- 1Department of Biosciences, COMSATS University Islamabad, Islamabad, Pakistan
- 2Department of Life Sciences, School of Science, University of Management and Technology, Lahore, Pakistan
- 3Department of Parasitology, Faculty of Veterinary Medicine, Firat University, Elazig, Turkey
- 4Department of Zoology, Abdul Wali Khan University Mardan, Mardan, Pakistan
- 5National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), Shanghai, China
- 6Key Laboratory of Parasite and Vector Biology, National Health Commission of the People's Republic of China, Shanghai, China
- 7WHO Collaborating Center for Tropical Diseases, Shanghai, China
- 8The School of Global Health, Chinese Center for Tropical Diseases Research, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Human alveolar echinococcosis (AE) is a neglected zoonotic disease. Prevalence of AE in humans is reported in Pakistan as a result of poor economic and sanitary conditions, close proximity to wildlife and limited knowledge of AE. Studies on the prevalence and transmission of AE have been limited, especially for rural Pakistan. The study objectives were to identify knowledge, attitudes and practices relating to AE, to determine awareness of the disease, and to identify knowledge about possible risk factors of infections involving the landscape epidemiological attributes of rural villages in Hunza, one of the districts of Gilgit-Baltistan, a region of Pakistan that borders China.
Methods: A community-based cross-sectional study was conducted among the general population of Hunza to establish the level of awareness, knowledge, attitudes, practices, landscape epidemiology, and disease management and control relating to AE in rural areas of Hunza. Data were collected by questionnaire.
Results: A total of 387 questionnaires was received. Statistical analysis showed that the population's knowledge about the disease was poor. The attitudes and practices of the participants indicated that their risk of infection was low. Knowledge of landscape epidemiology of the disease was poor but knowledge about AE disease management was good. The attitudes of residents toward disease treatment and control strategies were positive, although the overall knowledge of participants about prevention of infection was poor.
Conclusion: Knowledge of AE is poor among the residents of Hunza, Pakistan. Our study demands continued and strengthened awareness of the changes to lifestyle and practices associated with AE, not only in the study locality but throughout other areas of Pakistan.
Background
Echinococcosis is a zoonotic parasite diseases caused by tapeworm species of the genus Echinococcus spp. There are four types of echinococcosis: (1) infection with a species complex centered on Echinococcus granulosus sensu lato that causes cystic echinococcosis (CE), often known as hydatid disease or hydatidosis; (2) alveolar echinococcosis (AE), induced by Echinococcus multilocularis; (3) polycystic neotropical echinococcosis, caused by E. vogeli; and (4) unicystic echinococcosis, caused by E. oligarthrus. CE and AE are the two most common forms with medical and public health implications in humans. Foxes, coyotes and dogs are host to the adult of E. multilocularis, whereas small wild rodents host the metacestodes (larvae). Infected dogs and foxes are the main source of infection in humans (1). Echinococcus has two mammalian hosts in its life cycle. The adult tapeworm lives in the small intestine of a carnivore (the definitive host) and generates oncospheres in the form of eggs. The carnivore's digestive tract releases either cestode segments (gravid proglottids) holding eggs or free eggs into the environment. In a larval stage, the metacestode develops in internal organs after an intermediate host takes eggs orally. The metacestode typically produces a large number of protoscoleces, each of which has the potential to develop into an adult cestode once ingested by a suitable final host. Humans are accidental hosts who do not usually play a role in the development cycle of the parasite. However, infection in humans results in serious morbidity and mortality if left untreated, along with substantial social and economic consequences (2).
AE is characterized by an asymptomatic incubation period of 5–15 years and the slow development of a primary tumor-like lesion, which is usually located in the liver, although it can extend to other organs. Because the cysts grow slowly, infection with AE may not cause symptoms for several years. As the cysts grow, they may cause pain or discomfort in the upper abdomen, weakness, weight loss, general malaise and signs of hepatic failure (3). Humans can acquire E. multilocularis eggs by contact with infected definitive hosts or consuming food or water contaminated with eggs (4). The incidence of the infection and the dynamics of transmission are further affected by the hosts' intrinsic vulnerability, the nature of their interactions, host population densities, seasonal fluctuations, host age, food diversity and other factors (5).
Till 1980s, only four countries in central Europe were known to have E. multilocularis endemic areas: Austria, France, Germany and Switzerland (6). During 1999–2000, parasite has a much wider geographic range, including at least 12 European countries: Austria, Belgium, the Czech Republic, Denmark, France, Germany, Liechtenstein, Luxembourg, Poland, the Slovak Republic, the Netherlands and Switzerland (7, 8). The recent report of E. multilocularis in eastern Poland and the Slovak Republic supports the concept that the endemic zones in central and eastern Europe, which were previously thought to be distinct, are now linked. According to a communication from animal health authorities in Troms and Finnmark, Norway, E. multilocularis metacestodes were reported in rats on Spitsbergen Island, which is part of the Norwegian Svalbard Island group in the Barents Sea, in 1999 (9). Between 1934 and 1983, 157 human cases of AE were diagnosed, resulting in an average of 3.1 new cases per year in Turkey (10).
It has been reported that 22.9% of red foxes (Vulpes vulpes) and 16% of jackals (Canis aureus) were infected with adult stages of E. multilocularis in Ardabile province, northern Iran. In addition, a total of 37 human AE cases were diagnosed in various hospitals in Iran over 3.5 years, the majority of which were in the Ardabile province (11).
In China, the incidence of AE was 257 cases in Ningxia, 88 cases in Xinjiang, 71 cases in Gansu, 49 cases in Sichuan, 37 cases in Qinghai, 1 case in Heilongjiang Tibet provinces in 1992 (12). In recent years, over 350 AE cases have been detected in the Gansu region alone. Between 1991 and 1997, an ultrasonography mass screening survey with serological confirmation in south Gansu demonstrated a group prevalence of human AE of about 4% (135/3331). Given the size of the population in this rural location, this equates to a local group prevalence of around 200 cases per 100,000. However, because AE has a focused distribution, group or local prevalence may not be indicative of prevalence in wider regions (13).
Because it is a zoonotic infectious disease, the success of AE prevention and control programs depends on the cooperation of people living in areas where the disease is common. For this reason, knowing the knowledge, attitudes and practices of the society about the disease is one of the important determinants of social participation in the implementation of prevention and control programs. Thus, the objective of the current study was to assess knowledge, attitudes and practices (KAPs) regarding AE, to determine awareness of AE in various villages in Hunza province of Pakistan, which borders neighboring areas of China.
Methods
Study area
The study focused on rural areas of Gilgit-Baltistan, which is located in the northern part of Pakistan. It is a mountainous region and covers an area of 72,971 km2 (14). Gilgit-Baltistan is divided into three main regions: Gilgit, Baltistan and Diamer. The Gilgit region has 14 districts, one of which is Hunza. This study was performed specifically in Hunza and included both the central and upper regions of the district. Hunza covers an area of 7,900 km2 and has a total population of 70,000 (15). Hunza is bounded north and east by the Kashgar area of China's Xinjiang Uyghur Autonomous Region. We selected 25 villages for our study, of which 14 are located in upper Hunza and 11 in central Hunza.
Survey duration
Duration of the study was from August 2021 to December 2021. During this period, the various villages were visited to gather data on AE and identify AE risk factors.
Calculation of sample size
The total population of the Hunza valley is about 46,665, of whom 46.92% are males and 53.08% are females. In terms of age distribution, 25% are <12 years, 57% are between 12 and 60 years and 18% are >60 years (15). A recommended sample size of 382, calculated at a confidence level of 95%, was determined using Raosoft online sample size calculator (16). No survey had been conducted before on AE; thus we did not have any preliminary data on the public health impact of AE in Gilgit-Baltistan.
Study design
We used a community-based cross-sectional design for this study to determine awareness, prevalence and risk factors of AE disease in various rural areas of Hunza, Pakistan. A descriptive questionnaire was designed in such a way that students, professionals and villagers could complete it. Both qualitative and quantitative data were collected to establish the level of awareness and knowledge of AE disease among the population and their practices relating to AE.
Sampling procedure
A total of 387 questionnaires were filled by the participants. We carried out face to face interviews using structured and pretested questionnaires. Data were collected in randomly selected areas either with or without expected exposure factors such as proximity of people to dogs and other animals.
Inclusion and exclusion criteria
Individuals with or without any kind of animal association and from any occupational and educational background participated in our study. Children below the age of 15 years were excluded.
Data collection methods
A questionnaire was created to collect information on sociodemographic variables, and on knowledge, awareness, behaviors, attitudes, landscape epidemiology and disease management concerning AE. Data were collected to establish the components linked with knowledge, attitude, and awareness of AE. Because the condition does not have a distinct local label, the local language was used to describe it to participants, who were asked about their awareness of the disease. These were binary questions with “Yes” or “No” answers. The information was given in narrative text and is shown in Tables 1–6. The questionnaire included KAP items on AE management and control, household information, and dog management and care. Other questions related to risk factors, landscape epidemiology and AE disease management.
Questionnaire design
The questionnaire was designed to gather information on socio-demographics, KAPs, landscape epidemiology and AE disease management. A total of 73 questions were designed, of which 11 were on sociodemographic, 20 on knowledge, 6 on attitudes, 20 on practices, 6 on landscape epidemiology and 11 on disease management.
Statistical analysis
Data were entered in an MS Excel sheet and a database was established. Statistical analysis was performed using Jamovi 2.2.8 and 95% confidence interval was used to examine the factors involved in the prevalence of AE. The relationships between factors including KAPs, landscape epidemiology and disease management were analyzed by using logistic regression. A statistically significant difference was considered if P < 0.05.
Study variables
Both independent and dependent variables were included in this study. Dependent variables were knowledge about AE; practices that are associated with disease; attitude toward infection and exposure to dogs; landscape epidemiology; and management of AE. Independent variables included were gender, age, marital status, birthplace, number of family members, occupation, education, residency, religion, ethnicity, village and monthly income.
Results
Sociodemographic characteristics
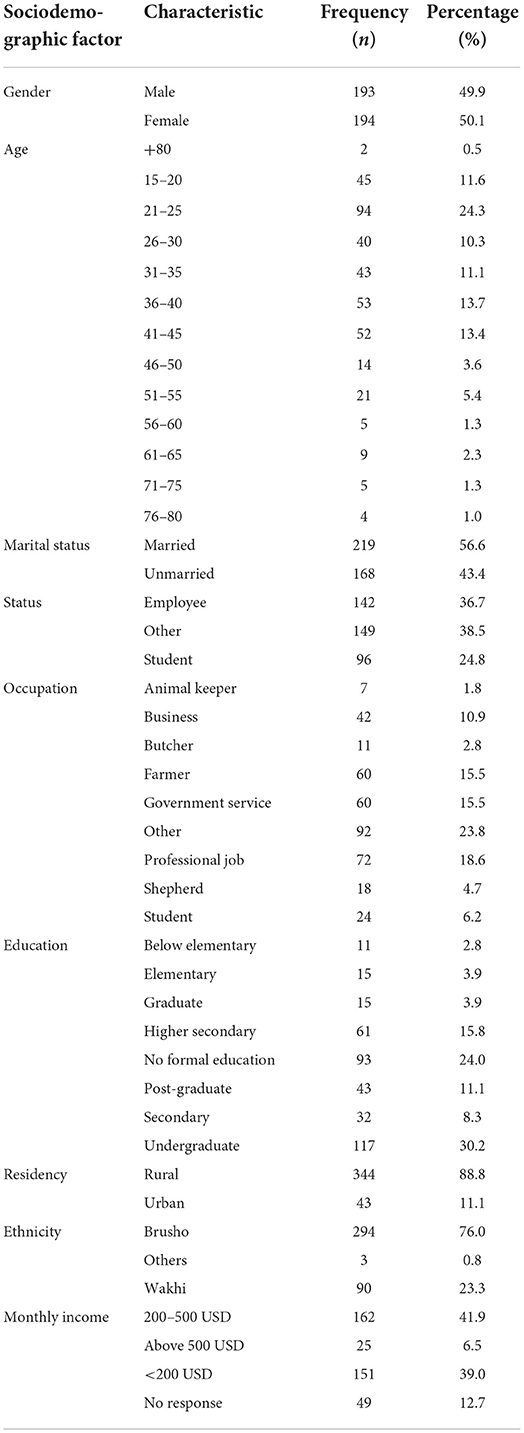
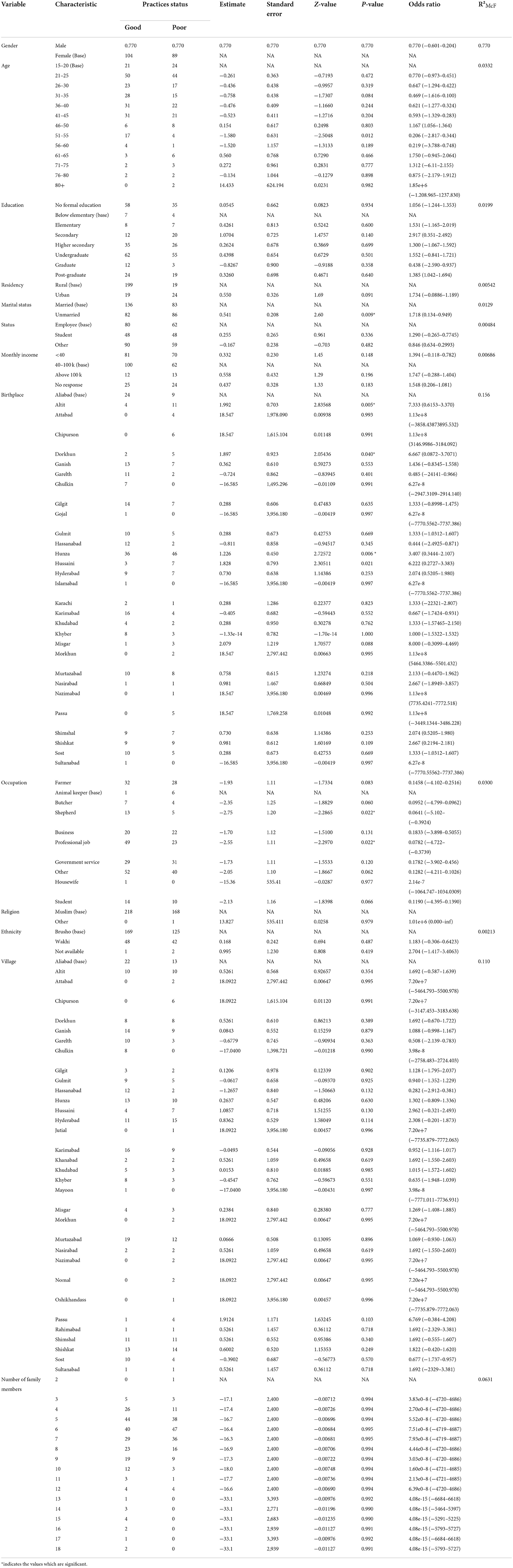
A total of 387 questionnaires were filled by participants targeted from the general population of villages in Hunza, Pakistan. The population sample, who were 15 to 80 years, comprised 49.9% (n = 193) males and 50.1% (n = 194) females. The major ethnic group was Brusho (76.0%) followed by Wakhi (23.3%). About 24.8% of the sample were in direct or indirect contact with livestock, including animal keepers (1.8%), butchers (2.8%), farmers (15.5%) and shepherds (4.7%). Regarding level of education, 45.2% were highly educated (undergraduate, graduate or post-graduate) whereas 30.8% were educated to secondary school level and 24.0% had no formal education. More details are shown in Table 1.
Knowledge about AE
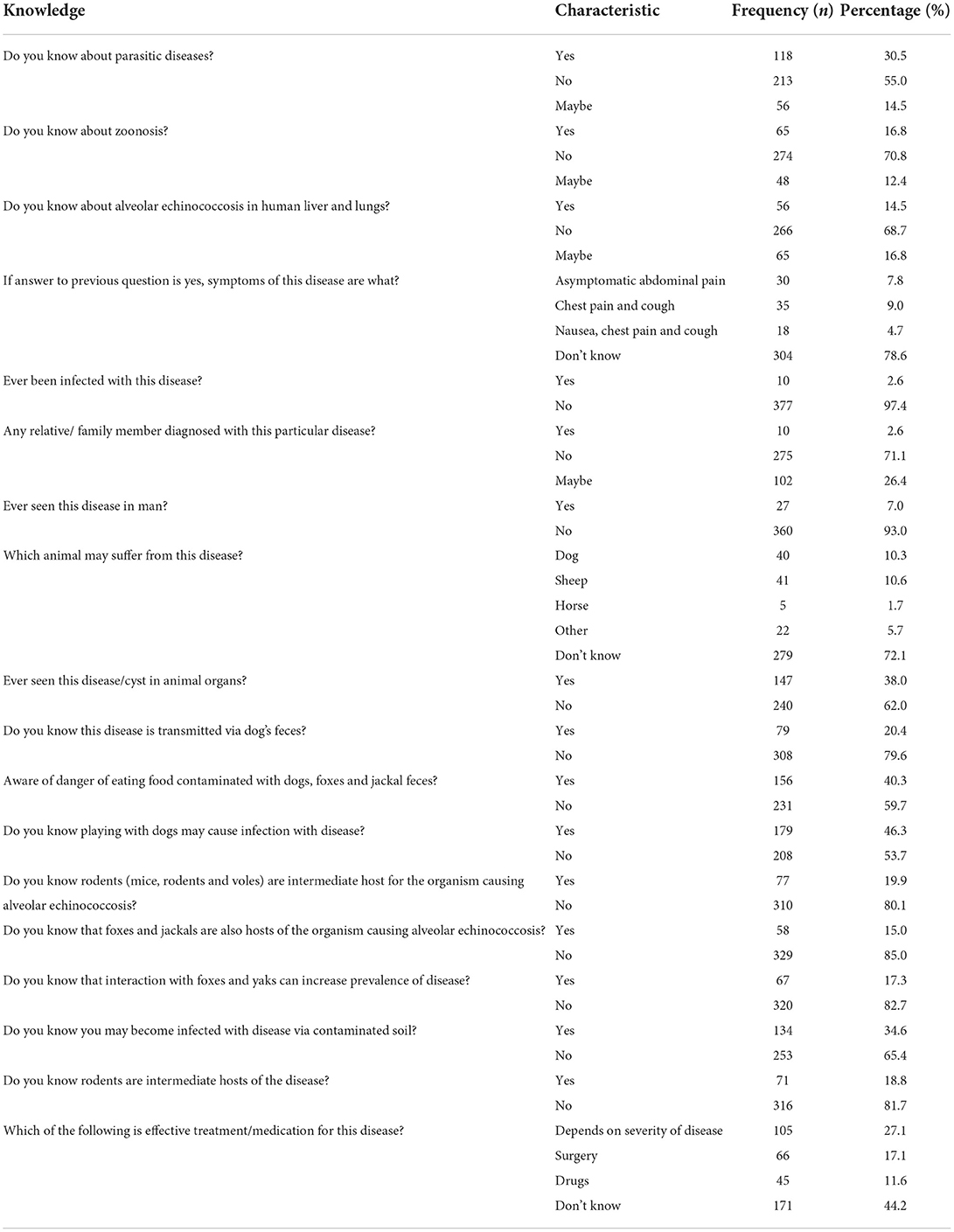
Nineteen questionnaire items of knowledge were surveyed in KAPs. Among the participants, 55.0% had never heard of any parasitic diseases and 70.8% had never heard of zoonosis. AE was not known by 68.7%, and the majority of participants had never seen the disease either in man or any animal. Of the participants, 79.6% did not know that AE could be transmitted via dog feces, and 59.7% were unaware of the danger of eating food contaminated with dog, fox or jackal feces. The majority of participants (80.1%) did not know about the host of E. multilocularis. More details are shown in Table 2.
Attitudes toward AE
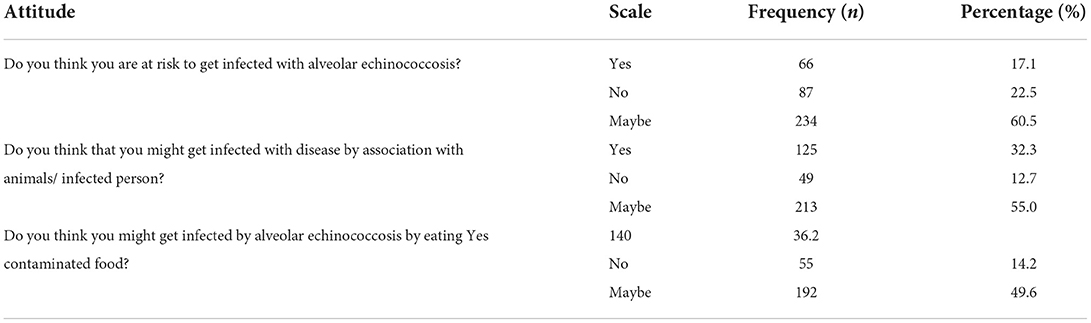
Six KAP questions concerned the attitude of the participants toward AE. Of the participants, 60.5% of the participants thought that they might be at risk of infection with AE, and 32.3% believed that they could become infected with the disease by association with animals or an infected person. Being infected with the disease by eating contaminated food was a belief expressed by 36.2%. More details are given in Table 3.
Practices related to AE
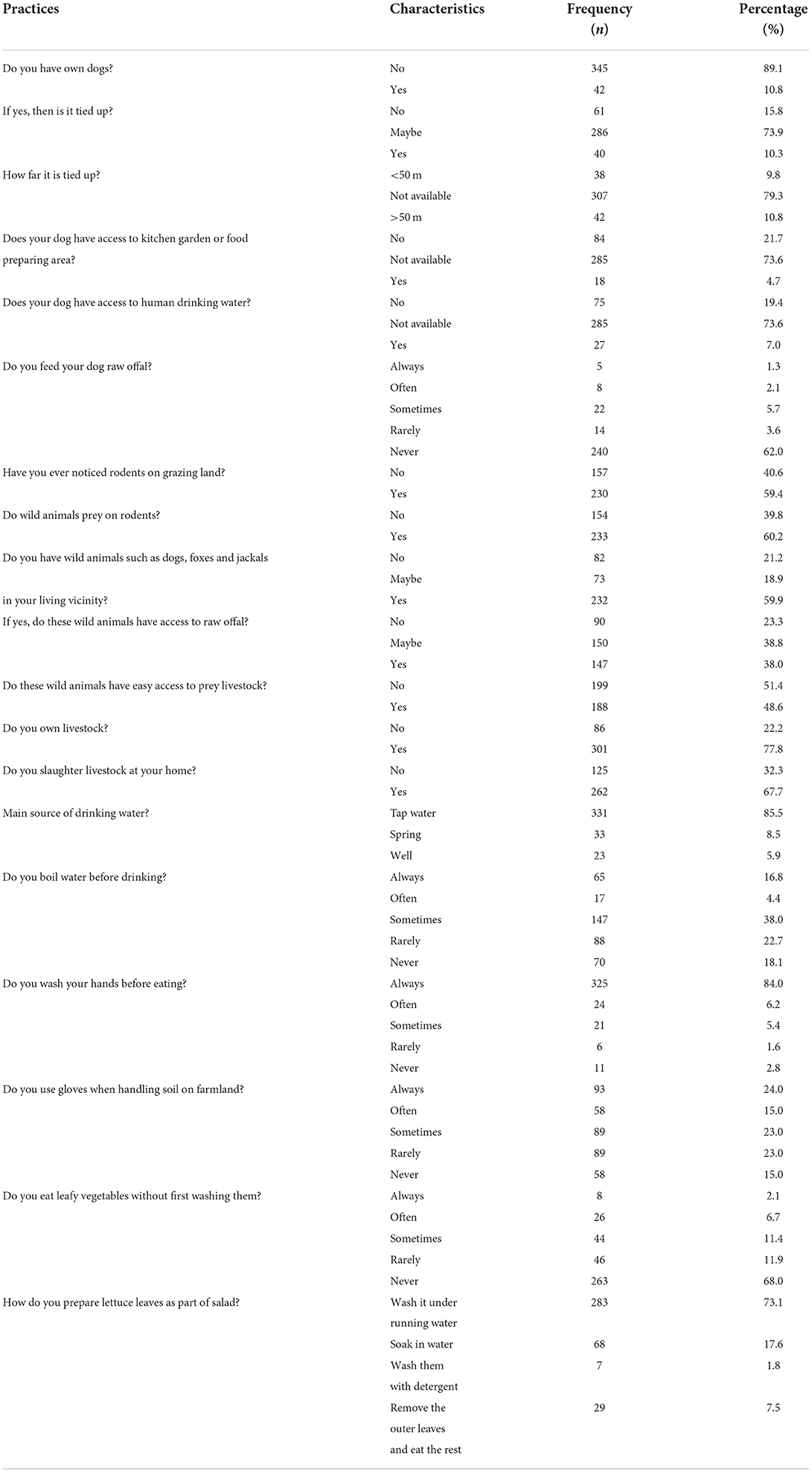
Among the participants, 10.8% owned a dog and 15.8% did not tie up their dogs; 21.7% of individuals who owned dogs did not give them access to the kitchen garden and 19.4% did not give excess to human drinking water, respectively. Interestingly, 59.4% of the participants noticed rodents on grazing land and 60.2% of the participants thought that wild animals prey on rodents. In addition, 59.9% of the participants reported seeing wild animals (foxes) in their locality. Participants believed that these wild animals had easy access to raw offal (38.0%) and that they preyed on livestock (48.6%). The majority of the participants (77.8%) kept livestock. Glaciers were the main source of drinking water for the participants (85.5%) and 16.8% always boiled water before drinking. Use of gloves when handling soil was reported by 24.0% of the participants and the majority (68.0%) reported never eating leafy vegetables without washing them. More details are given in Table 4.
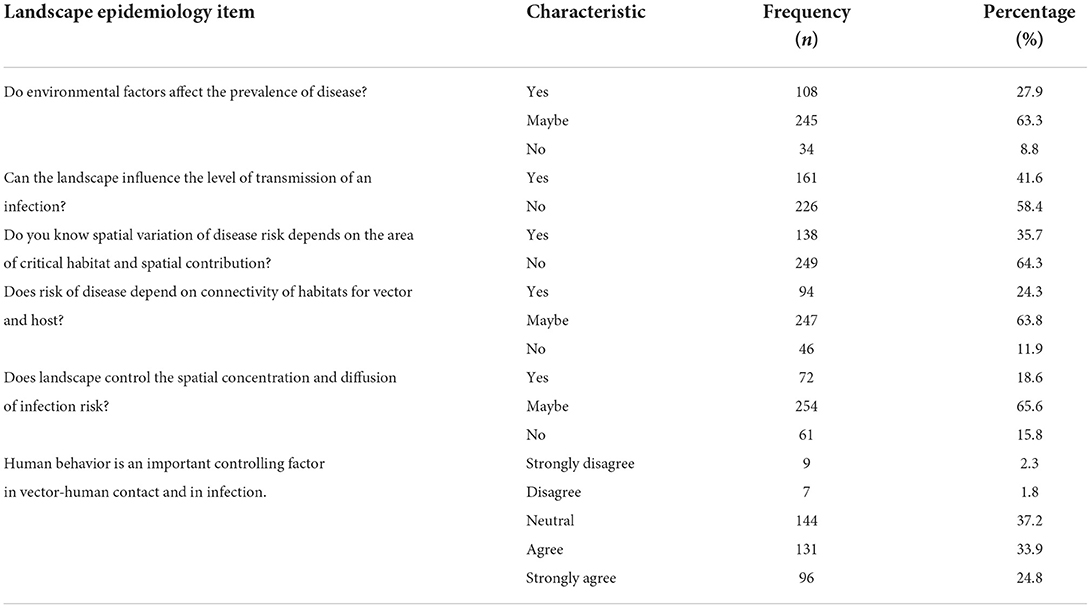
Landscape epidemiology of AE
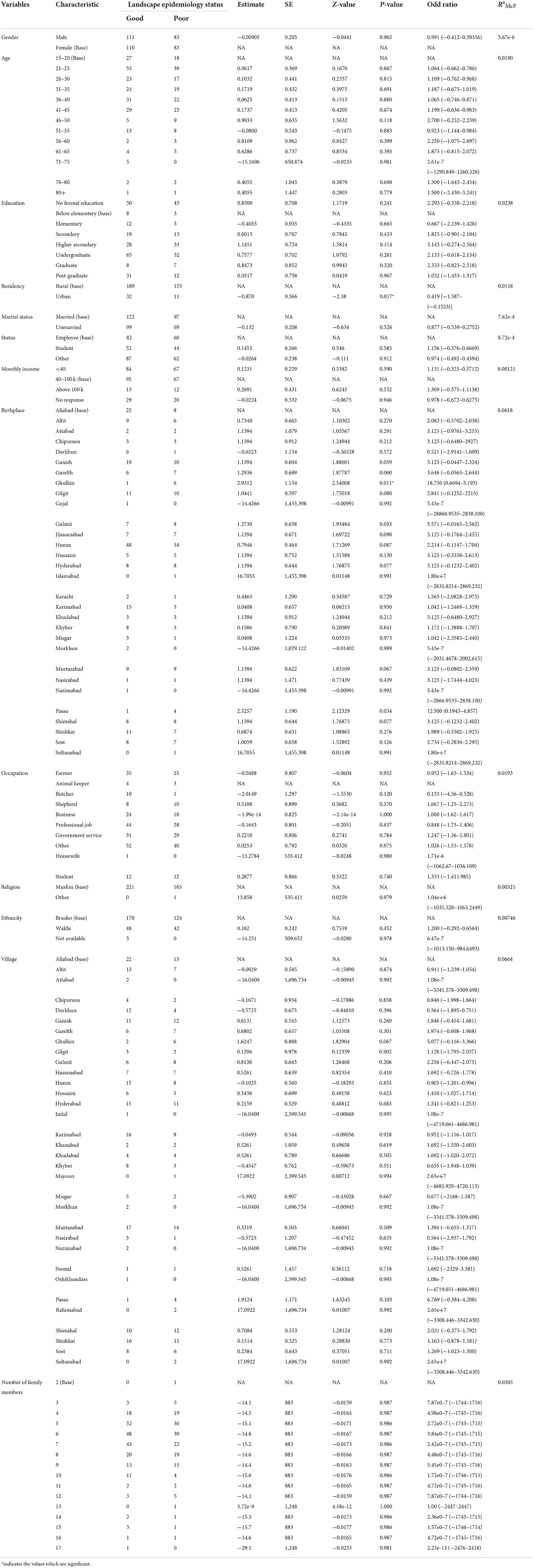
Six KAP questions were about landscape epidemiology of AE. Of the participants, 27.9% knew about the effect of environmental factors on the prevalence of AE. Only 41.6% of the participants knew that landscape influenced the level of transmission of infection. Another 35.7 and 24.3% knew about the spatial variation of the disease and the connectivity of habitat with intermediate and final hosts, respectively. Only 18.6% knew about landscape management, spatial concentration and infection diffusion risk. The details are shown in Table 5.
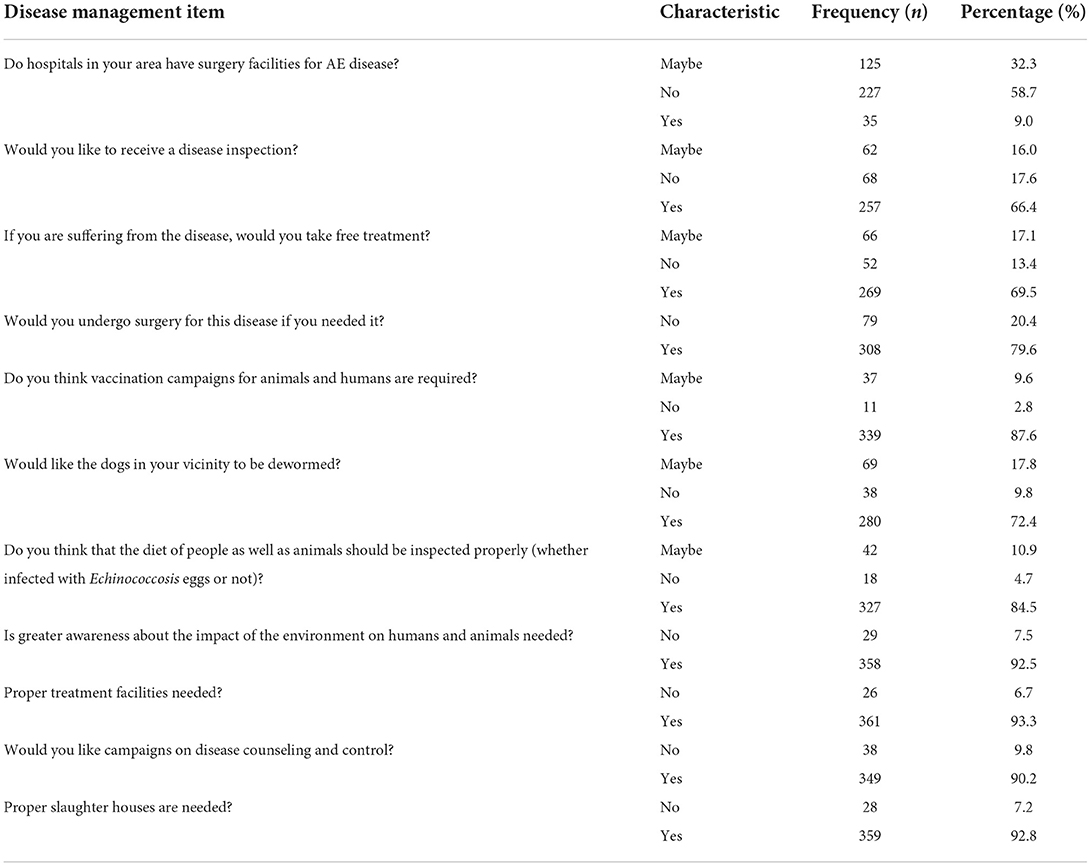
Disease management of AE
Eleven questions in the KAP study were about management of AE. Only 9.0% of the participants reported that hospitals in their area had surgical facilities to treat the disease. Most of the participants indicated they would like to be screened for disease (66.4%) and receive free treatment (69.5%). The majority (87.6%) thought that vaccination campaigns for animals and humans are required and (72.4%) of participants indicated they would like all dogs in the community to be dewormed. A large number of the participants (84.5%) indicated that they would like a proper diet inspection (whether food they are eating is at risk of contamination with E. multilocularis eggs) of both humans and animals. According to 92.5% of the participants, awareness about the disease is needed and the majority (93.3%) also thought that proper veterinary and sanitation facilities were required in their areas. Details are shown in Table 6.
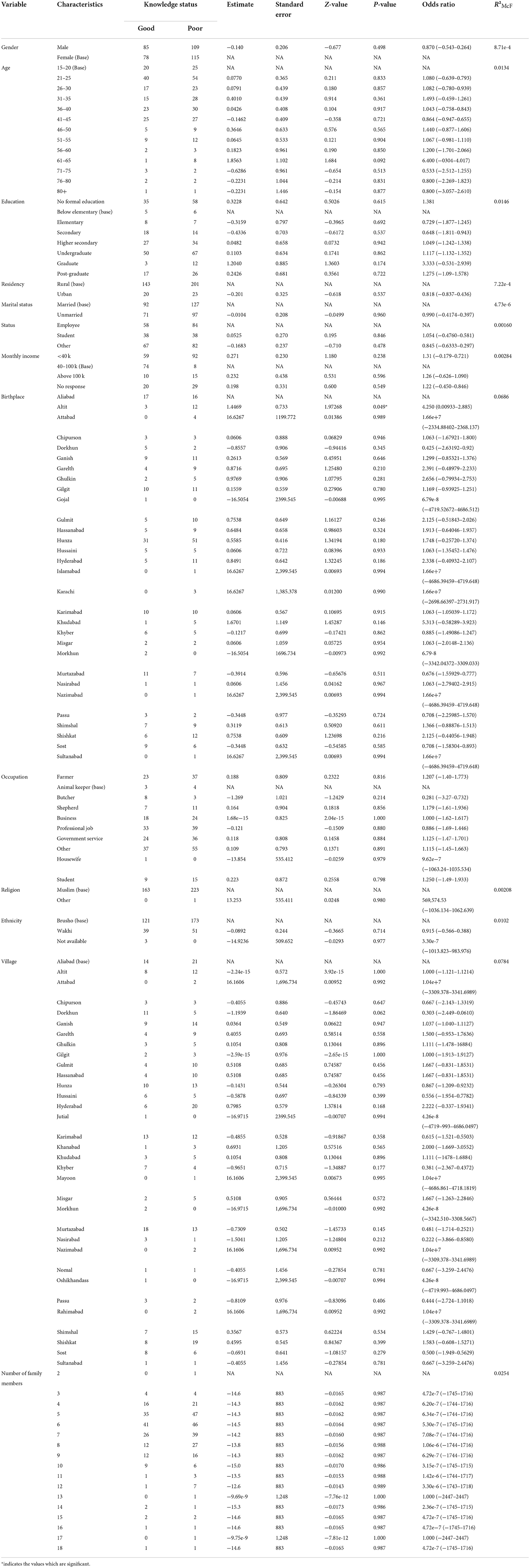
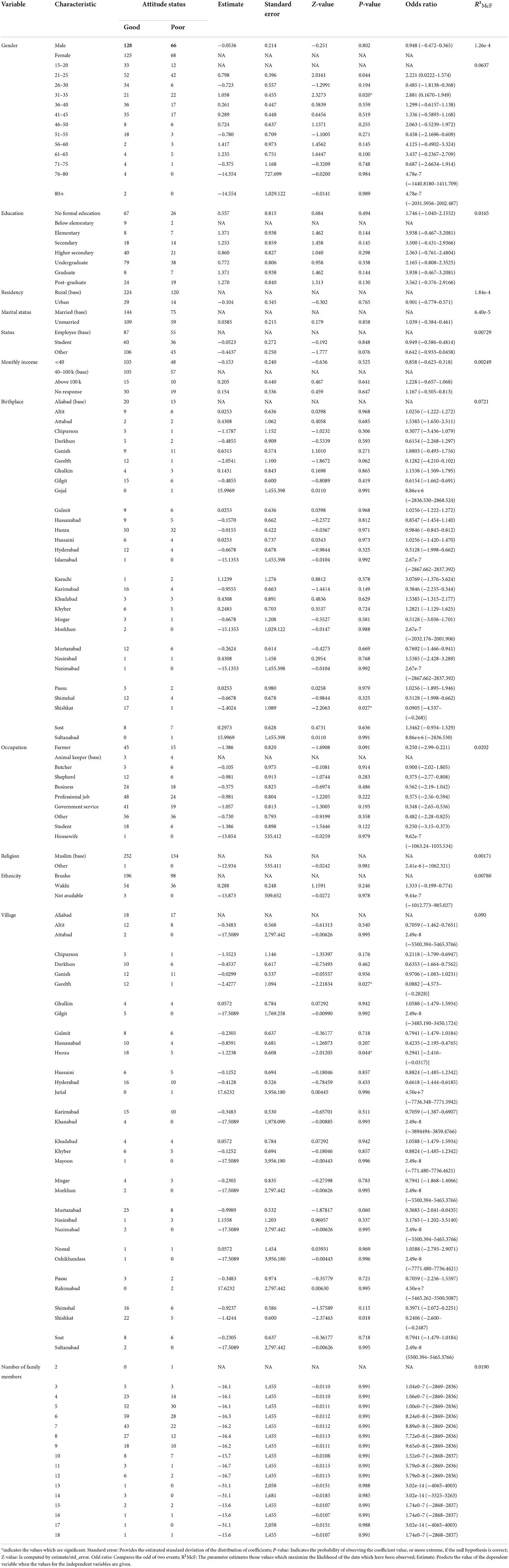
Statistical analysis of the relationship between KAPs, landscape epidemiology and disease management and the population sociodemographic characteristics
Using logistic regression, we assessed the association between the independent sociodemographic factors of age, gender, religion, ethnicity, education, marital status, occupation and income and the dependent factors of KAPs, landscape epidemiology and AE disease management, to determine the factors most associated with this disease.
The analysis results indicated that the study variables (KAPs, landscape epidemiology and AE disease management) had a moderate dependency on the defined sociodemographic aspects (age, gender, religion, ethnicity, education, marital status, occupation and income) of the study (Tables 7–11).
Discussion
AE is a zoonotic parasitic disease caused by E. multilocularis in humans and rodents. AE substantially endangers people's health and safety, and has an impact on social and economic development (17). Pakistan is a developing country of low socio-economic standing. The large majority of Pakistanis are involved in agriculture and small-scale dairy farming. Workers on these often small farms have direct contact with animals and, because health and hygiene principles are not adequately observed, residents of these places are at high risk of AE (18).
Sociodemographic factors
Age contributed to the overall models that determined knowledge and perceptions of AE. Elevated contaminations in the age range 20–59 years is most likely attributable to occupation and close interaction with canines and the environment (19). A comparable study in Jordan found that children had the highest risk of infection. Furthermore, Kyrgyzstan has its highest prevalence among children aged 16 years (20). According to a study conducted in 2004, farmers were at a significant risk of infection due to the exposure and contact with contaminated objects, which is consistent with other research that found that farming was responsible for 65% of cases (21). The other risk factors posed infection includes forestry, hunting, and simply living in a disease cluster or rural location (21, 22). According to a survey performed in east Gansu, China, females had a considerably higher prevalence of AE than males, which was consistent with findings in other endemic locations (23).
According to a study conducted in China's Tibet Autonomous Region, illiterate middle-aged and older women were more likely to have AE than the general population. Middle-aged and older women are designated vulnerable populations as they are more likely to come into contact with diseased pets and contaminated environments. Women in this community are more likely to perform housework, which includes tasks such as collecting and burning yak dung (the primary fuel in pastoral areas) and feeding dogs. In addition, young and middle-aged males in vulnerable communities may be predisposed to echinococcosis because of inappropriate behavior especially regarding poor habit of handwashing, poor knowledge and occupations like herdsmen and farmer which increase the risk of exposure to Echinococcus eggs (23, 24).
Sociodemographic factors such as gender, age, marital status, birthplace, occupation, residency, religion and income are important in determining the association of disease with KAPs.
Knowledge about AE
Our results indicated that knowledge about AE was poor in Hunza. The majority of the 387 respondents had never heard of any parasitic diseases. Knowledge about the cycle of transmission and infection was low among the participants, who lacked knowledge about the disease hosts. Knowledge about AE is important to effective prevention and control strategies and the participants were evidently unaware of the factors responsible for the prevalence and development of infection in either humans or animals.
Echinococcosis transmission has been demonstrated as strongly related to educational level, human behavior, lifestyle, living habits, and environmental factors (17). Health education plays a critical role in lowering echinococcosis transmission to humans (25). To prevent and control echinococcosis, raising awareness about lifestyle and living habits that increase susceptibility to echinococcosis in the general population is necessary. Residents need guidance to develop positive attitudes toward echinococcosis prevention and control, as well as to modify habits that increase risk of infection. Notably, echinococcosis is frequently endemic in underdeveloped or resource-poor regions where education is weak and illiteracy is rampant (26, 27).
A study from China likewise indicated that education level influenced views and practices regarding AE. Study data revealed that as education levels increased, the risk of echinococcosis is decreased. The study also found that just a tiny percentage of the interviewed locals were well versed in all aspects of echinococcosis. Many people were unaware of the routes of echinococcosis infection in humans or canines. The paucity of knowledge about echinococcosis infection routes resulted in a lack of knowledge about disease prevention. Changes in practice are heavily influenced by knowledge and attitudes. However, there is need of awareness to improve echinococcosis-related knowledge and change population attitudes in Pakistan (17, 23).
Attitudes toward AE
Perception about a disease can influence its epidemiology. If people perceive themselves at risk of acquiring the disease, they are more likely to protect themselves from infection with the disease and vice versa. The majority of respondents in our study did not believe themselves at risk of infection with E. multilocularis. However, they expressed a positive attitude toward preventing infection.
A study by Ahmed (28) that explored the relationship between dog populations and prevalence of echinococcal disease found that humans who come into contact with dogs are at least twice as likely to acquire an echinococcal infection than those who do not have contact with dogs. This is because infection is more likely in a polluted environment containing infected dog feces, which contain parasite eggs. In the same study, Khartoum city had the highest rate of echinococcosis seropositivity among humans (14.8%), most likely owing to the presence of a large dog population in the area. According to another study, AE is common among hunters, trappers, and others who work with fox fur. Hyperendemic foci have been seen in certain Inuit towns in the North American tundra, and in western China, where native dogs eat infected commensal rodents daily (29).
Another study reported that the Qinghai-Tibet Plateau has a vast landscape with diverse populations of wild canids, rats, and other wild animals, and, because the majority of the local people are herders, the natural AE transmission cycle is widespread. This region has many domestic and stray dogs, which can be considered a major source of infection as terminal hosts (30). These ecological factors could be key determinants of AE (31). Educating local people and primary-level health workers about AE will assist in promoting practices that prevent AE in humans.
Practices associated with AE
Several practices were associated with AE in our study. In the surveyed areas where stray dogs and wild canids were present thus risk of developing AE might be high. Of the total participants, 10.8% owned dogs. Most of the participants indicated the presence of rodents on grazing land. Rodents are intermediate hosts of E. multilocularis and stray and wild animals prey on these rodents, initiating the cycle of infection. Source drinking water was from glaciers. Participants indicated positively that they practiced self-hygiene such as washing hands, using gloves while handling soil and washing vegetables before eating.
Tibetan people keep large numbers of dogs, which are mostly employed to guard property and animals. According to one of previous research, 82.4% of the population of Tibet had dogs, with 21% owning three or more. Because it is against Buddhist doctrine to kill animals, including dogs, vast numbers of stray dogs cluster around temples and townships, where they are fed by monks and herders. Dogs are also predators of small mammals on nearby pastures. A necropsy of the intestines of stray dogs in this location revealed a prevalence of E. granulosus of 29.5% and E. multilocularis of 11.5% (31, 32).
Because of their frequent interaction with humans and contamination of soil around residences and in gardens, pet animals may constitute a risk for AE. We reported a link between dog ownership and AE, as well as a weaker but still significant link between having cats that wander freely outside or consume mice as shown in association table. “Dogs that killed game,” a rare action by individual dogs, was the factor in our survey that had the strongest link to the disease. As a result, the attributable risk was lower than for the other dog-related variables. Several other studies have found that dogs and cats are significant risk factors for AE, although results have been mixed (33, 34). The number of dogs owned over time and the degree of dog contact were the most relevant risk factors in a Chinese study of the Gansu-Han population with over 2,500 participants, including 86 individuals with AE (12).
The only two food consumption factors linked to AE were chewing grass and consuming unclean strawberries, in one study. Ingestion of eggs from infected plant parts or soil-stained hands also increased risk. Other garden food, as well as mushrooms from fields and meadows, were only eaten uncooked and unwashed on rare occasions (33).
Landscape epidemiology of AE
According to recent estimates, AE is subject to global spread and is an emerging and re-emerging problem in many areas. AE endemicity varies geographically, and it may be influenced by global environmental change over time. Landscape epidemiology provides a unique opportunity to assess and predict infection risk in several spatial and temporal dimensions (34). Environmental factors that support fluctuation in host population densities are likely to influence the risk of human infection (34, 35).
The participants in our study had poor knowledge of landscape epidemiology and its impact on the prevalence of infection in the area. They had poor knowledge of the environmental factors that affect AE prevalence and how environmental factors influence the level of transmission of infection and disease risk depending on critical habitat and spatial contribution. The majority of the participants were unaware of AE's dependency on the connectivity of vector, host and human behavior as an important controlling factor of vector-human contact and infection.
With respect to the transmission ecology of E. multilocularis and the epidemiology of human AE, the function of the physical environment, particularly landscape characteristics, is becoming increasingly important. In China, landscape ecology methodologies for assessing human AE risk have been examined (12, 17, 36). A study conducted to understand landscape epidemiology in southern Ningxia, China reported that in Xiji, possible E. multilocularis intermediate hosts could be found in every habitat. High densities of preferred susceptible prey provide ideal conditions for E. multilocularis transmission (36). Understanding the landscape epidemiology aspects of AE may also provide scientific information to enhance environmental policymaking and landscape planning procedures in echinococcosis-endemic locations. As a result, landscape epidemiology may be effective in promoting environmentally based interventions with minimum influence on Echinococcus spp. transmission dynamics. This is especially important in areas where climate change and landscape changes may be encouraging parasite transmission (35).
Disease management of AE
Human AE control and prevention methods can be implemented at many levels. Individually, hygiene-related measures and periodic deworming of domestic dogs are essential methods for reducing exposure to infective parasite eggs. On an environmental level, efforts to limit contamination with infective E. multilocularis eggs are aimed at either direct parasite management through deworming definitive hosts or wildlife host population suppression. In the past, population control tactics for the fox as a definitive host, such as hunting, trapping, and culling, have been advocated. When considering interventions in host populations, however, ecological changes and their impact on host populations should be considered (24). By deworming red foxes based on regular baiting campaigns, Japan and France have demonstrated the feasibility of lowering the infection pressure with E. multilocularis eggs.
In our study, the majority of respondents had a positive attitude toward receiving screening, free treatment and, if needed, surgery for the disease. Respondents had good knowledge about the importance of vaccination campaigns for humans and animals and the deworming of dogs. The population overall gave a positive response and indicated good knowledge about the importance of awareness and proper treatment in prevention of AE.
A previous study reported that different risk factors plays an important role toward the transmission of AE. The treatment of foxes with praziquantel-impregnated baits can disrupt the wildlife cycle (19). However, the feasibility of implementing such controls throughout wide swathes of AE-prevalent areas, such as the Tibetan plateau, is debatable. Although dog interaction has been identified as a risk factor for transmission to humans (12) and dogs are particularly vulnerable to infection with this parasite (37), periodic treatment of dogs will not disrupt the transmission cycle and will have considerably less impact on long-term transmission rates to humans (38, 39). Other studies suggest that improved control of food or water supplies that may be contaminated with parasite eggs is another way to reduce disease burden. The attributable percentage of disease burden owing to these transmission pathways, as well as the cost-efficacy of such intervention techniques, would determine such control (19). If populations in which the prevalence of AE is increasing take preventive measures such as self-hygiene, proper inspection and treatment for disease, food inspection and deworming of the wild animals around them, the prevalence of disease and transmission of infection from animals to humans may be more adequately controlled.
Conclusion
Our study indicated that awareness of AE was generally very low in the Hunza population of Pakistan. We found that the residents of Hunza did not know about AE, the threats associated with it, the life cycle of parasite E. multilocularis, its control or disease management. Many practices and factors were observed that could predispose the population to infection by AE. Wild animals were common in most villages. These factors favor the exposure of the population to E. multilocularis.
Despite low awareness of AE, positive attitudes toward the treatment of the disease were observed and the study population responded positively to questions that were important to controlling the disease. Interrupting the parasite life cycle and establishing and maintaining a proper surveillance system is crucial for the prevention and control of this disease. Creating awareness among residents of Hunza and other parts of Pakistan where the same risk factors are observed is necessary. As this disease is becoming more prevalent in Pakistan, improved awareness among the population will prevent future health problems. A proper surveillance system and further research on this zoonotic disease in humans are recommended.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of COMSATS University Islamabad (CUI), Islamabad under no. ERB/18/72. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
NJ collected the data and authored the paper following discussions with HA and JC. HA designed the study methodology. MSA, NJ, MA, SS, and JC helped in statistical analysis. MSA, SS, NJ, MA, CY, and JC revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81971969 and 81772225 to JC) and the Three-Year Public Health Action Plan (2020–2022) of Shanghai (No. GWV-10.1-XK13 to JC). The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Acknowledgments
We are grateful to all the members of the participants from villages Hunza for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AE, Alveolar echinococcosis; WHO, World Health Organization; P.R. China, People's Republic of China.
References
1. Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, et al. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. (2006) 55:S283–7. doi: 10.1016/j.parint.2005.11.041
2. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. (2004) 17:107–35. doi: 10.1128/CMR.17.1.107-135.2004
3. Palić J, Unterer S, Geisen V, Busch K. Pathology in practice. J Am Vet Med Assoc. (2022) 259:1–4. doi: 10.2460/javma.21.06.0297
4. Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroent. (2012) 18:1425. doi: 10.3748/wjg.v18.i13.1425
5. Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS, World Health Organization. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Organisation for Animal Health (2001).
6. Eckert, J. The “dangerous fox tapeworm” (Echinococcus multilocularis) and alveolar echinococcosis of humans in central Europe. Berl Munch Tierarztl Woch. (1996) 109:202–10.
7. Eckert J, Deplazes P. Alveolar echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol Today. (1999) 15:315–9. doi: 10.1016/S0169-4758(99)01476-3
8. Craig P, Pawlowski Z. Proceedings of the NATO Advanced Research Workshop on cestode zoonoses: echinococcosis and cysticercosis: an emergent and global problem, Poznan, Poland, 10-13 September 2000. In: Proceedings of the NATO Advanced Research Workshop on Cestode Zoonoses: Echinococcosis and Cysticercosis: An Emergent and Global Problem. Poznan (2002).
9. Slettbakk T, Karlsen E. Svalbard og tidligere funn av Echinococcus multilocularis pa mus. Orientiering til pressen, 15 October, Statens Dyrehelsetilsyn, Fylkesveterinaeren for Troms og Finmark, Harstad (1999).
12. Craig PS, Giraudoux P, Shi D, Bartholomot B, Barnish G, Delattre P, et al. An epidemiological and ecological study of human alveolar echinococcosis transmission in south Gansu, China. Acta Tropica. (2000)77:167–77. doi: 10.1016/S0001-706X(00)00134-0
13. Wang X, Dai G, Li M, Jia W, Guo Z, Lu, J. Prevalence of human alveolar echinococcosis in China: a systematic review and meta-analysis. BMC Public Health. (2020) 20:1–11. doi: 10.1186/s12889-020-08989-8
14. Kaufmann, Andreas, Auntof6, & Cerebellum. (2014). Index @ En.Wikipedia.Org. p. 5. Available online at: http://en.wikipedia.org/w/index.php?oldid=617123065 (accessed August 22, 2022).
15. Internet access: Arcgis. (2021). arcgis.com. Available online at: https://www.arcgis.com/apps/View/index.html?appid=06cb04a8960f411187a62e12e3aa1fee. (accessed August 22, 2022).
16. khush funer murtaza,. (2012). women empowerment through higher education in gilgit baltistan. p. 26. Available online at: https://ecommons.aku.edu/cgi/viewcontent.cgi?article=1027&context=pakistan_ied_pdcn#:~:text=Theliteracy rate in Gilgit-Baltistan is 37.8%25. (accessed August 22, 2022).
17. Ahmed H, Ali S, Afzal MS, Khan AA, Raza H, Shah ZH, et al. Why more research needs to be done on echinococcosis in Pakistan. Inf Dis Poverty. (2017) 6:1–5. doi: 10.1186/s40249-017-0309-z
18. Darani HY, Avijgan M, Karimi K, Manouchehri K, Masood J. Seroepidemiology of hydatid cyst in Chaharmahal va Bakhtiari Province, Iran. Irn JPublic Health, (2003) 32:31–3.
19. Torgerson PR. The use of mathematical models to simulate control options for echinococcosis. Acta Tropica. (2003) 85:211–21. doi: 10.1016/S0001-706X(02)00227-9
20. Kern P, Ammon A, Kron M, Sinn G, Sander S, Petersen LR, et al. Risk factors for alveolar echinococcosis in humans. Emerg Inf Dis. (2004) 10:2088. doi: 10.3201/eid1012.030773
21. Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, et al. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. JHepatol. (2011) 55:1025–33. doi: 10.1016/j.jhep.2011.02.018
22. Sen-Hai Y, Hu W, Xian-Hong W, Xiao M, Pei-Yun L, Yu-Fang L, et al. Cystic and alveolar echinococcosis: an epidemiological survey in a Tibetan population in southeast Qinghai, China. JapanJInfect Dis.(2008) 61:242. doi: 10.1017/S0022149X15000656
23. Yin J, Gongsang Q, Wang L, Li C, Wu X. Identification of vulnerable populations and knowledge, attitude, and practice analysis of echinococcosis in Tibet Autonomous Region of China. Environ Res. (2020) 190:110061. doi: 10.1016/j.envres.2020.110061
24. Craig PS, Hegglin D, Lightowlers MW, Torgerson PR, Wang Q. Echinococcosis: control and prevention. Adv Parasitol. (2017) 96:55–158. doi: 10.1016/bs.apar.2016.09.002
25. Ito A, Urbani C, Jiamin Q, Vuitton DA, Dongchuan Q, Heath DD, et al. Control of echinococcosis and cysticercosis: a public health challenge to international cooperation in China. Acta Tropica. (2003) 86:3–17. doi: 10.1016/S0001-706X(02)00269-3
26. Ahmed ME, Abdalla SS, Adam IA, Grobusch MP, Aradaib IE. Prevalence of cystic echinococcosis and associated risk factors among humans in Khartoum State, Central Sudan. Int Health. (2021) 13:327–33. doi: 10.1093/inthealth/ihaa059
27. Moro P, Schantz PM. Echinococcosis: a review. Int JInf Dis. (2009) 13:125–33. doi: 10.1016/j.ijid.2008.03.037
28. Vaniscotte A, Raoul F, Poulle ML, Romig T, Dinkel A, Takahashi K, et al. Role of dog behaviour and environmental fecal contamination in transmission of Echinococcus multilocularis in Tibetan communities. Parasitology. (2011) 138:1316–29. doi: 10.1017/S0031182011000874
29. Zheng C, Xue C, Han S, Li Z, Wang H, Wang L, et al. National alveolar echinococcosis distribution—China, 2012–2016. China CDC Weekly. (2020) 2:1–7. doi: 10.46234/ccdcw2020.001
30. Qiu JM, Qiu DC, Luo CX, Zhu YB, Chen XW. Survey on infective agent of alveolar echinococcosis in Ganzi Prefecture and experimental research in animal. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. (1989) 5:38–40.
31. Qiu JM. Epidemiological study on alveolar hydatid disease in Qinghai-Xizang plateau. J Pract Parasit Dis. (1995) 3:106–9.
32. Stehr-Green JK, Stehr-Green PA, Schantz PM, Wilson JF, Lanier A. Risk factors for infection with Echinococcus multilocularis in Alaska. Am J Trop Med Hyg. (1988) 38:380–5. doi: 10.4269/ajtmh.1988.38.380
33. Kreidl P, Allerberger F, Judmaier G, Auer H, Aspöck H, Hall AJ. Domestic pets as risk factors for alveolar hydatid disease in Austria. AmJ Epid. (1998) 147:978–81. doi: 10.1093/oxfordjournals.aje.a009388
34. Restrepo AMC, Yang YR, McManus DP, Gray DJ, Giraudoux P, Barnes T, et al. The landscape epidemiology of echinococcoses. Inf Dis o Poverty. (2016) 5:1–13. doi: 10.1186/s40249-016-0109-x
35. Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Inf Dis. (2007) 13:878. doi: 10.3201/eid1306.061074
36. Giraudoux P, Craig PS, Delattre P, Bao G, Bartholomot B, Harraga S, et al. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology. (2003) 127:S121–31. doi: 10.1017/S0031182003003512
37. No Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. (2006) 36:79–86. doi: 10.1016/j.ijpara.2005.08.012
38. Budke CM, Casulli A, Kern P, Vuitton DA. Cystic and alveolar echinococcosis: Successes and continuing challenges. PLoS Neglected Trop Dis. (2017) 11:e0005477. doi: 10.1371/journal.pntd.0005477
Keywords: alveolar echinococcosis, awareness, risk factors, disease management, Hunza, Pakistan
Citation: Jamill N, Ahmed H, Afzal MS, Simsek S, Ali A, Arshad M, Yu C and Cao J (2022) Assessment of risk, landscape epidemiology and management strategies to combat alveolar echinococcosis in the rural communities of Hunza, Pakistan. Front. Public Health 10:1015475. doi: 10.3389/fpubh.2022.1015475
Received: 09 August 2022; Accepted: 31 October 2022;
Published: 21 November 2022.
Edited by:
Alessandro Cassini, Direction générale de la santé, SwitzerlandCopyright © 2022 Jamill, Ahmed, Afzal, Simsek, Ali, Arshad, Yu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haroon Ahmed, aGFyb29uYWhtYWQxMkB5YWhvby5jb20=; Jianping Cao, Y2FvanBAY2hpbmFjZGMuY24=
 Naila Jamill
Naila Jamill Haroon Ahmed
Haroon Ahmed Muhammad Sohail Afzal
Muhammad Sohail Afzal Sami Simsek
Sami Simsek Abid Ali
Abid Ali Muhammad Arshad2
Muhammad Arshad2 Jianping Cao
Jianping Cao