- 1Shenzhen Nanshan Center for Chronic Disease Control, Shenzhen, China
- 2National Center for STD Control, Chinese Academy of Medical Sciences and Peking Union Medical College Institute of Dermatology, Nanjing, China
Background: Many studies have focused on the distribution and specific clinical symptoms caused by Chlamydia trachomatis. Still, relatively few studies have focused on the associations between Chlamydia trachomatis genotypes and cervical intraepithelial lesions.
Objectives: This study was conducted to determine the distribution of Chlamydia trachomatis genotypes and its associations with cervical intraepithelial lesions among women of reproductive age. The presence of other STIs coinfection was also evaluated.
Method: 375 Chlamydia trachomatis positive cervical swabs collected from women of reproductive age were analyzed though molecular assay. Multivariate logistic regression analyses (covariates include contraception, gravidity (≥1), abnormal vaginal discharge, adverse pregnancy outcomes, reproductive tract symptoms and abnormal cervical cytology) were performed to evaluate the associations between Chlamydia trachomatis genotypes and cervical intraepithelial lesions and genital clinical symptoms.
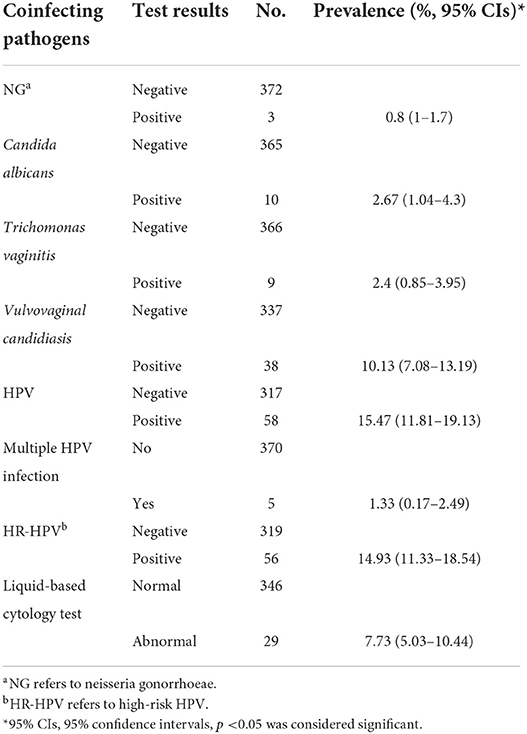
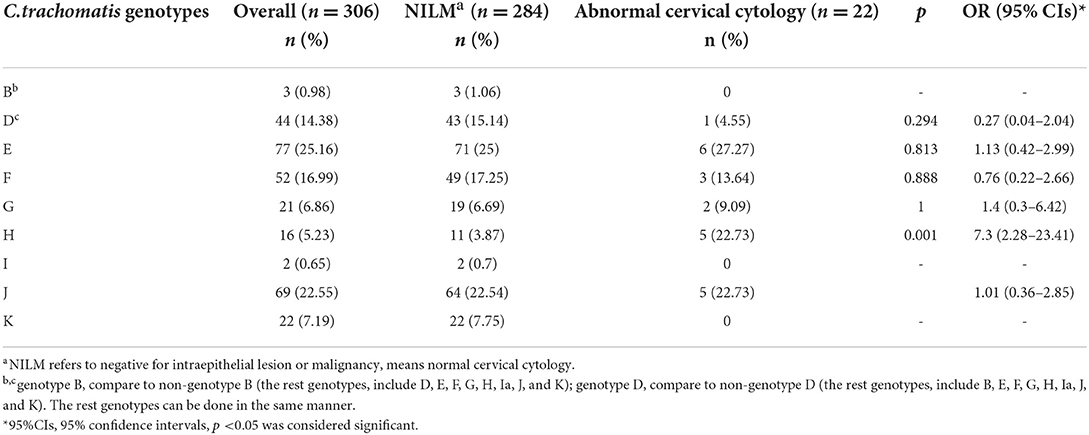
Results: Among 375 Chlamydia trachomatis positive cervical swabs, the prevalence of coinfection with Neisseria gonorrhoeae, Candida albicans, Trichomonas vaginitis, Vulvovaginal candidiasis, and HPV were 0.8%, 2.7%, 2.4%, 10.1% and 15.5%, respectively. 306 were genotyped successfully, and nine genotypes were identified. The most common genovar was E (25.16%, 77/306), followed by J (22.55%, 69/306), F (17%, 52/306), D (14.4%, 44/306), K (7.2%, 22/306), G (6.9%, 21/306), H (5.2%, 16/306), B (1.0%, 3/306), Ia (0.7%, 2/306). Genotype H was associated with abnormal cervical cytology [p = 0.006, aOR = 8.16 (1.86–36.6)]. However, this study observed no association between Chlamydia trachomatis genotypes and any genital clinical symptoms.
Conclusions: Chlamydia trachomatis genotype H may be a high risk factor for cervical intraepithelial lesions, which is useful for treatment and management measures for patients with cervical intraepithelial lesions.
Introduction
Chlamydia trachomatis (C.trachomatis), an obligate intracellular bacterium, remains the most frequent causative agent of sexually transmitted infections (STIs) worldwide, with a global incidence of 38 per 1,000 women and 33 per 1,000 men (1). 105 sentinel surveillance sites in China reported that C.trachomatis incidence increased from 35.8 per 100,000 in 2011 to 37.1 per 100,000 in 2015. Men who have sex with men (MSM) and female sex workers (FSW) have a higher risk of C.trachomatis infection, accounting for 6.5% and 17.3% of infections, respectively (2, 3). The true C.trachomatis prevalence may be underestimated because most cases are asymptomatic (about 70% of women and 50% of men don't have any clinical symptoms) (4).
Based on the major outer membrane protein (MOMP) encoded by the ompA gene, 19 C.trachomatis serovars have been classified into three clusters: genotype A-C (predominantly related to trachoma), genotype D-K (associated with urogenital infections), genotype L1-L3 (causing lymphogranuloma venereum) (5). Left untreated, C.trachomatis can lead to severe consequences, such as cervicitis, cervical ectopy and chronic pelvic inflammation in females, urethritis in males, and neonatal conjunctivitis in newborns (6).
Cervical cancer (CC) is the fourth most common cancer amongst women worldwide (7). The major risk factors associated with CC development include high-risk human papilloma virus (hrHPV) infection, age, smoking, childbirth, use of oral contraception, and diet (8–13). The association between certain hrHPV of HPV and cervical cancer is well established (14). However, previous studies show that only a few HPV infection cases will develop into CC (15). That means HPV alone is not sufficient for the development of CC.
CC arises from normal cervical epithelium through the progressive development of low grade and high grade cervical intraepithelial lesions (CINs) (16). And C.trachomatis is considered as a risk factor for abnormal cervical cytology in previous researches (17–19). However, it is unclear that which are high-risk and which are low-risk for CINs about 19 C.trachomatis genotypes, which may facilitate the prevention and treatment of C.trachomatis infection. Unfortunately, only a few studies have been conducted to evaluate the association between C.trachomatis genotypes and CINs (20). Thus, this study aims to determine the distribution of Chlamydia trachomatis genotypes and its associations with CINs among women of reproductive age.
Materials and methods
Study design
From March to August 2017, we recruited participants from 9,249 women who met eligibility criteria and provided informed consent in our previous study (21), and all of the women signed an informed consent to this study. Women who met any of the following exclusion criteria were not enrolled: pregnancy, without a history of sexual activity, sexual intercourse 3 days ago, menstrual period, previous hysterectomy, vaginal bleeding, vaginal douching or using a vaginal suppository, currently suffering from gynecological inflammation. The inclusion criteria were the same as inclusion criteria in our previous study (21): being a female resident, aged 20–60 years and living locally in Shenzhen city Nanshan District during the past 3 months. All participants signed informed consent and were interviewed using a structured questionnaire to collect socio-demographic and clinical information before enrollment. All participants voluntarily agreed to provide a self-administrated 3–5 mL first-catch urine specimen (Chlamydia trachomatis and Neisseria gonorrhoeae tests), a cervical swab (HPV tests), two vaginal swabs (gynecological examinations), and an exfoliated cervical cells specimen (liquid-based cervical cytology test).
Positive endocervical swabs samples
Out of the 9,249 women who met eligibility criteria and signed an informed consent, 9,090 (98.3%) women's specimens were successfully tested, and 375(4.13%, 375/9,090) were C.trachomatis positive.
375 positive endocervical swabs samples were used for C.trachomatis genotyping to evaluate association with CINs. More detailed study methods and epidemiological information about the study are available in a previously published article (21, 22).
C.trachomatis and neisseria gonorrhoeae DNA test
The method and procedures of C.trachomatis and Neisseria gonorrhoeae DNA test were described in our previous study (21).
C.trachomatis DNA extraction, OmpA PCR amplification, sequencing and genotyping
The positive endocervical swabs were each eluted with 1 ml sterile water and vortexed. Researchers took 200 μl of eluant from the samples for DNA extraction. DNAs were extracted by using QIAamp® cador® Pathogen Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. The remaining eluate was stored at −80°C.
Molecular genotyping of C.trachomatis was performed by ompA gene; the detailed information about ompA primers for the current nested PCR system is available at the Uppsala University C.trachomatis MLST database (http://mlstdb.bmc.uu.se). The nested PCR cycling protocol methods was previously described (23). The secondary PCR amplifications were purified and sent to be sequenced by HYK-High-throughput Biotechnology Institute (Shenzhen, China). All PCR amplifications were sequenced bidirectionally.
HPV DNA testing and genotype
HPV DNA testing and genotyping (14 HR-HPV including genotype 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 2 low-risk HPV including genotype 6 and 11) were conducted by the Beijing Genomics Institute according to operating instructions (24).
ThinPrep cytological test (TCT)
The cytobrush containing cervical exfoliated cells was collected and stained, and then fixed in TCT cytological solution for 15 min. TCT was performed by a SurePath liquid-based Pap test (BD, United States), according to the manufacturer's instructions. Cervical cytology test results were classified by the Bethesda System (TBS; 2001) criteria as follows: negative for intraepithelial lesion or malignancy (NILM); atypical glandular cells (AGC); squamous intraepithelial lesions (SIL) of low (LSIL) or high (HSIL) grade; atypical squamous cells (ASC) of undetermined significance (ASC-US) or not possible exclude HSIL (ASC-H); and squamous cell carcinoma (SCC) (25). In this study, cervical cytology was dichotomized into normal (NILM) and abnormal (≥ASC). More detailed information is available in our previous study (22).
Vaginal cleanliness evaluation
Vaginal smears on clean glass slides were observed under microscope at the hospital laboratory. The evaluation criteria of vaginal cleanliness was divided into 4 grades by the relative abundance of Lactobacillus spp., vaginal epithelial cells, pus cells and white blood cells per high power field (26). Grade I and grade II indicate normal vaginal cleanliness, whereas grade III and grade IV are abnormal, indicating the presence of inflammation or infection (22).
Gynecological examination
Vaginal secretions swab specimens were collected by two skilled gynecologists and were rolled on to a glass slide for Gram staining immediately. Vaginal cleanliness, detection of hyphae and spores of Candidiasis and clue cells were confirmed by Gram staining of vaginal secretions. Trichomonas Vaginalis was diagnosed by microscopic examination of wet mounts immediately once the vaginal secretions swab collected. Amine test, pH of vaginal secretions and leukocytes were further confirmed within 15 min. The diagnosis of bacterial vaginosis was based on Amsel's criteria (27), which was widely adopted to the clinical diagnosis of bacterial vaginosis. A positive diagnosis of bacterial vaginosis was made once three of the four following signs are present: the presence of clue cells, an adherent and homogenous grayish-white vaginal discharge, a vaginal pH exceeding a value of 4.5, a fishy or amine odor after the addition of a 10% potassium hydroxide solution. The method and procedures of gynecological examination were described in our previous study (22).
Statistical analyses
Raw data collection and statistical analysis were performed by Microsoft Excel 2016 and SPSS Statistics version.20.0 (SPSS Inc., Chicago, IL, United States), respectively. Abnormal cytology group (≥ASC-US was defined as women who had a diagnosis of the following cytology findings: ASC-US, ASC-H, LSIL, HSIL or AGC). C.trachomatis genotypes were divided into two group, genotype B group and non-B genotypes group, genotype D group and non-D genotypes group, genotype E group and non-E genotypes group, and so on. The chi-square test (χ2), or two-sided Fisher exact test for 2 × 2 contingency table was used to evaluate the associations between different C.trachomatis genotypes, sociodemographic characteristics, reproductive history, sexual behavior, and urogenital symptoms. Variables with a significance level of p < 0.2 were enrolled in the multivariate logistic regression model adjusted by potential confounders. Crude odds ratios (OR), adjusted odds ratio (AOR) and corresponding 95% CIs were calculated. A p < 0.05 was considered significant.
Ethics statement
The study was approved and overseen by Ethical Committee of the Shenzhen Nanshan Center for Chronic Disease Control (Approved No. LL20170017).
Results
Population characteristics
Socio-demographic and clinical information in patients with C.trachomatis infection were shown in Table 1. A total of 69.1% of C.trachomatis positive women were >35 years old; 73.9% of C.trachomatis positive women were middle and low-income. More than half of women were unemployed (63.5%). 94.1% of women were married, and 80.3% reported using condoms or other contraceptive methods, including oral contraceptive use and intrauterine devices; 54.7% had no reproductive tract symptoms.
A total of 24% of women had adverse pregnancy outcomes. Cesarean delivery accounted for 71.2%. 90.7% had never had a C.trachomatis test previously.
C.trachomatis coinfection with other STIs and Liquid-based cytology test results
As shown in Table 2, the most prevalent coinfection pathogen was HPV, which accounted for 15.47%, particularly high-risk HPV (96.55%, 56/58), followed by vulvovaginal candidiasis (10.13%), candida albicans (2.67%), trichomonas vaginitis (2.4%), and NG (0.8%).
The distribution of C.trachomatis genotypes by cytology status
As shown in Table 3, among 375 C.trachomatis positive cervical swabs, 306 were genotyped successfully, and 69 samples containing less C.trachomatis DNA were insufficient for genotyping. Out of 306 samples, nine genotypes were identified. The most common genotype was E (25.16%, 77/306), followed by J (22.55%, 69/306), F (17%, 52/306), D (14.4%, 44/306), K (7.2%, 22/306), G (6.9%, 21/306), H (5.2%, 16/306), B (1.0%, 3/306), and Ia (0.7%, 2/306).
22 (7.19%) samples had abnormal cervical cytology; The remaining were negative for intraepithelial lesion or malignancy (NILM) (92.81%, 284/306). We found a positive association between C.trachomatis genotype H infection and abnormal cervical cytology [p = 0.001, OR = 7.3 (95% CI: 2.28–23.41)].
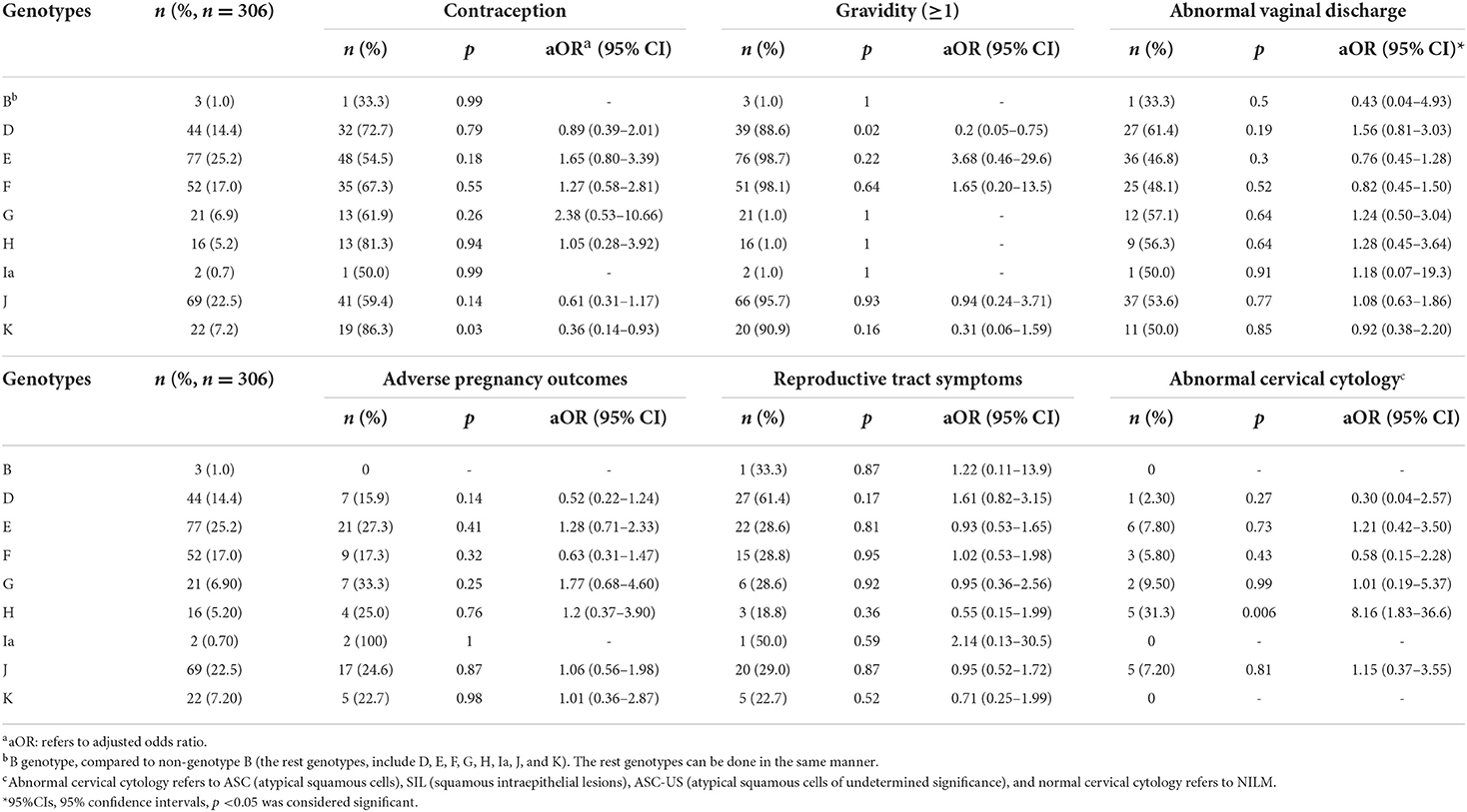
Associations between C.trachomatis genotypes and clinical characteristics
As shown in Table 4, after adjusting for potential confounders by multivariate logistic regression analysis, we found that no associations were observed between different C.trachomatis genotypes and gravidity, vaginal cleanliness, adverse pregnancy outcomes or reproductive tract symptoms. However, the association of genotype K with contraception, including condom use, oral contraceptive use, and intrauterine devices was observed (p = 0.03, aOR = 0.36, 95% CI: 0.14–0.93). Genotype D was associated with gravidity (p = 0.02, aOR = 0.2, 95% CI: 0.05–0.75). Most importantly, compared to other CT genotypes, there was a significant association between genotype H and abnormal cervical cytology (p = 0.006, aOR = 8.16, 95% CI: 1.83–36.6).
Discussion
In this study, 375 C.trachomatis positive cervical swabs were collected from a population-based cross-sectional survey with a relatively larger sample size (n = 9,090) (28). This study extended the existing literature by distribution of C.trachomatis ompA genotypes and its associations with abnormal cervical cytology.
In this study, we found that most participants had no C.trachomatis test previously, which indicated that they were unaware of the cervical lesions that C.trachomatis cause. And almost half reported that they had no vaginal discharge, which aligns with the fact that many infections are asymptomatic, thus delaying diagnosis.
As for coinfection, the most prevalent coinfection pathogen was HPV, especially high-risk HPV. The prevalence of coinfection with NG was relatively low in our study.
In this study, 306 samples were genotyped successfully and 69 samples containing less C.trachomatis DNA were insufficient for genotyping. The failure rate of C.trachomatis ompA gene sequencing in this study (18.4%, 69/375) was higher than in a study conducted in mainland China (5.83%, 14/240), but lower than other studies (Taiwan, 29.6%, 43/145; mainland China, 20.2%, 33/163) (29–31). The difference may be due to clinical samples (cervical swabs and urine) and different sequence methods performed in different studies.
Out of 306 successfully genotyped samples, nine different C.trachomatis genotypes from B and D-K were detected. The results in this study were diverse when compared with other studies (31, 32). We speculate that the specific location of the study, Shenzhen, may be linked to the diversity. Because Shenzhen is a modern city with rapid economic development and large unregistered, young, floating migrant population. It has been reported that C.trachomatis is the most common STI mainly affecting young individuals (1).Thus, it is reasonable that a diverse array of genotypes existed in our study. And similar to other studies, genotypes E, F, J, and D were the most prevalent in Guangdong (1).
Among nine C.trachomatis genotypes, E and J were the predominant genotypes in this study, which is slightly different when compared with two studies carried out in mainland China where F and E, D, and E genotypes were the most common, respectively (28, 31). Genotype E was one of the stable genotypes among the general population despite the existing difference, while G and D were identified as the most prevalent genotypes among MSM (33). Lymphogranuloma venereum genotype L1-L3 was absent in our study, but was also recognized in the MSM (34).
Interestingly, genotype B was detected in cervical swabs in our study, it was found associated with trachoma and neonatal conjunctivitis in other studies previously (5, 32, 35). This results indicated that genotype B may lead to multiple anatomical sites infection, not only caused eye infection, but also reproductive tract infection. To our knowledge, this was the first time that genotype B was found among women in Shenzhen, excluding a previous study conducted in MSM (33).
We observed that clinical manifestations including abnormal vaginal discharge, were not associated with any genotypes. These results are in accordance with some research, but different from others (1, 32, 36). Previous studies have shown that genotype K was associated with abnormal vaginal discharge, and genotype G was related to low abdominal pain (30, 37). Although the association between parity and STIs has been demonstrated, the association between parity and genotypes was not found in this study (17, 38).
The rate of abnormal cervical cytology in this study (7.2%) was higher than in a previous study (2.8%) (39). However, in terms of the relationship between C.trachomatis positive and cervical precancerous lesions, our findings varied with the previous literature (17, 18). A case-control study and a meta-analysis showed that C.trachomatis infection was a higher risk factor of CC, while other studies found no association between the C.trachomatis and CC or abnormal cervical cytology (20, 39, 40). Our result was similar to the latter.
In present study, genotype E and J are the most frequent genotypes, but these two was not significantly related to the abnormal cervical cytology, suggesting that genotype E may be not the pathogenic factor for abnormal cervical cytology. Genotype H was associated with abnormal cervical cytology, indicating that genotype H may be the pathogenic factor for abnormal cervical cytology, which is a little different from the previous study reported by Chen (1). They found that patients with genotype G infection commonly had abnormal cervical cytology (p = 0.029, OR = 1.868, 95% CI: 1.124–3.106). However, the association between genotype G and abnormal cervical cytology was not observed in our study (1). Genotypes B, D, G, and I were shown to be related to abnormal cervical cytology in a different case control study, which was also not found in this study (40).
Limitations
Several limitations of our study should be acknowledged. First, the genotyping method used in our study could not identify strains with multiple genotypes infections, despite being sensitive and specific. Second, the failure rate of C.trachomatis ompA gene sequencing in this study (18.4%, 69/375) was a little higher, which may influence the study's conclusion. Third, the sample size (n = 375) of this study was small, and samples collected in the study were limited to the Nanshan district, Shenzhen, so the representativeness of genotype distribution may be affected. Further research is needed to enroll a population comprising of both males and females and enlarge the target region.
Conclusions
In conclusion, C.trachomatis genotype H may increase the risk of cervical intraepithelial lesion in terms of cross-sectional epidemiology, which indicates that H is a higher risk C.trachomatis genotype. This provides some evidence for clinical diagnosis and treatment of C.trachomatis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved and overseen by Ethical Committee of the Shenzhen Nanshan Center for Chronic Disease Control (Approved No. LL20170017). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: X-sC, BL, Z-zL, L-lL, SS, LZ, Q-hW, and L-sT. Data curation: L-lL, Q-hW, and LZ. Formal analysis: L-lL, Q-hW, BL, and X-sC. Investigation: L-lL, Q-hW, LZ, and L-sT. Methodology: L-lL, SS, and Q-hW. Project administration and resources: Z-zL, L-lL, and SS. Software: L-lL and SS. Supervision: BL, Z-zL, L-sT, and X-sC. Validation: BL, X-sC, and Z-zL. Visualization: BL and X-sC. Writing—original draft and writing—review & editing: L-lL. All authors contributed to the article and approved the submitted version.
Funding
This study received financial support from the Shenzhen Nanshan Science and Technology Planning Project (SZ010).
Acknowledgments
The authors are grateful for the help received from nurse and doctors in Shenzhen Nanshan Maternity & Child Healthcare Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Y, Chen J, Yang L, Jiang Y, Li L, Yi W, et al. Distribution of chlamydia trachomatis genotypes in infective diseases of the female lower genital tract. Med Sci Monit: Int Med J Exp Clin Res. (2017) 23:4477–81. doi: 10.12659/MSM.902756
2. Chen XS, Yin YP, Liang GJ, Wang QQ, Jiang N, Liu Q, et al. The prevalences of Neisseria gonorrhoeae and Chlamydia trachomatis infections among female sex workers in China. BMC Pub Health. (2013) 13:121. doi: 10.1186/1471-2458-13-121
3. Fu GF, Jiang N, Hu HY, Mahapatra T, Yin YP, Mahapatra S, et al. The epidemic of HIV, syphilis, chlamydia and gonorrhea and the correlates of sexual transmitted infections among men who have sex with men in Jiangsu, China, 2009. PLoS ONE. (2015) 10:e0118863. doi: 10.1371/journal.pone.0118863
4. Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM. Chlamydia trachomatis: the persistent pathogen. Clin Vacc Immunol: CVI. (2017) 24:e00203–17. doi: 10.1128/CVI.00203-17
5. Piñeiro L, Isaksson J, Zapico M, Cilla G, Herrmann B. Chlamydia trachomatis genotypes A and B from urogenital specimens of patients in Spain: molecular characterization. Clin Microbiol Infect: Official Pub Eur Soc Clin Microbiol Infect Dis. (2018) 24:910.e5–8. doi: 10.1016/j.cmi.2018.01.025
6. Herrmann B, Isaksson J, Ryberg M, Tångrot J, Saleh I, Versteeg B, et al. Global multilocus sequence type analysis of chlamydia trachomatis strains from 16 countries. J Clin Microbiol. (2015) 53:2172–9. doi: 10.1128/JCM.00249-15
7. Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Nat Med Assoc. (2020) 112:229–32. doi: 10.1016/j.jnma.2020.03.002
8. Schiffman M, Solomon D. Clinical practice cervical-cancer screening with human papillomavirus and cytologic cotesting. New Eng J Med. (2013) 369:2324–31. doi: 10.1056/NEJMcp1210379
9. Gaffney DK, Hashibe M, Kepka D, Maurer KA, Werner TL. Too many women are dying from cervix cancer: problems and solutions. Gynecol Oncol. (2018) 151:547–54. doi: 10.1016/j.ygyno.2018.10.004
10. Crosbie E, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet (London, England). (2013) 382:889–99. doi: 10.1016/S0140-6736(13)60022-7
11. Dasgupta S, Chakraborty SB, Roy A, Roychowdhury S, Panda CK. Differential deletions of chromosome 3p are associated with the development of uterine cervical carcinoma in Indian patients. Mol Pathol: MP. (2003) 56:263–9. doi: 10.1136/mp.56.5.263
12. Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. New Engl J Med. (2009) 361:271–8. doi: 10.1056/NEJMct0806938
13. Tsu V, Jerónimo J. Saving the world's women from cervical cancer. New Eng J Med. (2016) 374:2509–11. doi: 10.1056/NEJMp1604113
14. Dyer O. Cervical cancer: deaths increase as HPV vaccine is underused, says WHO. BMJ (Clinical research ed). (2019) 364:l580. doi: 10.1136/bmj.l580
15. Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. (2018) 7:5217–36. doi: 10.1002/cam4.1501
16. Olusola P, Banerjee HN, Philley JV, Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. (2019) 8:622. doi: 10.3390/cells8060622
17. Jensen KE, Thomsen LT, Schmiedel S, Frederiksen K, Norrild B, van den Brule A, et al. Chlamydia trachomatis and risk of cervical intraepithelial neoplasia grade 3 or worse in women with persistent human papillomavirus infection: a cohort study. Sexually Trans Infect. (2014) 90:550–5. doi: 10.1136/sextrans-2013-051431
18. Zhu H, Shen Z, Luo H, Zhang W, Zhu X. Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine. (2016) 95:e3077. doi: 10.1097/MD.0000000000003077
19. Ji Y, Ma XX, Li Z, Peppelenbosch MP, Ma Z, Pan Q. The burden of human papillomavirus and chlamydia trachomatis coinfection in women: a large cohort study in inner Mongolia, China. J Infect Dis. (2019) 219:206–14. doi: 10.1093/infdis/jiy497
20. Robial R, Longatto-Filho A, Roteli-Martins CM, Silveira MF, Stauffert D, Ribeiro GG, et al. Frequency of Chlamydia trachomatis infection in cervical intraepithelial lesions and the status of cytological p16/Ki-67 dual-staining. Infect Agents Cancer. (2017) 12:3. doi: 10.1186/s13027-016-0111-8
21. Luo ZZ, Li W, Wu QH, Zhang L, Tian LS, Liu LL, et al. Population-based study of chlamydial and gonococcal infections among women in Shenzhen, China: implications for programme planning. PLoS ONE. (2018) 13:e0196516. doi: 10.1371/journal.pone.0196516
22. Li W, Liu LL, Luo ZZ, Han CY, Wu QH, Zhang L, et al. Associations of sexually transmitted infections and bacterial vaginosis with abnormal cervical cytology: a cross-sectional survey with 9090 community women in China. PLoS ONE. (2020) 15:e0230712. doi: 10.1371/journal.pone.0230712
23. Christerson L, Bom RJ, Bruisten SM, Yass R, Hardick J, Bratt G, et al. Chlamydia trachomatis strains show specific clustering for men who have sex with men compared to heterosexual populations in Sweden, the Netherlands, and the United States. J Clin Microbiol. (2012) 50:3548–55. doi: 10.1128/JCM.01713-12
24. Yi X, Zou J, Xu J, Liu T, Liu T, Hua S, et al. Development and validation of a new HPV genotyping assay based on next-generation sequencing. Am J Clin Pathol. (2014) 141:796–804. doi: 10.1309/AJCP9P2KJSXEKCJB
25. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 bethesda system: terminology for reporting results of cervical cytology. JAMA. (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
26. Yu F, Tang YT, Hu ZQ, Lin XN. Analysis of the vaginal microecological status and genital tract infection characteristics of 751 pregnant women. Med Sci Monitor: Int Med J Exp Clin Res. (2018) 24:5338–45. doi: 10.12659/MSM.909051
27. Verstraelen H, Verhelst R. Bacterial vaginosis: an update on diagnosis and treatment. Expert Rev Anti-Infect Therapy. (2009) 7:1109–24. doi: 10.1586/eri.09.87
28. Zhang JJ, Zhao GL, Wang F, Hong FC, Luo ZZ, Lan LN, et al. Molecular epidemiology of genital Chlamydia trachomatis infection in Shenzhen, China. Sex Trans Infect. (2012) 88:272–7. doi: 10.1136/sextrans-2011-050163
29. Hsu MC, Tsai PY, Chen KT Li LH, Chiang CC, Tsai JJ, et al. Genotyping of Chlamydia trachomatis from clinical specimens in Taiwan. J Med Microbiol. (2006) 55:301–8. doi: 10.1099/jmm.0.46262-0
30. Gao X, Chen XS, Yin YP, Zhong MY, Shi MQ Wei WH, et al. Distribution study of Chlamydia trachomatis serovars among high-risk women in China performed using PCR-restriction fragment length polymorphism genotyping. J Clin Microbiol. (2007) 45:1185–9. doi: 10.1128/JCM.02076-06
31. Xue Y, Zheng H, Tang W, Mai Z, Huang J, Huang S, et al. Prevalence and genotype distribution of chlamydia trachomatis in urine among men attending sexually transmitted disease clinics in guangdong province, China, in 2016. Jap J Infect Dis. (2018) 71:104–8. doi: 10.7883/yoken.JJID.2017.358
32. Casillas-Vega N, Morfín-Otero R. Frequency and genotypes of Chlamydia trachomatis in patients attending the obstetrics and gynecology clinics in Jalisco, Mexico and correlation with sociodemographic, behavioral, and biological factors. BMC Women's Health. (2017) 17:83. doi: 10.1186/s12905-017-0428-5
33. Li JH, Cai YM, Yin YP, Hong FC, Shi MQ, Feng TJ, et al. Prevalence of anorectal Chlamydia trachomatis infection and its genotype distribution among men who have sex with men in Shenzhen, China. Jap J Infect Dis. (2011) 64:143–6. doi: 10.7883/yoken.64.143
34. Rodríguez-Domínguez M, Puerta T, Menéndez B, González-Alba JM, Rodríguez C, Hellín T, et al. Clinical and epidemiological characterization of a lymphogranuloma venereum outbreak in Madrid, Spain: co-circulation of two variants. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. (2014) 20:219–25. doi: 10.1111/1469-0691.12256
35. Quint KD, Bom RJ, Bruisten SM, van Doorn LJ, Nassir Hajipour N, Melchers WJ, et al. Comparison of three genotyping methods to identify Chlamydia trachomatis genotypes in positive men and women. Mole Cell Probes. (2010) 24:266–70. doi: 10.1016/j.mcp.2010.04.007
36. Lysén M, Osterlund A, Rubin CJ, Persson T, Persson I, Herrmann B. Characterization of ompA genotypes by sequence analysis of DNA from all detected cases of Chlamydia trachomatis infections during 1 year of contact tracing in a Swedish County. J Clin Microbiol. (2004) 42:1641–7. doi: 10.1128/JCM.42.4.1641-1647.2004
37. Morré SA, Rozendaal L, van Valkengoed IG, Boeke AJ, van Voorst Vader PC, Schirm J, et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: an association with clinical manifestations? J Clin Microbiol. (2000) 38:2292–6. doi: 10.1128/.38.6.2292-2296.2000
38. Kleppa E, Holmen SD, Lillebø K, Kjetland EF, Gundersen SG, Taylor M, et al. Cervical ectopy: associations with sexually transmitted infections and HIV. A cross-sectional study of high school students in rural South Africa. Sex Trans Infect. (2015) 91:124–9. doi: 10.1136/sextrans-2014-051674
39. Oh JK, Franceschi S, Kim BK, Kim JY, Ju YH, Hong EK, et al. Prevalence of human papillomavirus and Chlamydia trachomatis infection among women attending cervical cancer screening in the Republic of Korea. Eur J Cancer Prev: Off J Eur Cancer Prev Organ (ECP). (2009) 18:56–61. doi: 10.1097/CEJ.0b013e328305a0a6
Keywords: cervical intraepithelial lesion, Chlamydia trachomatis, genotyping, human papillomavirus, women of reproductive age
Citation: Liu L-l, Sun S, Zhang L, Wu Q-h, Tian L-s, Li B, Chen X-s and Luo Z-z (2022) Distribution of Chlamydia trachomatis ompA genotypes and its association with abnormal cervical cytology among women of reproductive age in Shenzhen, China. Front. Public Health 10:1036264. doi: 10.3389/fpubh.2022.1036264
Received: 04 September 2022; Accepted: 17 October 2022;
Published: 31 October 2022.
Edited by:
Weiming Tang, University of North Carolina at Chapel Hill, United StatesReviewed by:
Haidong Lu, Yale University, United StatesYewei Xie, Duke-NUS Medical School, Singapore
Copyright © 2022 Liu, Sun, Zhang, Wu, Tian, Li, Chen and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-zhou Luo, cGF1bGx1bzk5MDlAMTYzLmNvbQ==
 Lan-lan Liu
Lan-lan Liu Si Sun
Si Sun Li Zhang1
Li Zhang1 Zhen-zhou Luo
Zhen-zhou Luo