
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 18 October 2022
Sec. Clinical Diabetes
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1036766
This article is part of the Research Topic Advanced Approaches in the Diagnosis and Treatment of Diabetes Mellitus and Secondary Complications View all 46 articles
Purpose: This meta-analysis aimed to explore the comparative short-term efficacy and safety of drug-coated balloon (DCB) vs. drug-eluting stent (DES) for treating small-vessel coronary artery lesions in diabetic patients.
Methods: We searched PubMed, EMBASE, the Cochrane Library, and China National Knowledgement Infrastructure (CNKI) for retrieving relevant studies regarding the comparison of DCB with DES in treating small-vessel coronary artery lesions in diabetic patients until May 31, 2022. Two independent authors screened study, extracted data, and assessed methodological quality. Then, the meta-analysis was conducted using RevMan software, version 5.4.
Results: We included 6 studies with 847 patients in this meta-analysis. Pooled results showed that DCB was associated with fewer major adverse cardiac events (MACE) [RR, 0.60; 95% confidence interval (CI), 0.39–0.93; p = 0.02], myocardial infarction (MI) (RR, 0.42; 95% CI, 0.19–0.94; p = 0.03), target lesion revascularization (TLR) (RR, 0.24; 95% CI, 0.08–0.69; p < 0.001), target vessel revascularization (TVR) (RR, 0.33; 95% CI, 0.18–0.63; p < 0.001), binary restenosis (RR, 0.27; 95% CI, 0.11–0.68; p = 0.005), and late lumen loss (LLL) [mean difference (MD), −0.31; 95% CI, −0.36 to −0.27; p < 0.001], but was comparable technique success rate, death, minimal lumen diameter (MLD), and net lumen gain (NLG) to DES. There was no difference in long-term outcomes between these two techniques.
Conclusions: This meta-analysis shows that DCB is better than DES in the short-term therapeutic efficacy and safety of small-vessel coronary artery lesions in diabetic patients. However, more studies are required to validate our findings and investigate the long-term effects and safety of DCB.
Patients who received percutaneous coronary interventions (PCI) usually present small-vessel coronary artery lesions, reporting an incidence of about 40% (1). Although significant advancements in therapeutic techniques, it remains challenging to treat small vessel coronary artery lesions resulting from a higher risk of technical failure, restenosis, and need for repeated revascularization (2, 3). Compared with non-diabetic patients, patients with diabetes mellitus suffered from worse clinical outcomes (e.g., binary restenosis and myocardial infarction) after PCI (4–7) owing to more challenging coronary anatomies (8–10), such as diffuse atherosclerotic plaques and higher frequency of thin-cap fibroatheroma and fibrocalcific atheroma (11).
The drug-eluting stent (DES) remains the cornerstone treatment for small-vessel coronary artery lesions (12) by reducing angiographic and clinical restenosis (13, 14). However, the presence of diabetes mellites significantly increases the risk of adverse outcomes as a significant predictor (15–18) because more stents of longer lengths and smaller diameters were usually required for PCI in diabetic patients (19). Therefore, the need to develop newer devices as alternatives to DES has been emphasized. As a result, drug-coated balloons (DCB) have attracted physicians' attention as a promising therapeutic modality for de novo lesions and small-vessel coronary artery lesions because they can deliver the antiproliferative drugs directly into the artery wall without the need for implanting metallic stents in the artery vessels (20).
Currently, several clinical trials and meta-analyses have evaluated the therapeutic role of DCB in treating small-vessel coronary artery lesions, indicating that the therapeutic efficacy and safety of DCB were not inferior to DES (21–25). However, only the meta-analysis by Razzack et al. (23) attempted to evaluate the therapeutic value of DCB in diabetic patients by introducing a subgroup analysis. Notably, this subgroup analysis involved only 3 eligible studies, which provided limited data to investigate the difference between DCB and DES in the treatment of small vessel coronary artery disease in diabetic patients. Meanwhile, most studies were underpowered to evaluate the differences between the DCB and DES in therapeutic efficacy and safety due to limited sample size (26–29). Therefore, we conducted this meta-analysis to investigate the comparative short-term therapeutic efficacy and safety of DCB vs. DES in diabetic patients with small-vessel coronary artery lesions.
We first designed this meta-analysis's methodological framework, referring to the Cochrane handbook for systematic reviewers (30). Finally, we reported the meta-analysis's results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (31). The present study did not require institutional review or patient's informed consent because it was a meta-analysis of published data. However, we must point out that the formal protocol of this meta-analysis was not registered on a public platform.
We systematically searched relevant studies on PubMed, EMBASE, and the Cochrane Library from their establishment date until May 31, 2022. We used the following major terms and their analogs to develop the basic search strategy, including “Coronary,” “diabetes,” “drug-eluting stent,” and “drug-eluting balloon.” We modified the basic search strategy to meet the requirements of each database. The detailed search strategy of each target database is summarized in Supplementary Table S1. In addition, we also checked the reference lists of studies included in this meta-analysis to identify those missing from the electronic literature search.
Two independent authors conducted the study selection by screening the titles, abstracts, and full texts of all retrieved studies according to the selection criteria were as follows: (1) Diabetic patients with small-vessel coronary artery lesions were treated with DCB or DES; (2) Studies reported at least one of the major adverse cardiac events (MACE) outcome, technique success rate, binary restenosis, minimal lumen diameter (MLD), late lumen loss (LLL), and net lumen gain (NLG); and (3) studies were published in English and Chinese, with full texts. We excluded ineligible studies following the exclusion criteria: (1) ineligible study designs, including case reports, experimental studies, reviews, and letters; (2) repeated publications of the same study; (3) essential data were not available after contacting the leading authors.
We defined the MACE as the primary endpoint, which was a composite outcome involving myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR), and death (32). In addition, we defined technique success rate, binary restenosis, MLD, LLL, and NLG as the secondary endpoints. TLR and TVR are treated as two separate outcomes in this meta-analysis; however, TLR is part of TVR. Specifically, TLR was defined as repeated PCI treatment within the target lesion stent or edge 5 mm, but TVR was defined as PCI in the target lesion coronary vessel outside the stent (33). All outcomes were reported within 12 months after treatment, which were used to reveal short-term therapeutic efficacy and safety.
Two independent authors conducted data extraction using the pre-designed standard information extraction sheet. The following data were extracted from all studies included in this meta-analysis, including the first author's name, publication year, country, study duration, study design, details of comparisons, sample size with the proportion of male patients, patients' mean age, basic reference vessel diameter (RVD), lesion length, diameter stenosis, the number of patients identified with American Heart Association (AHA) type B2/C lesion, and the information on the risk of bias. We calculated the transformed standard deviation (SD) based on the recognized formula (34) when the eligible study reported results as the interquartile range (IQR).
Two authors assessed the methodological quality of all retrieved studies using the Cochrane risk of bias assessment tool version 2.0 (RoB 2.0) (35). Specifically, the RoB 2.0 quantified the overall methodological quality of a study from five areas, including “randomization process,” “deviations from the intended interventions,” “missing outcome data,” “measurement of the outcome,” and “selection of the reported result.” Using the RoB 2.0 tool, the overall methodological quality of one study was labeled with “low,” “high,” or “some concerns.” The results of the risk of bias assessment were graphically presented using the “robvis” command (36).
For dichotomous variables, including the MACE outcome including MI, TLR, TVR, and death, technique success rate, and binary restenosis, relative risk (RR) with a 95% confidence interval (CI) was used to express the pooled estimate; however, for continuous variables, including MLD, LLL, and NLG, mean difference (MD) with a 95% CI was used to express the pooled estimate (37). We first tested the level of the statistical heterogeneity across studies using the Cochrane Q test (38) and I2 statistic (39). Significant heterogeneity was considered if p < 0.1 and I2 ≥ 50% (40) and random-effects model was selected for meta-analysis. On the contrary, the fixed-effects model was selected for meta-analysis when p > 0.1 and I2 < 50% (40). The publication bias examination was not conducted because only six eligible studies were included in this meta-analysis, which did not meet the criteria for constructing a funnel plot (41). Meta-analysis was conducted using RevMan software, version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) (42, 43).
We retrieved 499 studies from the electronic literature search, and one additional study was identified from the reference list. Using the EndNote software, 77 duplicate studies were removed. After screening the titles and abstracts of 423 retaining studies, we excluded 402 ineligible studies. We accessed and screened the full texts of 21 studies, and 15 studies were excluded due to three reasons, including unrelated to the topic (n = 11), conference abstract without sufficient data (n = 3), and duplicate publication (n = 1). Finally, as shown in Figure 1, we included 6 studies (26–29, 44, 45) in this study for meta-analysis.
All studies were randomized controlled trials (RCTs) and were published between 2017 and 2021. Three studies were conducted in China (27–29), two studies in Italy (26, 44), and one study in Germany (45). Three studies (26, 44, 45) were conducted in multiple centers; however, other three studies (27–29) were conducted in a single center. The sample size of individual study ranged from 70 to 252, with an accumulated number of 847. Among the 6 included studies, five studies (26, 27, 29, 44, 45) reported MACE outcome, all studies (26–29, 44, 45) reported technique success, two studies (26, 27) reported binary restenosis, four studies (26–29) reported MLD and LLL, and three studies (26, 27, 29) reported NLG. We can access the remaining basic information of all studies in Table 1.
One study was high risk in the randomization process, three studies were high risk in the deviations from intended interventions, two studies were high risk in the missing outcome data, and all studies were low or some concerns in the remaining two domains. Finally, the overall methodological quality was rated to be low to moderate. The results of the risk of bias assessment are depicted in Figure 2.
Among the 6 studies included in this meta-analysis, five studies (26, 27, 29, 44, 45) reported the data on the MACE outcome. There was no significant statistical heterogeneity across studies (p = 0.87, I2 = 0%), so we selected a fixed-effects model for meta-analysis. The meta-analysis suggested that DCB was associated with a decreased risk of MACE outcome compared to DES (RR, 0.60; 95% CI, 0.39–0.93; p = 0.02; Figure 3).
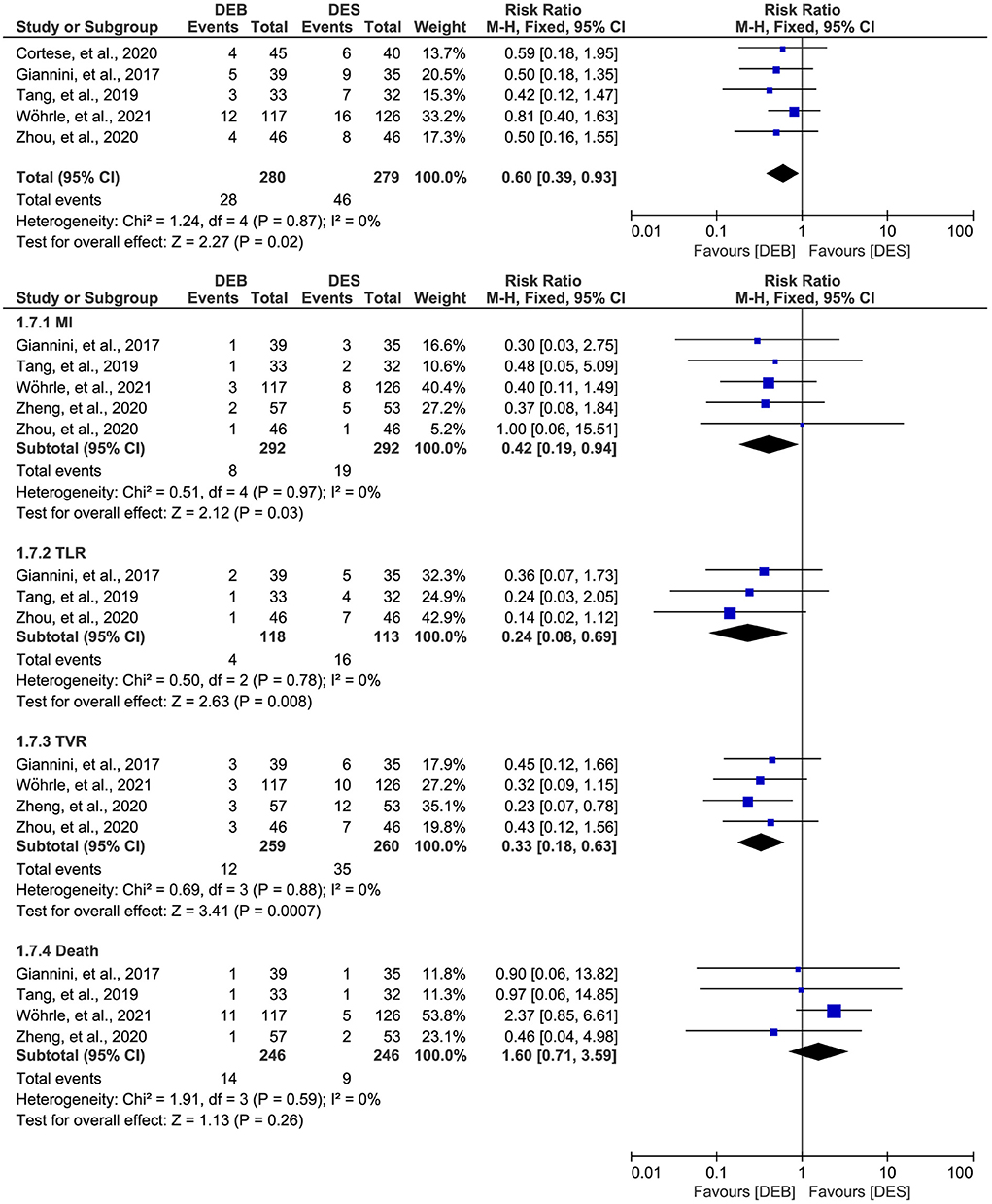
Figure 3. Meta-analysis of the MACE outcome. The black diamond represents the pooled result. If the black diamonds are completely to the left of the null line (“1”), it means that DEB is better than DES in terms of MACE results, MI, TLR, TVR, and death; if the black diamonds are completely to the right of the null line (“1”), it means that DEB is inferior to DES in terms of all outcomes; and if the black diamonds crossed through the null line (“1”), it means that DEB is comparable to DES in terms of all outcomes. MACE, major adverse cardiac events; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization; DCB, drug-eluting balloon; DES, drug-eluting stent; M-H, Mantel-Haenszel.
Furthermore, we conducted a subgroup analysis to investigate the difference between DCB and DES in a single MACE outcome. There was no significant statistical heterogeneity across studies, so we selected a fixed-effects model for meta-analysis. The results of subgroup analysis suggested significant difference between the two techniques in MI (RR, 0.42; 95% CI, 0.19–0.94; p = 0.03), TLR (RR, 0.24; 95% CI, 0.08–0.69; p = 0.008), and TVR (RR, 0.33; 95% CI, 0.18–0.63; p = 0.007), but not in death (RR, 1.60; 95% CI, 0.71–3.59; p = 0.26).
In addition, one study (45) also reported the MACE outcome at the 3-years follow-up. However, as shown in Supplementary Figure S1, there was no statistical difference between the techniques regarding the MACE outcome (RR, 0.87; 95% CI, 0.52–1.45; p = 0.59) and the single MACE outcome, including MI (RR, 0.65; 95% CI, 0.27–1.60; p = 0.35), TVR (RR, 0.56; 95% CI, 0.26–1.19; p = 0.13), and death (RR, 1.27; 95% CI, 0.67–2.42; p = 0.47).
All included studies (26–29, 44, 45) reported the data on the technique success rate, and there was no significant statistical heterogeneity across studies (p = 0.17, I2 = 36%). Therefore, we selected a fixed-effects model for meta-analysis, and the pooled result suggested a comparable technique success rate between the two techniques (RR, 1.01; 95% CI, 0.98–1.05; p = 0.50; Figure 4). Moreover, two studies (26, 27) reported the data on the binary restenosis. There was no significant statistical heterogeneity across studies (p = 0.91, I2 = 0%), so we selected a fixed-effects model for meta-analysis. The pooled result suggested that DCB was associated with a lower binary restenosis rate than DES (RR, 0.27; 95% CI, 0.11–0.68; p = 0.005; Figure 4).
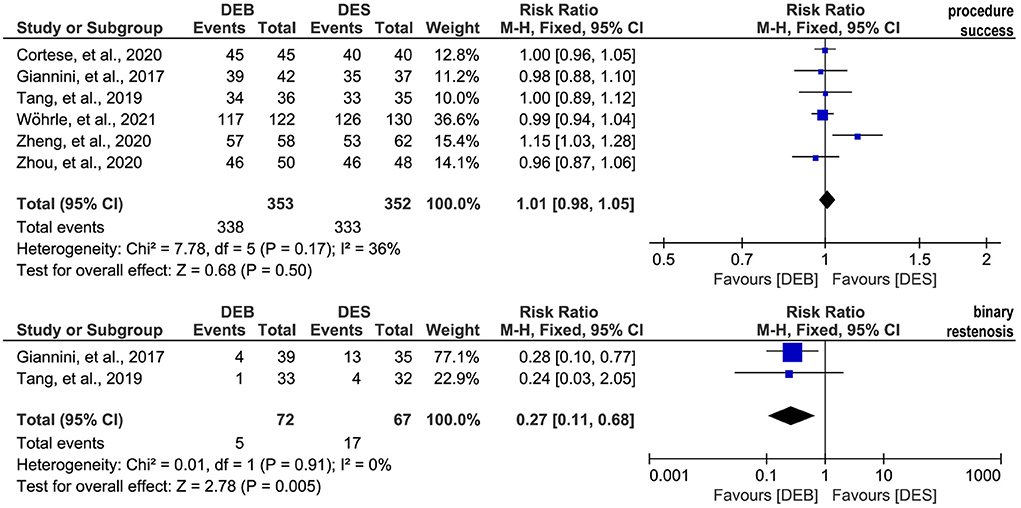
Figure 4. Meta-analysis of the procedure success rate and binary restenosis. The black diamond represents the pooled result. If the black diamonds are completely to the left of the null line (“1”), it means that DEB is better than DES in terms of the procedure success and binary restenosis; if the black diamonds are completely to the right of the null line (“1”), it means that DEB is inferior to DES in terms of all outcomes; and if the black diamonds crossed through the null line (“1”), it means that DEB is comparable to DES in terms of all outcomes. DCB, drug-eluting balloon; DES, drug-eluting stent; M-H, Mantel-Haenszel.
Four studies (26–29) reported the data on the MLD, and there was significant statistical heterogeneity across studies (p = 0.07, I2 = 57%). Therefore, we selected a random-effects model for meta-analysis, and the pooled result suggested no statistical difference between the two techniques in the MLD (MD, 0.05; 95% CI, −0.06 to 0.16; p = 0.34, Figure 5). The same four studies (26–29) also reported the data on the LLL, and there was no significant statistical heterogeneity across studies (p = 0.56, I2 = 0%). The result of the meta-analysis based on a fixed-effects model suggested that patients receiving DCB had fewer LLL than patients treated by DES (MD, −0.31; 95% CI, −0.36 to −0.27; p < 0.001; Figure 5). In addition, three studies (26, 27, 29) reported the data on the NLG. There was significant statistical heterogeneity across studies (p < 0.1, I2 = 86%), so we selected a random-effects model for meta-analysis. The pooled result suggested no statistical difference between the two techniques regarding the NLG (MD, −0.01; 95% CI, −0.30 to 0.29; p = 0.95; Figure 5).
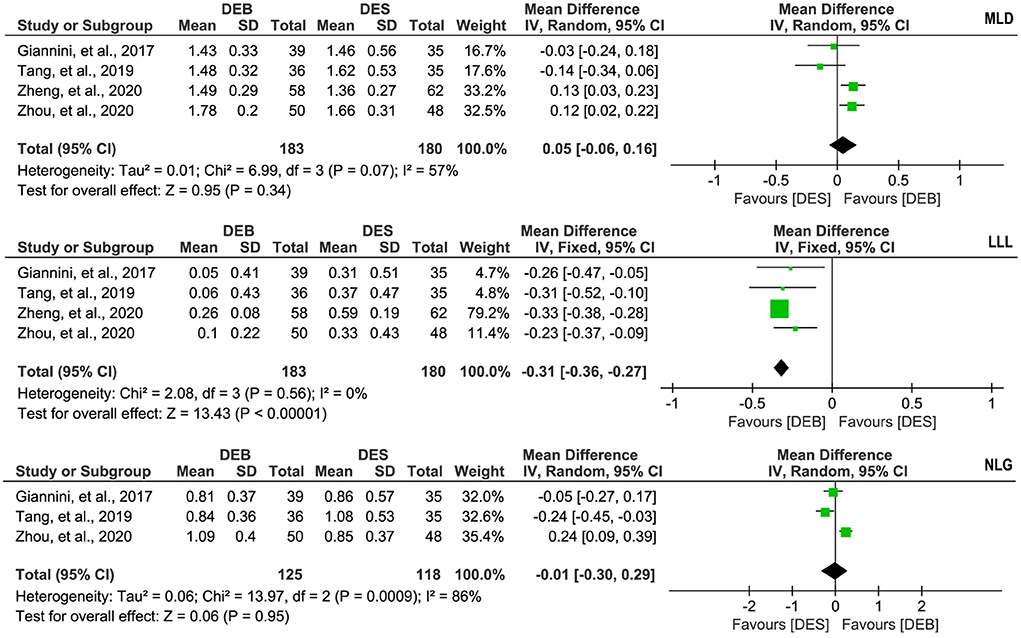
Figure 5. Meta-analysis of MLD, LLL, and NLG. The black diamond represents the pooled result. If the black diamonds are completely to the left of the null line (“0”), it means that DEB is better than DES in terms of MLD, LLL, and NLG; if the black diamonds are completely to the right of the null line (“0”), it means that DEB is inferior to DES in terms of all outcomes; and if the black diamonds crossed through the null line (“0”), it means that DEB is comparable to DES in terms of all outcomes. MLD, minimal lumen diameter; LLL, late lumen loss; NLG, net lumen gain; DCB, drug-eluting balloon; DES, drug-eluting stent; IV, inverse variance; SD, standard deviation.
In the present meta-analysis, we systematically retrieved all relevant studies comparing DCB with DES in small-vessel coronary artery lesions among patients with diabetes mellitus. In the final data analysis, we included 6 low to moderate quality studies, accumulating a total of 847 patients. The pooled results showed that DCB was comparable to DES regarding technique success rate, MLD, and NLG; however, DCB had a lower risk in MACE outcome and binary restenosis. Subgroup analysis further indicated that DCB had a lower incidence in MI, TLR, and TVR than DES but comparable death to DES. The result from only one study suggested comparable MACE outcomes between the techniques at 3-years follow-up.
Interventional treatment of small-vessel coronary artery lesions is still challenging due to an increased risk of technical failure, restenosis, and the need for repeated revascularization (24), which is especially prominent in diabetic patients (11). DES remains the normative therapeutic strategy for PCI (46); however, implantation of DES will cause arterial wall injury to initiate vascular-proliferative cascade with smooth muscle cell proliferation and migration, resulting in neointimal hyperplasia (47). Compared to DES, DCB can deliver the antiproliferative drug into the vessel wall without the need for the implantation of metal struts, therefore directly inhibiting endothelial proliferation and adverse remodeling (20). From the theoretical perspective, the implantation of DCB will be superior to DES for treating small-vessel coronary artery lesions, which also interprets why the present meta-analysis found that DCB was associated with fewer binary restenosis, LLL, and single MACE outcome.
Currently, several studies (21–25) have investigated the comparative efficacy and safety of DCB vs. DES for treating de novo lesions in small-vessel coronary disease using the meta-analytic technique. However, the meta-analyses by Li et al. (21) did not isolate diabetic patients from general populations, although authors found that DCB was non-inferior to DES, delivering a good outcome in non-fatal MI, and can be recommended as an optimal treatment strategy in patients with de novo small-vessel coronary artery diseases. In addition, the meta-analysis by Elgendy et al. (22) assessed the differences in reducing TLR between DCB and DES in novo small-vessel coronary artery by introducing subgroup analysis; however, this meta-analysis did not also isolate diabetic patients form general populations. Another meta-analysis by Razzack et al. (23) included eight studies first to investigate the difference in therapeutic efficacy and safety between DCB and DES in treating de novo lesions in small-vessel coronary disease. Then, the authors evaluated the therapeutic value of DCB in diabetic patients by introducing a subgroup analysis involving 3 studies, indicating no statistical difference between DCB and DES regarding the MACE outcome [odds ratio (OR), 1.34; 95% CI, 0.73–2.46; p = 0.34], inconsistent with our finding.
In the present meta-analysis, we specifically evaluated the therapeutic efficacy and safety of DCB vs. DES for treating small-vessel coronary artery lesions in diabetic patients. The results of our meta-analysis provided more specific evidence-based information for practitioners dedicated to treating small coronary vessel lesions in diabetic patients compared with that meta-analyses reported by Li et al. (21) and Elgendy et al. (22). In addition, 6 eligible studies were included in our meta-analysis. Therefore, the statistical power of this meta-analysis was significantly higher than the meta-analysis by Razzack et al. (23), generating more reliable results. As a result, we can have the confidence to convince that DCB is associated with fewer MACE outcomes than DES in treating small-vessel coronary artery lesions in diabetic patients. More importantly, the present meta-analysis not only included the MACE outcome, but also considered other outcomes, including technique success rate, binary restenosis, MLD, LLL, and NLG, which benefited us to evaluate the therapeutic efficacy and safety of DCB more comprehensively for small-vessel coronary artery lesions in diabetic patients.
Although this meta-analysis included RCTs to enhance the reliability of the pooled results, we cannot ignore that it faced some limitations. First, although 6 eligible studies were included in the final analysis, not all studies reported all outcomes. Therefore, studies included for individual outcome remains limit, which may adversely impact the robustness of the pooled results. Second, the results of the risk of bias assessment suggested that the overall methodological quality of 6 included studies was low to moderate. Therefore, we cannot eliminate the negative impact of low methodological quality on the robustness of the pooled results. Third, we detected significant statistical heterogeneity for meta-analyses of some outcomes. However, we could not conduct a sensitivity analysis to test the robustness of the results due to limited studies. As a result, we should cautiously interpret the results with significant statistical heterogeneity. Fourth, only one study reported outcomes in the long-term follow-up; therefore, we could not adequately evaluate the differences in long-term therapeutic efficacy and safety between the two techniques. Fifth, we could not assess the potential differences in treatment effect among different type of DCB because limited data are available. Sixth, our finding should be interpreted with caution because the criteria of small vessel and the follow-up duration varied slightly between included studies (as shown in Table 1). Finally, the formal protocol of this meta-analysis was not registered publicly although we conducted it in strict accordance with the process of a meta-analysis.
In conclusion, the present meta-analysis suggested that DCB is better than DES in the short-term therapeutic efficacy and safety of small-vessel coronary artery lesions in diabetic patients because DCB can significantly decrease the LLL and reduce the risk of binary restenosis, and it is also associated with fewer risk of MI, TLR, and TVR. However, all findings of this meta-analysis are generated from studies with low to moderate quality. Meanwhile, only one study evaluates the long-term therapeutic efficacy and safety. Therefore, more multi-center, large-scale, and high-quality studies are needed to validate our findings and investigate the difference between the two techniques in the long-term outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
KL: conceptualization, formal analysis, and writing original draft preparation. KL and KC: methodology and validation. KC: resources and project administration. JF and XP: data curation. KL, JF, and XP: writing review and editing. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1036766/full#supplementary-material
Supplementary Figure S1. Meta-analysis of the MACE outcome at 3-years follow-up. The black diamond represents the pooled result. If the black diamonds are completely to the left of the null line (“1”), it means that DEB is better than DES in terms of MACE outcome; if the black diamonds are completely to the right of the null line (“1”), it means that DEB is inferior to DES in terms of MACE outcome; and if the black diamonds crossed through the null line (“1”), it means that DEB is comparable to DES in terms of MACE outcome. MACE, major adverse cardiac events; MI, myocardial infarction; TVR, target vessel revascularization; DCB, drug-eluting balloon; DES, drug-eluting stent; M-H, Mantel-Haenszel.
Supplementary Table S1. Detailed search strategies of target databases.
PCI, percutaneous coronary interventions; DCB, drug-coated balloons; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MACE, major adverse cardiac events; MLD, minimal lumen diameter; LLL, late lumen loss; NLG, net lumen gain; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
1. Akiyama T, Moussa I, Reimers B, Ferraro M, Kobayashi Y, Blengino S, et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. (1998) 32:1610–8. doi: 10.1016/S0735-1097(98)00444-6
2. Godino C, Furuichi S, Latib A, Morici N, Chieffo A, Romagnoli E, et al. Clinical and angiographic follow-up of small vessel lesions treated with paclitaxel-eluting stents (from the TRUE Registry). Am J Cardiol. (2008) 102:1002–8. doi: 10.1016/j.amjcard.2008.05.052
3. Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. (2014) 100:153–9. doi: 10.1136/heartjnl-2013-304933
4. Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. (2000) 102:2180–4. doi: 10.1161/01.CIR.102.18.2180
5. Mathew V, Holmes DR. Outcomes in diabetics undergoing revascularization: the long and the short of it. J Am Coll Cardiol. (2002) 40:424–7. doi: 10.1016/S0735-1097(02)02025-9
6. Smith SCJr, Faxon D, Cascio W, Schaff H, Gardner T, Jacobs A, et al. Prevention conference VI: diabetes and cardiovascular disease: writing group VI: revascularization in diabetic patients. Circulation. (2002) 105:e165–169. doi: 10.1161/01.CIR.0000013957.30622.05
7. Van Belle E, Périé M, Braune D, Chmaït A, Meurice T, Abolmaali K, et al. Effects of coronary stenting on vessel patency and long-term clinical outcome after percutaneous coronary revascularization in diabetic patients. J Am Coll Cardiol. (2002) 40:410–7. doi: 10.1016/S0735-1097(02)01971-X
8. Jiménez-Quevedo P, Sabaté M, Angiolillo D, Alfonso F, Hernández-Antolín R, Bañuelos C, et al. LDL-cholesterol predicts negative coronary artery remodelling in diabetic patients: an intravascular ultrasound study. Eur Heart J. (2005) 26:2307–12. doi: 10.1093/eurheartj/ehi420
9. Nasu K, Tsuchikane E, Katoh O, Fujita H, Surmely JF, Ehara M, et al. Plaque characterisation by Virtual Histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. (2008) 94:429–33. doi: 10.1136/hrt.2007.118950
10. Jiménez-Quevedo P, Suzuki N, Corros C, Ferrer C, Angiolillo DJ, Alfonso F, et al. Vessel shrinkage as a sign of atherosclerosis progression in type 2 diabetes: a serial intravascular ultrasound analysis. Diabetes. (2009) 58:209–14. doi: 10.2337/db08-0376
11. Sabaté M. Drug-coated balloon for diabetic patients with small coronary vessels: is it the way to go? JACC Cardiovasc Interv. (2021) 14:1799–800. doi: 10.1016/j.jcin.2021.07.011
12. Chichareon P, Katagiri Y, Asano T, Takahashi K, Kogame N, Modolo R, et al. Mechanical properties and performances of contemporary drug-eluting stent: focus on the metallic backbone. Expert Rev Med Devices. (2019) 16:211–28. doi: 10.1080/17434440.2019.1573142
13. Moussa I, Leon MB, Baim DS, O'Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. (2004) 109:2273–8. doi: 10.1161/01.CIR.0000129767.45513.71
14. Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. (2005) 45:1172–9. doi: 10.1016/j.jacc.2004.10.075
15. Iijima R, Ndrepepa G, Mehilli J, Markwardt C, Bruskina O, Pache J, et al. Impact of diabetes mellitus on long-term outcomes in the drug-eluting stent era. Am Heart J. (2007) 154:688–93. doi: 10.1016/j.ahj.2007.06.005
16. Claessen BE, Smits PC, Kereiakes DJ, Parise H, Fahy M, Kedhi E, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. (2011) 4:1209–15. doi: 10.1016/j.jcin.2011.07.016
17. Cannon LA, Simon DI, Kereiakes D, Jones J, Mehran R, Kusano H, et al. The XIENCE nano everolimus eluting coronary stent system for the treatment of small coronary arteries: the SPIRIT Small Vessel trial. Catheter Cardiovasc Interv. (2012) 80:546–53. doi: 10.1002/ccd.23397
18. van der Heijden LC, Kok MM, Danse PW, Schramm AR, Hartmann M, Löwik MM, et al. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. (2016) 176:28–35. doi: 10.1016/j.ahj.2016.02.020
19. Jiménez-Quevedo P, Sabaté M, Angiolillo DJ, Alfonso F, Hernández-Antolín R, Gómez-Hospital JA, et al. Efficacy of sirolimus-eluting stent implantation in diabetic patients with very small vessels (< or = 2.25 mm). Insights from the DIABETES trial. Rev Esp Cardiol. (2006) 59:1000–7. doi: 10.1157/13093976
20. Kleber FX, Rittger H, Bonaventura K, Zeymer U, Wöhrle J, Jeger R, et al. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol. (2013) 102:785–97. doi: 10.1007/s00392-013-0609-7
21. Li M, Guo C, Lv YH, Zhang MB, Wang ZL. Drug-coated balloon versus drug-eluting stent in de novo small coronary vessel disease: a systematic review and meta-analysis. Medicine. (2019) 98:e15622. doi: 10.1097/MD.0000000000015622
22. Elgendy IY, Gad MM, Elgendy AY, Mahmoud A, Mahmoud AN, Cuesta J, et al. Clinical and angiographic outcomes with drug-coated balloons for de novo coronary lesions: a meta-analysis of randomized clinical trials. J Am Heart Assoc. (2020) 9:e016224. doi: 10.1161/JAHA.120.016224
23. Razzack A, Pothuru S, Hassan SA, Mandava S, Castellanos D, Reddy KT, et al. Long-term efficacy and safety of drug-eluting stent versus drug-coated balloon for de novo lesions in small-vessel coronary disease: a systematic review and meta-analysis. Cardiovasc Revascularization Med. (2021) 28:S22. doi: 10.1016/j.carrev.2021.06.051
24. Sanz Sánchez J, Chiarito M, Cortese B, Moretti A, Pagnotta P, Reimers B, et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease: a meta-analysis of randomized trials. Catheter Cardiovasc Interv. (2021) 98:66–75. doi: 10.1002/ccd.29111
25. Wu X, Li L, He L. Drug-coated balloon versus drug-eluting stent in patients with small-vessel coronary artery disease: a meta-analysis of randomized controlled trials. Cardiol Res Pract. (2021) 2021:1647635. doi: 10.1155/2021/1647635
26. Giannini F, Latib A, Jabbour RJ, Costopoulos C, Chieffo A, Carlino M, et al. Comparison of paclitaxel drug-eluting balloon and paclitaxel-eluting stent in small coronary vessels in diabetic and nondiabetic patients – results from the BELLO (balloon elution and late loss optimization) trial. Cardiovasc Revascularization Med. (2017) 18:4–9. doi: 10.1016/j.carrev.2016.12.008
27. Tang KH, Gao JF, Ren JP. Application of drug-eluting balloon in diabetes patients with primary coronary stenosis of small vessel (Chinese). Shaanxi Med J. (2019) 48:432–4. doi: 10.3969/j.issn.1000-7377.2019.04.006
28. Zheng HJ, Jin H, Cui HL, Zhu YK, Zeng H, Han FJ, et al. Safety of drug-coated balloon versus drug-eluting stents in the treatment of type 2 diabetes mellitus complicated by coronary artery small vessel disease in older adult patients (Chinese). Chinese J Tissue Eng Res. (2020) 24:4573–9. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwOTAxEg14ZGtmMjAyMDI4MDIyGggyNXpuZG1ubA%3D%3D
29. Zhou MK, Liu XJ, Zhang DD, Du H, Pan PY, Wang R, et al. Outcome comparison of drug-eluting stent and drug-eluting balloon treating the small vessel disease in patients with coronary heart disease and diabetes (Chinese). J Cardiovasc Pulmon Dis. (2020) 39:1420–3. doi: 10.3969/j.issn.1007-5062.2020.12.002
30. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 63. Chichester: Cochrane (2022).
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
32. Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8, 2006. Circulation. (2007) 115:2352–7. doi: 10.1161/CIRCULATIONAHA.107.688416
33. Zhang R, Tao Z, Gong J, Ji Z, Yang M, Ma G, et al. Albumin to globulin ratio was associated with in-stent restenosis and revascularization events after percutaneous coronary intervention. Clin Transl Sci. (2022) 15:1187–95. doi: 10.1111/cts.13236
34. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
35. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
36. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12:55–61. doi: 10.1002/jrsm.1411
37. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
38. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
39. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
40. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
41. Palma Perez S, Delgado Rodriguez M. Practical considerations on detection of publication bias. Gac Sanit. (2006) 20 (Suppl. 3):10–6. doi: 10.1157/13101085
42. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
43. Spineli LM, Pandis N. Meta-analysis: random-effects model. Am J Orthod Dentofacial Orthop. (2020) 157:280–2. doi: 10.1016/j.ajodo.2019.10.007
44. Cortese B, Di Palma G, Guimaraes MG, Piraino D, Orrego PS, Buccheri D, et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc Intervent. (2020) 13:2840–9. doi: 10.1016/j.jcin.2020.08.035
45. Wöhrle J, Scheller B, Seeger J, Farah A, Ohlow MA, Mangner N, et al. Impact of diabetes on outcome with drug-coated balloons versus drug-eluting stents: the BASKET-SMALL 2 trial. JACC Cardiovasc Interven. (2021) 14:1789–98. doi: 10.1093/eurheartj/ehab724.2107
46. Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. (2015) 65:2496–507. doi: 10.1016/j.jacc.2015.04.017
47. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145:e18–114. doi: 10.1161/CIR.0000000000001038
Keywords: small-vessel coronary artery, diabetes mellitus, drug-coated balloon, drug-eluting stent, meta-analysis
Citation: Li K, Cui K, Dan X, Feng J and Pu X (2022) The comparative short-term efficacy and safety of drug-coated balloon vs. drug-eluting stent for treating small-vessel coronary artery lesions in diabetic patients. Front. Public Health 10:1036766. doi: 10.3389/fpubh.2022.1036766
Received: 05 September 2022; Accepted: 26 September 2022;
Published: 18 October 2022.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Feng Hu, Shanghai Jiao Tong University, ChinaCopyright © 2022 Li, Cui, Dan, Feng and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijun Cui, Y3Vpa2FpanVuc2N1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.