- 1Chongqing Key Laboratory of Infectious Diseases and Parasitic Diseases, Department of Infectious Diseases, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Thoracic Surgery, Daping Hospital, Army Medical University, Chongqing, China
- 3Department of Ultrasound, The 941st Hospital of the People's Liberation Army Joint Logistic Support Force, Xining, China
- 4Department of Geriatrics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Carcinosarcoma is a rare biphasic tumor composed of both carcinoma and sarcoma elements, which occurs at various sites. Most studies are case reports or small population-based studies for a single disease site, so comprehensive evaluations of epidemiology and prognostic factors for carcinosarcoma are needed.
Methods: Surveillance, Epidemiology, and End Results (SEER)-8 (1975–2019) provided data for the epidemiological analysis. SEER-17 (2000–2019) provided data on the primary tumor sites, initial treatment, construction, and validation of the nomogram.
Results: The age-adjusted incidence per 100,000 persons of carcinosarcoma increased significantly from 0.46 to 0.91 [1975–2019; average annual percent change (AAPC): 1.3%, P = 0.006], with localized stage increasing from 0.14 to 0.26 [2005–2015; annual percent change (APC): 4.2%]. The 20-year limited-duration prevalence per 100,000 increased from 0.47 to 3.36 (1999–2018). The mortality per 100,000 increased significantly from 0.16 to 0.51 (1975–2019; AAPC: 1.9%, P < 0.001). The 5-year relative survival was 32.8%. The greatest number of carcinosarcomas were from the uterus (68.7%), ovary (17.8%), lung and bronchus (2.3%). The main treatment is comprehensive treatment based on surgery; however, surgery alone is preferred in older patients. In multivariate analysis (N = 11,424), age, sex, race, year of diagnosis, disease stage, tumor site, and treatment were associated with survival. A nomogram was established to predict 1-, 3-, and 5-year survival, and the C-indexes were 0.732 and 0.748 for the training and testing sets, respectively. The receiver operating characteristic curve demonstrated that the nomogram provided a comprehensive and accurate prediction [1-year area under the curve (AUC): 0.782 vs. 0.796; 3-year AUC: 0.771 vs. 0.798; 5-year AUC: 0.777 vs. 0.810].
Conclusions: In this study, the incidence, prevalence, and mortality of carcinosarcoma have increased over the past decades. There was a rapid rise in the incidence of localized stage in recent years, which reflected improved early detection. The prognosis of carcinosarcoma remains poor, signifying the urgency of exploring targeted cancer control treatments. Explicating distribution and gender disparities of carcinosarcoma may facilitate disease screening and medical surveillance. The nomogram demonstrated good predictive capacity and facilitated clinical decision-making.
Introduction
Carcinosarcoma is a rare biphasic tumor composed of both carcinoma and sarcoma elements; this is the most lethal malignancy (1–3). Carcinosarcoma occurs at various sites, mainly in the uterus and ovary, but also in the lung, bladder, peritoneum, and gallbladder (4–9). Due to the highly aggressive behavior of carcinosarcomas, advanced stage at diagnosis and frequent recurrences may explain the poor prognosis (1, 5, 9).
Most studies on carcinosarcoma focused on gynecological carcinosarcoma and suggested that the incidence of carcinosarcoma gradually increased in recent years (5, 10). The vast majority of studies are case reports or small population-based studies for a single disease site (11–14). Previous researches on single pathological types indicated that primary tumor site could affect prognosis (15–17). In multisite tumors (MDM2-amplified liposarcoma, neuroendocrine tumor, undifferentiated multitype sarcoma, cutaneous melanoma), tumor site was a significant variable in survival prediction model (18–21). A study on liposarcoma proposed that anatomic localization and histological grade, and not tumor size, should be included in liposarcoma-specific staging system (18). Hence, it is necessary to explore the effect of tumor site on prognosis, especially for rare tumors. To date, there is insufficient systematic studies on carcinosarcoma based on large sample sizes. Distribution and prognosis of tumor site remain unclear. Using the Surveillance, Epidemiology, and End Results (SEER) program, we performed a large population and multi-primary tumor site epidemiological and clinical analysis of carcinosarcoma to provide more clinical evidence.
Because of the rarity and aggressiveness of carcinosarcoma, clinical trials are difficult to conduct, and comprehensive analysis of treatments is lacking; hence, there is no standard consensus for treatment guidelines (4, 9, 22, 23). The prognosis of carcinosarcoma is difficult to judge because of its complexity (5, 9). Therefore, this study analyzed treatment strategies and established a nomogram combining various factors to predict the survival probability based on a large population of patients with carcinosarcoma.
Methods
Data source
The data were obtained from the SEER database (www.seer.cancer.gov) of the National Cancer Institute using the SEER*Stat software (SEER*Stat 8.4.0). The SEER 8 registries program is a unique record for long-term (1975–2019) incidence, prevalence, mortality, and relative survival, representing about 8.3% of the US population, released April 2022. Analysis of ten leading specific tumor sites, initial treatment, as well as construction and validation of nomogram were based on the SEER 17 registries program (2000–2019); covering ~26.5% of the US population, released April 2022.
Study population and variables
Carcinosarcoma (8,950/3, 8,951/3, 8,980/3, 8,981/3) was identified according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). Meanwhile, diagnoses were confirmed by positive histology; only one primary and the type of reporting source was not autopsy or death certificate.
The variables included age, sex, race, year of diagnosis, disease stage, site record, treatment, survival time, and vital status (alive/dead). The year of diagnosis was divided into four periods, including 2000–2004, 2005–2009, 2010–2014, and 2014–2019. Vital status was used as study endpoint, defined as any patient who died after the follow-up cut-off date was recorded to alive as of the cut-off date. Overall survival (OS) is defined as the period from the date of diagnosis to death. In the epidemiology section, the disease stage was referenced to SEER historic stage A (1975–2015); in the specific patient list, the disease stage was a combination of SEER historic stage A (1975–2015) and combined summary stage (2016–2019). Treatment was divided into no treatment/unknown; single treatment, chemotherapy; single treatment, radiotherapy; single treatment, surgery; chemotherapy + radiotherapy; chemotherapy + surgery; radiotherapy + surgery; and chemotherapy + radiotherapy + surgery by calculation.
Study design
The study design is presented in a flowchart (Figure 1). The age-adjusted incidence, prevalence, and age-adjusted mortality of carcinosarcoma were obtained from the SEER 8 database and expressed per 100,000 persons, referring to the 2,000 US standard population. The trends of incidence and mortality, including the annual percent change (APC) and average annual percent change (AAPC), were quantified using the Joinpoint Regression Program (version 4.7), allowing up to two joinpoints. The last 20-year prevalence and the 1-, 3-, and 5-year relative survival for carcinosarcoma were calculated using the SEER*Stat 8.4.0.
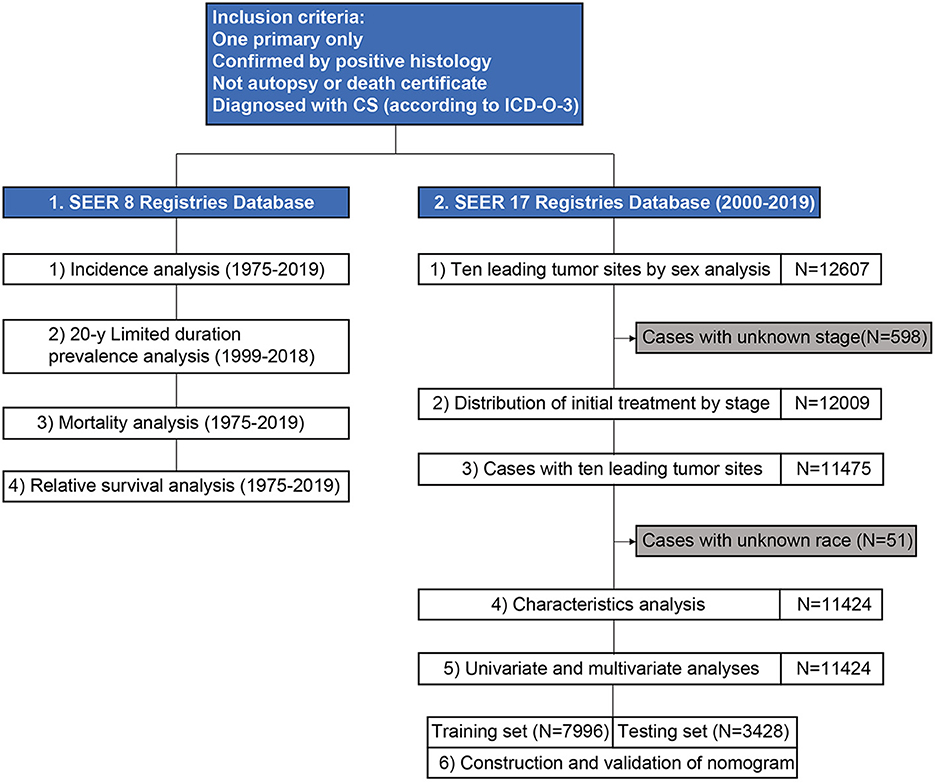
Figure 1. A flowchart of study design and patient selection. CS, carcinosarcoma; ICD-O-3, International Classification of Disease for Oncology, 3rd edition; SEER, surveillance, epidemiology, and end results.
A detailed patient list was obtained from SEER 17. Ten leading specific tumor sites by sex were analyzed, and the distribution of initial treatment by stage and age was assessed. This study included patients with carcinosarcoma of the ten leading specific tumor sites. Patient characteristics were presented, and univariate and multivariate analysis were performed to investigate prognostic factors associated with survival. Patients were randomly divided into training and testing sets at a ratio of 7:3. A nomogram to predict 1-, 3-, and 5-year survival for carcinosarcoma was constructed based on the prognostic factors in the training set, and concordance indexes (C-index) and area under the curve (AUC) were used to evaluate the nomogram.
Statistical analysis
Variables were presented as frequency and percentage and were compared using Pearson's chi-square test. Univariate and multivariate Cox proportional hazards models were used to assess the prognostic factors associated with OS by calculating the hazard ratio (HR) and 95% confidence interval (CI). C-index and calibration curves were used to evaluate the discriminative ability in the training and testing sets. The total nomogram score for each patient was obtained, and the corresponding AUC was used to estimate accuracy. Statistical analyses were performed using the R software (version 4.0.5; http://www.r-project.org/). All tests of statistical significance were 2-sided, and a P-value < 0.05 was considered statistically significant.
Results
Annual incidence
Using population data obtained from SEER 8, the age-adjusted incidence of all (both sexes) cases showed a marked increase from 0.46 in 1975 to 0.91 per 100,000 persons in 2019 (AAPC: 1.3%, P = 0.006), largely because of a rapid increase among cases in women (AAPC: 1.5%, P < 0.001) rather than in men (AAPC: 0.0%, P = 0.986; 1.73 vs. 0.07 per 100,000 persons in 2019, respectively; Figure 2A). As for age at diagnosis, the incidence was highest in ≥70, followed by 60–69, 50–59, and ≤ 49 years (3.33 vs. 3.25 vs. 1.31 vs. 0.06 per 100,000 persons in 2019), with an AAPC of 0.7% (P = 0.143), 1.9% (P = 0.073), 0.9% (P = 0.003), and 1.6% (P = 0.001), respectively (Figure 2B). The incidence rates of white, black, and other races were rising over the past decades, with an AAPC of 1.3% (P < 0.001), 1.8% (P = 0.005), and 2.5% (P < 0.001), respectively. Among black populations, a marked increase in incidence was observed from 1998 to 2019 (0.38–1.40 per 100,000 persons), with an APC of 4.7% (Figure 2C). From 1975 to 2015, the incidence of localized (AAPC: 0.9%, P = 0.045), regional (AAPC: 3.3%, P < 0.001), and distant stages (AAPC: 1.9%, P < 0.001) had a rising trend; among them, localized stage had increased the most in recent years (2005–2015, APC 4.2%). Patients with unknown stage decreased from 1975 to 2015, with an AAPC of −3.3% (P < 0.001; Figure 2D). Detailed data are shown in eTables 1, 2.
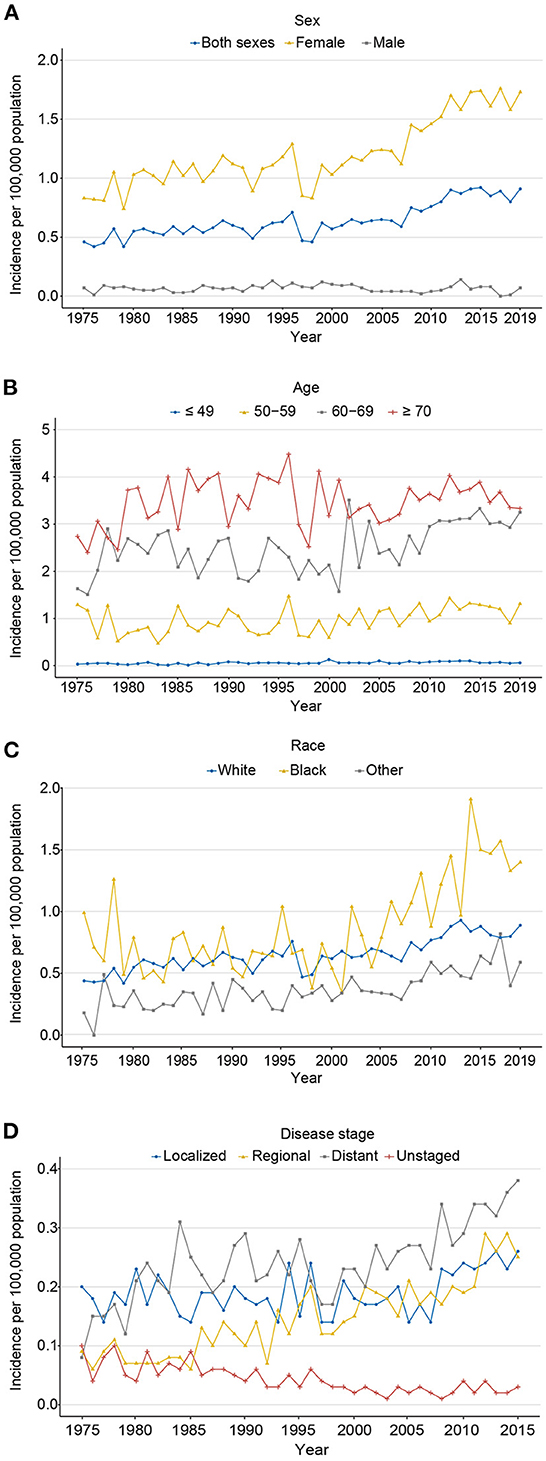
Figure 2. The age-adjusted incidence of carcinosarcoma, SEER-8. (A) for sex (1975–2019); (B) for age (1975–2019); (C) for race (1975–2019); and (D) for disease stage (1975–2015).
Twenty-year limited-duration prevalence
The 20-year limited-duration prevalence of all carcinosarcomas increased from 0.47 to 3.36 per 100,000 persons from 1999 to 2018 (Figure 3A; eTable 3). The prevalence in women was dramatically higher than that in men (6.20 vs. 0.10 per 100,000 persons in 2018). For age groups, the prevalence in 60–69 years was the highest, followed by ≥70, 50–59, and ≤ 49 years (1.25 vs. 0.92 vs, 0.78 vs, 0.40 per 100,000 persons in 2018, respectively; Figure 3B). Among different races, prevalence increased the most in the black population from 1.05 to 6.26 per 100,000 persons from 1999 to 2018, followed by white and other races (Figure 3C). For stage groups, the prevalence increased most in localized, followed by regional, distant, and unknown stages (1.53 vs. 0.92 vs. 0.75 vs. 0.08 per 100,000 persons in 2015, respectively; Figure 3D).
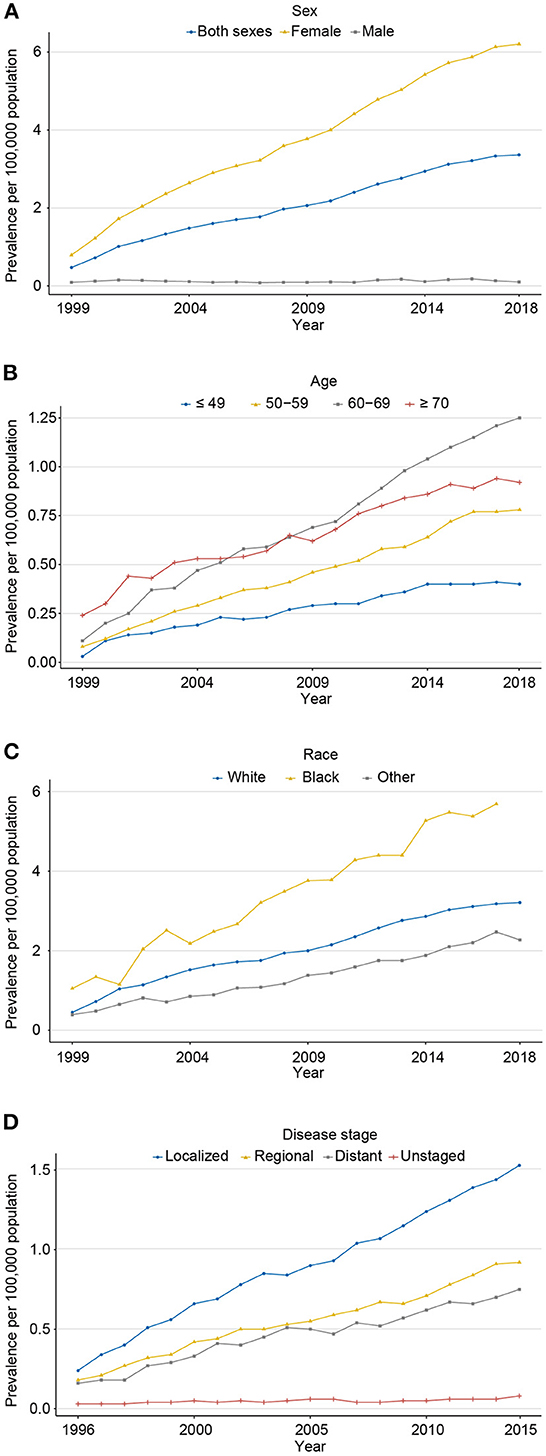
Figure 3. The 20-year limited-duration prevalence of carcinosarcoma, SEER-8. (A) for sex (1999–2018); (B) for age (1999–2018); (C) race (1999–2018); and (D) disease stage (1996–2015).
Annual mortality
Contrary to the rapid progress against cancer, the mortality of carcinosarcoma increased significantly from 0.16 to 0.51 per 100,000 persons from 1975 to 2019, with an AAPC of 1.9% (P < 0.001; Figure 4A; eTables 4, 5). The mortality rate for women still increased from 2015 to 2019, with an AAPC of 0.5% (P = 0.001); fortunately for men, it decreased sharply, with an AAPC of −4.1% (P = 0.003). Patients aged 70 years or older had the highest death rate, followed by 60–69, 50–59, and ≤ 49 years (0.22 vs. 0.17 vs. 0.07 vs. 0.04 per 100,000 persons in 2019, respectively), with an AAPC of 1.6% (P = 0.022), 5.6% (P = 0.120), 0.8% (P = 0.013), and 2.0% (P < 0.001), respectively (Figure 4B). The mortality rate in black populations edged up from 0.55 in 1975 to 0.78 per 100,000 persons in 2019, with an AAPC of −0.5% (P = 0.524; Figure 4C). Meanwhile, steady increases in mortality for white and other races were observed from 1975 to 2019, with an AAPC of 3.0% (P = 0.036) and 1.3% (P = 0.029), respectively. Notably, from 1975 to 2019, mortality only declined in unknown stage (AAPC −3.4%, P < 0.001), stabilized at localized stage (AAPC 2.1%, P = 0.124), and increased in regional (AAPC 2.9%, P < 0.001) and distant stages (AAPC 2.9%, P < 0.001; Figure 4D).
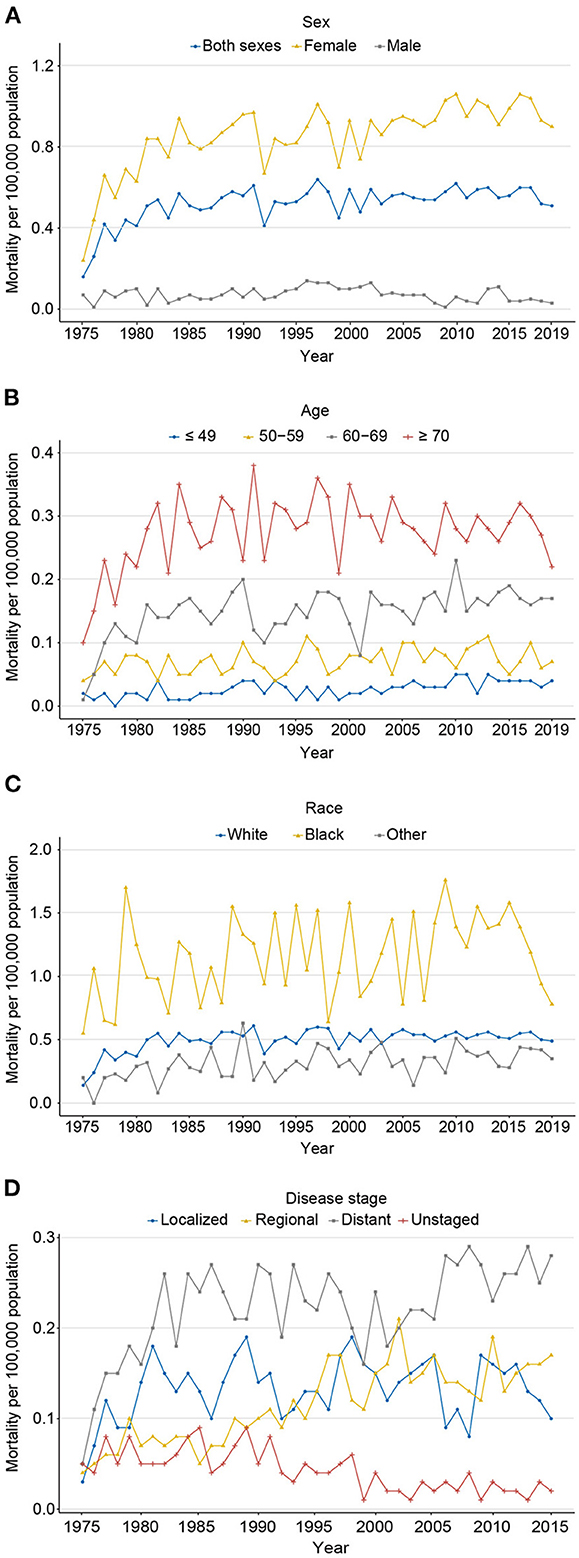
Figure 4. The age-adjusted mortality of carcinosarcoma, SEER-8. (A) for sex (1975–2019); (B) for age (1975–2019); (C) for race (1975–2019); and (D) for disease stage (1975–2015).
Relative survival analysis
The 1-, 3-, and 5-year relative survival rate for all carcinosarcomas based on SEER 8 from 1975 to 2019 were 64.2, 38.9, and 32.8%, respectively (Figure 5). Survival rates were close to overall for women (1-year, 65.2%; 3-year, 39.7%; 5-year, 33.5%) and much worse for men (1-year, 43.0%; 3-year, 22.9%; 5-year, 18.7%). For age groups, survival rate was highest for ≤49 years (1-year, 74.5%; 3-year, 54.4%; 5-year, 49.1%) and lowest for ≥70 years (1-year, 58.2%; 3-year, 33.0%; 5-year, 28.2%). Among different races, black patients (1-year, 58.3%; 3-year, 32.8%; 5-year, 27.3%) had the worst prognosis than white (1-year, 64.9%; 3-year, 39.6%; 5-year, 33.4%) and other races (1-year, 66.1%; 3-year, 42.1%; 5-year, 36.0%). Survival was the best in the localized stage (1-year, 84.1%; 3-year, 65.5%; 5-year, 60.1%), followed by the regional stage (1-year, 67.2%; 3-year, 38.7%; 5-year, 31.9%), and the worst in the distant stage (1-year, 47.5%; 3-year, 19.3%; 5-year, 12.9%).
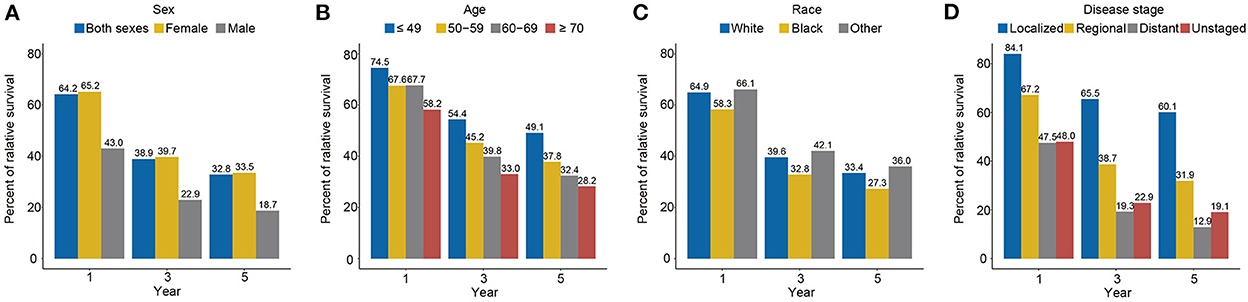
Figure 5. The 1-, 3-, and 5-year relative survival rates of carcinosarcoma by sex (A, 1975–2019), age (B, 1975–2019), race (C, 1975–2019), and disease stage (D, 1975–2015), SEER-8.
Ten leading specific tumor sites by sex
Using a detailed patient list from SEER 17, Figure 6 presents the ten leading specific tumor sites for carcinosarcoma by sex. Of 12,607 cases, the greatest number of carcinosarcomas were from the uterus (68.7%), ovary (17.8%), lung and bronchus (2.3%), breast (1.5%), and urinary bladder (1.2%). In total, uterus, ovary, and breast accounted for 88.0% of all cases (Figure 6A). In women, uterus (71.8%), ovary (18.6%), and breast (1.6%) accounted for 92.0% of all female carcinosarcomas (Figure 6B). In men, lung and bronchus (32.1%), urinary bladder (17.9%), and salivary gland (5.2%) were the three most common sites (Figure 6C).
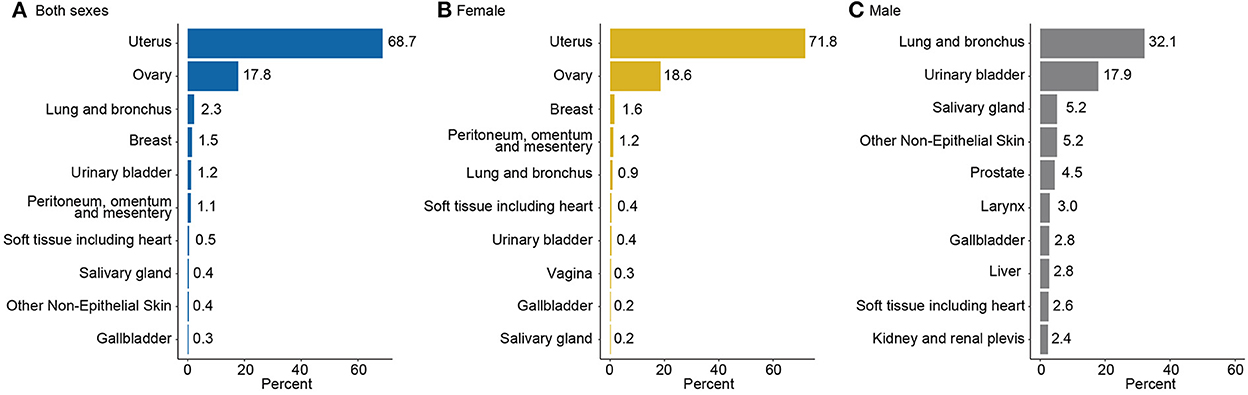
Figure 6. Ten leading specific tumor sites of carcinosarcoma by sex, SEER-17, 2000–2019. (A) Both sexes, (B) for female, and (C) for male.
Initial treatment for carcinosarcoma by stage and age
From 2000 to 2019, 2.4% of localized, 3.1% of regional, and 9.5% of distant stage carcinosarcoma cases (12,009) were classified as receiving no treatment/unknown (Figure 7A). Receiving single surgery or surgery combined with other comprehensive modality treatments (including chemotherapy and radiotherapy) played the most important role in all stages of all carcinosarcomas (localized, 96.2%; regional, 91.5%; distant, 77.8%). In contrast, only a minority of the patients received a single treatment. The proportion of receiving no treatment/unknown was higher in the older patients (≥70 years) than in younger patients (<70 years) at every stage (localized, 4.5 vs. 1.0%; regional, 5.0 vs. 1.8%; distant, 13.9 vs. 6.7%; Figures 7B,C). A higher proportion of older patients received single surgery treatment than younger patients (localized, 43.2 vs. 30.7%; regional, 34.1 vs. 19.4%; distant, 26.5 vs. 16.9%), whereas a lower proportion of older than younger patients received surgery combined with chemotherapy and/or radiotherapy (localized, 31.1 vs. 51.0%; regional, 41.2 vs. 63.7%; distant, 43.8 vs. 61.3%).
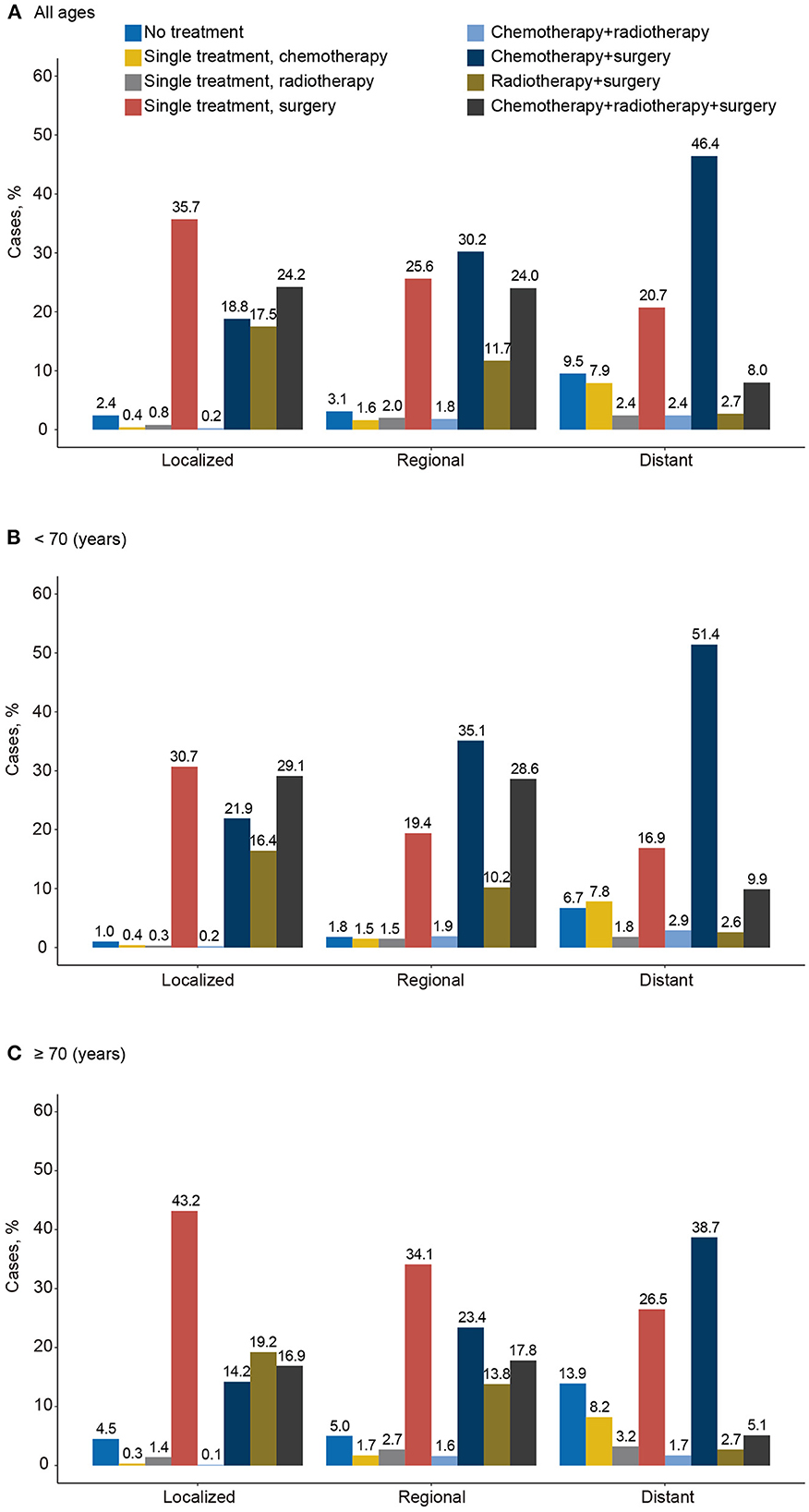
Figure 7. Distribution of initial treatment of carcinosarcoma by disease stage and age, SEER-17, 2000–2019. (A) All ages, (B) for <70 years, and (C) for ≥70 years.
Patient characteristics, univariate and multivariate analyses
A total of 11,424 patients with carcinosarcoma of the ten leading specific tumor sites were identified in the following analysis (Table 1). Among these patients, 40.6% (4,639) were aged 70 years or older. Most of the patients were women (11,088, 97.1%) and white population (8,290, 72.6%). For year of diagnosis, 18.4% (2,105) were diagnosed in 2000–2004, 22.6% (2,580) were diagnosed in 2005–2009, 27.7% (3,161) were diagnosed in 2010–2014, and 31.3% (3,578) were diagnosed in 2015–2019. As for disease stage, 3,499 (30.6%) were localized, 3,453 (30.2%) were regional, and 4,472 (39.1%) were distant stage. Furthermore, the uterus (8,317, 72.8%) was the most common primary tumor site of carcinosarcoma, followed by ovary (2,187, 19.1%) and lung and bronchus (280, 2.5%). This was generally consistent with previous rankings. Receiving surgical treatment (3,048, 26.7%) or surgery combined with chemotherapy, radiotherapy, or chemoradiotherapy (7,016, 61.5%) dominated the treatment modalities.
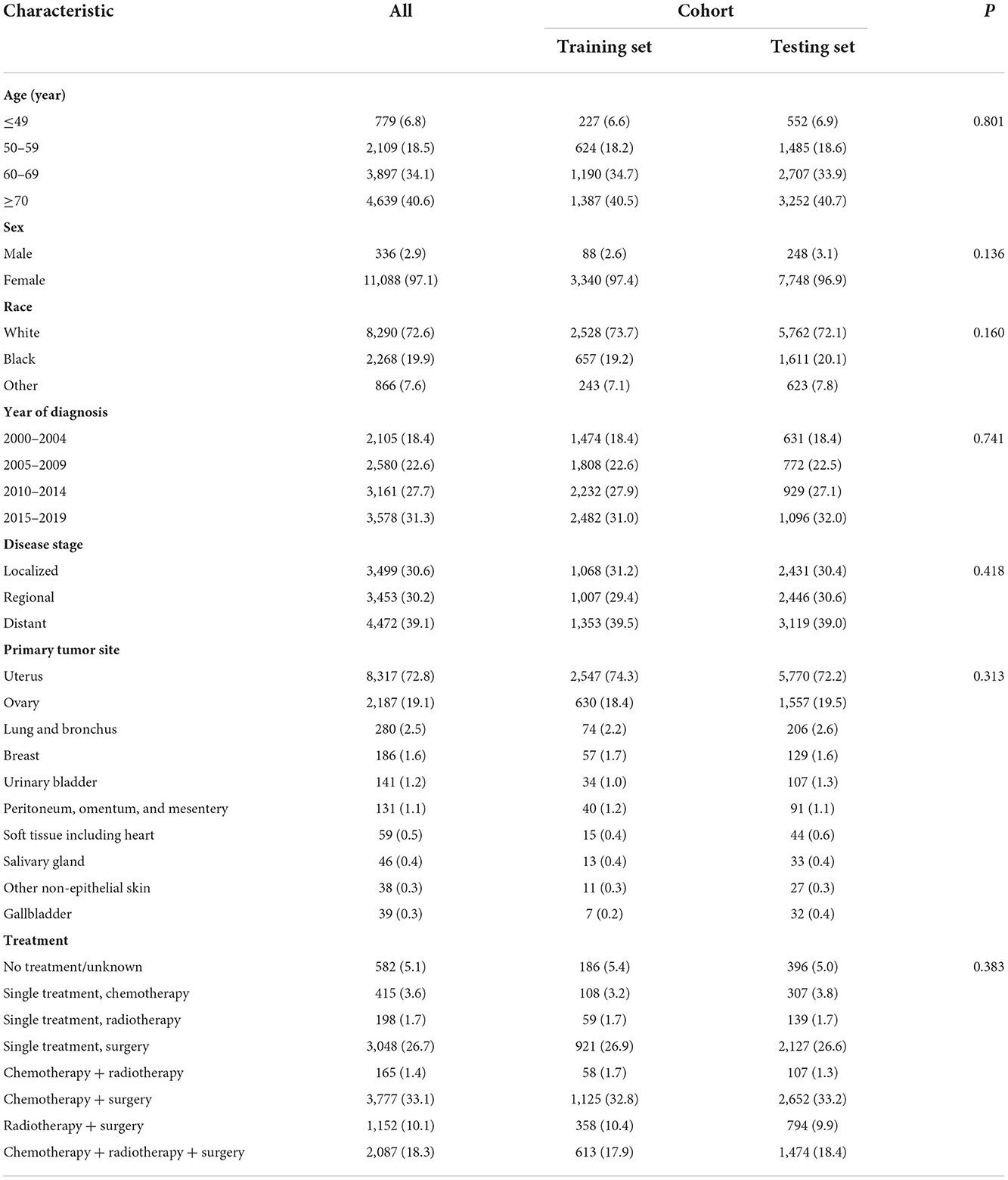
Table 1. Baseline clinicopathological characteristics for all carcinosarcoma, training set, and testing set.
Univariate and multivariate analyses were performed to investigate prognostic factors associated with survival. In univariate analysis, age, sex, race, year of diagnosis, disease stage, primary tumor site and treatment were all statistically significant (eTable 6). In multivariate analysis, the disease stage and treatment were the most relevant prognostic factors (eTable 6). Compared with localized, the significant increase in mortality was observed in distant (HR: 4.57, 95% CI: 4.26–4.90; P < 0.001) and regional stages (HR: 2.41, 95% CI: 2.26–2.58; P < 0.001). All treatment modalities were effective, with triple therapies (HR: 0.13, 95% CI: 0.11–0.14; P < 0.001) being the most effective factor in improving OS. Compared with the uterus, the gallbladder (HR: 1.57, 95% CI: 1.09–2.27; P = 0.016) and soft tissues including heart (HR: 1.45, 95% CI: 1.09–1.94; P = 0.012) were associated with inferior survival. Other non-epithelial skin (HR: 0.52, 95% CI: 0.34–0.80; P = 0.003), salivary gland (HR: 0.56, 95% CI: 0.37–0.85; P = 0.007), and ovary (HR: 0.78, 95% CI: 0.73– 0.83; P < 0.001) were associated with superior survival. The other sites showed no significant differences. In addition, age (50–59 vs. 0–49 years: HR: 1.34, 95% CI: 1.19–1.49, P < 0.001; 60–69 vs. 0–49 years: HR: 1.49, 95% CI: 1.34–1.65, P < 0.001; ≥70 vs. 0–49 years: HR: 1.86, 95% CI: 1.68–2.07, P < 0.001), sex (female vs. male: HR: 0.70, 95% CI: 0.58–0.85, P < 0.001), race (black vs. white: HR: 1.17, 95% CI: 1.10–1.24; P < 0.001), and year of diagnosis (2005–2009 vs. 2000–2004: HR: 0.90, 95% CI: 0.84–0.96, P < 0.001; 2010–2014 vs. 2000–2004: HR: 0.88, 95% CI: 0.83–0.94, P < 0.001; 2015–2019 vs. 2000–2004: HR: 0.87, 95% CI: 0.81–0.93, P < 0.001) were significantly associated with survival.
Construction and validation of nomogram
At a ratio of 7:3, patients were randomly assigned to the training (7,996) and testing sets (3,428, Table 1). There were no significant differences between the two sets. A nomogram for predicting 1-, 3-, and 5-year survival probability was constructed by including prognostic factors in the multivariate analysis based on the training set (Figure 8A). Consistent with previous results, treatment was the most significant factor associated with survival, followed by disease stage and primary tumor site. Age, sex, race and year of diagnosis were also included in the nomogram. The detailed scores for each characteristic are presented in eTable 7.
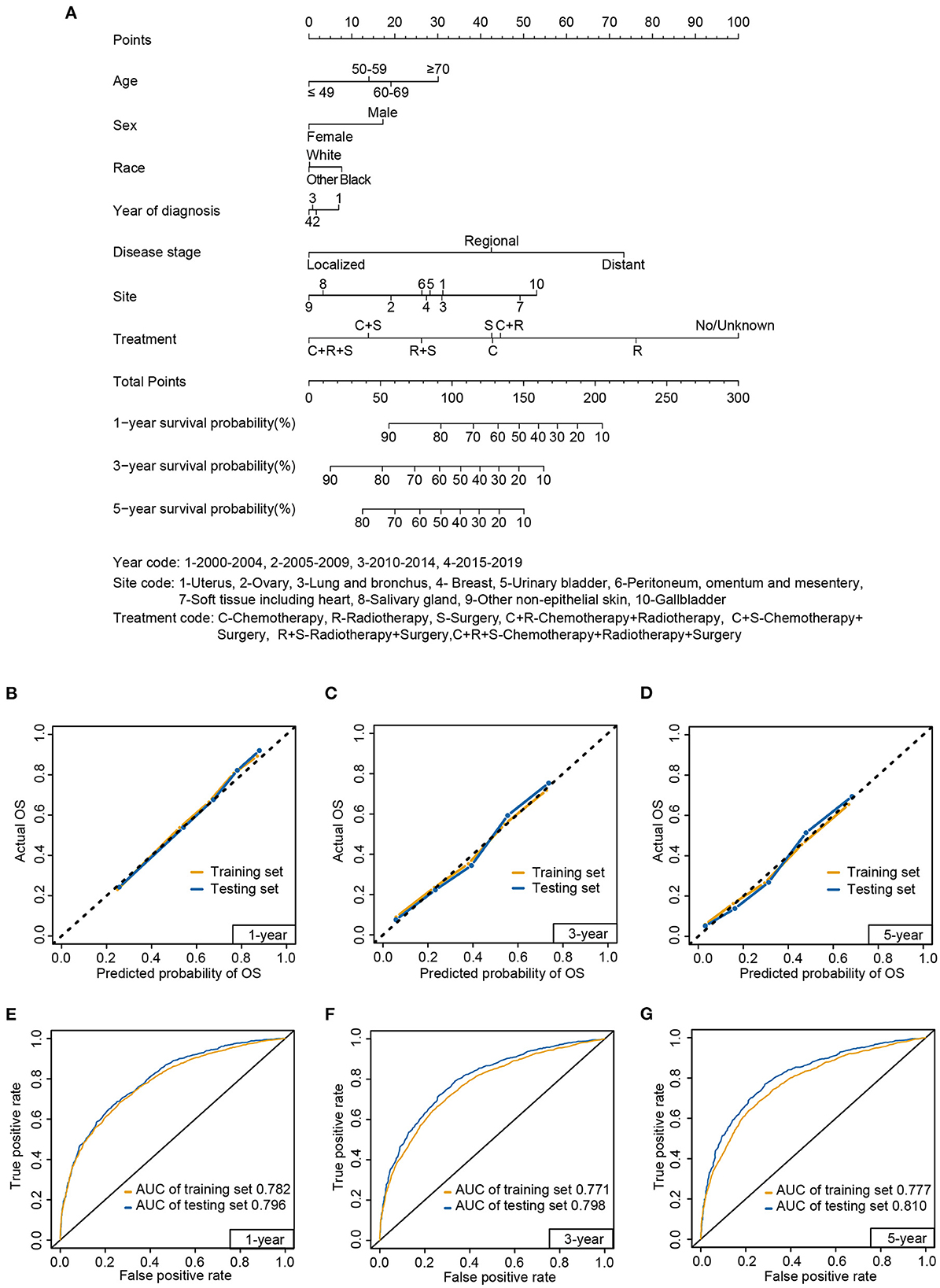
Figure 8. Nomogram (A) to predict 1-, 3-, and 5-year survival probabilities for carcinosarcoma, calibration curve (B–D), and receiver operating characteristic (E–G) curve of the nomogram in the training set and testing set. AUC, area under the curve.
The calibration curves displayed high internal and external consistency with the actual observations for the survival probability of the training (C-index: 0.732) and testing sets (C-index: 0.748; Figures 8B–D). Meanwhile, for predicting 1-year survival, the AUCs of the training and testing sets were 0.782 and 0.796, respectively (Figure 8E). For predicting 3-year survival, the AUCs of the training and testing sets were 0.771 and 0.798, respectively (Figure 8F). For predicting 5-year survival, the AUCs of the training and testing sets were 0.777 and 0.810, respectively (Figure 8G).
Discussion
In this large population-based study, the incidence of carcinosarcoma has continued to increase in the past decades, reaching a plateau from 2012 to 2019. This may reflect changes in medical practice, such as the more extensive use of cancer screening, biopsy, and better recognition by pathologists (5, 10, 24). The sex disparity in incidence has increased over time, mainly because of the dramatic increase in the female incidence rate. Carcinosarcoma mainly occurs in older patients, but its incidence continues to increase in younger patients. Previous studies demonstrated that black women have an increased risk of uterine carcinosarcoma, which may suggest a possible future demographic change in carcinosarcoma because the uterus is the dominant site of carcinosarcoma (10, 25, 26). Another possible explanation for the steep increase in incidence in the black population may be socioeconomic disparities (including low income, insurance status, health services) (25, 27, 28). With the rapid development of health inspection and imaging modalities, the incidence of patients diagnosed with unknown stage declined over the past decades. The most rapid increase in the incidence of localized stage tumors was accompanied by an increase in regional and distant stage tumors, which suggests the significance of prevention and early detection of carcinosarcoma. Mortality patterns reflect incidence trends and treatment effectiveness, with increased slowing for women, white population, and distant stage, and stabilizing rate for regional stage, while decreasing for ≥70 years old. The rapid decline in mortality among men may be due in part to reduction in smoking (29). Thus, while medical advances have slowed the trend of rising mortality, targeted cancer control treatments remain urgent (11, 25, 27, 28, 30). Consistent with the overall rising incidence and slowing death trend, the prevalence of carcinosarcoma has increased year-by-year, mainly in women, elderly, blacks, and localized stage. The prognosis of carcinosarcoma is far worse than that of most other solid tumors (29). The 5-year survival rate of carcinosarcoma was only 32.8%, similar to the previous studies of uterine and ovarian carcinosarcoma (29.8–37%) (5, 6, 23, 27, 31). Hence, effective prevention and treatment for carcinosarcoma are lacking and urgently needed.
In women, the uterus and ovary are the main sites of carcinosarcoma, which accounted for approximately 5% of all uterine cancers and 1–3% of all malignant ovarian tumors (3, 23, 31). In men, the lung and bronchus is the most common primary site of carcinosarcoma, followed by the urinary bladder and salivary gland. Although there have been some case reports and analyses of single-site carcinosarcoma, distribution and gender disparities of carcinosarcoma are new and comprehensive understanding of carcinosarcoma, facilitating disease screening and medical surveillance for future research (6, 7, 9, 32–34).
Despite the increasing interest of the medical community toward carcinosarcoma, there is still a scarcity of specific guidelines for its management (9, 27, 35). Surgery remains the predominant initial treatment for localized stage, whereas due to the high recurrence rate of carcinosarcoma, the proportion of combination therapy is more pronounced in regional and distant stages. Most studies support an improved survival benefit with combination therapy, but the incidence of carcinosarcoma is too low to provide prospective clinical trial support (22, 24, 36–41). Older patients prefer surgery alone rather than adjuvant chemotherapy or chemoradiotherapy after surgical resection. Meanwhile, a substantial proportion of patients were undertreated, especially older patients (≥70 years) with distant stage. Older patients with more comorbidities and worse performance scores limit aggressive treatment, and these differences may lead to poorer survival (41). However, these data should be interpreted with caution for most cancer types, as the SEER database provides only partial treatment information (chemotherapy, radiotherapy, and surgery) and does not include targeted therapy and immunotherapy (42). Of note, targeted therapy or immunotherapy was not available as a first-line option for carcinosarcoma to date, and thus would not affect the data analysis in this study (27, 43).
To further explore the risk factors for patients with carcinosarcoma, age, sex, race, year of diagnosis, disease stage, primary tumor site, and treatment correlated with OS. Older age, male sex, black, earlier year of diagnosis, advanced stage, and carcinosarcoma originating from the gallbladder and soft tissue including heart were associated with poorer prognosis. Identifying risk factors is emphasized to improve the outcome of carcinosarcoma. Explicating prognostic differences due to primary site will provide the basis for treatment and follow-up strategies. Survival benefits from all treatment modalities, especially triple therapies. This result demonstrates the importance of aggressive treatment in fighting carcinosarcoma invasion (2, 4, 12, 22, 33). A prognostic nomogram was constructed based on these seven risk factors. The total score was calculated using the quantitative score of each factor, and the 1-, 3-, and 5-year survival rate were scientifically and accurately predicted. Due to the inclusion of the primary tumor site, the predictive model is applicable to a wider population of patients and has a better predictive probability than other single-site predictive models (11, 30). In summary, this simple but effective model could be used to individualize prognostic assessment of carcinosarcoma and will facilitate clinical decision-making.
Limitations and strengths
This study had several limitations. First, the analysis based on the SEER database was retrospective, and some information, such as specific chemotherapy drugs was lacking. Second, there was a lag in the data, with some variables censored after 2015. Finally, as definitive diagnosis of carcinosarcoma is difficult, the incidence and prevalence may be underestimated. Despite some limitations, this study offered the advantage of providing nationally representative epidemiological data, as well as survival data. To the best of our knowledge, this is the first, largest, and most up-to-date comprehensive study of carcinosarcoma that integrates multiple primary tumor sites, presenting a detailed analysis of treatment and prognosis. Therefore, this study provides significant and comprehensive information for studying carcinosarcoma.
Conclusions
In this study, the incidence has continued to increase over the past decades, with increased acceleration in the localized stage, reflecting improved early detection. The increasing trend in mortality has slowed and declined rapidly among men; hence, the prevalence of carcinosarcoma has increased. Nonetheless, the survival of patients with carcinosarcoma remains poor, reflecting the urgency to improve early detection and explore targeted cancer control treatments. Explicating distribution and gender disparities of carcinosarcoma may facilitate disease screening and medical surveillance. All treatment modalities offer survival benefits, especially triple therapy. Differences in treatment patterns, comorbidities, and performance scores may explain the inferior prognosis of older patients. Furthermore, according to the risk factors in the multivariate analysis, a nomogram was constructed to predict the survival probability of carcinosarcoma, which demonstrated a good predictive capacity and could guide the surveillance, treatment, and follow-up strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MC and WH: full access to all of the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, concept and design, administrative, and technical or material support. MC, XH, QY, JZ, JP, DW, and KT: acquisition and analysis or interpretation of data. MC: drafting of the manuscript. MC, XH, and QY: statistical analysis. WH: supervision. All authors: critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are grateful to all the staff in the National Cancer Institute (USA) for their contribution to the SEER program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1038211/full#supplementary-material
Abbreviations
AAPC, average annual percent change; APC, annual percent change; AUC, area under the curve; CI, confidence interval; C-index, concordance indexes; HR, hazard ratio; ICD-O-3, International Classification of Diseases for Oncology, 3rd edition; OS, overall survival; ROC, receiver operating characteristic; SEER, Surveillance, Epidemiology, and End Results.
References
1. Gotoh O, Sugiyama Y, Takazawa Y, Kato K, Tanaka N, Omatsu K, et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat Commun. (2019) 10:4965. doi: 10.1038/s41467-019-12985-x
2. Barker HE, Scott CL. Genomics of gynaecological carcinosarcomas and future treatment options. Semin Cancer Biol. (2020) 61:110–20. doi: 10.1016/j.semcancer.2019.10.006
3. Kurman RJ, International International Agency for Research on Cancer World Health Organization. Who Classification of Tumours of Female Reproductive Organs. 4th ed. Lyon: International Agency for Research on Cancer (2014). 307 p.
4. Elshaikh MA, Modh A, Jhingran A, Biagioli MC, Coleman RL, Gaffney DK, et al. Executive summary of the American Radium Society(R) appropriate use criteria for management of uterine carcinosarcoma. Gynecol Oncol. (2020) 158:460–6. doi: 10.1016/j.ygyno.2020.04.683
5. Matsuzaki S, Klar M, Matsuzaki S, Roman LD, Sood AK, Matsuo K. Uterine carcinosarcoma: contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol. (2021) 160:586–601. doi: 10.1016/j.ygyno.2020.10.043
6. Ciccarone F, Biscione A, Moro F, Fischerova D, Savelli L, Munaretto M, et al. Imaging in gynecological disease: clinical and ultrasound characteristics of ovarian carcinosarcoma. Ultrasound Obstet Gynecol. (2022) 59:241–7. doi: 10.1002/uog.23733
7. Sun L, Dai J, Wang X, Jiang G, Gonzalez-Rivas D, Song J, et al. Pulmonary carcinosarcoma: analysis from the surveillance, epidemiology and end results database. Interact Cardiovasc Thorac Surg. (2020) 30:4–10. doi: 10.1093/icvts/ivz215
8. Liu L, Zhu JL, Tian Y. Carcinosarcoma is an aggressive subtype of bladder cancer: a population-based study. Cancer Med-Us. (2022) 11:2216–23. doi: 10.1002/cam4.4611
9. Chen X, Zhou Y, Shu X, Wei G, Qiu M. Gallbladder carcinosarcoma: current perspectives and new development. Expert Rev Gastroenterol Hepatol. (2021) 15:1107–14. doi: 10.1080/17474124.2021.1919509
10. Matsuo K, Ross MS, Machida H, Blake EA, Roman LD. Trends of uterine carcinosarcoma in the United States. J Gynecol Oncol. (2018) 29:e22. doi: 10.3802/jgo.2018.29.e22
11. Ren F, Wang S, Li F, Gao J, Xu H, Li X, et al. Clinical nomograms for predicting the overall survival and cancer-specific survival of patients with ovarian carcinosarcoma patients after primary surgery. J Cancer. (2021) 12:7223–36. doi: 10.7150/jca.63224
12. Alhatem A, Quinn PL, Xia W, Chokshi RJ. Pancreatic carcinosarcoma clinical outcome analysis of the National Cancer Institute Database. J Surg Res. (2021) 259:62–70. doi: 10.1016/j.jss.2020.11.033
13. Lin S, Liu C, Tao Z, Zhang J, Hu X. Clinicopathological characteristics and survival outcomes in breast carcinosarcoma: a seer population-based study. Breast. (2020) 49:157–64. doi: 10.1016/j.breast.2019.11.008
14. Liu QQ, Lin HM, Han HW, Yang CN, Liu C, Zhang R. Complete response to combined chemotherapy and Anti-Pd-1 therapy for recurrent gallbladder carcinosarcoma: a case report and literature review. Front Oncol. (2022) 12:803454. doi: 10.3389/fonc.2022.803454
15. Smith VA, MaDan OP, Lentsch EJ. tumor location is an independent prognostic factor in head and neck merkel cell carcinoma. Otolaryng Head Neck. (2012) 146:403–8. doi: 10.1177/0194599811431789
16. Chweya CM, Low CM, Van Gompel JJ, Van Abel KM, Stokken JK, Choby G. Population-based analysis on the effect of nodal and distant metastases in sinonasal adenocarcinoma. Head Neck-J Sci Spec. (2021) 43:128–36. doi: 10.1002/hed.26457
17. Ran JC, Zou HH, Li XY, Guo F, Xu WG, Han W. A population-based competing risk survival analysis of patients with salivary duct carcinoma. Ann Transl Med. (2020) 8:1355. doi: 10.21037/atm-20-1849
18. Tucci JJ, Dashti NK, Cates JMM. A proposed staging system for improved prognostication of Mdm2-amplified liposarcoma. Am J Surg Pathol. (2021) 45:101–7. doi: 10.1097/PAS.0000000000001554
19. Xu ZH, Wang L, Dai S, Chen MJ, Li F, Sun JG, et al. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw Open. (2021) 4:24750. doi: 10.1001/jamanetworkopen.2021.24750
20. Xu FS, Zhao FF, Feng XJ, Li CZ, Han DD, Zheng S, et al. Nomogram for predicting cancer-specific survival in undifferentiated pleomorphic sarcoma: a surveillance, epidemiology, and end results-based study. Cancer Control. (2021) 28:10732748211036775. doi: 10.1177/10732748211036775
21. Li W, Xu XW, Zhang YG. Novel prognostic models predicting the cancer-specific survival in patients with cutaneous melanoma based on metastatic lymph node status. Ann Surg Oncol. (2021) 28:4572–81. doi: 10.1245/s10434-020-09556-6
22. Chow S, Chan JK, Kapp DS, Mann A, Liou WS, Liao CI. Does chemotherapy or radiation benefit surgical stage I uterine carcinosarcoma patients? Am J Obstet Gynecol. (2020) 222:383–4. doi: 10.1016/j.ajog.2019.11.1260
23. Romeo C, Le Saux O, Jacobs M, Joly F, Ferron G, Favier L, et al. Therapeutic challenges in patients with gynecologic carcinosarcomas: analysis of a multicenter national cohort study from the french prospective tmrg network. Cancers. (2022) 14:354. doi: 10.3390/cancers14020354
24. van der Horst RL, van der Hel O, Lutgens L, van der Aa M, Slangen B, Kruitwagen R, et al. The role of multimodal adjuvant therapy for Figo I-Ii carcinosarcoma of the uterus: a systematic review. Crit Rev Oncol Hematol. (2022) 175:103701. doi: 10.1016/j.critrevonc.2022.103701
25. Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. (2003) 98:176–86. doi: 10.1002/cncr.11484
26. Wilhite AM, Baca Y, Xiu J, Paladugu R, ElNaggar AC, Brown J, et al. Molecular profiles of endometrial cancer tumors among black patients. Gynecol Oncol. (2022) 166:108–16. doi: 10.1016/j.ygyno.2022.04.014
27. Pezzicoli G, Moscaritolo F, Silvestris E, Silvestris F, Cormio G, Porta C, et al. Uterine carcinosarcoma: an overview. Crit Rev Oncol Hematol. (2021) 163:103369. doi: 10.1016/j.critrevonc.2021.103369
28. Rojas C, Tian C, Powell MA, Chan JK, Bateman NW, Conrads TP, et al. Racial disparities in uterine and ovarian carcinosarcoma: a population-based analysis of treatment and survival. Gynecol Oncol. (2020) 157:67–77. doi: 10.1016/j.ygyno.2020.01.017
29. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
30. Gao L, Lyu J, Luo X, Zhang D, Jiang G, Zhang X, et al. Nomogram to predict overall survival based on the log odds of positive lymph nodes for patients with endometrial carcinosarcoma after surgery. BMC Cancer. (2021) 21:1149. doi: 10.1186/s12885-021-08888-0
31. Karpathiou G, Chauleur C, Dal Col P, Dridi M, Hathroubi S, Mobarki M, et al. An Immunohistochemical analysis of Cd3, Pd-L1, and Ctla-4 expression in carcinosarcomas of the gynecological tract and their metastases. Pathol Res Pract. (2020) 216:153028. doi: 10.1016/j.prp.2020.153028
32. Wang J, Wang FW, Lagrange CA, Hemstreet Iii GP, Kessinger A. Clinical features of sarcomatoid carcinoma (carcinosarcoma) of the urinary bladder: analysis of 221 cases. Sarcoma. (2010) 2010:454792. doi: 10.1155/2010/454792
33. Vordermark D, Medenwald D, Izaguirre V, Sieker F, Marnitz S. The role of postoperative radiotherapy for carcinosarcoma of the uterus. Cancers. (2020) 12:3573. doi: 10.3390/cancers12123573
34. Oldan JD, Chin BB. Fdg Pet/Ct imaging of prostate carcinosarcoma. Clin Nucl Med. (2016) 41:629–31. doi: 10.1097/RLU.0000000000001250
35. Denschlag D, Ulrich UA. Uterine carcinosarcomas - diagnosis and management. Oncol Res Treat. (2018) 41:675–9. doi: 10.1159/000494335
36. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, Version 1.2018, Nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:170–99. doi: 10.6004/jnccn.2018.0006
37. Ravishankar P, Smith DA, Avril S, Kikano E, Ramaiya NH. Uterine carcinosarcoma: a primer for radiologists. Abdom Radiol. (2019) 44:2874–85. doi: 10.1007/s00261-019-02038-8
38. Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, Leitao MM, Powell MA, Poveda A, et al. Gynecologic cancer intergroup (Gcig) consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer. (2014) 24(9 Suppl. 3):S55–60. doi: 10.1097/IGC.0000000000000228
39. Rauh-Hain JA, Birrer M, Del Carmen MG. Carcinosarcoma of the ovary, fallopian tube, and peritoneum: prognostic factors and treatment modalities. Gynecol Oncol. (2016) 142:248–54. doi: 10.1016/j.ygyno.2016.06.003
40. del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol. (2012) 125:271–7. doi: 10.1016/j.ygyno.2011.12.418
41. Rauh-Hain JA, Starbuck KD, Meyer LA, Clemmer J, Schorge JO, Lu KH, et al. Patterns of care, predictors and outcomes of chemotherapy for uterine carcinosarcoma: a national cancer database analysis. Gynecol Oncol. (2015) 139:84–9. doi: 10.1016/j.ygyno.2015.08.014
42. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. (2021) 7:1824–32. doi: 10.1001/jamaoncol.2021.4932
Keywords: carcinosarcoma, SEER database, epidemiology, initial treatment, nomogram
Citation: Chen M, He X, Yang Q, Zhang J, Peng J, Wang D, Tong K and Huang W (2022) Epidemiology and prediction model of patients with carcinosarcoma in the United States. Front. Public Health 10:1038211. doi: 10.3389/fpubh.2022.1038211
Received: 07 September 2022; Accepted: 15 November 2022;
Published: 28 November 2022.
Edited by:
Xuexiao Ma, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Jingkun Qu, The Second Affiliated Hospital of Xi'an Jiaotong University, ChinaMeng Yang, Huazhong University of Science and Technology, China
Copyright © 2022 Chen, He, Yang, Zhang, Peng, Wang, Tong and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiang Huang, MjAxODE3QGNxbXUuZWR1LmNu
 Mingjing Chen
Mingjing Chen Xiandong He
Xiandong He Qiao Yang
Qiao Yang Jia Zhang4
Jia Zhang4