
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 22 November 2022
Sec. Aging and Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1035762
This article is part of the Research TopicAging and Chronic Disease: Public Health Challenge and Education ReformView all 37 articles
Background: Older adults with mild cognitive impairment (MCI) have the possibility of reverting to normal cognitive function. Leisure activity engagement (LAE) plays a critical role in the progress of the cognitive function. A better understanding of the dynamic relationship between LAE and MCI reversion would inform the implementation of preclinical dementia interventions. This study aimed to investigate the association between change patterns of LAE and MCI reversion among older adults using the Chinese Longitudinal Healthy Longevity Survey (CLHLS) database.
Study design: Longitudinal population-based study.
Methods: Older adults with MCI at the baseline were enrolled in this study. Information about cognitive function, overall, cognitively stimulating, physically active/demanding, and socially engaged LAE was collected at baseline and follow-up. Adjusted hazard ratios (HRs) for reversion and 95% confidence intervals (CIs) were calculated by Cox hazard models with time as the underlying time metric. We also assessed potential effect modifications by creating a cross-product of the stratifying variable with LAE change patterns in the fully adjusted model.
Results: The restricted cubic spline showed that the association between LAE change scores and MCI reversion rate was statistically significant and nonlinear (p<0.01). Taking participants in the low–low group as a reference, participants in the low–medium, low–high, medium–medium, medium–high, high–medium, and high–high groups had increased possibilities of MCI reversion with HRs (95% CI) of 2.19 (1.57–3.06), 2.97 (2.13–4.13), 0.87 (0.64–1.19), 2.28 (1.71–3.03), 2.78 (2.10–3.69), 1.93 (1.43–2.59), and 2.74 (2.09–3.60), respectively. Further stratified models showed that the impact of LAE change patterns on MCI reversion varied in different ages (nonagenarian, octogenarian, and younger elderly) and gender.
Conclusions: Participants who maintained the highest LAE had the greatest possibility of MCI reversion. Meanwhile, a higher level of LAE maintenance was associated with the increased possibility of MCI reversion. These results provide a practical message to older adults about how dynamic changes in LAE are associated with improved cognitive function.
Mild cognitive impairment (MCI) is the transitional stage between normal aging and dementia in which individuals demonstrate objective cognitive impairment that does not interfere with daily functional independence (1). Recent evidence reported that the prevalence of MCI was around 14.8% in people aged 75–79 years and 25.2% in people aged 80–84 years (2). Even though older adults with MCI have a higher risk of progressing to dementia than individuals with normal cognitive function (3, 4), nearly 30% of them could still revert to normal cognitive function (5).
To date, few studies have focused on individuals who reverted to normal cognitive function after a diagnosis of MCI. Knowing the predictors of MCI reversion in older adults with the increasing need to treat dementia at an early stage is important. In addition, most previous studies investigated the association between MCI reversion and non-modifiable factors and lifelong factors that cannot be changed in later life (e.g., gender, age, educational level, economic status, and living place) (6, 7). Evidence on modifiable predictors for MCI reversion is still limited.
Leisure activity engagement (LAE) plays a critical role in the progress of cognitive function (8). Many resources found that LAE may delay or prevent Alzheimer's disease (AD) or related dementia (9). However, few researchers studied the impact of LAE on MCI reversion. It is critical to investigate the impact of LAE on MCI reversion among community-dwelling older adults to prevent dementia at an early stage. It is also valuable to differentiate the types of leisure activities, that is, cognitive, physical, and social when studying their effects on MCI reversion since these three subtypes of LAE have varied pathways which converge within three major etiological hypotheses (such as the cognitive reserve hypothesis, the vascular hypothesis, and the stress hypothesis) for dementia and other cognitive disorders (10).
Although a cohort study identified some specific activities positively associated with MCI reversion (4), the LAE and cognitive ability measurements were conducted at a given age. They only assessed the change in cognitive ability from the point award, which ignored dynamic features of LAE over time and could introduce some measurement errors (11, 12). It is critical to capture changes in LAE since they reflect the associated risks when individuals change their lifestyle in the real world from a public health perspective (13). A better understanding of the dynamic relationship between LAE and MCI reversion would inform the implementation of preclinical dementia interventions and improve our understanding of the aging mind and the brain.
The association between changes in LAE, such as changing from a higher engagement at the baseline to a lower engagement at follow-up, and MCI reversion rate remains unclear, and we aimed to fill in this blank. To address this question, we investigated the association between change patterns of overall, cognitive-based, physical-based, and social-based LAE and MCI reversion among older adults by using the data from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) database.
Participants were selected from older adults enrolled in the population-based cohort study titled CLHLS. The CLHLS was a nationwide prospective cohort study that enrolled individuals aged 65 years or older. The sample was randomly selected from 806 cities and counties in 23 provinces of China using multi-stage stratified sampling, covering about half of the cities and counties in each province (14). More detailed information on the study design and data quality assessment of the CLHLS has been presented in previous studies (15). All baseline and follow-up surveys were administered at the homes of participants by trained interviewers with a structured questionnaire. Proxy respondents, usually a spouse or other close family members, were interviewed when the participants were unable to answer questions, but questions regarding cognitive function were answered by the participants themselves.
In this study, we included participants who had MCI at the baseline. The baseline exclusion criteria were health problems, such as clinical diagnosis of dementia, missing data regarding the exclusion criteria, relocation or death during the follow-up period, without MCI (MMSE<18 or MMSE>23) at the baseline. Among 35,474 participants enrolled in the CLHLS from 2002 to 2014, 31,930 participants were excluded according to the exclusion criteria. Finally, 3,544 participants with MCI at the baseline were enrolled in the study (Figure 1).
The Chinese Longitudinal Healthy Longevity Survey study was approved by the Institutional Review Board of Duke University (Pro00062871) and the Biomedical Ethics Committee of Peking University (IRB00001052-13074). Each participant signed written informed consent.
The frequency of engaging in eight typical leisure activities: housework, personal outdoor activities, garden work, reading newspapers/books, raising domestic animals, playing cards and/or Mahjong, watching TV and/or listening to the radio, social activities (organized) was recorded as “almost every day,” “not daily, but once a week,” “not weekly, but at least once a month,” “not monthly, but sometimes,” or “never.” The frequency of engaging in each activity was recoded on a three-point Likert scale: frequently, occasionally, and rarely, and they were scored as 2, 1, and 0, respectively. The classification resembles that used in a study by Fernández-Mayoralas, Rojo-Pérez (16), which categorized participation in leisure activities as “active,” “moderately active,” and “inactive.” This categorization has drawn out more differences among the three categories, as opposed to categorizing participation into a two-category variable (“active” and “inactive”).
According to the predominant element of each activity, these activities were categorized into cognitively stimulating, physically active/demanding, and socially engaged (17). The range of overall, cognitively stimulating (such as reading newspapers/books and watching TV/listening to the radio), physically active/demanding (such as housework, personal outdoor activities, and garden work), and socially engaged (such as playing cards/Mahjong, engagement in social activities, and raise domestic animals) were 0–16, 0–4, 0–6, and 0–6, respectively, following established studies (18).
Leisure activity engagement (LAE) change patterns of overall, cognitively stimulating, physically active/demanding, and socially engaged were calculated according to LAE from the baseline to follow-up. The overall LAE was categorized into three groups [low (0–2 score), medium (3–5 score), and high (6–16 score] according to the distribution of participants. Then, nine relative LAE change patterns were created: low–low, low–medium, low–high, medium–low, medium –medium, medium–high, high–low, high–medium, and high–high. Accordingly, cognitively stimulating LAE was categorized into low, medium, and high with 0, 1–2, and 3-−4, and physically active/demanding LAE was categorized into three groups with 0, 1–3, and 4–6, and socially engaged LAE was categorized into three groups with 0, 1, and 2–6.
The mild cognitive impairment was measured by the Chinese version of the Mini-Mental State Examination (MMSE) and adapted and validated from the scale developed by Folstein et al. (19). The Chinese version of the MMSE considers the cultural and socioeconomic conditions of the older Chinese adults so that all question items in the test could be easily comprehended and answered by a survey participant with normal cognitive functioning (20). The Chinese version of the Mini-Mental State Examination (CMMSE) made small modifications based on the social-cultural differences of the Chinese population. According to regional divisions of China, test items for the orientation to the place were adapted as to province, district, street, place, and floor to replace the phrase country, town, street, place, and floor. The reliability and validity of the CMMSE in the CLHLS have been established in prior studies (21, 22). In particular, previous research showed that participants were more likely to be unable to answer relatively difficult tasks when they exhibited poor health and/or existing cognitive limitations (23). Therefore, following prior research, we categorized responses of “unable to answer” as incorrect answers. This approach has been widely used in previous studies and will not introduce potential bias (24). The MMSE measured 5 aspects of cognitive function (such as orientation, reaction, attention and calculation, recall, and language) by 24 items. The total score ranged from 0 to 30 and a higher score indicated better cognitive function. Mild cognitive impairment (MCI) was defined as a CMMSE score between 18 and 23 (25, 26), and reversion was defined as a participant with MCI reaching 24 or above in the follow-up tests for the first time. Reliable change in MMSE should be at least 2 to 4 points (27), and we added an additional restriction of MMSE change≥3 points to the definition of MCI reversion. Sensitivity analysis was also conducted for other thresholds (≥4).
The following covariates were assessed: age, sex, residence (urban or rural), education (years of schooling), marital status (married, divorced/widowed/never), economic status (favorable or unfavorable), living pattern (living with family members, alone, or at the nursing home), number of people living with, smoke at present (yes or no), drinking alcohol at present (yes or no), exercise at present (yes or no), activity of daily living (ADL), instrumental ADL (IADL), and chronic diseases including hypertension, diabetes, heart disease, stroke or cardiovascular disease (CVD), cataract, digestive system diseases, arthritis, and Parkinson's disease were coded into yes and no.
The activity of daily living (ADL) was measured at each wave using six items (such as dressing, bathing, indoor transferring, toileting, continence, and feeding). Participants were asked if they needed assistance with each of the six activities. Instrumental ADL (IADL) was composed of eight items (such as shopping, visiting neighbors, washing clothes, making food, walking 1 km, crouching and standing [repeated three times], carrying 5 kg weight, and taking public transport). Respondents were categorized as having an IADL disability if they needed help in performing at least one of the eight items according to the Lawton scale (28).
Baseline characteristics are presented as the mean (standard deviation [SD]) for continuous variables and the number (percentages [%]) for categorical variables. Pearson's Chi-squared test and t-test were used to examine the difference in the baseline characteristics of participants in different LAE change patterns. The reversion rate was calculated during the follow-up assessments. We applied Cox hazard models with time as the underlying time metric to calculate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) for analyzing the association between LAE changes and MCI reversion. We examined the proportional hazards assumption by creating a cross-product of follow-up time and LAE change patterns. Possible nonlinear relationships by nonparametrically restricted cubic splines were analyzed between the continuous LAE change points and MCI reversion (29, 30).
Demographic variables, functional ability, and chronic medical illness were listed as possible covariates. The association between changes in LAE and MCI reversion was investigated in three models: Model 1, adjusted for sex and age; Model 2, further adjusted for residence, years of schooling, marital status, economic status, living pattern, and the number of people living with based on Model 1; Model 3, further adjusted for smoking, alcohol drinking, ADL, IADL, and chronic diseases (such as hypertension, diabetes, heart disease, stroke or CVD, cataract, digestive system diseases, arthritis, and Parkinson's disease) based on Model 2. Adjusted hazard ratios (HRs) for reversion and 95% confidence intervals (CIs) were calculated.
We performed stratified analyses to evaluate potential effect modifications by baseline age (younger elderly 65–79 years, octogenarian 80–89 years, nonagenarian: ≥90 years), sex (male or female), residence (urban or rural), marital status (married, divorced/widowed/never), economic status (favorable or unfavorable), living pattern (living with family members, alone, or at the nursing home). We assessed potential effect modifications by creating a cross-product of the stratifying variable with LAE change patterns in the fully adjusted model.
To assess the possibility of reverse confounding between LAE and MCI reversion, people who can do more leisure activity may have less severe MCI (than others with similar CMMSE scores) and therefore, a better chance of reversion. We included the baseline MMSE score in the multivariate Cox hazard model for the sensitivity analysis.
Analyses were performed by using IBM SPSS v26.0 and R 4.2.1. Statistical tests were two-sided, and the p-values of < 0.05 were considered to indicate statistical significance.
Among 3,544 participants, the mean age was 86.7 years (SD, 9.6 years) at the baseline and 30.1% of participants were male. Over a mean of 3.8 years (SD, 1.7 years) of follow-up, 1,742 participants (49.2%) reverted to normal cognitive function over 6,045 person-years. Table 1 presents the baseline characteristics of the participants by the LAE change patterns. Most participants were in the low–low group (599, 16.9%), and the low–high group had the least participants (115, 3.2%). Participants in the high–high group were more likely to be younger elderly, female, and have lower ADL and IADL scores. Supplementary Table S1 presents the baseline characteristics of the participants by the MCI reverting status.
After adjusting for sex, age, residence, years of schooling, marital status, economic status, living pattern, number of people living with, smoking, alcohol drinking, ADL, IADL, and chronic disease (such as hypertension, diabetes, heart disease, stroke or CVD, cataract, digestive system diseases, arthritis, and Parkinson's disease), the restricted cubic spline model showed a nonlinear relationship between LAE change patterns and MCI reversion (Figure 2 nonlinear test, χ2 = 157.97, Pnon−linearity <0.001).
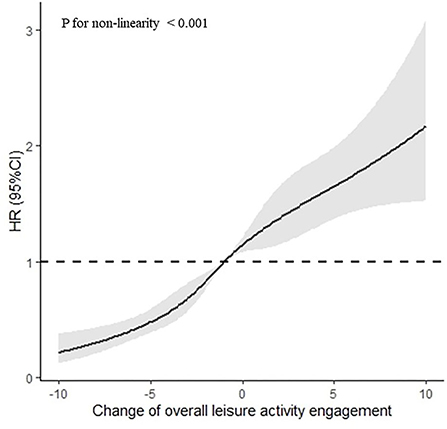
Figure 2. Association between the LAE change points and MCI reversion rate based on restricted cubic spline model. The results derived from the full-adjusted models were presented as hazard ratios with 95% confidential intervals. The model adjusted for sex, age, residence, years of schooling, marital status, economic status, living pattern, number of people living with, smoking, alcohol drinking, activity of daily living (ADL), instrumental activity of daily living (IADL), and chronic disease (such as hypertension, diabetes, heart disease, stroke or CVD, cataract, digestive system diseases, arthritis, and Parkinson's disease).
The high–high group has the highest MCI reversion rate at 84.8%, and the low–low group has the lowest reversion rate at 23.4%. Figure 3 and Supplementary Table S2 show the association between overall LAE change patterns and the reversion rate. Taking participants in the low–low group as a reference, participants in the low–medium, low–high, medium–medium, medium–high, high–medium, and high–high groups had increased possibilities of MCI reversion with HRs (95% CI) of 2.19 (1.57–3.06), 2.97 (2.13–4.13), 2.28 (1.71–3.03), 2.78 (2.10–3.69), 1.93 (1.43–2.59), and 2.74 (2.09–3.60), respectively.
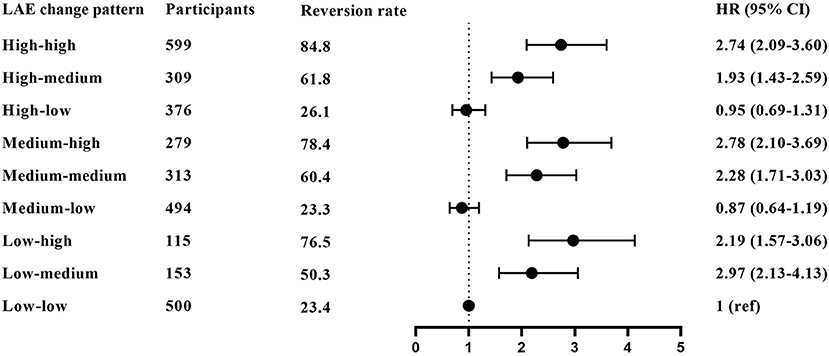
Figure 3. The association between overall LAE change patterns and MCI reversion. The results derived from the full-adjusted models were presented as hazard ratios with 95% confidential intervals. The model adjusted for sex, age, residence, years of schooling, marital status, economic status, living pattern, number of people living with, smoking, alcohol drinking, activity of daily living (ADL), instrumental activity of daily living (IADL), and chronic disease (such as hypertension, diabetes, heart disease, stroke or CVD, cataract, digestive system diseases, arthritis, and Parkinson's disease).
The association among cognitively stimulating, physically active/demanding, and socially engaged LAE change patterns and MCI reversion is also shown in Table 2. The associations between cognitively stimulating LAE change patterns and MCI reversion were similar to the main results, as physically active/demanding LAE change patterns. However, physically active/demanding LAE showed a larger effect on MCI reversion than cognitively stimulating LAE. For social-based LAE change patterns, participants in the medium–medium group and the high–medium group did not have increased possibilities of MCI reversion with HRs (95% CI) of 1.26 (0.75–2.11) and 1.30 (0.94–1.80), respectively.
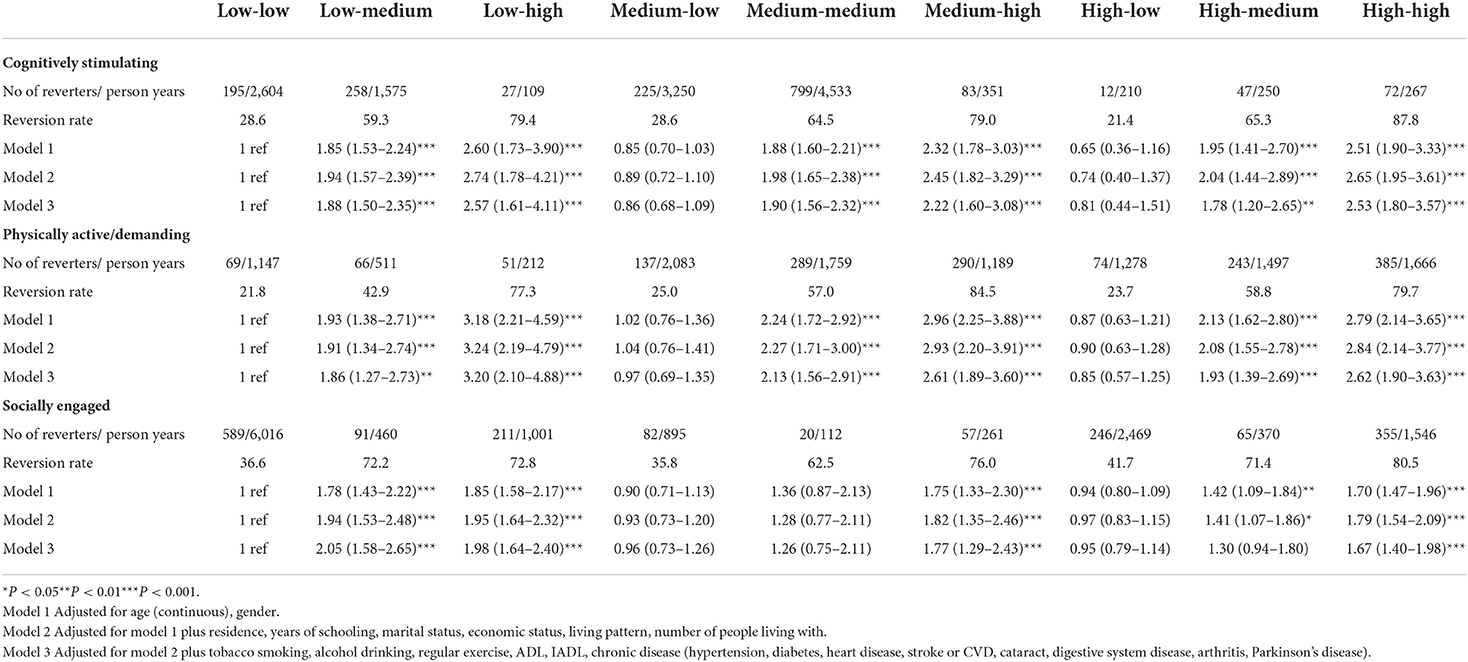
Table 2. The association between cognitively stimulating, physically active/demanding, and socially engaged LAE change patterns and MCI reversion.
In addition, the associations between absolute LAE change patterns and MCI reversion in eight activities are shown in Table 3. For most of the leisure activities, compared with participants in the rarely–rarely group, participants in the rarely–frequently, and frequently–frequently groups had increased possibilities of MCI reversion.
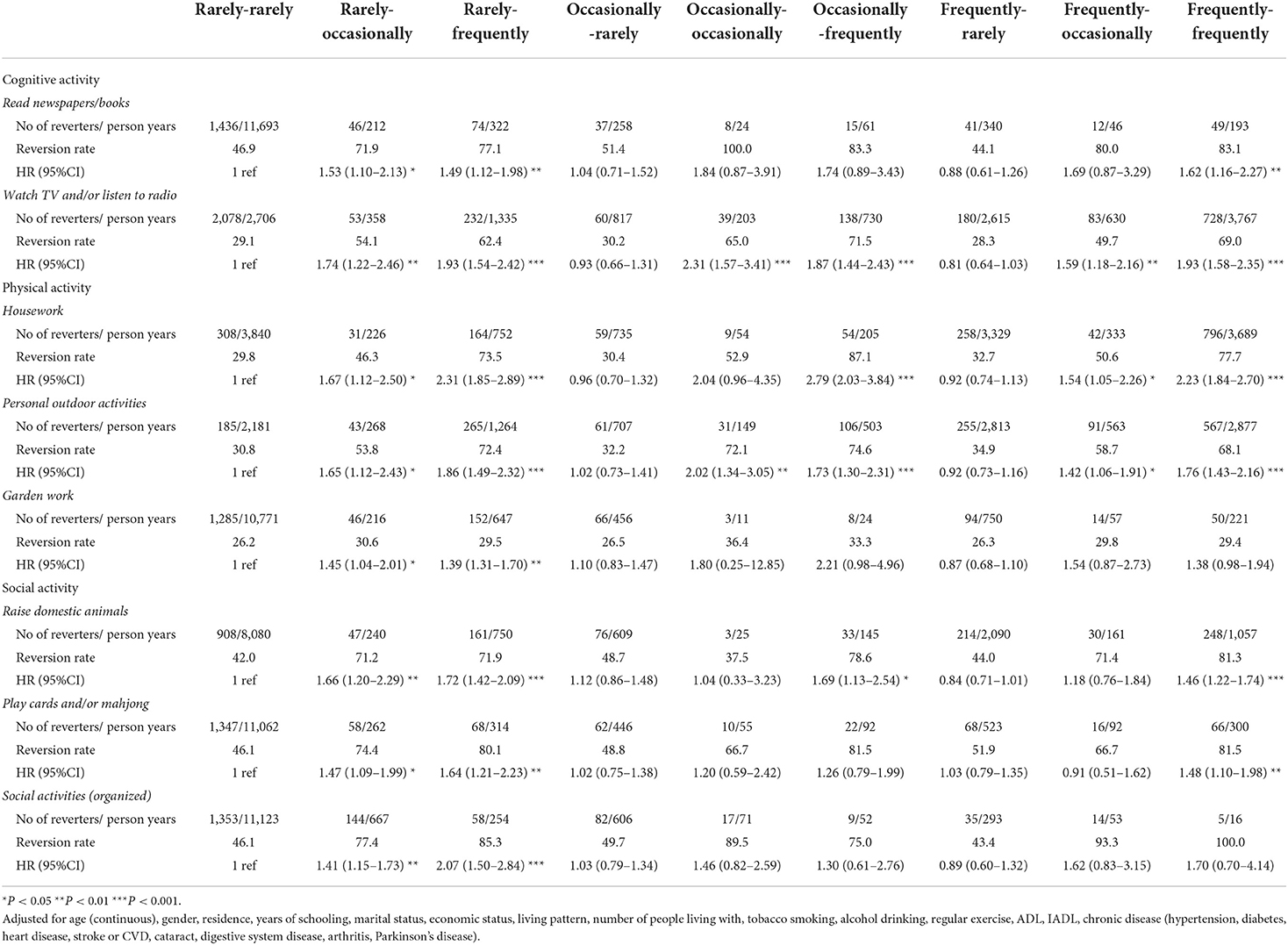
Table 3. The association between absolute LAE change patterns and MCI reversion in eight activities.
In the stratified analysis (Table 4), among participants with unfavorable economic status, compared with those in the low–low group, only participants in the medium–high and high–high groups had increased possibilities with HRs (95% CI) of 2.44 (1.29–4.60) and 2.17 (1.18–3.98), respectively. Among participants living alone or living at nursing homes, the association between LAE change patterns and MCI reversion was insignificant. Among participants who were divorced/widowed/never married, compared with participants in the low–low group, participants in the low–medium, low–high, medium–medium, medium–high, and high–high groups had increased possibilities with HRs (95%CI) of 2.45 (1.65–3.62), 3.30 (2.20–4.93), 2.19 (1.56–3.07), 2.86 (2.04–4.00), 1.88 (1.32–2.70), and 3.01 (2.17–4.17), respectively. In addition, LAE presented a larger effect on MCI reversion among urban residents than rural residents.
When stratified by age (Supplementary Table S3), the associations between overall LAE and MCI reversion were insignificant among the younger elderly. For cognitively stimulating LAE, compared to younger elderly in the low–low group, those in the low–high and high–high groups had increased possibilities of MCI reversion with HRs (95% CI) of 2.61 (1.23–5.55) and 1.96 (1.09–3.52), respectively. In addition, an octogenarian in the medium–low group had a statistically significant lower possibility of MCI reversion with an HR (95% CI) of 0.59 (0.41–0.85). For physically active/demanding LAE, compared with the low–low group, nonagenarians in the medium–medium, medium–high, and high–high groups had increased possibilities of MCI reversion with HRs (95% CI) of 1.71 (1.09–2.70), 2.98 (1.82–4.90), 2.72 (1.55–4.78), respectively. The associations between overall physically active/demanding and MCI reversion were not significant among the younger elderly. For socially engaged LAE, only nonagenarians did not show a statistically significant result in the medium–medium group.
When stratified by sex (Supplementary Table S4), the associations between different types of LAE change patterns and MCI reversion among female participants were similar to the main results. For physically active/demanding LAE, male participants in the low–high, medium–high, and high–high groups have higher possibilities of MCI reversion with HRs (95% CI) of 2.32 (1.22–4.40), 1.95 (1.17–3.24), and 3.28 (1.73–6.23), respectively.
Supplementary Table S5 shows the associations among overall, cognitively stimulating, physically active/demanding, and socially engaged LAE changes patterns and MCI reversion after setting MMSE change≥4 points as MCI reverted status. All of the associations were similar to results when setting MMSE change≥3 points as MCI reverted to normal cognitive function status.
Supplementary Table S6 shows the associations between all variables and MCI reversion after adding the baseline MMSE score as a covariate. All of the associations were similar to the main results. Taking participants in the low–low group as a reference, participants in the low–medium, low–high, medium–medium, medium–high, high–medium, and high–high groups had increased possibilities of MCI reversion with HRs (95% CI) of 2.42 (1.64–3.58), 3.58 (2.49–5.15), 2.80 (2.02–3.89), 3.34 (2.42–4.62), 2.36 (1.68–3.31), and 3.46 (2.54–4.73), respectively. Meanwhile, the baseline MMSE score was not significantly associated with MCI reversion.
In this prospective study, we found that participants who maintained the highest level of LAE had the greatest possibility of MCI reversion. A higher level of LAE maintenance was also associated with the increased possibility of MCI reversion. In contrast, maintaining lower LAE was not associated with MCI reversion among older adults in subsequent years. The results of this study could provide practical instructions to older adults on how dynamic changes in LAE are associated with cognitive ability. These findings highlighted the significance of addressing higher LAE among older adults for promoting MCI reversion in later life.
Taking the general association between LAE and cognitive ability first, the current findings replicated those widely reported in the literature. Individuals participating in more leisure activities generally score higher on cognitive ability tests. However, the association between LAE changes over time and MCI reversion is still unclear. Our study found that participants with MCI who maintained higher LAE had a greater possibility to reverse to normal cognitive function. These findings were in line with the previous study, which suggested that community-dwelling older adults with MCI who continued their multi-domain lifestyle activities (such as cognitive, social, and productive) were more likely to revert to normal cognitive function (31). However, this previous study did not investigate which level of LAE is mostly beneficial for cognitive ability. Our study showed that maintaining the highest level of most types of LAE is positively associated with MCI reversion in various populations, which has important public health implications for older adults.
Results from the subgroup analysis of three sub-types of LAE showed that physically active/ demanding LAE had the most robust association with a higher possibility of MCI reversion compared with cognitively stimulating and socially engaged LAE. Previous studies have shown that physical activities were positively associated with a reduced risk of cognitive decline and dementia (32). For example, in a Swedish cohort, physical activity positively predicted changes in cognitive ability (33). In older adults of the offspring cohort, total physical activity (measured in steps/day) was associated with better executive function (34). In addition, the positive effect of high-intensity exercise on cognitive ability was also reported in some interventional studies (35, 36). Some evidence showed that household physical activity benefits the brain volume, especially the gray matter volume, which might delay the decline in memory function and executive control process among older adults (37). Physical activity, which might more directly contribute to the levels of brain structure and function than cognitive and social activities among older adults with MCI (38, 39), may be able to stop the neuronal decline caused by age and aid in the growth of capillaries in the brain (40).
Meanwhile, our findings also reinforced the arguments that changing to or maintaining a higher level of cognitive-based LAE is beneficial for MCI reversion. Even though previous findings suggested that more frequent cognitive leisure activities were associated with a higher level of cognitive function in later life (41, 42), few reported the association between changes in cognitively stimulating LAE and cognitive ability. Our study found that if a participant changed their frequency of watching TV and/or listening to the radio from “low–low” to “low–high,” the possibilities of MCI reversion of this participant will increase. This goal is achievable for almost all older people since these activities are frequently carried out daily. Intellectual stimulation has been found to strengthen synaptic transmission (i.e., neural plasticity) and increase cognitive reserve (43). Therefore, it might be desirable for older adults with MCI to participate in more cognitive leisure activities later in life.
Another interesting finding was that even though the MCI reversion rates of younger elderly were higher than octogenarians and nonagenarians, the impact of LAE on MCI reversion was more evident among octogenarians and nonagenarians than younger elderly. We have also found that the impact of improvement in cognitively stimulating LAE on MCI reversion was most evident in nonagenarians, who were nonagenarian adults. Some studies have reported that younger age was positively associated with the possibility of MCI reversion (5, 26). However, few studies investigated the moderating effect of age on the relationship between leisure activity and MCI reversion. Bielak et al. found that nonagenarian adults showed a stronger relationship between cognitive-based LAE level and cognitive ability than young–old adults (44). This finding may indicate that there appears to be something unique about how cognitive-based LAE and perceptual speed ability change over time for nonagenarian individuals compared with young–old individuals. Later-life LAE among older adults with MCI may predict later cognitive status, but individuals might respond differently to such effects (45). In addition, older adults with younger age tend to participate in more challenging activities than those in later older years. In fact, the nonagenarian adults are more likely to preoccupy themselves with passive activities, such as watching TV or listening to the radio (46). The results implicated that an age-stratified policy to prevent the decline of cognitive function while promoting lifestyle adjustment is needed in older Chinese adults. For example, simple cognitive leisure activities are encouraged in nonagenarian adults to improve their cognitive functions. However, Ihle et al. suggested that mental leisure activity only supported cognitive ability in young–old individuals (47). Another possible reason for the different relationships between LAE and cognitive ability among older adults in different age groups was that nonagenarians accounted for the largest proportion of participants, which might pose significant results. Age is typically overlooked as a possible moderator in the relationship between LAE and cognitive ability. More studies are necessary to determine how the relationship between LAE and cognitive ability differ according to age.
The leisure activity engagement-cognitive ability relations may also be modified by sex. In our study, the relations were less significant in male participants than in female participants. Compared to maintaining low–low physically active/demanding LAE, only maintaining or changing to a high level of participation could increase the possibility of MCI reversion for male participants. The most recent work considered gender difference was on physical activity. Since activities included in physical-based LAE were mainly about domestic activity, which was usually finished by older female adults under traditional Chinese culture (48). As noted by Biek (49), gender differences in LAE-cognitive ability relations may be partially explained by traditional roles (50). Fellendorf et al. also found that female individuals in the vigorous physical activity group performed significantly higher in most cognitive domains than female individuals with moderate or low physical activity (51). However, this significant difference was not found in male participants. It is important to consider gender-specific intervention in leisure activities to improve cognitive function among older adults.
We also found that compared to older adults with unfavorable economic status, those with favorable economic status were more likely to revert to normal cognitive function when they increased their LAE. In addition, older adults who lived with family members had a higher possibility of MCI reversion than older adults who lived alone or stayed at nursing homes. Meanwhile, LAE presented a larger effect on MCI reversion among urban residents than rural residents. These results showed that older adults of higher socioeconomic status could enjoy extra benefits from leisure activities. A recent study detected a substantial interaction between life course economic status and LAE on cognitive function (52). Even though older adults selected the same activity category, those with higher socioeconomic status might choose specific types that were more cognitively engaging. For example, Di Liegro et al. (53) reported that the brain-derived neurotrophic factor (BDNF), which is positively related to cognition, tends to be elevated more by open-skill exercise (e.g., basketball and badminton) than by closed-skill exercise (e.g., walking and running), probably due to additional attention required when facing ever-changing situations (53). However, open-skill exercise requires specific equipment and facilities, which needs investment. Therefore, older adults with higher socioeconomic status may benefit more from LAE.
Even though this study provides clear implications for the reversion from MCI to normal cognitive function in Chinese older adults, some limitations still exist. In this study, MCI was measured by MMSE and its sensitivity in screening MCI is lower than Montreal Cognitive Assessment (MOCA). However, MMSE still has comparable performance in the detection of MCI with 0.62 sensitivity and 0.87 specificity in the Chinese population (54). As the definition of MCI in this study was a bit different from the diagnostic criteria by Petersen et al. (55), participants without IADL disability at the baseline were used to assess sensitivity. The results were largely similar (Supplementary Table S7). The measure of leisure activities is also limited in its scope. Many activities not included, such as traveling, entertaining, and volunteering (56), and there is no measure of the intensity, duration, or quality of included activities. Meanwhile, the classification may not be perfectly mutually exclusive for some activities that can encompass one or more cognitively stimulating, physically active/demanding, and socially engaged domains. For example, playing Mahjong may fall into overlapping classes of cognitively stimulating and socially engaging activities, whereas raising domestic animals involves physical and social elements. However, the index of these leisure activities in each activity dimension could not be calculated without measuring the intensity, duration, and quality. Further studies are needed to investigate LAE in a more detailed way to avoid the underestimation or overestimation of effects (57).
In conclusion, maintaining or improving to a higher overall, cognitively stimulating, physically active/demanding, and socially engaged LAE was associated with a significantly higher MCI reversion rate in subsequent years among older adults. These results provided a practical message to the older adults about how dynamic changes in LAE were associated with improved cognitive function. Since the sample included in this study was mainly extremely aged, more studies between young and older adults were suggested. These findings also emphasized the importance of promoting a higher LAE across old age for preventing dementia in later life.
Publicly available datasets from The Chinese Longitudinal Healthy Longevity Survey (CLHLS)-Longitudinal Data repository were used in this study. These are available via, https://opendata.pku.edu.cn/dataset.xhtml?persistentId=doi:10.18170/DVN/WBO7LK. Further queries can be directed to the corresponding author.
The studies involving human participants were reviewed and the CLHLS study was approved by the Institutional Review Board of Duke University (Pro00062871) and the Biomedical Ethics Committee of Peking University (IRB00001052-13074). The patients/participants provided their written informed consent to participate in this study.
QT contributed to the study design, interpretation of the findings, and revision of the manuscript. XX contributed to data analysis, interpretation of the findings, and drafting of the manuscript. SW contributed interpretation of the findings and revision of the manuscript. LN contributed to data cleaning and revision of the manuscript. IL contributed to data analysis and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (Grant nos. 72204075 and 72204228), Hebei Provincial Postdoctoral Science Foundation (Grant no. B2022003032), Postdoctoral Research Funding of Hebei Medical University, and Hebei Province Social Science Development Research Project (Grant no. 20220202303).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1035762/full#supplementary-material
1. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. (2006) 367:1262–70. doi: 10.1016/S0140-6736(06)68542-5
2. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
3. Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. (2020) 73:1157–66. doi: 10.3233/JAD-191092
4. Shimada H, Doi T, Lee S, Makizako H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: a 4-year longitudinal study. Alzheimers Res Therapy. (2019) 11:24. doi: 10.1186/s13195-019-0480-5
5. Xue H, Hou P, Li Y. Mao Xe, Wu L, Liu Y. Factors for predicting reversion from mild cognitive impairment to normal cognition: a meta-analysis. Int J Geriatr Psychiatry. (2019) 34:1361–8. doi: 10.1002/gps.5159
6. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PLoS ONE. (2013) 8:e59649. doi: 10.1371/journal.pone.0059649
7. Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of reversion from mild cognitive impairment to normal cognition. Dement Geriatr Cogn Disord. (2017) 43:204–14. doi: 10.1159/000456070
8. Fancourt D, Aughterson H, Finn S, Walker E, Steptoe A. How leisure activities affect health: a narrative review and multi-level theoretical framework of mechanisms of action. Lancet Psychiatry. (2021) 8:329–39. doi: 10.1016/S2215-0366(20)30384-9
9. Almeida-Meza P, Steptoe A, Cadar D. Is engagement in intellectual and social leisure activities protective against dementia risk? Evidence from the english longitudinal study of ageing. J Alzheimers Dis. (2021) 80:555–65. doi: 10.3233/JAD-200952
10. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. (2004) 3:343–53. doi: 10.1016/S1474-4422(04)00767-7
11. Li W, Sun H, Xu W, Ma W, Yuan X, Wu H, et al. Leisure activity and cognitive function among Chinese old adults: The multiple mediation effect of anxiety and loneliness. J Affect Disord. (2021) 294:137–42. doi: 10.1016/j.jad.2021.07.051
12. Zhang W, Feng Q, Fong JH, Chen H. Leisure participation and cognitive impairment among healthy older adults in China. Res Aging. (2022). doi: 10.1177/01640275221082151. [Epub ahead of print].
13. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. (2017) 377:143–53. doi: 10.1056/NEJMoa1613502
14. Yi Z. Introduction to the Chinese Longitudinal Healthy Longevity Survey (CLHLS). In:Yi Z, Poston DL, Vlosky DA, Gu D, , editors. Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions. Dordrecht: Springer Netherlands. (2008). p. 23–38.
15. Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. (2017) 389:1619–29. doi: 10.1016/S0140-6736(17)30548-2
16. Fernández-Mayoralas G, Rojo-Pérez F, Martínez-Martín P, Prieto-Flores ME, Rodríguez-Blázquez C, Martín-García S, et al. Active ageing and quality of life: factors associated with participation in leisure activities among institutionalized older adults, with and without dementia. Aging Ment Health. (2015) 19:1031–41. doi: 10.1080/13607863.2014.996734
17. Zhang W, Chen H, Feng Q. Education and psychological distress of older Chinese: Exploring the longitudinal relationship and its subgroup variations. J Aging Health. (2015) 27:1170–98. doi: 10.1177/0898264315577589
18. Zhang Y, Fu S, Ding D, Lutz MW, Zeng Y, Yao Y. Leisure activities, APOE ε4, and cognitive decline: a longitudinal cohort study. Front Aging Neurosci. (2021) 13:594. doi: 10.3389/fnagi.2021.736201
19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
20. Jia X, Wang Z, Huang F, Su C, Du W, Jiang H, et al. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. (2021) 21:485. doi: 10.1186/s12888-021-03495-6
21. Yi Z, Vaupel JW. Functional capacity and self–evaluation of health and life of oldest old in China. J Soc Issues. (2002) 58:733–48. doi: 10.1111/1540-4560.00287
22. Zhang Z, Gu D, Hayward MD. Early life influences on cognitive impairment among oldest old Chinese. J Gerontol Ser B. (2008) 63:S25–33. doi: 10.1093/geronb/63.1.S25
23. Xu H, Dupre ME, Gu D, Wu B. The impact of residential status on cognitive decline among older adults in China: results from a longitudinal study. BMC Geriatr. (2017) 17:107. doi: 10.1186/s12877-017-0501-9
24. Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol Ser B. (1997) 52B(Special_Issue):37–48. doi: 10.1093/geronb/52B.Special_Issue.37
25. An R, Liu GG. Cognitive impairment and mortality among the oldest-old Chinese. Int J Geriatr Psychiatry. (2016) 31:1345–53. doi: 10.1002/gps.4442
26. Sha F, Zhao Z, Wei C, Li B. Modifiable factors associated with reversion from mild cognitive impairment to cognitively normal status: a prospective cohort study. J Alzheimers Dis. (2022) 86:1897–906. doi: 10.3233/JAD-215677
27. Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. (2007) 78:1298. doi: 10.1136/jnnp.2006.109074
28. Gu D, Brown BL, Qiu L. Self-perceived uselessness is associated with lower likelihood of successful aging among older adults in China. BMC Geriatr. (2016) 16:172. doi: 10.1186/s12877-016-0348-5
29. Malloy EJ, Spiegelman D, Eisen EA. Comparing measures of model selection for penalized splines in Cox models. Comput Stat Data Anal. (2009) 53:2605–16. doi: 10.1016/j.csda.2008.12.008
30. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. doi: 10.1002/sim.4780080504
31. Katayama O, Lee S, Bae S, Makino K, Shinkai Y, Chiba I, et al. Lifestyle changes and outcomes of older adults with mild cognitive impairment: a 4-year longitudinal study. Arch Gerontol Geriatr. (2021) 94:104376. doi: 10.1016/j.archger.2021.104376
32. Wang S, Bressington DT, Leung AYM, Davidson PM, Cheung DSK. The effects of bibliotherapy on the mental well-being of informal caregivers of people with neurocognitive disorder: a systematic review and meta-analysis. Int J Nurs Stud. (2020) 109:103643. doi: 10.1016/j.ijnurstu.2020.103643
33. Stenling A, Sörman DE, Lindwall M, Hansson P, Körning Ljungberg J, Machado L. Physical activity and cognitive function: between-person and within-person associations and moderators. Aging Neuropsychol Cogn. (2021) 28:392–417. doi: 10.1080/13825585.2020.1779646
34. Spartano NL, Demissie S, Himali JJ, Dukes KA, Murabito JM, Vasan RS, et al. Accelerometer-determined physical activity and cognitive function in middle-aged and older adults from two generations of the Framingham Heart Study. Alzheimers Dement Transl Res Clin Interv. (2019) 5:618–26. doi: 10.1016/j.trci.2019.08.007
35. Brown BM, Frost N, Rainey-Smith SR, Doecke J, Markovic S, Gordon N, et al. High-intensity exercise and cognitive function in cognitively normal older adults: a pilot randomised clinical trial. Alzheimers Res Therapy. (2021) 13:33. doi: 10.1186/s13195-021-00774-y
36. Espeland MA, Lipska K, Miller ME, Rushing J, Cohen RA, Verghese J, et al. Effects of physical activity intervention on physical and cognitive function in sedentary adults With and without diabetes. J Gerontol Ser A. (2017) 72:861–6. doi: 10.1093/gerona/glw179
37. Koblinsky ND, Meusel L-AC, Greenwood CE, Anderson ND. Household physical activity is positively associated with gray matter volume in older adults. BMC Geriatr. (2021) 21:104. doi: 10.1186/s12877-021-02054-8
38. Domingos C, Pêgo JM, Santos NC. Effects of physical activity on brain function and structure in older adults: a systematic review. Behav Brain Res. (2021) 402:113061. doi: 10.1016/j.bbr.2020.113061
39. Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements – a systematic review. Eur Rev Aging Phys Act. (2019) 16:10. doi: 10.1186/s11556-019-0217-2
40. Srinivas NS, Vimalan V, Padmanabhan P, Gulyás B. An overview on cognitive function enhancement through physical exercises. Brain Sci. (2021) 11:1289. doi: 10.3390/brainsci11101289
41. Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, Kennison RF, et al. Cognitively stimulating activities: effects on cognition across four studies with up to 21 years of longitudinal data. J Aging Res. (2012) 2012:461592. doi: 10.1155/2012/461592
42. Iizuka A, Suzuki H, Ogawa S, Kobayashi-Cuya KE, Kobayashi M, Takebayashi T, et al. Can cognitive leisure activity prevent cognitive decline in older adults? A systematic review of intervention studies. Geriatr Gerontol Int. (2019) 19:469–82. doi: 10.1111/ggi.13671
43. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. (2006) 36:441–54. doi: 10.1017/S0033291705006264
44. Bielak AAM, Gerstorf D, Anstey KJ, Luszcz MA. Longitudinal associations between activity and cognition vary by age, activity type, and cognitive domain. Psychol Aging. (2014) 29:863–72. doi: 10.1037/a0036960
45. Bielak AAM, Gow AJ. A decade later on how to “use it” so we don't “lose it”: An update on the unanswered questions about the influence of activity participation on cognitive performance in older age. Gerontology. (2022). doi: 10.1159/000524666. [Epub ahead of print].
46. Paillard-Borg S, Wang HX, Winblad B, Fratiglioni L. Pattern of participation in leisure activities among older people in relation to their health conditions and contextual factors: a survey in a Swedish urban area. Ageing Soc. (2009) 29:803–21. doi: 10.1017/S0144686X08008337
47. Ihle A, Fagot D, Vallet F, Ballhausen N, Mella N, Baeriswyl M, et al. Cross-lagged relation of leisure activity participation to Trail Making Test performance 6 years later: Differential patterns in old age and very old age. Neuropsychology. (2019) 33:234–44. doi: 10.1037/neu0000497
48. Xu XY, Kwan RYC, Miao J, Chai A, Leung AYM. Telephone-based Behavioral Activation for improving sleep quality in family caregivers of people with dementia: a pilot randomized controlled trial. Behav Ther. (2022) 53:887–99. doi: 10.1016/j.beth.2022.02.007
49. Bielak AAM. How can we not 'lose it' if we still don't understand how to 'use it'? Unanswered questions about the influence of activity participation on cognitive performance in older age–a mini-review. Gerontology. (2010) 56:507–19. doi: 10.1159/000264918
50. Hassing LB. Gender differences in the association between leisure activity in adulthood and cognitive function in old age: a prospective longitudinal population-based study. J Gerontol Ser B. (2020) 75:11–20. doi: 10.1093/geronb/gbx170
51. Fellendorf FT, Kainzbauer N, Platzer M, Dalkner N, Bengesser SA, Birner A, et al. Gender differences in the association between physical activity and cognitive function in individuals with bipolar disorder. J Affect Disord. (2017) 221:232–7. doi: 10.1016/j.jad.2017.06.048
52. Sun R, Zhang Z. Leisure activities and cognitive impairment in old age: the role of life course socioeconomic status. Aging Ment Health. (2022). doi: 10.1080/13607863.2022.2046694. [Epub ahead of print].
53. Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical Activity and Brain Health. Genes. (2019) 10:720. doi: 10.3390/genes10090720
54. Tsoi KKF, Chan JYC, Hirai HW, Wong SYS, Kwok TCY. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. (2015) 175:1450–8. doi: 10.1001/jamainternmed.2015.2152
55. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. (2014) 275:214–28. doi: 10.1111/joim.12190
56. Thomas PA. Trajectories of social engagement and limitations in late life. J Health Soc Behav. (2011) 52:430–43. doi: 10.1177/0022146511411922
Keywords: leisure activity, mild cognitive impairment, reversion, older adults, longitudinal study
Citation: Xu XY, Wang SS, Niu L, Leung ISH and Tian QB (2022) Association of leisure activity changes and reversion from mild cognitive impairment to normal cognitive function among older adults: A prospective cohort study. Front. Public Health 10:1035762. doi: 10.3389/fpubh.2022.1035762
Received: 03 September 2022; Accepted: 31 October 2022;
Published: 22 November 2022.
Edited by:
Xuan Li, University of Mississippi Medical Center, United StatesReviewed by:
Bingyu Li, Shenzhen University, ChinaCopyright © 2022 Xu, Wang, Niu, Leung and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Bao Tian, dHFiMTk4MEBoZWJtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.