
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 07 November 2022
Sec. Public Mental Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1021309
This article is part of the Research TopicCurrent Trends and Challenges in the Assessment of Suicidal Behavior: A Psychometric ApproachView all 8 articles
 Yayun Xu1,2,3
Yayun Xu1,2,3 Jun Liang4,5,6,7
Jun Liang4,5,6,7 Wenfan Gao4,5,6,7
Wenfan Gao4,5,6,7 Yanhong Sun4,5,6,7
Yanhong Sun4,5,6,7 Yuanyuan Zhang4,5,6,7
Yuanyuan Zhang4,5,6,7 Feng Shan4,5,6,7
Feng Shan4,5,6,7 Jinfang Ge1,2,8*
Jinfang Ge1,2,8* Qingrong Xia4,5,6,7*
Qingrong Xia4,5,6,7*Objective: Major Depressive Disorder (MDD) is a leading cause of disability, with a high risk of suicidal ideation (SI). Few studies have evaluated the potential of multiple cytokines as biomarkers for SI in patients with MDD. In the present study, we examined the serum levels of multiple cytokines in patients with first-episode drug-naïve MDD, with the aim to discover and identify serum cytokines-based biomarkers for identification of SI in MDD.
Methods: A total of 55 patients with first-episode drug-naïve MDD were enrolled and divided into two groups: 26 MDD patients without SI and 29 MDD patients with SI. Beck Scale for Suicide Ideation was used to estimate SI. A total of 37 cytokines were measured using Multiplex Luminex Assays. The levels of serum cytokines between MDD patients without SI and MDD patients with SI were compared and diagnostic values of different cytokines were evaluated using the receiver operating characteristic (ROC) curve method for discriminating MDD patients with SI from MDD patients without SI. The relationship between the group and the abnormal cytokines were investigated in multiple linear regression models, with adjustments for age, gender, BMI, smoking, and Hamilton Depression Rating Scale-24 (HAMD-24) scores.
Results: The levels of CCL26 and VEGF in MDD patients with SI were significantly lower than those in MDD patients without SI (all P < 0.05). On the contrary, the levels of IL-17C, CXCL10, and TNF-β in MDD patients with SI were significantly higher than those in MDD patients without SI (all P < 0.05). Moreover, the results of multiple linear regression revealed that group was a significant independent predictor of serum IL-17C, CCL-26, VEGF, and TNF-β levels (all P < 0.05). In terms of CXC10, group was also likely to be a significant independent predictor (β = 0.257, P = 0.063). Furthermore, the AUC values of IL-17C and TNF-β were 0.728 and 0.732, respectively. Additionally, a combined panel of IL-17C and TNF-β achieved a high accuracy in discriminating MDD patients with SI from MDD patients without SI (AUC = 0.848, sensitivity = 75.9%, specificity = 72.7%).
Conclusions: These results suggested that circulating IL-17C and TNF-β may hold promise in the discovery of biomarkers for identification of SI in MDD.
Major Depressive Disorder (MDD), one of the most common psychiatric disorders, is associated with increased risk of suicidal ideation (SI), suicide attempts and completed suicide (1, 2). According to the World Health Organization (WHO), MDD affects 300 million people globally. It has been reported that 58% of MDD patients have SI and 15% have attempted suicide (3, 4). Due to its high morbidity, mortality and disability rate, MDD places a very heavy burden on families and society. Currently, the diagnosis of depression and SI primarily depends on subjective symptoms/manifestations, with uncertainties as high as 40% as a consequence (5). Therefore, the use of objective biomarkers to identify subgroups at high risk of suicide in patients with MDD has important clinical implications for suicide prevention in the clinical setting.
Several lines of evidence indicate a close relationship between cytokines and MDD. It has been demonstrated that patients with MDD manifested elevated peripheral levels of interleukin-6 (IL-6) and C-reactive protein (CRP) in a cumulative meta-analysis (6). Moreover, increased levels of peripheral CRP and IL-6 were associated with an increased risk of depressive episodes and subsequent development of depressive symptoms (7, 8). Furthermore, depressed patients who are resistant to conventional antidepressants showed higher peripheral blood levels of pro-inflammatory cytokines (9, 10). Additionally, alterations in peripheral cytokine levels were associated with antidepressant treatment outcomes in MDD (11). These findings suggest that peripheral cytokines are implicated in the pathophysiology of depression and may hold significant promise as potential biomarkers for identification of SI in MDD.
Although no objective biomarkers of SI risk currently exist, numerous evidence has suggested several biomarkers that hold promise as predictive indicators of SI. A recent review summarized the last 5 years of research into suicide-associated biomarkers and found that the serotonergic system, inflammation, hypothalamic-pituitary-adrenal axis, lipids, and endocannabinoids emerged as the most promising diagnostic, predictive, and therapeutic indicators (12). Moreover, another meta-analysis evaluated the relationship between blood hormone levels and suicidal behavior (13). The results suggested that blood thyrotropin stimulating hormone (TSH), leptin and dehydroepiandrosterone sulfate (DHEAS) levels were associated with suicide attempts, and progesterone levels were associated with SI (13). Furthermore, biochemical indicators including alpha 1-antitrypsin (AAT), transferring, high-density lipoprotein cholesterol, and apolipoprotein A1 were identified to be potential biomarkers of SI (14). It is noteworthy that the biological mechanisms of suicidality may be related to stress, inflammation and apoptosis (15). Additionally, several proinflammatory cytokines have been reported to be related to suicidality. Specifically, it has been shown that the serum IL-1β level was lowest in suicide attempters, differentiating them from suicide ideators or healthy controls (16). A systematic review found that blood IL-2, IL-6, IL-8, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) levels were altered in patients with suicidal behavior (17). In MDD patients, increased baseline TNF-α might predict changes of SI intensity (18). Taken together, few studies have evaluated the potential of multiple cytokines as biomarkers for SI in patients with MDD.
In the present study, we examined the serum levels of multiple cytokines in patients with MDD, with the aim to discover and identify serum cytokines-based biomarkers in discriminating MDD patients with SI from MDD patients without SI. A total of 37 cytokines in patients with MDD were measured and Beck Scale for Suicide Ideation was used to estimate suicidal ideation. Subsequently, the levels of serum cytokines between MDD patients without SI and MDD patients with SI were compared and diagnostic values of different cytokines were evaluated using the receiver operating characteristic (ROC) curve method for discriminating MDD patients with SI from MDD patients without SI.
This study was conducted at Anhui Mental Health Center between August 2020 and June 2022. Fifty-six patients with first-episode drug-naïve MDD were diagnosed by trained psychiatrists according to the Diagnostic and Statistical Manual for Psychiatric Disorders-Fifth Version (DSM-V). Common criteria for patient inclusion were as follows: (1) being between the ages of 18–65; (2) meeting DSM-V criteria for depression; (3) Hamilton Depression Rating Scale-24 (HAMD-24) scores higher than 20; and (4) receiving no treatment with antidepressants, anti-inflammatory agents or other psychotropic drugs in the previous 3 months. Common criteria for patient exclusion were as follows: (1) current or lifetime history of major neurological disorder or other psychiatric disorders; (2) current or lifetime history of substance abuse other than tobacco; (3) chronic infections, inflammatory, or immune disorders; (4) currently receiving anti-inflammatory treatment. A full medical examination and a detailed medical history inquiry were recorded to fulfill the inclusion/exclusion criteria. This procedure was approved by the ethics committee of the Anhui Mental Health Center (registration number HFSY-IRB-PJ-XQR-2020001) and was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all the participants.
Suicidal ideation was estimated by the 19-item Beck Scale for Suicide Ideation (19). Individuals who scored > 0 on either item 4 or 5 were considered to be currently suicidal. Individuals who received a score of 0 for both these items were considered to be currently non-suicidal (20). In the present study, 55 MDD patients were divided into two groups accordingly: 26 MDD patients without SI and 29 MDD patients with SI. HAMD-24 was used to evaluate the severity of depressive symptoms in all participants.
The blood samples from the subjects were collected between 7:00 and 8:00 o'clock, centrifuged at 1,200 g for 10 min at 4°C. The supernatant was used as serum samples, which were maintained at−80°C until detection. The blood samples were collected at baseline before treatment. A total of 37 serum cytokines, including IL-1α (also called IL-1F1), IL-1β (also called IL-1F2), IL-1RA (also called IL-1F3), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8 (also called C-X-C motif chemokine ligand 8, CXCL8), IL-10, IL-12 (also called IL-23 p40), IL-12 p70, IL-13, IL-15, IL-16, IL-17C, IL-27, IL-31, C-C motif chemokine ligand 3 (CCL3; also called macrophage inflammatory protein 1α, MIP-1α), CCL4 (also called MIP-1β), CCL11 (also called eotaxin), CCL17 (also called thymus and activation regulated chemokine, TRAC), CCL26 (also called eotaxin-3), CXCL10 (also called interferon-inducible Protein 10, IP-10; cytokine responsive gene-2, CRG-2), vascular endothelial growth factor (VEGF), VEGF-C, VEGFR1 (also called Flt1), TNF-α, TNF-β (also called lymphotoxin), Tie-2, interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), fibroblast growth factor-basic (FGF basic, also called FGF2/bFGF), thymic stromal lymphopoietin (TSLP), intercellular cell adhesion molecule-1 (ICAM-1), and placenta growth factor (PIGF) were measured by the multiplex bead immunoassay (LXSAHM-10 and LXSAHM-27, R&D system for antibody detection, Shanghai Universal Biotech Co., Ltd) according to the manufacturer's instructions.
Statistical analysis was calculated using SPSS (version 17.0; IBM Corp., Armonk, NY, USA). The data are shown as mean ± standard error of the mean (SEM), and the statistical significance was set at P < 0.05. The normality of continuous variable was evaluated using the Kolmogorov-Smirnov normality test. Normally distributed data were compared using the Student's t-test, whereas data following a non-normal distribution were analyzed using a non-parametric test (Mann-Whitney U test). Chi-squared test was used to determine the difference between the two groups with respect to sex, marital status, and smoking status. Two-group comparisons of the serum cytokines were performed using Student's t-test or Mann-Whitney U test and false discovery rate (FDR) adjustment using the Benjamini-Hochberg (BH) adjusted P-value, correcting for multiple testing. The relationship between the group and the abnormal cytokines were investigated in multiple linear regression models, with adjustments for age, gender, BMI, smoking, and HAMD-24 scores. The correlation between the severity of suicidal ideation (as measured by the 19-item Beck Scale for Suicide Ideation) and the cytokines identified as being elevated in MDD patients with SI was evaluated by Spearman's correlation test. The diagnostic performance of serum cytokines was estimated using the receiver operating characteristic (ROC) curve analysis and calculating the area under the curve (AUC) values in discriminating MDD patients with SI from MDD patients without SI.
Table 1 summarizes the demographic and clinical characteristics of MDD patients without SI and MDD patients with SI. There were no significant differences in age, sex BMI, years of education, marital status, smoking status, or HAMD scores between the two groups (Table 1).
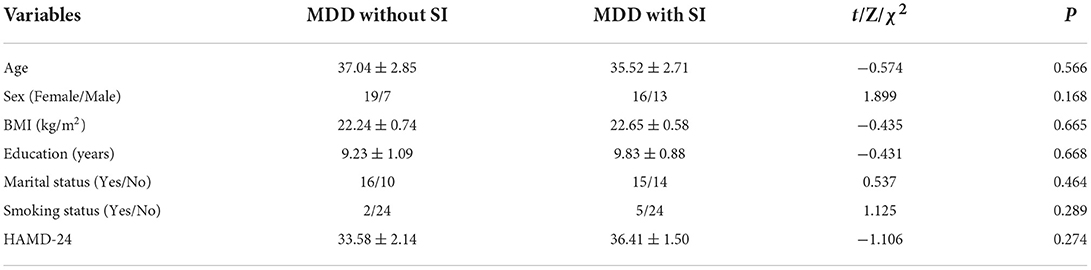
Table 1. Demographic and clinical characteristics of MDD patients without SI and MDD patients with SI.
As shown in Table 2, the levels of CCL26 and VEGF in MDD patients with SI were significantly lower than those in MDD patients without SI (all P < 0.05). On the contrary, the levels of IL-17C, CXCL10, and TNF-β in MDD patients with SI were significantly higher than those in MDD patients without SI (all P < 0.05). After applying the Benjamini-Hochberger correction for multiple measurements, none of these differences persisted (all P > 0.05).
There were no significant differences in other cytokines levels including IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-12 p70, IL-13, IL-15, IL-16, IL-27, IL-31, CCL3, CCL4, CCL11, CCL17, VEGF-C, VEGFR1, TNF-α, Tie-2, IFN-γ, GM-CSF, FGF basic, TSLP, ICAM-1, and PIGF between the two groups (Table 2).
The relationship between the group and the abnormal cytokines was investigated in multiple linear regression models, with adjustments for age, gender, BMI, smoking, and HAMD scores (Table 3). The results revealed that group was a significant independent predictor of serum IL-17C, CCL-26, VEGF, and TNF-β levels, while controlling for other independent variables including age, gender, BMI, smoking, and HAMD-24 scores (all P < 0.05). In terms of CXC10, group was also likely to be a significant independent predictor (β = 0.257, P = 0.063).
The correlation between the severity of suicidal ideation (as measured by the 19-item Beck Scale for Suicide Ideation) and the cytokines identified as being elevated in MDD patients with SI was evaluated by Spearman's correlation test. The results showed that no significant relationship was observed between the severity of SI and serum IL-17C (r = −0.159, P = 0.409), CXCL10 (r = −0.145, P = 0.453), and TNF-β (r = −0.131, P = 0.497) levels in MDD patients with SI.
The diagnostic performance of different cytokines in discriminating MDD patients with SI from MDD patients without SI were performed by ROC curve analysis (Table 4). Among the 9 different cytokines between the two groups, the AUC values of 2 cytokines including IL-17C and TNF-β were 0.728 and 0.732, respectively.
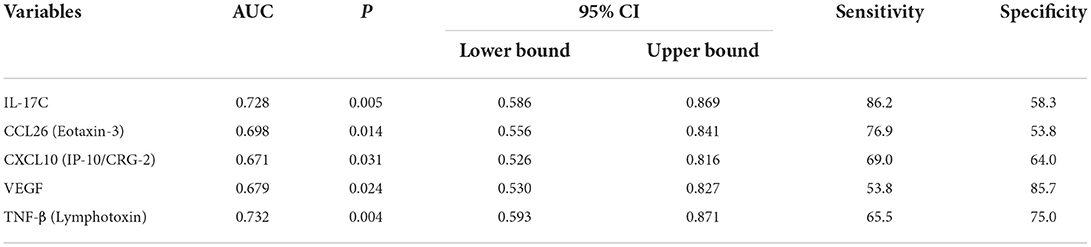
Table 4. ROC analysis of different cytokines in discriminating MDD patients with SI from MDD patients without SI.
Combining detection of multiple serum proteins as a single panel can improve the sensitivity or specificity of a single biomarker (21). As shown in Figure 1, the ROC curve analysis demonstrated that a combined panel of IL-17C and TNF-β achieved a high accuracy in discriminating MDD patients with SI from MDD patients without SI (AUC = 0.848, sensitivity = 75.9%, specificity = 72.7%).
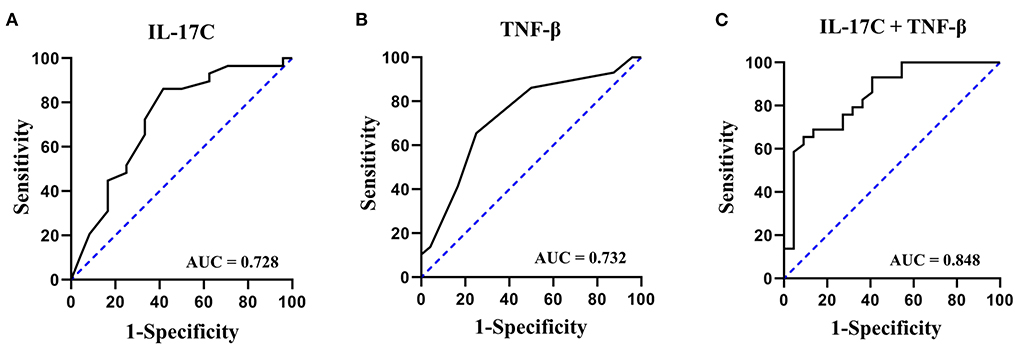
Figure 1. ROC curves of IL-17C, TNF-β, and a combined panel of IL-17C and TNF-β in discriminating MDD patients with SI from MDD patients without SI. (A) ROC curve of IL-17C; (B) ROC curve of TNF-β; (C) ROC curve of a combined panel of IL-17C and TNF-β.
The present study investigated the serum levels of 37 cytokines in patients with MDD, with the aim to identify serum cytokines-based biomarkers for identification of SI in MDD. Two main findings emerged from the present study. First, MDD patients with SI showed higher levels of IL-17C, CXCL10, and TNF-β, and lower levels of CCL26 and VEGF compared to MDD patients without SI. Second, a combined ROC analysis using IL-17C and TNF-β revealed an AUC of 0.848 with a sensitivity of 75.9% and a specificity of 72.7% in separating MDD patients with SI from MDD patients without SI.
Cytokines are a class of multifunctional proteins involved in cellular communication and activation, that can be used as markers for micro-inflammation (22). Numerous studies have demonstrated the abnormal levels of cytokines in patients with depression. It has been reported that compared to healthy controls, depressed patients exhibited higher levels of IL-6 and TNF-α (23). Further studies have indicated that patients with MDD had increased levels of IL-6, IL-10, IL-12, IL-13, and TNF-α (24). Additionally, abnormal levels of other cytokines including IL-1β, IL-2, IL-4, IL-8, IL-15, CCL3, CCL4, CCL11, and FGF basic have been found in depressed patients in several studies (25–29). These studies focus on evaluating differences in peripheral cytokines between depressed patients and healthy subjects. It is noteworthy that several cytokines including IL-2, IL-6, IL-8, TNF-α and VEGF are reported to be related to the suicidal behavior (17). Early recognition of SI before suicidal behavior in patients with MDD plays an important role in reducing the mortality caused by suicide. Therefore, in the present study, we further compared the differences in serum levels of 37 cytokines between patients without SI and MDD patients with SI. The results confirmed that MDD patients with SI showed higher levels of IL-17C, CXCL10, and TNF-β, and lower levels of CCL26 and VEGF compared to MDD patients without SI. However, after applying the Benjamini-Hochberger correction for multiple measurements, none of these differences persisted. Thus, multicenter large sample studies are required to further confirm these findings. Taken together, these findings link peripheral cytokines to the pathogenesis of depression and SI in depressed patients. Given that this study is a cross-sectional study, the causal relationship between these cytokines and SI needs further studies to explore.
It has been demonstrated that the severity of depressive symptoms may be associated with alterations in cytokine levels (30). Taken together the possible effect of age (31), gender, BMI (32, 33), and smoking (34) on the serum cytokines, the relationship between the group and the abnormal cytokines was investigated in multiple linear regression models, with adjustments for age, gender, BMI, smoking, and HAMD-24 scores. The results revealed that group was a significant independent predictor of serum IL-17C, CCL-26, CXC10, VEGF, and TNF-β levels, further linking these cytokines to the SI of MDD patients. Additionally, there were 3 subjects over 60 years old (2 MDD patients without SI and 1 MDD patients with SI). After we excluded the data of these 3 subjects, the results of this study have not been altered.
We further explored the potential values of these aberrant cytokines as diagnostic biomarkers of SI in MDD. Given that AUC value in ROC analysis should be > 0.7 to be of clinical value for screening (35), IL-17C and TGF-β meet this condition.
IL-17C is a member of the IL-17 family that is selectively induced in epithelia by bacterial challenge and inflammatory stimuli (36). Prior studies showed a crucial role of IL-17C in the pathogenesis of immune-mediated skin diseases (37), autoimmune hepatitis (38), and acute pneumonia (39). To the best of our knowledge, few studies have evaluated the changes in peripheral blood levels of IL-17C in patients with depression. In the present study, we firstly found that the serum concentration of TGF-β was significantly higher in MDD patients with SI compared to MDD patients without SI. Moreover, IL-17C revealed an AUC of 0.728 with a sensitivity of 86.2% and a specificity of 58.3% in separating MDD patients with SI from MDD patients without SI. Since this study is a single-center study and the sample size is relatively small, the aberrant level and the diagnostic value of IL-17C in MDD patients with SI should be confirmed by multicentric studies.
TGF-β is a cytokine with a role in the differentiation of Th17 and T regulatory lymphocytes (40). Previous studies have indicated a crucial role of TGF-β in the pathophysiology of depression. Animal studies have demonstrated an increased TGF-β level in chronic unpredictable mild stress (CUMS) mice, a realistic animal model of depression (41). Moreover, TGF-β was believed to be a key factor responsible for the imbalance between Th17 and Treg cells related to the depression-like behavioral changes in CUMS mice (41). Clinical studies have reported that the TGF-β levels were significantly higher in MDD patients compared to controls (42, 43). After 6 weeks of antidepressant treatment, TGF-β production were significantly lower than before treatment in MDD patients (44). Additionally, the concentration of TGF-β was significantly higher in patients with MDD with childhood maltreatment (CM) history, compared to MDD patients with no CM (45). Combined with the elevated TGF-β levels in MDD patients with SI and the potential diagnostic value of TGF-β in separating MDD patients with SI in the present study, these findings provide more data linking peripheral TGF-β to the disease severity and SI in MDD.
It has been shown that combined detection of multiple serum proteins as a single panel can increase the sensitivity or specificity of a single biomarker (21). Therefore, we further evaluated the potential of a combined panel of IL-17C and TNF-β for the diagnosis of SI in MDD. The results showed that the AUC value of this panel increased to 0.848 with a moderate sensitivity of 75.9% and a moderate specificity of 72.7% in separating MDD patients with SI from MDD patients without SI.
There are some limitations of this study. Firstly, this was a cross-sectional single-center study with a relatively small sample size and the generalizability of the findings was uncertain, which is a significant flaw, and further prospective studies with larger sample sizes are required to confirm our present findings. Secondly, this study is a cross-sectional study, the causal relationship between these cytokines and SI needs further studies to explore. Thirdly, SI was self-reported and may have been subject to reporting bias. Fourthly, the present study only evaluated the SI of MDD patients, not their suicide attempts. As suicide attempt indicates a more severe level of suicidality, further studies are needed to investigate the potential of multiple cytokines as biomarkers for suicide attempt in patients with MDD.
Several public health implications should be highlighted. (1) Given the fact that approximately one million people die of suicide worldwide each year, it is of great clinical significance to identify the patients with SI in a timely and early manner. However, the current diagnosis of SI primarily depends on subjective symptoms/manifestations, with uncertainties as high as 40% as a consequence. Our study for the first time confirms a combined panel of IL-17C and TNF-β for the diagnosis of SI in MDD and may therefore help to identify potentially suicidal patients, especially those patients who are unwilling to disclose suicidal ideation during standard screening. (2) Exercise has been shown to increase growth factor secretion of important cytokines, such as VEGF (46). In the present study, the serum VEGF levels were significantly lower in MDD patients with SI compared to MDD patients without SI. Thus, whether moderate exercise, a public health-related behavior, can improve the SI of MDD patients by increasing the level of blood VEGF needs further studies to investigate. (3) Our results demonstrated that a combined panel of IL-17C and TNF-β achieved a high accuracy in discriminating MDD patients with SI from MDD patients without SI. These two cytokines can be detected by Enzyme-linked immunosorbent assay (ELISA). This detection method is cheap, simple, reliable and fast, which can be set up in all levels of health care facilities including resource limited areas, especially in low to middle income countries.
In conclusion, the present study reveals that MDD patients with SI showed higher levels of IL-17C, CXCL10, and TNF-β, and lower levels of CCL26 and VEGF compared to MDD patients without SI. Our study for the first time confirms a combined panel of IL-17C and TNF-β for the diagnosis of SI in first-episode drug-naïve MDD. These findings provide evidence that alterations in peripheral cytokines levels hold significant promise as biomarkers for identification of SI in MDD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Anhui Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
YX and QX conceived the study. YX, JG, and QX wrote the protocol. YX, JL, WG, YS, YZ, and FS performed the analyses. YX wrote the first draft. All authors read and commented the manuscript and agreed on the final version.
This study was provided by the National Natural Science Foundation of China (81870403), Key Research and Development Program of Anhui Province (202004j07020001), and Hefei Sixth Cycle Key Medical Specialty.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li X-Y, Tabarak S, Su X-R, Qin Z, Chai Y, Zhang S, et al. Identifying clinical risk factors correlate with suicide attempts in patients with first episode major depressive disorder. J Affect Disord. (2021) 295:264–70. doi: 10.1016/j.jad.2021.08.028
2. Zhou SC, Luo D, Wang XQ, Zhu J, Wu S, Sun T, et al. Suicidal ideation in college students having major depressive disorder: role of childhood trauma, personality and dysfunctional attitudes. J Affect Disord. (2022) 311:311–8. doi: 10.1016/j.jad.2022.05.085
3. Sokero TP, Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Isometsä ET. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry. (2003) 64:1094–100. doi: 10.4088/JCP.v64n0916
4. Jeon HJ, Lee J-Y, Lee YM, Hong JP, Won S-H, Cho S-J, et al. Unplanned versus planned suicide attempters, precipitants, methods, and an association with mental disorders in a Korea-based community sample. J Affect Disord. (2010) 127:274–80. doi: 10.1016/j.jad.2010.05.027
5. Mogi T, Toda H, Yoshino A. Clinical characteristics of patients with diagnostic uncertainty of major depressive disorder. Asian J Psychiatr. (2017) 30:159–62. doi: 10.1016/j.ajp.2017.10.001
6. Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. (2015) 49:206–15. doi: 10.1016/j.bbi.2015.06.001
7. Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, et al. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav Immun. (2021) 93:214–25. doi: 10.1016/j.bbi.2021.01.016
8. Valkanova V, Ebmeier K, Allan C. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. (2013) 150:736–44. doi: 10.1016/j.jad.2013.06.004
9. Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:445–50. doi: 10.1016/j.pnpbp.2007.09.015
10. Amitai M, Taler M, Carmel M, Michaelovsky E, Eilat T, Yablonski M, et al. The relationship between plasma cytokine levels and response to selective serotonin reuptake inhibitor treatment in children and adolescents with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. (2016) 26:727–32. doi: 10.1089/cap.2015.0147
11. Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:339–50. doi: 10.1038/s41380-019-0474-5
12. Johnston JN, Campbell D, Caruncho HJ, Henter ID, Ballard ED, Zarate CA. Suicide biomarkers to predict risk, classify diagnostic subtypes, and identify novel therapeutic targets: 5 years of promising research. Int J Neuropsychopharmacol. (2022) 25:197–214. doi: 10.1093/ijnp/pyab083
13. Fu X-L Li X, Ji J-M, Wu H, Chen H-L. Blood hormones and suicidal behaviour: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 139:104725. doi: 10.1016/j.neubiorev.2022.104725
14. Bai S, Fang L, Xie J, Bai H, Wang W, Chen J-J, et al. Potential biomarkers for diagnosing major depressive disorder patients with suicidal ideation. J Inflamm Res. (2021) 14:495–503. doi: 10.2147/JIR.S297930
15. Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. (2013) 18:1249–64. doi: 10.1038/mp.2013.95
16. Ganança L, Galfalvy HC, Cisneros-Trujillo S, Basseda Z, Cooper TB, Ren X, et al. Relationships between inflammatory markers and suicide risk status in major depression. J Psychiatr Res. (2021) 134:192–9. doi: 10.1016/j.jpsychires.2020.12.029
17. Serafini G, Pompili M, Elena Seretti M, Stefani H, Palermo M, Coryell W, et al. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur Neuropsychopharmacol. (2013) 23:1672–86. doi: 10.1016/j.euroneuro.2013.06.002
18. Choi KW, Jang EH, Kim AY, Kim H, Park MJ, Byun S, et al. Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorder and panic disorder: a 12-week follow-up study. J Psychiatr Res. (2021) 133:73–81. doi: 10.1016/j.jpsychires.2020.12.011
19. Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol. (1979) 47:343–52. doi: 10.1037/0022-006X.47.2.343
20. Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. (2005) 112:294–301. doi: 10.1111/j.1600-0447.2005.00585.x
21. Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. (2018) 67:662–75. doi: 10.1002/hep.29561
22. Kim Y-K, Na K-S, Myint A-M, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 64:277–84. doi: 10.1016/j.pnpbp.2015.06.008
23. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
24. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
25. Mota R, Gazal M, Acosta BA, de Leon PB, Jansen K, Pinheiro RT, et al. Interleukin-1β is associated with depressive episode in major depression but not in bipolar disorder. J Psychiatr Res. (2013) 47:2011–4. doi: 10.1016/j.jpsychires.2013.08.020
26. Jeenger J, Singroha V, Sharma M, Mathur DM. C-reactive protein, brain-derived neurotrophic factor, interleukin-2, and stressful life events in drug-naive first-episode and recurrent depression: a cross-sectional study. Indian J Psychiatry. (2018) 60:334–9. doi: 10.4103/psychiatry.IndianJPsychiatry_169_18
27. Pérez-Sánchez G, Becerril-Villanueva E, Arreola R, Martínez-Levy G, Hernández-Gutiérrez ME, Velasco-Velásquez MA, et al. Inflammatory profiles in depressed adolescents treated with fluoxetine: an 8-week follow-up open study. Mediators Inflamm. (2018) 2018:4074051. doi: 10.1155/2018/4074051
28. Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. (2008) 18:230–3. doi: 10.1016/j.euroneuro.2007.06.004
29. Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. (2018) 23:48–58. doi: 10.1038/mp.2017.205
30. Giollabhui NM, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. (2021) 26:3302–14. doi: 10.1038/s41380-020-00867-4
31. Larsson A, Carlsson L, Gordh T, Lind A-L, Thulin M, Kamali-Moghaddam M, et al. The effects of age and gender on plasma levels of 63 cytokines. J Immunol Methods. (2015) 425:58–61. doi: 10.1016/j.jim.2015.06.009
32. Dalamaga M, Liu J. A chromatin remodeling checkpoint of diet-induced macrophage activation in adipose tissue. Metabol Open. (2022) 15:100204. doi: 10.1016/j.metop.2022.100204
33. Pischon N, Heng N, Bernimoulin J-P, Kleber B-M, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res. (2007) 86:400–9. doi: 10.1177/154405910708600503
34. Keulen HVV, Gomes AS, Toffolo MCF, Oliveira EE, Silva LC, Alves CS, et al. Serum levels of nitric oxide and cytokines in smokers at the beginning and after 4months of treatment for smoking cessation. Int J Cardiol. (2017) 230:327–31. doi: 10.1016/j.ijcard.2016.12.111
35. Swets J. Measuring the accuracy of diagnostic systems. Science. (1988) 240:1285–93. doi: 10.1126/science.3287615
36. Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. (2011) 12:1159–66. doi: 10.1038/ni.2156
37. Monin L, Gudjonsson JE, Childs EE, Amatya N, Xing X, Verma AH, et al. MCPIP1/Regnase-1 restricts IL-17A- and IL-17C-dependent skin inflammation. J Immunol. (2017) 198:767–75. doi: 10.4049/jimmunol.1601551
38. Huang J, Yuan Q, Zhu H, Yin L, Hong S, Dong Z, et al. IL-17C/IL-17RE Augments T cell function in autoimmune hepatitis. J Immunol. (2017) 198:669–80. doi: 10.4049/jimmunol.1600977
39. Steck P, Ritzmann F, Honecker A, Vella G, Herr C, Gaupp R, et al. Streptococcus pneumoniaeInterleukin 17 Receptor E (IL-17RE) and IL-17C mediate the recruitment of neutrophils during acute pneumonia. Infect Immun. (2019) 87:e00329–19. doi: 10.1128/IAI.00329-19
40. Eisenstein E, Williams C. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. (2009) 65:26R–31R. doi: 10.1203/PDR.0b013e31819e76c7
41. Hong M, Zheng J, Ding Z-y, Chen J-h, Yu L, Niu Y, et al. Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation. (2013) 20:39–50. doi: 10.1159/000343100
42. Davami MH, Baharlou R, Vasmehjani AA, Ghanizadeh A, Keshtkar M, Dezhkam I, et al. Elevated IL-17 and TGF-β serum levels: a positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin Neurosci. (2016) 7:137–42. doi: 10.15412/J.BCN.03070207
43. Kim Y-K, Lee S-W, Kim S-H, Shim S-H, Han S-W, Choi S-H, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:356–61. doi: 10.1016/j.pnpbp.2007.08.041
44. Kim Y-K, Na K-S, Shin K-H, Jung H-Y, Choi S-H, Kim J-B. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2007) 31:1044–53. doi: 10.1016/j.pnpbp.2007.03.004
45. Jovanovic AM, Mitkovic-Voncina M, Kostic M, Jeremic M, Todorovic J, Popadic D, et al. Childhood maltreatment correlates with higher concentration of transforming growth factor beta (TGF-β) in adult patients with major depressive disorder. Psychiatry Res. (2021) 301:113987. doi: 10.1016/j.psychres.2021.113987
Keywords: cytokines, biomarker, diagnosis, serum, major depressive disorder, suicidal ideation
Citation: Xu Y, Liang J, Gao W, Sun Y, Zhang Y, Shan F, Ge J and Xia Q (2022) Peripheral blood cytokines as potential diagnostic biomarkers of suicidal ideation in patients with first-episode drug-naïve major depressive disorder. Front. Public Health 10:1021309. doi: 10.3389/fpubh.2022.1021309
Received: 17 August 2022; Accepted: 24 October 2022;
Published: 07 November 2022.
Edited by:
S. M. Yasir Arafat, Enam Medical College, BangladeshReviewed by:
Ravi Philip Rajkumar, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaCopyright © 2022 Xu, Liang, Gao, Sun, Zhang, Shan, Ge and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinfang Ge, Z2VqaW5mYW5nQGFobXUuZWR1LmNu; Qingrong Xia, YWhtY3hxckAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.