
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 05 January 2023
Sec. Environmental Health and Exposome
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1018327
 Hongyun Li1,2
Hongyun Li1,2 Ying Liang2
Ying Liang2 Li Dong2,3
Li Dong2,3 Cancan Li4
Cancan Li4 Lu Zhang2
Lu Zhang2 Bin Wang2,5
Bin Wang2,5 Delong Ma6
Delong Ma6 Qunzheng Mu2
Qunzheng Mu2 Jun Wang2
Jun Wang2 Haifeng Hou7*
Haifeng Hou7* Qiyong Liu2,8*
Qiyong Liu2,8*Background: Peromyscopsylla hesperomys and Orchopeas sexdentatus are regarded to be representative plague vectors in the United States. The incidence of plague is rising globally, possibly due to climate change and environmental damage. Environmental factors such as temperature and precipitation have a significant impact on the temporal and spatial distribution of plague vectors.
Methods: Maximum entropy models (MaxEnt) were utilized to predict the distributions of these two fleas and their trends into the future. The main environmental factors influencing the distribution of these two fleas were analyzed. A risk assessment system was constructed to calculate the invasion risk values of the species.
Results: Temperature has a significant effect on the distribution of the potentially suitable areas for P. hesperomys and O. sexdentatus. They have the potential to survive in suitable areas of China in the future. The risk assessment system indicated that the risk level for the invasion of these two species into China was moderate.
Conclusion: In order to achieve early detection, early interception, and early management, China should perfect its monitoring infrastructure and develop scientific prevention and control strategies to prevent the invasion of foreign flea vectors.
Globally, flea-borne infectious diseases, for instance, plague, are creating a comeback and their incidence is on the rise, which may be related to global climate change and environmental damage (1). Climate change is defined as a modification of temperature and weather patterns over a long-term cycle. Global warming is an incontrovertible fact, with the temperatures of global lands and oceans increasing by an average of 0.89°C (0.69–1.08°C) from 1901 to 2012 (2).
The World Health Organization estimates that the number of excess deaths attributable to global climate change will reach 250,000 per year in 2030–2050. Moreover, global warming has vital implications for vector-borne infectious diseases: First, the temporal and geographic distribution of vector organisms is susceptible to climatic and environmental factors, and a tiny increase in temperature could cause obvious changes in the population size and distribution range of vector organisms. Second, climatic factors influence the reproduction of pathogenic microorganisms in vector organisms considerably (3). Due to future climate change, the risk of plague in the temperate zone might grow significantly (4). The occurrence of extreme weather events and natural disasters increases the likelihood of large-scale outbreaks and epidemics of plague (5).
Fleas are vectors of the plague bacterium (6). They transmit plague via the formation of Yersinia pestis bacterial embolus, and the speed of this process is regulated by temperature (7, 8). Climate change impacts the fluctuations of the flea population via variations in temperature and humidity and elevates the risk of biological invasion (9). The risk assessment of biological invasion includes invasion risk, colonization risk, spread risk, and damage consequences. The premise of colonization is that the alien species has a suitable zone. The ecological niche model (ENM) provides an important quantitative analysis tool for predicting the distribution of specie's suitable zones, which uses distribution data of known species and relevant environmental variables to determine the ecological requirements of species based on the constructed model and projects the results into different spatial and temporal dimensions (10). The maximum entropy (MaxEnt) model has better predictive performance than other ecological niche models (11), and it can predict the future distribution of species' suitable zones, while others cannot, therefore, we chose the MaxEnt model. The model is an algorithm for predicting species' suitable areas based on the probability distribution and has the advantages of wide application, high prediction accuracy, and low requirements for sample content (12–16).
In total, 464 human plague cases were reported in the United States (U.S.) from 1950 to 2009 (17). In the U.S., most human cases are normally associated with flea bites (18). Data indicated that the U.S. is the first exporter to China and the third importer to China in 2020. With the development of global economic integration, the increasing flow of cargo between China and the U.S. has added the risk of the entry of plague vector fleas because fleas can be attached to host, clothing, and textiles imported by vehicles/ships, which has the potential to cause an epidemic of plague in China. Therefore, it is significant to study the global distribution of U.S. plague vector fleas and the risk of them invading China by using the MaxEnt model to prevent the introduction of plague into China.
In this research, Peromyscopsylla hesperomys (P. hesperomys) and Orchopeas sexdentatus (O. sexdentatus) were selected for the predicting suitable areas and the invasion risk assessment.
Literature searches were conducted using China National Knowledge Infrastructure (CNKI), PubMed, Web of Science, Embase, and Google Scholar. Global Invasive Species Database (GISD), Invasive Species Compendium (ISC), European and Mediterranean Plant Protection Organization (EPPO), World Health Organization (WHO), and the U.S. Centers for Disease Control and Prevention (CDC) were used to screen for fleas with invasive risk and positive for plague Yersinia pestis (19). Two representative U.S. fleas: P. hesperomys and O. sexdentatus, were identified as vectors of plague and selected for the study.
Occurrence points of P. hesperomys and O. sexdentatus were obtained from the Global Biodiversity Information Facility (GBIF) (https://www.gbif.org; accessed on 5 April 2022). The accuracy of the coordinate points was checked. When the species distribution data were too dense in a particular area, ENMTools software (https://www.activestate.com/products/perl/downloads; accessed on 12 April 2022) is used to keep only one distribution point within the same raster to avoid result overfitting (20), and we obtained 39 occurrence points for P. hesperomys (Figure 1) and 65 for O. sexdentatus (Figure 2).
Environmental variables, acquired from the near-current climate scenario (1970–2000) and future climate scenarios (2021–2040, 2041–2060, 2061–2080, and 2081–2100), were collected from WorldClim (version 2.1, http://www.worldclim.org; accessed on 5 April 2022) by a spatial resolution of 5 arc-min, containing bioclimatic variables (Bio1–Bio19), monthly maximum temperature (Tmax1–Tmax12), monthly minimum temperature (Tmin1–Tmin12), monthly precipitation (Prec1–Prec12), and elevation (Ele). They were processed by the BCC-CSM2-MR (Medium-Resolution Beijing Climate Center Climate System Model version 2) under four different socio-economic models driven by CO2: shared socio-economic pathways (SSPs) 126, 245, 370, and 585 (21).
To avoid the overfitting of environmental variables, Maxent was used to get the contribution rate of environmental variables. R software was used (version 4.1.0 https://www.r-project.org; accessed on 15 February 2022) to conduct Pearson's correlation analysis on environmental variables. If the absolute value of the correlation coefficient between two variables was >0.8, the variable with a smaller contribution was removed. If the absolute value of the correlation coefficient was < 0.8, both variables were retained. In the end, 10 variables were filtered as predictors of P. hesperomys, and 8 variables were filtered as predictors of O. sexdentatus (Table 1).
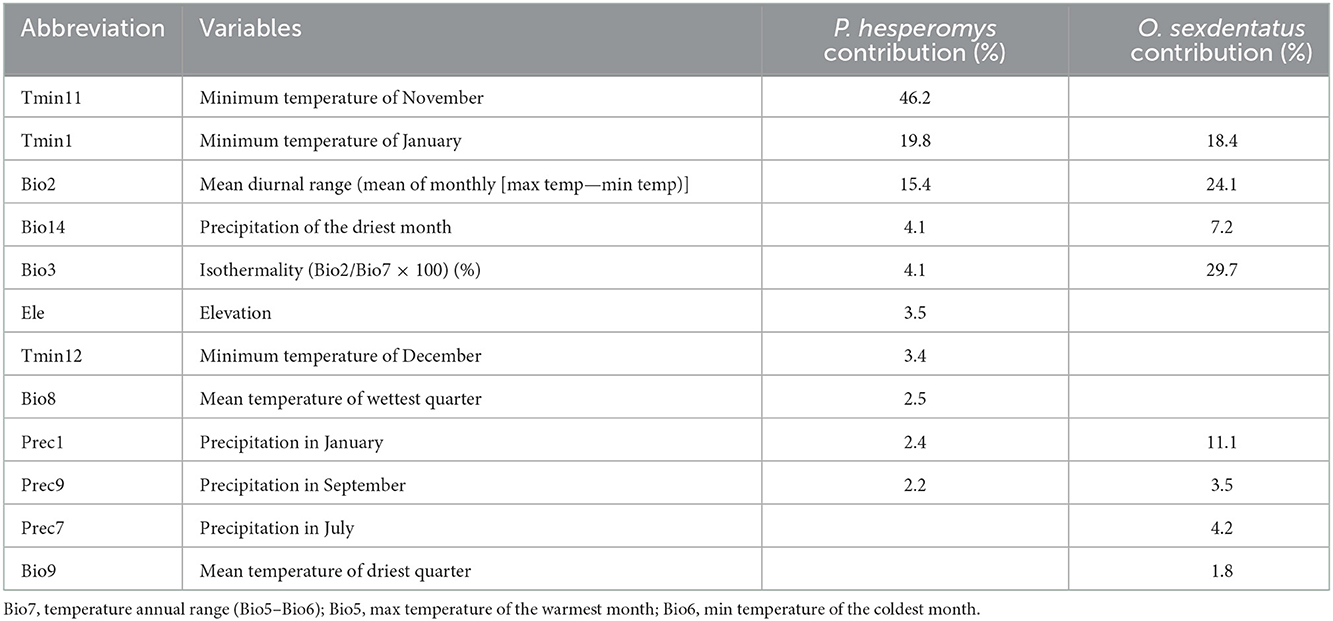
Table 1. Percentage contributions of environmental variables in Maxent for P. hesperomys and O. sexdentatus.
The China map (scale: 1:4,000,000) is from the National Geomatics Center of China (http://www.ngcc.cn/ngcc; accessed on 12 April 2022), and the world map (scale: 1:10,000,000) is from the Natural Earth (https://www.naturalearthdata.com/downloads; accessed on 12 April 2022).
MaxEnt software (version 3.4.1, https://biodiversityinformatics.amnh.org; accessed on 5 April 2022) was used to predict the suitable areas for P. hesperomys and O. sexdentatus under near-current and future climate scenario model. The training and testing data sets were randomly generated, with 75% of the distribution points for training and 25% for testing.
The regularization multiplier (RM) and feature combination (FC) affected the predictive performance and accuracy of the MaxEnt model (22). R software was utilized to calculate RM and FC to optimize the MaxEnt model parameters (23). The RM parameter was usually set to eight levels: 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4. The FC levels usually set five characteristic parameters: automatic linear (L), quadratic (Q), fragmentation (hinge, H), product (P), and threshold (T) to obtain eight features (L, LQ, LQP, QHP, LQH, LQHP, QHPT, and LQHPT). We used the R software package “ENMeval” to obtain the best model by Akaike's information criterion (AIC). When deltaAICc is equal to 0, the model is deemed to be the most appropriate (24). The RM and FC in this study were LQP and 1 in P. hesperomys, and QHP and 1 in O. sexdentatus, respectively. Jackknife tests were used to assess contribution rates of environmental variables, and the receiver operating characteristic (ROC) curve was used to evaluate the performance of the prediction model. The area under the curve (AUC) value, which can judge the model accuracy, is defined as the area under the ROC curve. The judgment standard: AUC (0.5–0.7), low predictive; AUC (0.7–0.9), middle predictive; AUC (0.9–1), highly predictive.
ArcGIS software (version 10.5) was purchased by the Department of Vector Biological and Control of the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention (ICDC). Based on the natural break points, the probability of presence was classified into four categories: unsuitable area, low suitable, middle suitable, and high suitable, using ArcGIS's reclassification tool with complementary cloglog values (25).
In this research, a multi-indicator invasion risk assessment system was designed by referring to the previous invasion risk assessment index system (26, 27). An assessment index system of the invasion risk, colonization risk, spread risk, and damage consequences was conducted (Supplementary Table S1). Experts in the fields of biology, ecology, environmental science, and public health were invited to score each research index of the assessed species, and the assessment results were used to calculate the comprehensive invasion risk (R) value of the species as the risk for them invading China.
Based on the general process of foreign biological invasion (i.e., entry, colonization, spread, and damage), risk factors that affect biological invasion are divided into three categories, including the possibility of entry (P1), the possibility of colonization and spread (P2), and the damage consequences (P3). Each assessment factor is quantitatively or qualitatively described by specific elements.
Based on fuzzy mathematical principles, a uniform calculation was performed to classify each reference indicator into five grades according to the risk level, and a range was set for each level in the order of none [0], weak (0 < x ≤ 0.25), moderate (0.25 < x ≤ 0.5), strong (0.5 < x ≤ 0.75), and extremely strong (0.75 < x ≤ 1). Scores of quantitative indicators are assigned values based on specific situations, and qualitative indicators are scored by experts based on their professional knowledge and research experience. The R-value is calculated by P1, P2, and P3, which are logically related to each other. According to the multiplicative principle, the calculated formula is as follows: R = .
The calculated risk value ranged from 0 to 1, which was classified as extremely low risk (0.01–0.05), low risk (0.05–0.3), moderate risk (0.3–0.7), and high risk (0.7–1).
The weight coefficients of the indicators were determined in accordance with the multiplication principle.
The entry possibility calculated formula is as follows:
Colonization and spread possibility calculated formulas are as follows:
The damage consequences calculated formula is as follows:
Based on the output of MaxEnt, the global suitable areas of P. hesperomys under the near-current climate scenario were classified as unsuitable areas, low suitable areas, middle suitable areas, and high suitable areas. The suitable areas are mainly on the west coast of Canada, most of the states in the U.S. mainland and Alaska state, Mexico, and countries along the Andes Mountains. In addition, suitable areas are distributed in European including western Russia, North African countries (Libya, Algeria, Egypt, Morocco), west and Central Asian countries (Iraq, Iran, Afghanistan), and east Asia countries (China, Japan, and Korean Peninsula). The total global area of the suitable areas is 25.11 × 106 km2 (Figure 3).
The potentially suitable areas for O. sexdentatus under near-current climate scenarios were mainly located in the west coast and Midwest of the U.S., the Southeastern U.S., northern Mexico, and southern Argentina in the Americas. In addition, the suitable areas for O. sexdentatus are located in Europe (Spain, Portugal, France, the United Kingdom, and Central and Eastern European countries), Africa (South Africa and the Mediterranean coastal areas countries), and Asia (Pakistan, Saudi Arabia, Iran, and China and other Central Asian countries). The total area of the global suitable areas is 9.11 × 106 km2 (Figure 4).
Under each future climate scenario model, the potentially suitable areas for P. hesperomys in China are distributed in southern Tibet, Xinjiang, Yunnan, and western Sichuan. The low suitable area is mainly distributed in the Yellow River basin provinces of Qinghai, Sichuan, Ningxia, Inner Mongolia, Shaanxi, Shanxi, Henan, and Shandong and to a lesser extent in Anhui, Jiangsu, Zhejiang, Hubei, Beijing, Tianjin, and Hebei (Figure 5). As shown in Table 2, under ssp2.4-5 scenario models from 2021 to 2040, the suitable area for P. hesperomys has the largest range in China (404.94 × 104 km2). The area potentially suitable for P. hesperomys under each climate scenario reveals an overall increasing trend in the future (Figure 6).
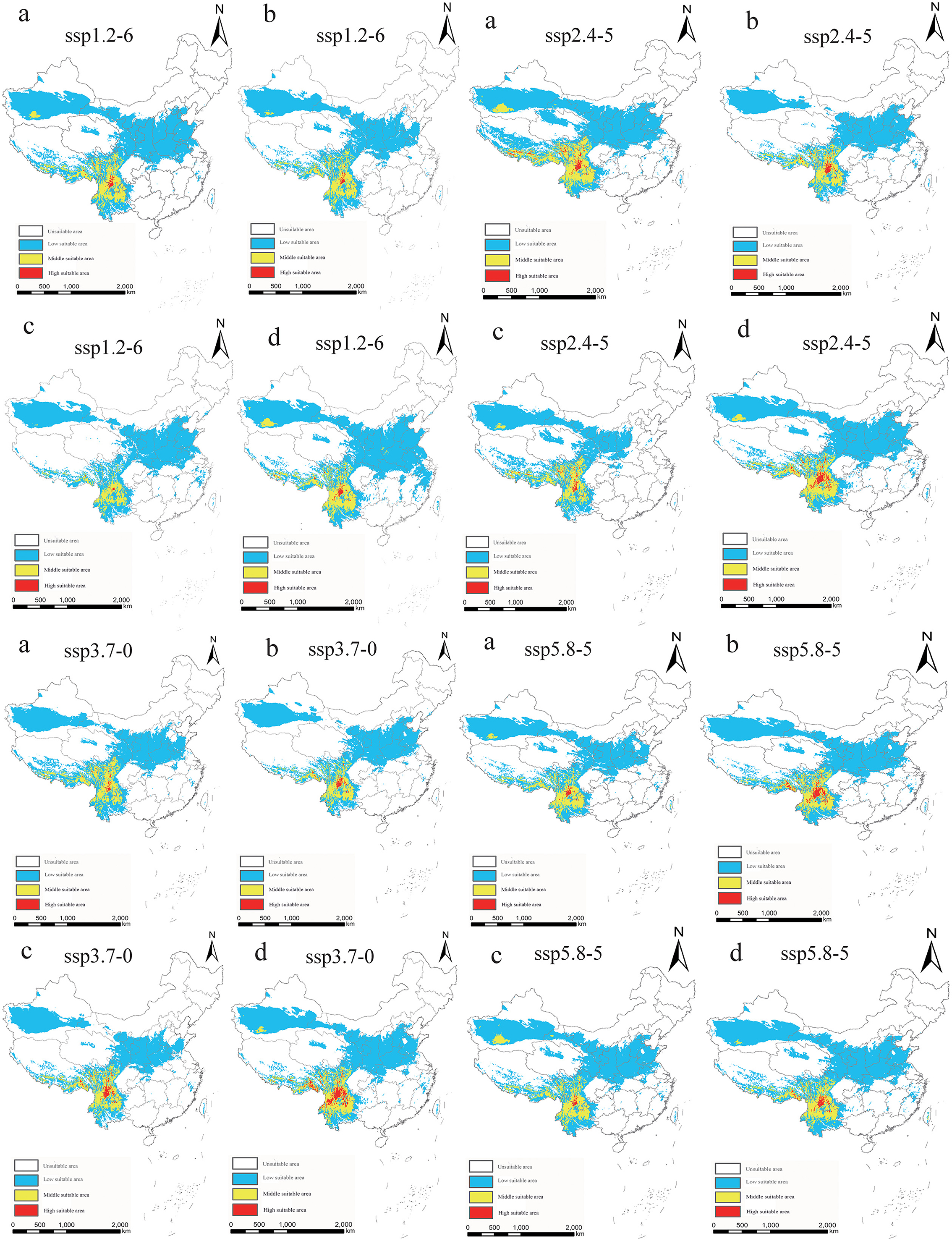
Figure 5. Suitable areas in China for P. hesperomys under different future climate scenario models (a: 2021–2040, b: 2041–2060, c: 2061–2080, and d: 2081–2100).
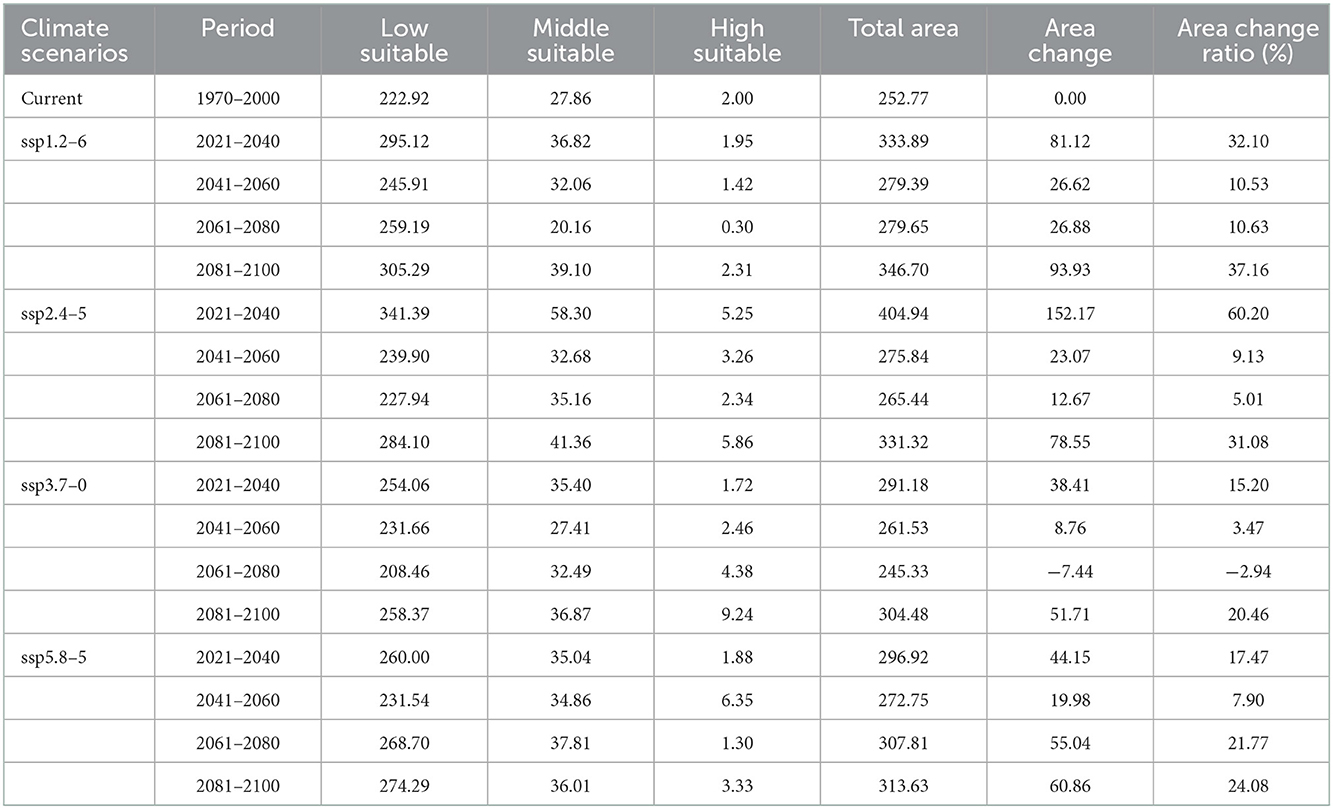
Table 2. Suitable areas of P. hesperomys in China under current and future climate scenarios (×104 km2).
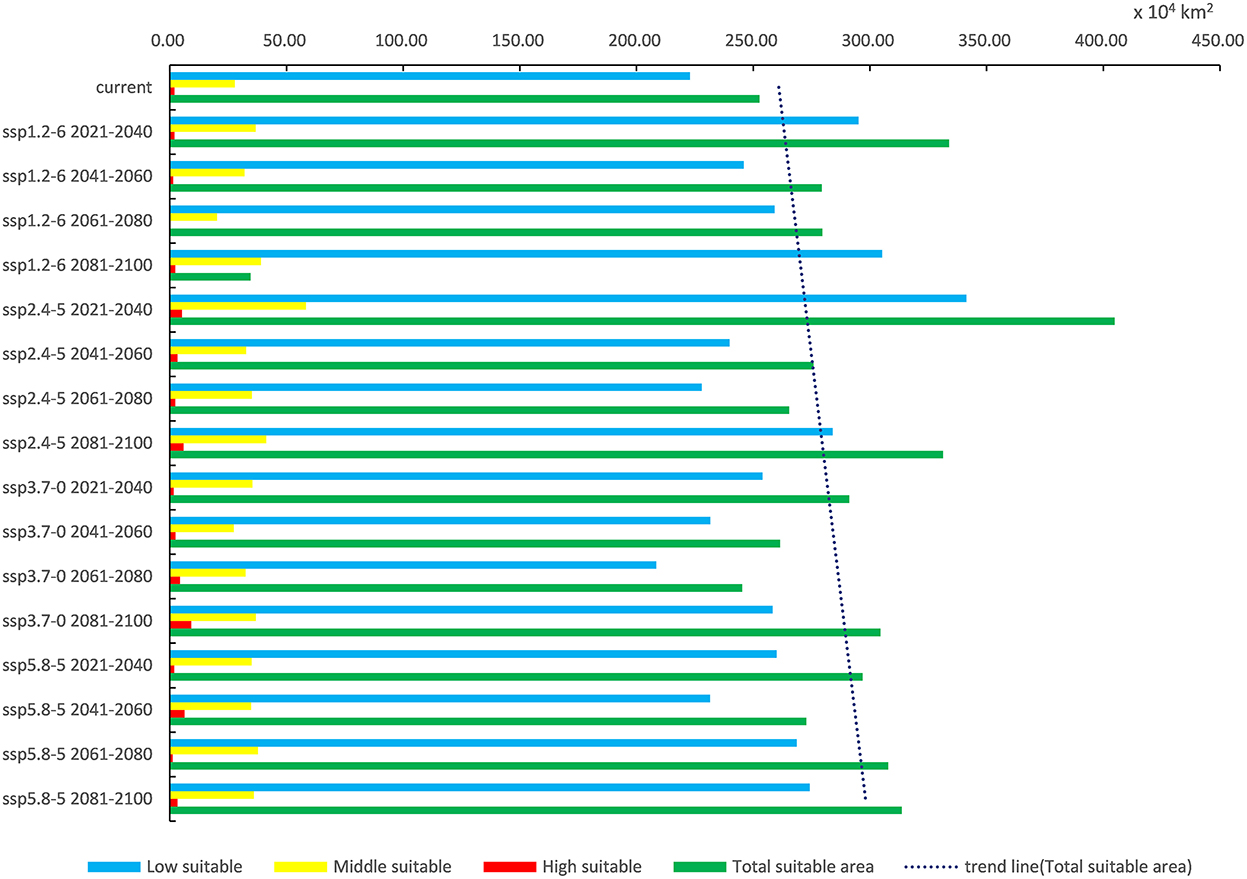
Figure 6. Variation trend of suitable areas for P. hesperomys in China under current and future climate scenarios.
In light of the predictions for different future climate scenario models, the middle and high suitable areas for O. sexdentatus in China are distributed in southern Tibet and the northwestern part of Yunnan, and the low suitable areas are mainly distributed in Yunnan, Sichuan, and Tibet (Figure 7). As shown in Table 3, under the ssp3.7-0 climate scenario model from 2061 to 2080, O. sexdentatus has the largest suitable area in China (72.54 × 104 km2). The overall trend of the suitable area in China is decreasing (Figure 8).
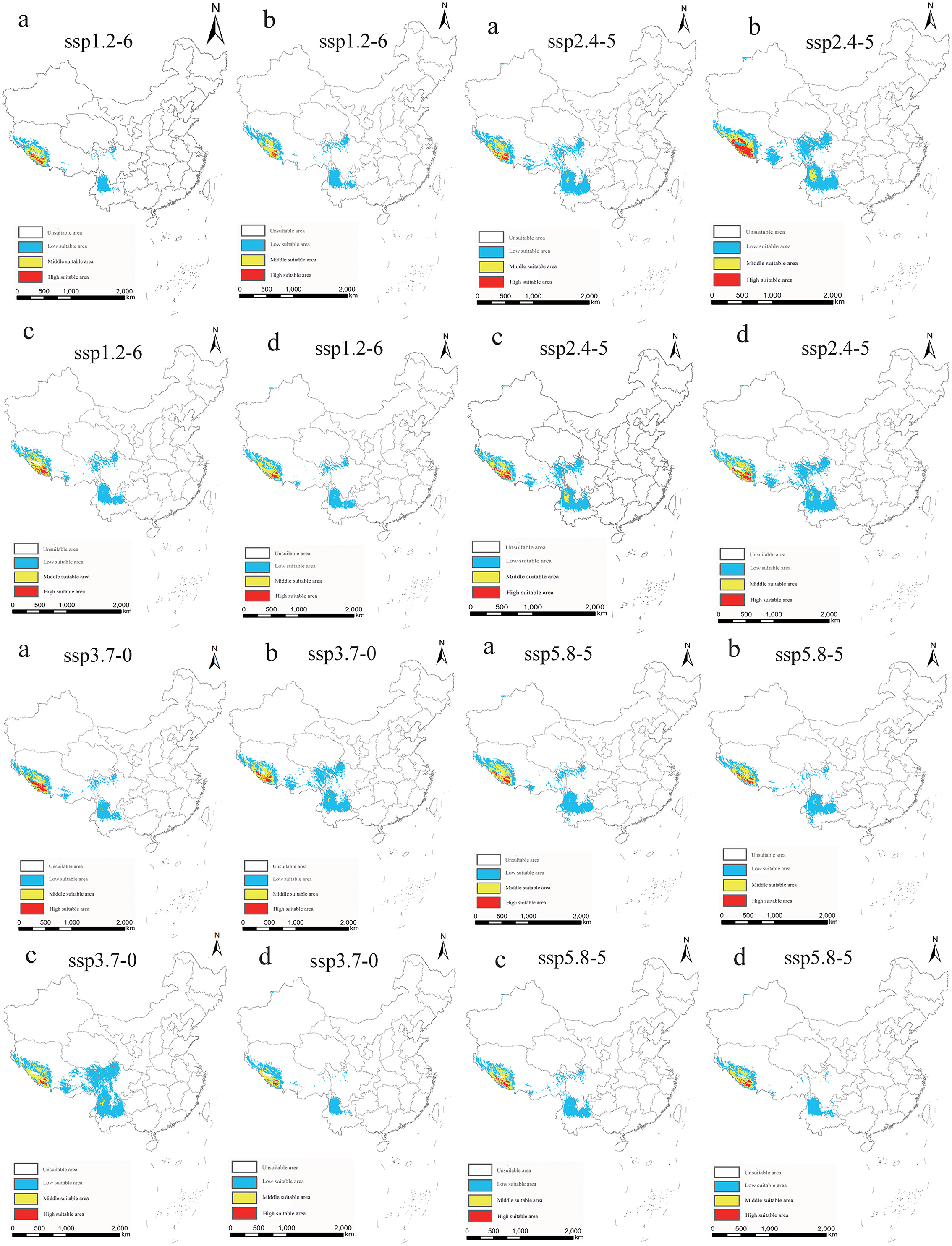
Figure 7. Suitable areas in China for O. sexdentatus under different future climate scenario models (a: 2021–2040, b: 2041–2060, c: 2061–2080, and d: 2081–2100).
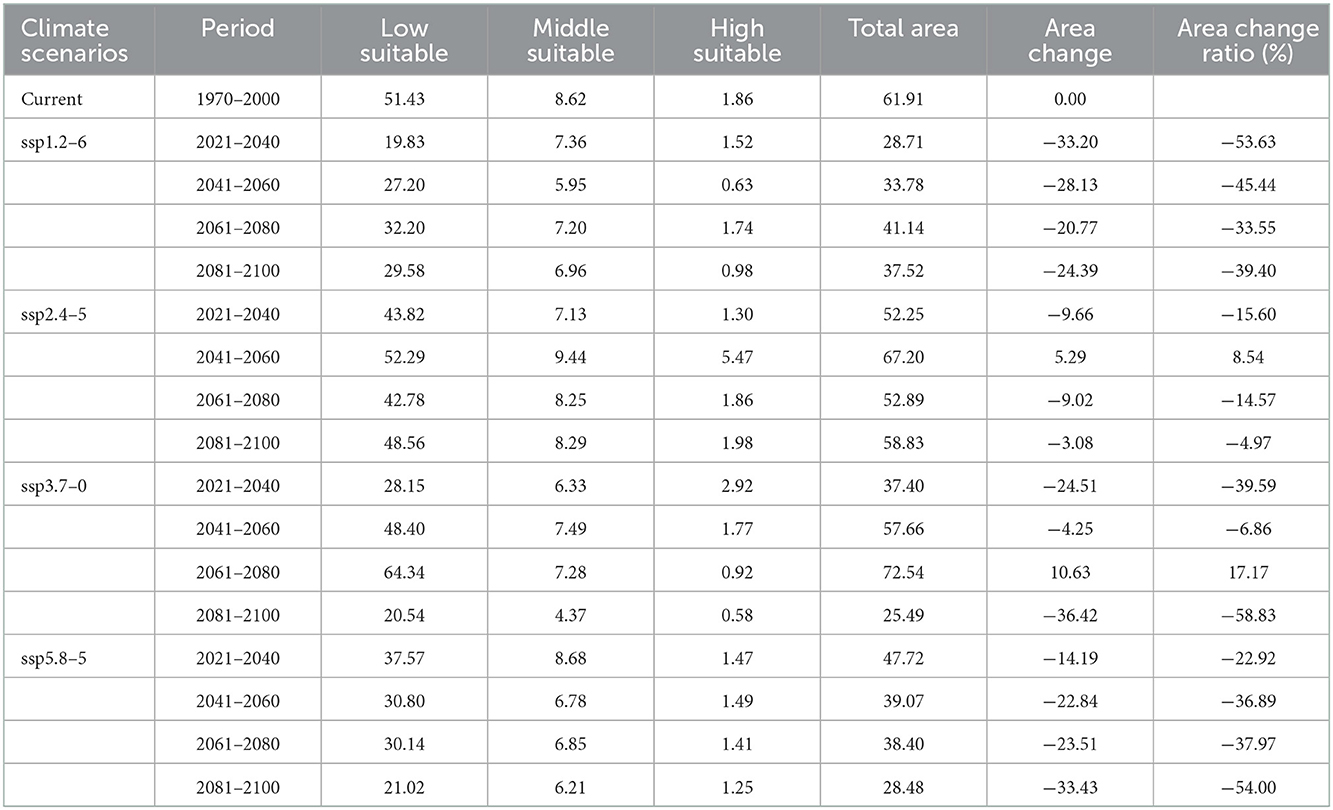
Table 3. Suitable areas of O. sexdentatus in China under current and future climate scenarios (×104 km2).
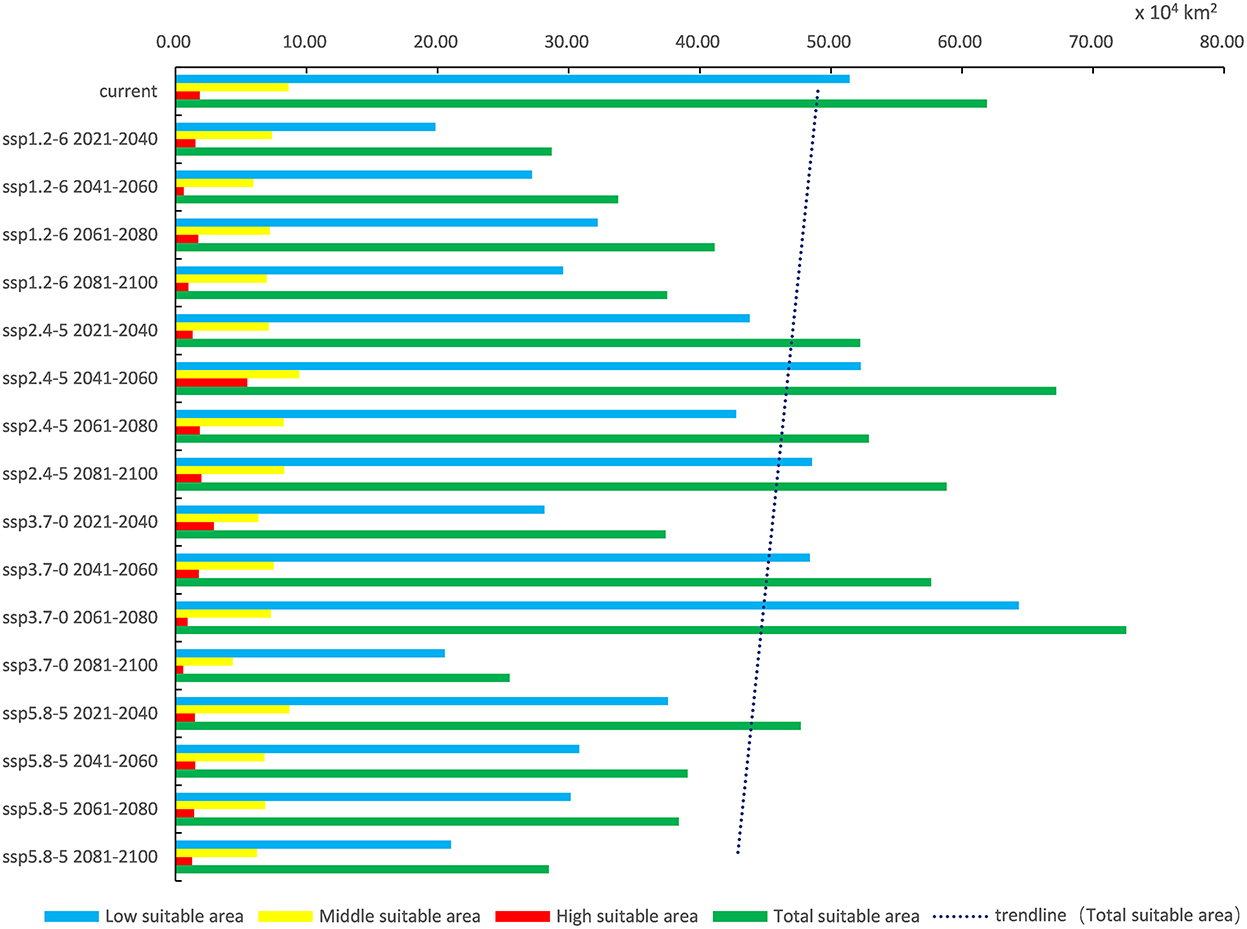
Figure 8. Variation trend of suitable areas for O. sexdentatus in China under current and future climate scenarios.
We evaluated the model's overall performance using a ROC curve (Figure 9). The accuracy of the predicted results was estimated by the AUC value. The AUC value of the MaxEnt model for P. hesperomys and O. sexdentatus s was 0.978 and 0.990, which revealed that the accuracy of the models was high.
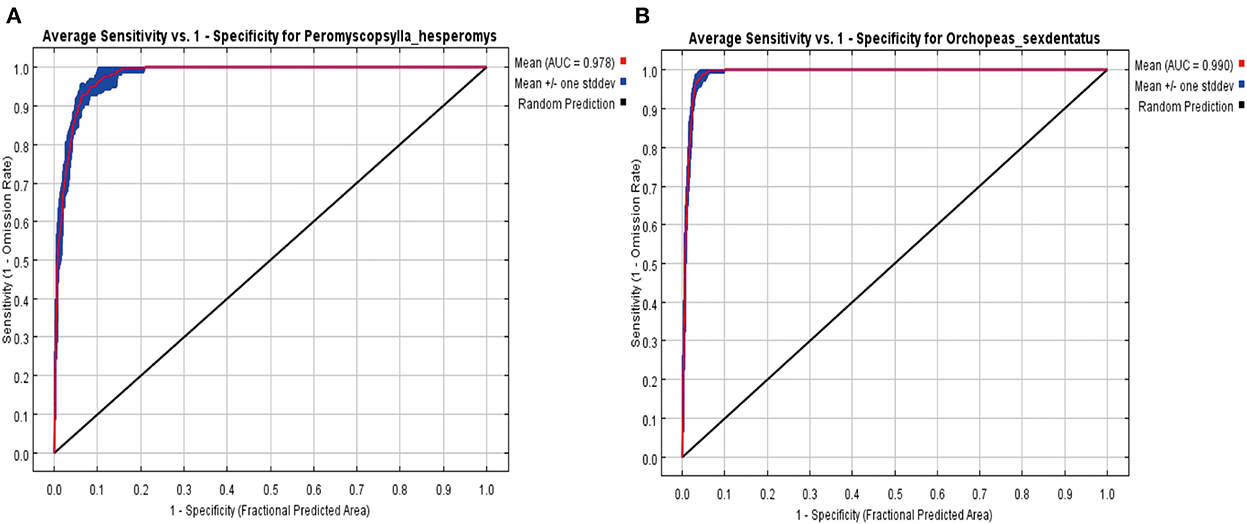
Figure 9. Receiver operating characteristic (ROC) curve by the MaxEnt for P. hesperomys (A) and O. sexdentatus (B).
According to the constructed multi-indicator invasion risk assessment system and scoring criteria of each indicator, the comprehensive risk value of two representative U.S. plague vector fleas for invading China was calculated (Supplementary Table S2). The R-value of P. hesperomys and O. sexdentatus was 0.399 and 0.393, respectively, indicating that the risk levels of them invading China are moderate.
We used the MaxEnt model with optimized parameters to predict the suitable areas of plague vector fleas within China in the future under four SSPs scenarios, which served as an early warning. It provides the basis for preventing the plague vector fleas from invading China and developing relevant custom inspection and quarantine policies.
In the context of climate change, the total suitable area for P. hesperomys in China showed an overall trend of expansion, which may be due to global warming. The suitable areas of P. hesperomys in different periods under each climate scenario were higher than those under the current climate scenario. The large scale of suitable areas that appeared in China suggests that the prevention and control of P. hesperomys should be promoted. During the 2081–2100 period of the ssp1.2-6 climate scenario, the main range of suitable zones of P. hesperomys in China will extend from the Yellow River basin provinces to Zhejiang, Anhui, Jiangsu, Hubei, Chongqing, and a little in Guizhou, Hunan, Jiangxi, and Fujian. Increased monitoring of fleas in these areas is recommended to lower the risk of invasion and colonization. Our study found that suitable areas of P. hesperomys and O. sexdentatus in China include Yunnan, Sichuan, Chongqing, Tibet, Qinghai, Inner Mongolia, Guizhou, Xinjiang, Hebei, Gansu, Shaanxi, and Liaoning, which overlap with the distribution of their hosts (i.e., small rodents and woodrats) (28). Therefore, the risks of invasion, colonization, and the spread of the plague vector flea in these areas of China are relatively high.
According to the Köppen climate classification, the climate type of P. hesperomys distribution zones in the southeastern U.S. is the same as that of southern China for a temperate climate with hot summers but without any dry seasons (Cfa). Its climate is characterized by a minimum monthly mean temperature in the range of 0 to 18°C and a maximum monthly mean temperature of 22°C or higher within 1 year and high humidity. The Cfa has shown a significant expanding trend during the past 40 years in China (29), which is consistent with the results of our study: the suitable areas for P. hesperomys have a tendency to expand in southern China in the future. The expansion of the suitable area implies a rising public health risk for the population in the area, so it is necessary to strengthen health education on flea prevention and control of the population in the potentially suitable areas. In addition, the irregular geographical distribution of precipitation and high occurrences of extreme weather might have an impact on the distribution patterns for P. hesperomys.
The efficiency of plague vector transmission for fleas is influenced by temperature, precipitation, and relative humidity (30, 31). Temperature is the primary factor influencing the growth of the plague vector flea, by accelerating flea growth observed at both larvae and pupae stages at higher temperatures (32). Therefore, global warming is conducive to the survival and activity of the plague vector flea. The main environmental variables influencing the distribution of potentially suitable areas for P. hesperomys were found to be Tmin11 (minimum temperature in November) and Tmin1 (minimum temperature in January), while the main environmental variables affecting the distribution of potentially suitable areas for O. sexdentatus were Bio3 (isothermality) and Bio2 (mean diurnal range). It also illustrated that high temperatures had a dramatic effect on the survival of vector fleas. Furthermore, a longer survival time of fleas was found at elevated humidity conditions. With rising humidity, the density of the host increases, and fleas may be more effective in transmitting Yersinia pestis (33). As the Cfa is characterized by high temperatures and humidity, the expansion of Cfa in southern China will increase the suitability of plague vector fleas as well as the risk of colonization and the ability to spread the plague.
In the background of climate change and global warming, originally unsuitable areas for biological survival are gradually transformed into suitable areas for survival, which lays the foundation for the invasion of alien species. The phenomenon of biological invasion becomes more frequent and serious (9). At present, invasive species have been discovered in all provincial-level administrative regions of China, and the southwest and southeast coasts are severe hazard areas (34).
With the implementation of China's strategies “The Silk Road Economic Belt” and “21st-Century Maritime Silk Road,” the risk of biological invasion in China continued to ascend. Our study revealed that the provinces along the Silk Road Economic Belt: Xinjiang, Gansu, Shaanxi, Chongqing, Sichuan, Inner Mongolia, Henan, Hebei, Qinghai, Beijing, and Tianjin were distributed with suitable areas for the plague vector flea. Furthermore, there are widespread suitable areas for vector fleas in Europe and Central Asia along the Silk Road. Thus, China Railway Express along the Silk Road Economic Belt offers a possible path for fleas to invade China. The third plague pandemic was spread by sea from India to the United States. The 21st-Century Maritime Silk Road starts in southern Europe and ends at the China port cities of Beihai, Haikou, Guangzhou, and Zhanjiang. Therefore, vector fleas invading China via shipping is possible.
The invasion risk assessment system reveals that two representative U.S. plague vector fleas are at moderate risk for invading China. Owing to the two species' significant potential for invasive damage in China, it is essential to bolster monitoring initiatives to prevent entry, colonization, and future spreading. We advise taking the following actions to prevent and control the vector flea: First, regulations control: departments for specific plague control in middle or high suitable areas and harbor cities with low suitable areas (i.e., Beijing, Tianjin, Qingdao, Rizhao, and Lianyungang) are needed to prevent the introduction of rodents and fleas. Second, use an early warning platform to master the dynamics of flea population. Flea surveillance has to be upped, especially in locations that are moderately or highly suitable for the two fleas. Third, environmental control: (1) hang sticky papers in the room to capture fleas, and (2) vacuum rooms infested with fleas, which can reduce 60% of eggs, 27% of larvae, and 95% of adults. Finally, chemical control: (1) clothing against fleas: spray 0.1% of dichlorvos on the bedding, and (2) to kill fleas on pigs, dogs, cats, and apply 1% permethrin or trichlorfon to the animal's body.
Innovations: First, there is no study on the risk of vector fleas invading China at present. This research provides a systematic research method for the visual screening of the key invasive vector fleas and their invasion risks in China. Second, this study found many potentially suitable areas for fleas with the risk of invading China. Although they have not been intercepted and discovered in China at present, they may still colonize and localize under certain conditions. Finally, the suitable areas for vector fleas are gradually expanding to southern China with global warming and the dramatic increase of the Cfa climate in China.
Constrained by historical climate data (1970–2000), there is a lack of the latest climate data to estimate current suitability zones, so there are limitations in this study. The MaxEnt model used in this study only considered the environmental adaptability of the species and only fitted selected environmental variables, such as temperature, precipitation, and elevation. A more accurate estimation of distribution in suitable areas requires additional variables such as topographic factors, soil moisture, interspecific relationships, vegetation cover type, population density, host density, land use, and economic indicators like GDP per capita. While this invasion risk assessment system involves a large number of related indicators, it still cannot fully and objectively represent the actual invasion risk of the species. Therefore, further optimization in the construction and screen of the indicator system is required.
From a worldwide perspective, our research offered scientific data about the distribution of the suitable areas of P. hesperomys and O. sexdentatus. Both species have large potential suitable areas in China, the temperature has a vital impact on the distribution of suitable areas, and the suitable areas of P. hesperomys are expanding into the southern part of China under global warming. The invasion risk assessment system was adopted, and both species were found to be of moderate invasion risk for China, which served as an early warning for biological invasion in China. China should develop scientific prevention and control strategies and strengthen surveillance of its related alarm systems to prevent the invasion of overseas flea vectors.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
HH and QL: conception, design of the research, and critical revision of the manuscript for intellectual content. HL, YL, LD, CL, LZ, BW, DM, QM, and JW: literature review and acquisition of data. HL: analysis and interpretation of the data and writing of the manuscript. All authors read and approved the final draft.
This study was supported by the National Key R&D Program of China (No. 2020YFC1200101) and the National Natural Science Foundation of China (No. 32090023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1018327/full#supplementary-material
Supplementary Table 1. Invasion risk assessment system for vector biology and reference standard of index assignment.
Supplementary Table 2. Two vector fleas risk assessment indicator score.
Supplementary Table 3. Occurrence points of Peromyscopsylla hesperomys.
Supplementary Table 4. Occurrence points of Orchopeas sexdentatus.
2. Ren G, Guo J, Xu M. Climate changes of China mainland over the past half century. Acta Meteorol Sin. (2005) 63:15.
3. Liu Q. Impact of climate change on vector-borne diseases and related responsestrategies in china: major research findings and recommendations for future research. Chin J Vector Biol Control. (2021) 32:11. doi: 10.11853/j.issn.1003.8280.2021.01.001
4. Kausrud KL, Viljugrein H, Frigessi A, Begon M, Davis S, Leirs H, et al. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proceed Biol Sci. (2007) 274:1963–9. doi: 10.1098/rspb.2007.0568
6. El Hamzaoui B, Laroche M, Almeras L, Berenger JM, Raoult D, Parola P. Detection of bartonella spp. In Fleas by Maldi-Tof Ms. PLoS Negl Trop Dis. (2018) 12:e0006189. doi: 10.1371/journal.pntd.0006189
7. Stenseth NC, Samia NI, Viljugrein H, Kausrud KL, Begon M, Davis S, et al. Plague dynamics are driven by climate variation. Proc Natl Acad Sci U S A. (2006) 103:13110–5. doi: 10.1073/pnas.0602447103
8. Ben-Ari T, Neerinckx S, Agier L, Cazelles B, Xu L, Zhang Z, et al. Identification of Chinese plague foci from long-term epidemiological data. Proc Natl Acad Sci U S A. (2012) 109:8196–201. doi: 10.1073/pnas.1110585109
9. Weber BE. Plant invasions in China: what is to be expected in the wake of economic development? BioSci. (2008) 58: 437–44. doi: 10.1641/B580511
10. Kulhanek SA, Ricciardi LA. Using ecological niche models to predict the abundance and impact of invasive species: application to the common carp. Ecol Appl. (2011) 21:203–13. doi: 10.1890/09-1639.1
11. Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol S. (2009) 40:677–97. doi: 10.1146/annurev.ecolsys.110308.120159
12. Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. (2006) 190:231–59. doi: 10.1016/j.ecolmodel.2005.03.026
13. Merow C, Smith MJ, Silander Jr JA. A practical guide to maxent for modeling species' distributions: what it does, and why inputs and settings matter. Ecography. (2013) 36:1058–69. doi: 10.1111/j.1600-0587.2013.07872.x
14. Phillips SJ, Dudík M. Modeling of species distributions with maxent: new extensions and a comprehensive evaluation. Ecography. (2008) 31:161–75. doi: 10.1111/j.0906-7590.2008.5203.x
15. Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ, et al. Statistical explanation of maxent for ecologists. Divers Distrib. (2011) 17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x
16. Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A. Original article: predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in madagascar. J Biogeogr. (2006) 34:102–17. doi: 10.1111/j.1365-2699.2006.01594.x
17. Brown HE, Ettestad P, Reynolds PJ, Brown TL, Hatton ES, Holmes JL, et al. Climatic predictors of the intra-and inter-annual distributions of plague cases in New Mexico based on 29 years of animal-based surveillance data. Am J Trop Med Hyg. (2010) 82:95–102. doi: 10.4269/ajtmh.2010.09-0247
18. Bevins SN, Baroch JA, Nolte DL, Zhang M, He H. Yersinia pestis: examining wildlife plague surveillance in China and the USA. Integr Zool. (2012) 7:99–109. doi: 10.1111/j.1749-4877.2011.00277.x
19. Colman RE, Vogler AJ, Lowell JL, Gage KL, Morway C, Reynolds PJ, et al. Fine-scale identification of the most likely source of a human plague infection. Emerg Infect Dis. (2009) 15:1623–5. doi: 10.3201/eid1510.090188
20. Warren DL, Glor RE, Turelli M. Enmtools: a toolbox for comparative studies of environmental niche models. Ecography. (2010) (33):607–11. doi: 10.1111/j.1600-0587.2009.06142.x
21. Lu Y, Wu T, Li Y, Yang B. Mitigation of the double itcz syndrome in Bcc-Csm2-Mr through improving parameterizations of boundary-layer turbulence and shallow convection. Geosci Model Dev. (2021) 14:5183–204. doi: 10.5194/gmd-14-5183-2021
22. Ji W, Gao G, Wei J. Potential global distribution of daktulosphaira vitifoliae under climate change based on maxent. Insects. (2021) 12:347. doi: 10.3390/insects12040347
23. Gao T, Shi J. The potential global distribution of Sirex juvencus (Hymenoptera: Siricidae) under near current and future climatic conditions as predicted by the maximum entropy model. Insects. (2021) 12:222. doi: 10.3390/insects12030222
24. Dan LW, Seifert SN. Ecological niche modeling in maxent: the importance of model complexity and the performance of model selection criteria. Ecol Appl. (2011) 21:335–42. doi: 10.1890/10-1171.1
25. Zhou R, Gao Y, Chang N, Gao T, Ma D, Li C, et al. Projecting the potential distribution of glossinamorsitans (diptera: glossinidae) under climate change using the maxent model. Biology (Basel). (2021) 10:1150. doi: 10.3390/biology10111150
26. Wang R, Huang P, Fu J. Invasion risk assessment, early warning and monitoring of major invasivepests introduced with fruit and vegetables from Taiwan to Mainland China. J Biosafety. (2019) 28:11.
27. Li C, Gao Y, Chang N, Ma D, Zhou R, Zhao Z, et al. Risk assessment of anopheles philippinensis and anopheles nivipes (diptera: culicidae) invading China under climate change. Biology (Basel). (2021) 10:998. doi: 10.3390/biology10100998
28. China National Bureau of Statistics: 9-7 Forest Pest Bio Control by Region (2014) Cont. Table [Eb/Ol] (2016-09-13). Available online at: http://www.stats.gov.cn/ztjc/ztsj/hjtjzl/2014/201609/t20160913_1399631.html (accessed November 9, 2022).
29. Feng Y, Du S. Climate changes and landscape responses of China during the past 40 years (1979–2018) under köppen-geiger climate classification. ISPRS. (2020) 3:731–7. doi: 10.5194/isprs-annals-V-3-2020-731-2020
30. Cordes LS, Blumstein DT, Armitage KB, CaraDonna PJ, Childs DZ, Gerber BD, et al. Contrasting effects of climate change on seasonal survival of a hibernating mammal. PNAS. (2020) 117:18119–26. doi: 10.1073/pnas.1918584117
31. Ben Ari T, Neerinckx S, Gage KL, Kreppel K, Laudisoit A, Leirs H, et al. Plague and climate: scales matter. PLoS Pathog. (2011) 7:e1002160. doi: 10.1371/journal.ppat.1002160
32. Kreppel KS, Telfer S, Rajerison M, Morse A, Baylis M. Effect of temperature and relative humidity on the development times and survival of synopsyllus fonquerniei and xenopsylla cheopis, the flea vectors of plague in madagascar. Parasit Vectors. (2016) 9:82. doi: 10.1186/s13071-016-1366-z
33. Eads DA. (editor) Factors Affecting Flea Densities in Prairie Dog Colonies: Implications for the Maintenance and Spread of Plague. Colorado State University (2014).
Keywords: global distribution, climate change, potential suitable areas, risk assessment, plague vector fleas
Citation: Li H, Liang Y, Dong L, Li C, Zhang L, Wang B, Ma D, Mu Q, Wang J, Hou H and Liu Q (2023) Predicting global potential distribution of Peromyscopsylla hesperomys and Orchopeas sexdentatus and risk assessment for invading China under climate change. Front. Public Health 10:1018327. doi: 10.3389/fpubh.2022.1018327
Received: 13 October 2022; Accepted: 28 November 2022;
Published: 05 January 2023.
Edited by:
Ciro Fernando Bustillo-Lecompte, Ryerson University, CanadaReviewed by:
David Eads, United States Department of the Interior, United StatesCopyright © 2023 Li, Liang, Dong, Li, Zhang, Wang, Ma, Mu, Wang, Hou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Hou,  aGZob3VAc2RmbXUuZWR1LmNu; Qiyong Liu,
aGZob3VAc2RmbXUuZWR1LmNu; Qiyong Liu,  bGl1cWl5b25nQGljZGMuY24=
bGl1cWl5b25nQGljZGMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.