- 1Department of Emergency Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Department of Psychiatry, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 3Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 4Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 6Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 7Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 8Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 9Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung, Taiwan
Introduction: We aim to explore the association between chronic kidney disease (CKD) and cataracts.
Methods: A total of 121,380 participants with adequate information collected from 29 community-based recruitment centers since 2008 were analyzed. The association between CKD and self-reported diagnosed cataracts was examined in a cross-sectional cohort and was validated in a longitudinal cohort of 25,263 participants without cataracts at baseline.
Results and discussion: Of all participants, cataracts occurred in 503/1,947 (26%) and 10,464/119,433 (9%) subjects in the CKD and non-CKD groups, respectively. Multivariate logistic regression showed that CKD was significantly associated with a higher prevalence of self-reported diagnosed cataracts. In the validation cohort, a higher incidence of cataracts was also noted in the CKD group (65/317, 21%) compared to the non-CKD group (1,964/24,252, 8%) during a mean 47-month follow-up. After adjusting for confounders, subjects with CKD had a 1.498-fold higher risk of incident cataracts than those without CKD (95% confidence interval = 1.114 to 2.013, p value = 0.007). We found that CKD was associated with a higher prevalence of cataracts as well as incident cataracts, which suggests CKD patients and their primary physicians should be aware of this disease and can provide a clue for further exploration of the possible mechanisms and treatments.
Introduction
Cataracts, or opacification of the lens, are a significant cause of blindness especially in middle to low-income countries. It is estimated that over 15.2 million people are blind and over 78.8 million people are moderate to severe vision impaired secondary to cataracts worldwide (1). Despite advances in cataract surgery, cataracts continue to be a public health and financial burden due to the extended life expectancy and growing population. In order to identify the reversible risk factors of cataracts, it is important to halt the disease's development.
Chronic kidney disease (CKD), defined as decreased kidney function or presence of kidney damage for 3 or more months, is associated with several diseases, including hypertension (2), diabetes mellitus (DM) (3), and cerebral vascular disease (4). Moreover, CKD shares common pathogenetic mechanisms with various eye diseases, such as microvascular dysfunction, epithelial dysfunction, oxidative stress an inflammation (5). Recent studies have examined the association between CKD and cataracts (6, 7), but lack evidence of large-scale cross-sectional and longitudinal cohort studies. Here, we present major evidence for the association between CKD and cataracts.
Materials and methods
Data source and study population
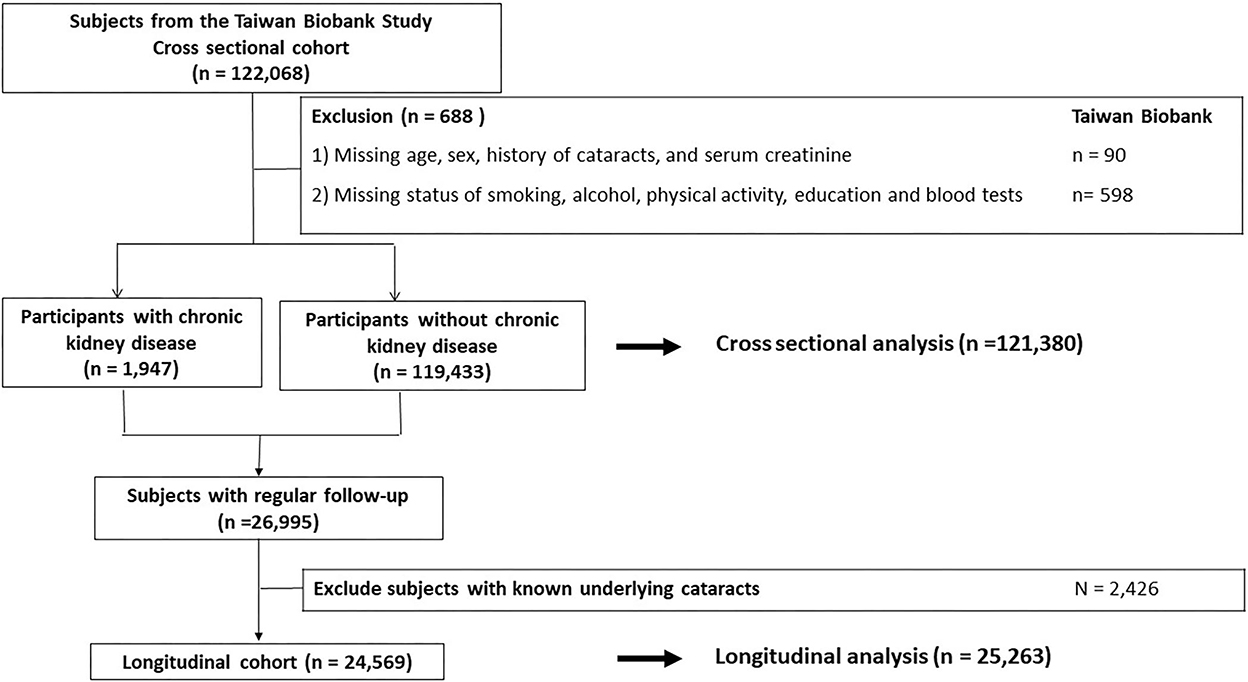
All participants were enrolled at 29 community-based recruitment centers in Taiwan since 2008. They were between 30 and 70 years old and had no history of malignancies when joining the cohort. Other detailed information has been described previously (8–10). In the cross-sectional cohort, a total of 121,380 participants with adequate information about age, sex, serum creatinine and history of cataracts, and possible confounders were analyzed (Figure 1). Then, a longitudinal cohort enrolled 25,263 participants without cataracts at baseline and with adequate long-term follow-up was used to validate the results of the cross-sectional analysis (Figure 1). The investigations followed the Declaration of Helsinki and participants signed a consent form. This study was approved by our institute (KMUHIRB-E(I)-20210058).
Definition of CKD
In the present study, CKD is defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. Based on eGFR, we divided participants into CKD and non-CKD groups. Equation used to calculate eGFR (ml/min/1.73 m2) is [(140–age) × body weight / (0.814 × Serum creatinine in μmol/L)] × (0.85 if female).
Self-reported diagnosed cataracts
Firstly, participants were asked, “Have you ever been diagnosed with cataracts?” Self-reported diagnosed cataracts were defined as participants answering “Yes” to this question. For those that have a past history of cataracts, they would be asked “Which eye was diagnosed with cataracts?” and “When were you diagnosed with cataracts?” Researchers would repeat these questions to ensure consistent responses.
Variables and variables selection
All the variables in this study came from questionnaires, physical examination, and blood draws. The potential risk factors for the association between CKD and cataracts were identified through literature reviews, including age (11, 12), gender (13), body mass index (14), smoking status, alcohol status (15), education status, hypertension (including systolic and diastolic pressures) (16), diabetes mellitus (including fasting glucose), dyslipidemia (including total cholesterol and triglyceride), hemoglobin (17), albumin (18) and uric acid (19). To further identify the possible confounders, these variables were further investigated for their association with cataracts through univariate logistic regression, and then put into multivariate analysis if they were significant.
Statistical analyses
In the present study, participants were classified as CKD and non-CKD groups. Their clinical characteristics were described as percentages for qualitative variables and mean ± standard deviation for quantitative variables. The differences between groups were calculated by using Pearson χ2 test or independent t-test, depending on the types of variables. To determine the association between the presence of CKD and cataracts, univariate and multivariate logistic regression analyses were used with odds ratios (ORs) and 95% confidence intervals (CIs). SPSS 20.0 (?IBM Corp, Armonk, NY, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Wien, Austria) were two statistical tools we used and a p-value of <0.05 was regarded as statistically significant.
Results
Clinical characteristics of the study participants in the cross sectional cohort
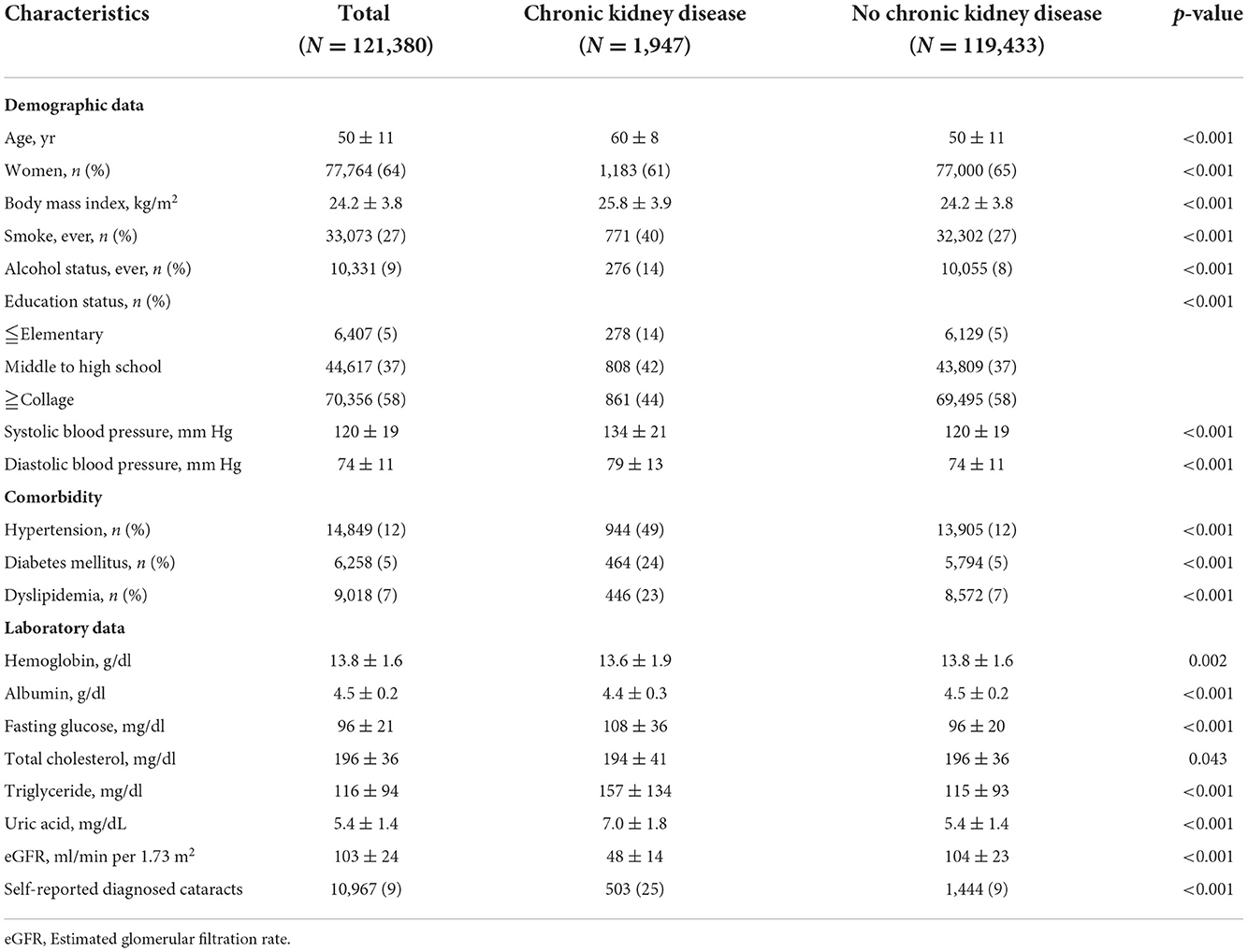
The clinical characteristics for all participants, those with CKD and those without CKD are shown in Table 1. Of the 121,380 participants, 77,764 (64%) were female, and 1,947 (2%) and 119,433 (98%) were classified as CKD and non-CKD groups, respectively. The subjects with CKD were more likely to be male gender, older, had higher body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP), and a higher prevalence of smoking, drinking, past history of hypertension, past history of DM, and past history of dyslipidemia compared to the men without CKD (Table 1). In addition, self-reported diagnosed cataracts occurred in 503/1,947 (26%) and 10,464/119,433 (9%) subjects in the CKD and non-CKD groups, respectively (Table 1).
The association between CKD and prevalence of cataracts
To examine the association between CKD and self-reported diagnosed cataracts, logistic regression analyses were used. Univariate logistic regression showed that age, sex, BMI, smoking, drinking, educational status, SBP, DBP, past history of hypertension, past history of DM, past history of dyslipidemia, serum hemoglobin, albumin, fasting glucose, total cholesterol, triglyceride, and CKD were significantly associated with self-reported diagnosed cataracts (Table 2). After adjustment for confounders, age, sex, BMI, smoking, DBP, past history of hypertension, past history of DM, past history of dyslipidemia, serum albumin and CKD were still significantly related to cataracts (Table 2). Compared with those in the non-CKD group, the odds of cataracts in the CKD group were 3.627 (95% CI = 3.271–4.023, p-value = <0.001) and 1.335 (95% CI = 1.189–1.500, p-value = <0.001) in univariate and multivariate analyses, respectively. We further divided participants by age (<60 years old and ≧60 years old) and the results remained the same (Supplementary Table 1).
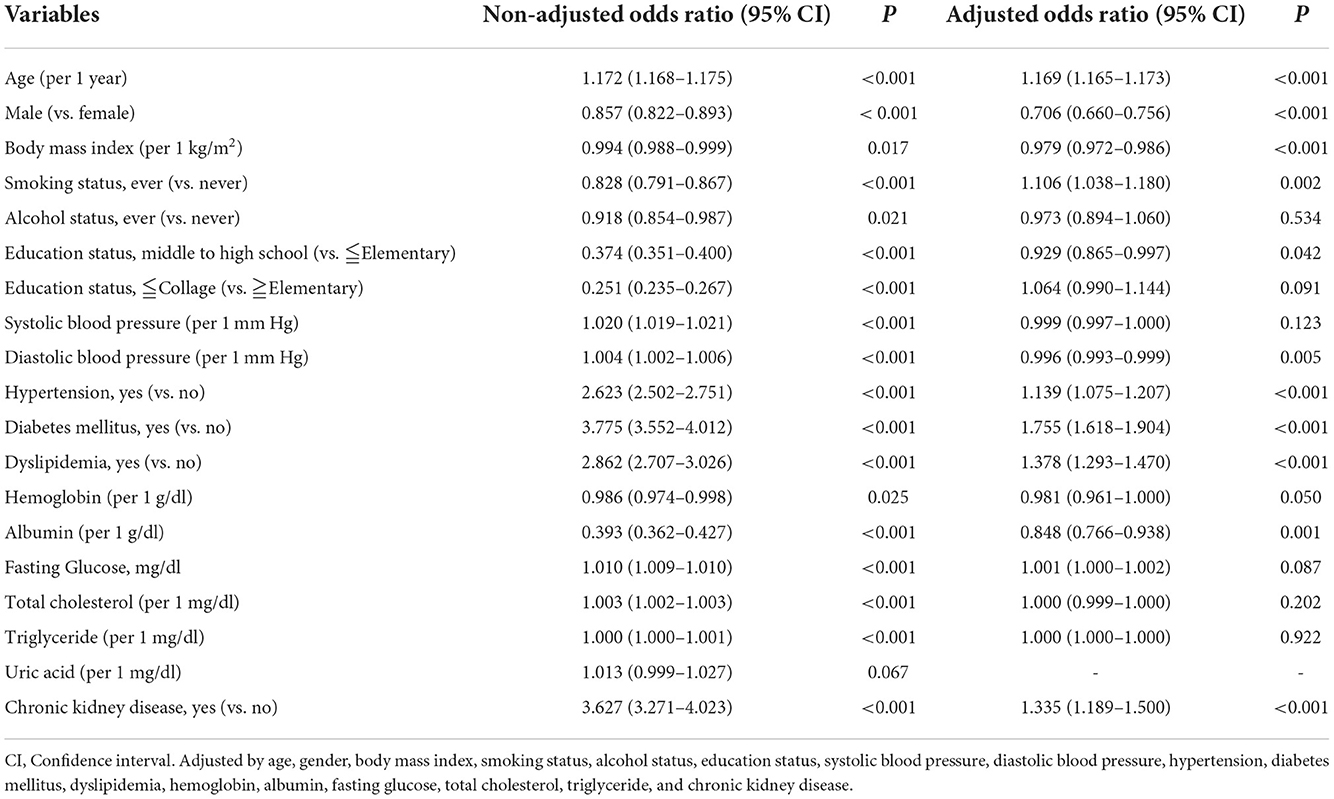
Table 2. Odds ratios for self-reported diagnosed cataracts in univariable and multivariable binary logistic analysis in the cross-sectional cohort (n = 121,380).
The association between CKD and the development of cataracts
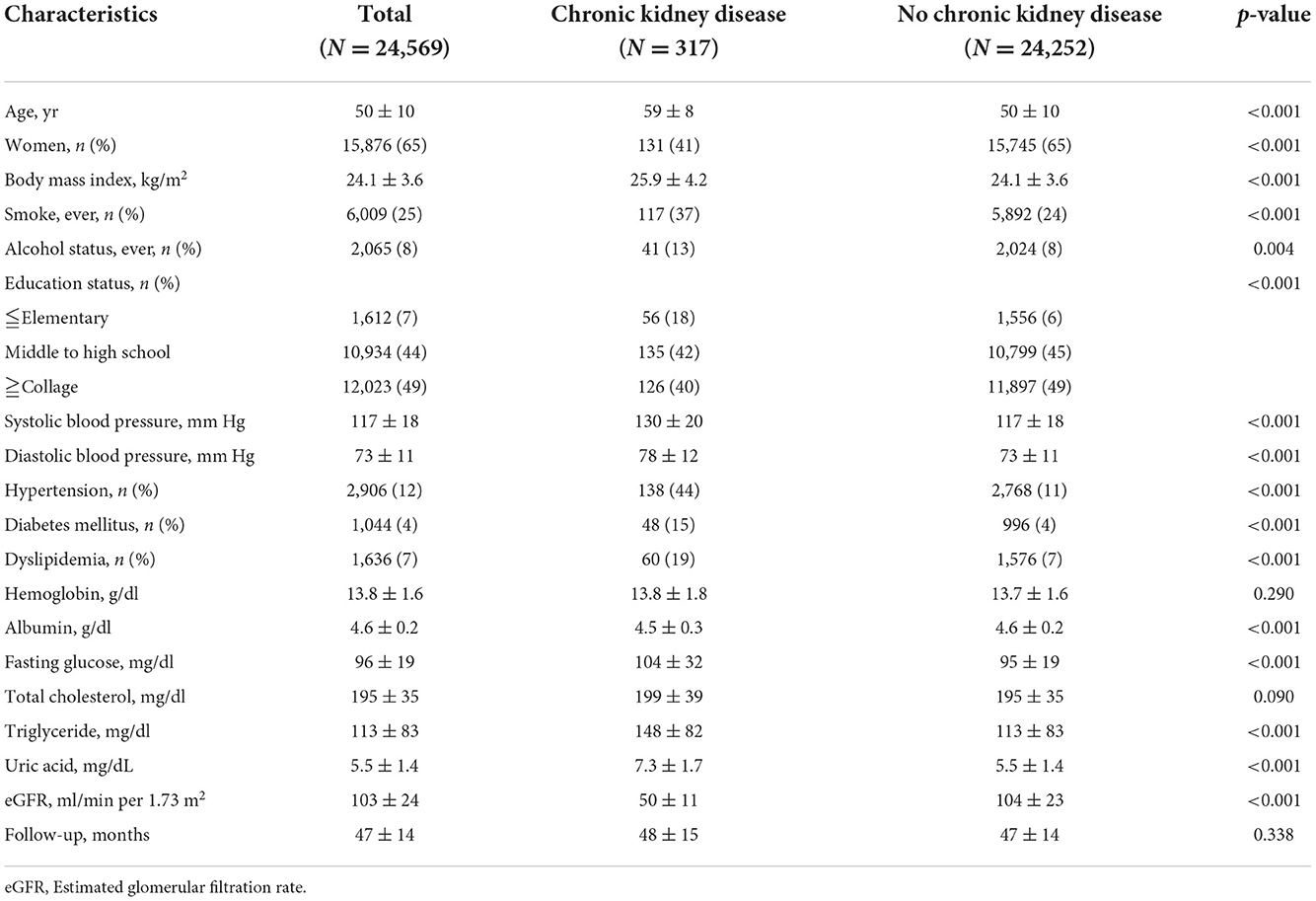
To further validate the association between CKD and cataracts, the risk of CKD for developing cataracts was evaluated in a longitudinal subgroup of 24,569 participants who had no history of cataracts at baseline (Table 3). In this cohort, 15,876 (65%) were female and incident cataracts occurred in 2,029 (8%) participants during a mean 47-month follow-up. The subjects with CKD were more likely to be male gender, older, had higher BMI, SBP, and DBP, and a higher prevalence of smoking, drinking, past history of hypertension, past history of DM, and past history of dyslipidemia compared to the men without CKD (Table 3). A higher incidence of cataracts was noted in the CKD group (65/317, 21%) compared to the non-CKD group (1,964/24,252, 8%). Univariate logistic regression showed that age, sex, smoking, drinking, SBP, past history of hypertension, past history of DM, past history of dyslipidemia, serum albumin, fasting glucose, total cholesterol, triglyceride, and CKD were significantly associated with incident cataracts (Table 4). In multivariate analysis, the risk of developing cataracts was still higher in participants with older age, past history of DM, past history of dyslipidemia, and CKD (Table 4). Compared with those in the non-CKD group, the odds of cataracts in the CKD group were 2.927 (95% CI = 2.220–3.860, p-value = <0.001) and 1.498 (1.114–2.013, p-value = 0.007) in univariate and multivariate analyses, respectively. We further divided participants by age (<60 years old and >60 years old) and the results for those ≧60 years old were similar to the entire cohort; however, for those <60 years old, the relationship between CKD and cataracts was not statistically significant (Supplementary Table 2).
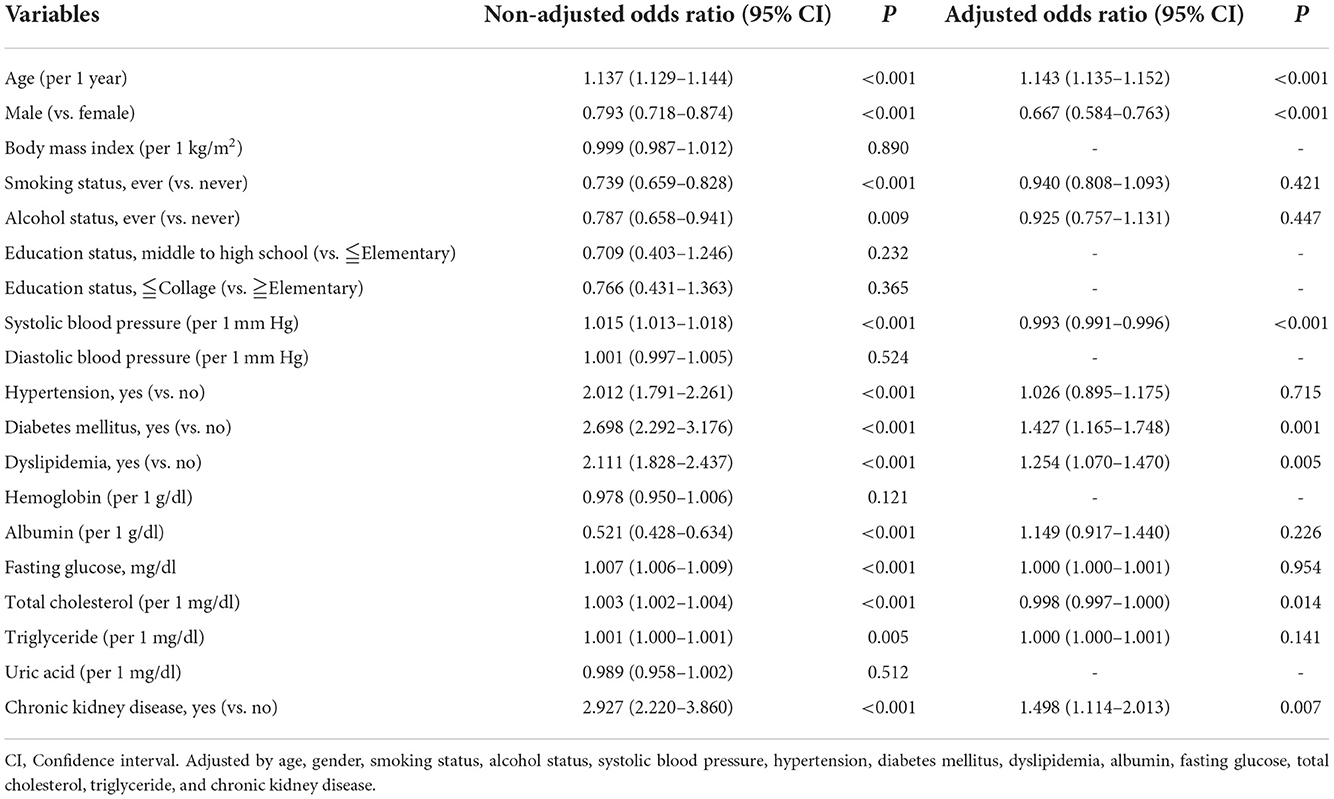
Table 4. Odds ratios for self-reported diagnosed cataracts in univariable and multivariable binary logistic analysis in the longitudinal cohort (n = 24,569).
Discussion
In this study, we found that the risk of cataracts was higher in CKD patients (OR = 1.335; 95% CI, 1.189–1.500) in the cross-sectional cohort after adjusting for confounders. Furthermore, we examined 24,569 participants who had mean follow up periods of 47-months from 29 community-based recruitment centers without a history of cataracts at baseline and found that the incidence of developing cataracts (OR = 1.498; 95% CI, 1.114–2.013) is significantly higher in patients with CKD. To the best of our knowledge, this is the first large-scale cross-sectional and longitudinal study to investigate the relationship between CKD and risk of cataracts.
There were several previous cohort studies evaluating the association between CKD and cataracts but the results varied. Klein et al. (20) conducted a prospective study over a 5 years period in 1988 and found that renal function abnormalities were not significantly associated with incidence of cataracts after controlling for age and gender. Huynh et al. (21) found that there were no significant effects of CKD stage on the incidence of any type of cataract in agreement with that of Klein et al. However, they reported increased odds of incident cataract surgery in patients with moderate to severe renal dysfunction younger than 60 years of age. The authors attributed this effect to additional factors involving patients undergoing cataract surgery. Klein et al. (22) further investigated the relationship between cystatin C and the 15-year incidence of age-related cataracts in 2008. They concluded with contradictory results that increasing cystatin C levels was associated with an increased risk of age-related cataracts, especially cortical and posterior subcapsular subtypes. In recent years, Liu et al. (7) retrospectively analyzed 1-million subjects from a Taiwanese health insurance database. They found that the risk of cataract was higher in CKD group (adjusted hazard ratio 1.86) than non-CKD group and an even higher risk of cataracts in end stage renal disease group (adjusted hazard ratio 2.33), which indicated more severe renal dysfunction increased the risk of cataracts. However, the accuracy of the diagnosis of CKD and cataracts recorded by the International Classification of Disease Codes (ICD) has been questioned. The study performed by Rim et al. (23) also concluded that end stage renal disease patients who started hemodialysis were more likely to undergo cataract surgery. The results of our study were consistent with Liu et al. but constructed on more solid evidence by combining cross-sectional and longitudinal cohorts. In addition, patients diagnosed with CKD were based on eGFR in our study compared to Liu et al. which used ICDs.
Age-related cataracts comprised the vast majority of cataracts, but the risk factors were different from early-onset cataracts. Some known risk factors for early-onset cataracts included trauma, ultraviolet-B exposure, steroid use, diabetes mellitus and myotonic dystrophy, but not CKD (24–26). In our longitudinal cohort, we divided participants by age (<60 years old and ≧60 years old) and found that the relationship between CKD and cataracts was not statistically significant in those <60 years old, supporting the previous results.
In addition to CKD, we found that diabetes mellitus and dyslipidemia were associated with an increasing risk of cataracts. Our investigation was similar to the results of Liu et al. (7) in which diabetes and hyperlipidemia were independent risk factors associated with cataracts but contradictory results of alcohol use. Moreover, several previous studies have been performed which verified the association between diabetes and cataracts (27–31), and dyslipidemia and cataracts (32–34).
Proven by Liu et al. (7) the risk for cataracts increased with the severity of renal impairment. The results indicated that prevention of CKD progression and early eye screening programs might decrease the incidence of cataracts. As for diabetes, previous studies showed better glycaemic control was associated with a lower incidence of cataracts (34–36). A meta-analysis concluded that statin therapy could protect against cataract formation (37). Our study further validated the association between cataracts and CKD, diabetes mellitus and dyslipidemia which provided physicians perspectives on early screening and prevention of cataracts in these populations.
Several mechanisms were proposed to contribute to the relationship between CKD and cataracts. Urea sequestration in the lens with subsequent water accumulation and formation of osmotic cataracts was one of the possible mechanisms (38). Another possible mechanism was oxidative stress in CKD patients forming advanced glycation end products (AGEs) and leading to cataract formation (39, 40). AGEs acting on endothelial cells could lead to apoptosis, cell cycle arrest and generation of proinflammatory cytokines. These factors cause insolubility of lens proteins, accumulation of fluorescent products in the lens nucleus and the lens eventually losing its transparency.
This is the first study combining cross-sectional and longitudinal cohorts to analyze the association between CKD and risk of cataracts. Additionally, the diagnosis of CKD was based on eGFR, not by coding. Despite these strengths, the present study has several limitations. Firstly, data on cataracts were obtained from questionnaires, thus the diagnosis of cataracts lacked objective comprehensive ophthalmic examinations, and we did not have patient data for cataract subtypes and grades, nor for surgical modality and surgery-related complications; however, this substitution has been validated in other large-scale studies (41–43). Secondly, the database did not include other risk factors of cataracts, such as myopia, eye trauma, steroid use, ultraviolet light exposure and uveitis but recruited as many confounders as possible to strengthen the results. Thirdly, this study only involved Asians, so the results might not be representative of other ethnicities. Fourthly, we were unable to analyze the association between severity of CKD and cataracts because we had fewer than 100 subjects with end-stage renal disease. Fifthly, a rigorous definition of CKD should include follow-up eGFR data over 3-months and evidence of proteinuria, as opposed to a single eGFR at the time of admission. However, this substitution has been validated in other large-scale studies (44, 45). Finally, information regarding genetic testing was lacking, but most of the subjects in this study did not have congenital cataracts (46).
Conclusion
We found that CKD was associated with a higher prevalence of cataracts as well as incident cataracts, which suggests CKD patients and their primary physicians should be aware of this disease and can provide a clue for further exploration of the possible mechanisms and treatments.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data underlying this study are from the Taiwan Biobank. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to S-CC (c2NhcmNoZW5vbmVAeWFob28uY29tLnR3), Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, methodology, software, data curation, writing—review and editing, visualization, and project administration: J-HG. Formal analysis: J-IL and J-HG. Investigation: C-WC and Y-HL. Validation, resources, supervision, and funding acquisition: S-PH and S-CC. Writing–original draft preparation: C-YH. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported partially by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC111A01-1 and KMUTC111IFSP01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1029962/full#supplementary-material
References
1. GBD 2019 Blindness and Vision Impairment Collaborators Vision Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. (2021) 9:e144–60. doi: 10.1016/S2214-109X(20)30489-7
2. Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. (2011) 2011:132405. doi: 10.4061/2011/132405
3. Allison SJ. Urea inhibits insulin secretion in Ckd. Nat Rev Nephrol. (2016) 12:581. doi: 10.1038/nrneph.2016.131
4. Lau WL, Huisa BN, Fisher M. The cerebrovascular-chronic kidney disease connection: perspectives and mechanisms. Transl Stroke Res. (2017) 8:67–76. doi: 10.1007/s12975-016-0499-x
5. Wong CW, Wong TY, Cheng CY, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. (2014) 85:1290–302. doi: 10.1038/ki.2013.491
6. Wong CW, Lamoureux EL, Cheng CY, Cheung GC, Tai ES, Wong TY, et al. Increased burden of vision impairment and eye diseases in persons with chronic kidney disease - a population-based study. EBioMedicine. (2016) 5:193–7. doi: 10.1016/j.ebiom.2016.01.023
7. Liu YT, Hung TY, Lee YK, Huang MY, Hsu CY, Su YC. Association between chronic kidney disease and risk of cataract: a nationwide retrospective cohort study. Am J Nephrol. (2017) 45:524–31. doi: 10.1159/000475555
8. Lee MR, Ke HL, Huang JC, Huang SP, Geng JH. Obesity-related indices and its association with kidney stone disease: a cross-sectional and longitudinal cohort study. Urolithiasis. (2022) 50:55–63. doi: 10.1007/s00240-021-01288-w
9. Chen CH, Lee JI, Jhan JH, Lee YC, Geng JH, Chen SC, et al. Secondhand smoke increases the risk of developing kidney stone disease. Sci Rep. (2021) 11:17694. doi: 10.1038/s41598-021-97254-y
10. Chang CW, Ke HL, Lee JI, Lee YC, Jhan JH, Wang HS, et al. Metabolic syndrome increases the risk of kidney stone disease: a cross-sectional and longitudinal cohort study. J Pers Med. (2021) 11:1154. doi: 10.3390/jpm11111154
11. Chang JR, Koo E, Agrón E, Hallak J, Clemons T, Azar D, et al. Risk factors associated with incident cataracts and cataract surgery in the age-related eye disease study (Areds): areds report number 32. Ophthalmology. (2011) 118:2113–9. doi: 10.1016/j.ophtha.2011.03.032
12. Foster PJ, Wong TY, Machin D, Johnson GJ, Seah SK. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the chinese population of singapore: the tanjong pagar survey. Br J Ophthalmol. (2003) 87:1112–20. doi: 10.1136/bjo.87.9.1112
13. Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of cataract and associated risk factors: the visual impairment project. Arch Ophthalmol. (2006) 124:79–85. doi: 10.1001/archopht.124.1.79
14. Azim SF, Akhter QS, Akter T, Farid F. Obesity and dyslipidemia: risk factors for development of senile cataract. J Bangladesh Soc Physiol. (2018) 13:29–34. doi: 10.3329/jbsp.v13i1.37846
15. Drews RC. Alcohol and cataract. Arch Ophthalmol. (1993) 111:1312. doi: 10.1001/archopht.1993.01090100018002
16. Yu X, Lyu D, Dong X, He J, Yao K. Hypertension and risk of cataract: a meta-analysis. PLoS ONE. (2014) 9:e114012. doi: 10.1371/journal.pone.0114012
17. Alharbi AMD, Alhazmi AMS. Prevalence, risk factors, and patient awareness of diabetic retinopathy in saudi arabia: a review of the literature. Cureus. (2020) 12:e11991. doi: 10.7759/cureus.11991
18. Delcourt C, Dupuy AM, Carriere I, Lacroux A, Cristol JP. Albumin and transthyretin as risk factors for cataract: the pola study. Arch Ophthalmol. (2005) 123:225–32. doi: 10.1001/archopht.123.2.225
19. Sharon Y, Schlesinger N. Beyond joints: a review of ocular abnormalities in gout and hyperuricemia. Curr Rheumatol Rep. (2016) 18:37. doi: 10.1007/s11926-016-0586-8
20. Klein BE, Klein R, Lee KE. Renal function abnormalities and incident cataract after a five-year interval: the beaver dam eye study. Curr Eye Res. (1998) 17:720–5. doi: 10.1080/02713689808951248
21. Huynh SC, Kifley A, Strippoli GF, Mitchell P. Is renal impairment a predictor of the incidence of cataract or cataract surgery? findings from a population-based study. Ophthalmology. (2005) 112:293–300. doi: 10.1016/j.ophtha.2004.09.014
22. Klein BE, Knudtson MD, Brazy P, Lee KE, Klein R. Cystatin C, other markers of kidney disease, and incidence of age-related cataract. Arch Ophthalmol. (2008) 126:1724–30. doi: 10.1001/archophthalmol.2008.502
23. Rim TH, Yoon CY, Park HW, Chung EJ. Association between starting hemodialysis for end-stage renal disease and incident cataract surgery: a 12-year nationwide cohort study. Invest Ophthalmol Vis Sci. (2016) 57:1112–9. doi: 10.1167/iovs.15-18276
24. Praveen MR, Shah GD, Vasavada AR, Mehta PG, Gilbert C, Bhagat G, et al. Study to explore the risk factors for the early onset of cataract in India. Eye. (2010) 24:686–94. doi: 10.1038/eye.2009.137
25. Jyothi R, Sathyan S. Etiopathogenesis of presenile cataracts in central kerala: a cross-sectional observational study. Kerala J Ophthalmol. (2017) 29:179–83. doi: 10.4103/kjo.kjo_102_17
26. Wang W, Shang X, Zhang L, Li L, He M. Risk factors for early-onset cataracts treated surgically in Australian adults. Investig Ophthalmol Vis Sci. (2018) 59:3795.
27. Klein BE, Klein R, Wang Q, Moss SE. Older-Onset diabetes and lens opacities. the beaver dam eye study. Ophthalmic Epidemiol. (1995) 2:49–55. doi: 10.3109/09286589509071451
28. Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the wisconsin epidemiologic study of diabetic retinopathy. Am J Ophthalmol. (1995) 119:295–300. doi: 10.1016/S0002-9394(14)71170-5
29. Saxena S, Mitchell P, Rochtchina E. Five-year incidence of cataract in older persons with diabetes and pre-diabetes. Ophthalmic Epidemiol. (2004) 11:271–7. doi: 10.1080/09286580490510733
30. Drinkwater JJ, Davis WA, Davis TME. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. (2019) 35:e3073. doi: 10.1002/dmrr.3073
31. Kiziltoprak H, Tekin K, Inanc M, Goker YS. Cataract in diabetes mellitus. World J Diabetes. (2019) 10:140–53. doi: 10.4239/wjd.v10.i3.140
32. Hiller R, Sperduto RD, Reed GF, D'Agostino RB, Wilson PW. Serum lipids and age-related lens opacities: a longitudinal investigation: the framingham studies. Ophthalmology. (2003) 110:578–83. doi: 10.1016/S0161-6420(02)01762-1
33. Tung TH, Liu JH, Lee FL, Chen SJ, Tsai CY, Chou P. Community-based study of cataracts among type 2 diabetics in Kinmen. Eur J Epidemiol. (2005) 20:435–41. doi: 10.1007/s10654-004-7537-9
34. Becker C, Schneider C, Aballéa S, Bailey C, Bourne R, Jick S, et al. Cataract in patients with diabetes mellitus-incidence rates in the uk and risk factors. Eye. (2018) 32:1028–35. doi: 10.1038/s41433-017-0003-1
35. Srinivasan S, Raman R, Swaminathan G, Ganesan S, Kulothungan V, Sharma T. Incidence, progression, and risk factors for cataract in type 2 diabetes. Invest Ophthalmol Vis Sci. (2017) 58:5921–9. doi: 10.1167/iovs.17-22264
36. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (Ukpds 35): prospective observational study. BMJ. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
37. Kostis JB, Dobrzynski JM. Prevention of cataracts by statins: a meta-analysis. J Cardiovasc Pharmacol Ther. (2014) 19:191–200. doi: 10.1177/1074248413511690
38. Berlyne GM, Ari JB, Danovitch GM, Blumenthal M. Cataracts of chronic renal failure. Lancet. (1972) 1:509–11. doi: 10.1016/S0140-6736(72)90175-4
39. Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G. Increased levels of advanced glycation end products in human cataractous lenses. J Cataract Refract Surg. (2003) 29:998–1004. doi: 10.1016/S0886-3350(02)01841-2
40. Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of maillard reaction in the aging eye. Amino Acids. (2012) 42:1205–20. doi: 10.1007/s00726-010-0778-x
41. Khoza LB, Nunu WN, Tshivhase SE, Murwira TS, Mambanga P, Ramakuela NJ, et al. Survey on prevalence of cataract in selected communities in limpopo province of South Africa. Sci Afr. (2020) 8:e00352. doi: 10.1016/j.sciaf.2020.e00352
42. Gothwal VK, Wright TA, Lamoureux EL, Lundström M, Pesudovs K. Catquest questionnaire: re-validation in an australian cataract population. Clin Experiment Ophthalmol. (2009) 37:785–94. doi: 10.1111/j.1442-9071.2009.02133.x
43. Stocks N, Patel R, Sparrow J, Davey-Smith G. Prevalence of cataract in the speedwell cardiovascular study: a cross-sectional survey of men aged 65–83. Eye. (2002) 16:275–80. doi: 10.1038/sj.eye.6700106
44. Sullivan MK, Jani BD, McConnachie A, Hanlon P, McLoone P, Nicholl BI, et al. Hospitalisation events in people with chronic kidney disease as a component of multimorbidity: parallel cohort studies in research and routine care settings. BMC Med. (2021) 19:278. doi: 10.1186/s12916-021-02147-6
45. Geng T, Li X, Ma H, Heianza Y, Qi L. Adherence to a healthy sleep pattern and risk of chronic kidney disease: the uk biobank study. Mayo Clin Proc. (2022) 97:68–77. doi: 10.1016/j.mayocp.2021.08.028
Keywords: chronic kidney disease, cataract, risk factors, epidemiology, longitudinal cohort, cross-sectional cohort, glomerular filtration rate
Citation: Huang C-Y, Lee J-I, Chang C-W, Liu Y-H, Huang S-P, Chen S-C and Geng J-H (2022) Chronic kidney disease and its association with cataracts–A cross-sectional and longitudinal study. Front. Public Health 10:1029962. doi: 10.3389/fpubh.2022.1029962
Received: 28 August 2022; Accepted: 17 November 2022;
Published: 07 December 2022.
Edited by:
Xiaodong Sun, Affiliated Hospital of Weifang Medical University, ChinaReviewed by:
Sonia Sharma, Monash University, AustraliaPriyanka Kokil, Indian Institute of Information Technology, Design and Manufacturing, Kancheepuram, India
Copyright © 2022 Huang, Lee, Chang, Liu, Huang, Chen and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiun-Hung Geng, dTkwMDEwOTBAaG90bWFpbC5jb20=
 Chun-Yen Huang1
Chun-Yen Huang1 Shu-Pin Huang
Shu-Pin Huang Szu-Chia Chen
Szu-Chia Chen Jiun-Hung Geng
Jiun-Hung Geng