregnancy. Obstet Med. (2010) 3:17–24. doi: 10.1258/om.2010.100004
82. De Benoist B, Cogswell M, Egli I, McLean E. Worldwide Prevalence of Anaemia 1993-2005; WHO Global Database of Anaemia (2008).
Keywords: anemia, pregnancy, systematic review, nutritional factors, evidence
Citation: Zhang J, Li Q, Song Y, Fang L, Huang L and Sun Y (2022) Nutritional factors for anemia in pregnancy: A systematic review with meta-analysis. Front. Public Health 10:1041136. doi: 10.3389/fpubh.2022.1041136
Received: 10 September 2022; Accepted: 26 September 2022;
Published: 14 October 2022.
Edited by:
António Raposo, CBIOS, Universidade Lusófona Research Center for Biosciences and Health Technologies, PortugalReviewed by:
Zayed Alsharari, University of Tabuk, Saudi ArabiaCopyright © 2022 Zhang, Li, Song, Fang, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Sun, c3VueXU1Mjc4MjY1NjhAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.